Abstract
Understanding how memory breaks down in the earliest stages of the Alzheimer’s disease (AD) process has significant implications, both clinically and with respect to intervention development. Previous work has highlighted a robust picture superiority effect in patients with amnestic mild cognitive impairment (aMCI). However, it remains unclear as to how pictures improve memory compared to words in this patient population. In the current study, we utilized receiver operating characteristic (ROC) curves to obtain estimates of familiarity and recollection for pictures and words in patients with aMCI and healthy older controls. Analysis of accuracy shows that even when performance is matched between pictures and words in the healthy control group, patients with aMCI continue to show a significant picture superiority effect. The results of the ROC analysis showed that patients demonstrated significantly impaired recollection and familiarity for words compared controls. In contrast, patients with aMCI demonstrated impaired recollection, but intact familiarity for pictures, compared to controls. Based on previous work from our lab, we speculate that patients can utilize the rich conceptual information provided by pictures to enhance familiarity, and perceptual information may allow for post-retrieval monitoring or verification of the enhanced sense of familiarity. Alternatively, the combination of enhanced conceptual and perceptual fluency of the test item might drive a stronger or more robust sense of familiarity that can be accurately attributed to a studied item.
Keywords: Alzheimer’s disease, recognition memory, familiarity, picture superiority effect, semantic memory, conceptual fluency
Introduction
In addition to aiding in accurate and early diagnoses, understanding how memory breaks down in AD identifies areas of intact cognition, which could provide the basis of behavioral or pharmacologic intervention. One recent surprising finding in patients with AD and amnestic mild cognitive impairment (aMCI) is a robust picture superiority effect (Ally, Gold, & Budson, 2009a). That is, the difference in accuracy between pictures and words is larger in patients than in controls, even in the earliest stages of the disease. MCI is thought to represent a transitional stage between healthy aging and dementia, and the amnestic subtype has been associated with an estimated tenfold increase in yearly conversion rate to AD compared to age-matched controls with no cognitive impairment (Petersen, 2004). Studying this population can likely give us insight into memory deficits at the earliest clinical manifestation of the disease course. Although the scientific and medical community has evaluated patients with MCI for more than a decade, a full understanding of how memory is impaired in this patient group is yet to be realized. The overarching aim of the current investigation is to determine whether the robust picture superiority effect in patients with aMCI is due to intact familiarity for pictures but impaired familiarity for words compared to healthy older adults with no cognitive impairment.
Dual-process models of recognition memory contend that two separable processes support accurate recognition decisions: recollection and familiarity (see Yonelinas, 2002 for review). Recollection refers to the retrieval of information about a previous event or item, bound to the context in which it was previously experienced (e.g. source, time, spatial location, etc). In contrast, familiarity is often described as a relatively fast and automatic process in which a vague general sense of prior encounter occurs but is void of any contextual information. This distinction can be easily described with the common experience of seeing an acquaintance outside of a traditional setting and experiencing a feeling of familiarity, but being unable to recollect the context in which the person was previously encountered (“I recognize him, but who is he? Where do I know him from?”). However, after a moment the contextual information regarding the person’s identity may become clear (“Oh, it is John from the gym!”). Familiarity is the initial feeling of knowing without being able to identify the contextual details regarding a previous encounter, whereas the subsequent realization of contextual information represents recollection.
Studies of the picture superiority effect have found that pictures enhance recollection compared to words in healthy young (Ally & Budson, 2007; Curran & Doyle, 2011; Rajaram, 1996) and older adults (Ally et al., 2008). However, behavioral and event-related potential (ERP) work with patients with aMCI has revealed significantly impaired recollection (Ally, Gold, & Budson, 2009b; Ally, McKeever et al., 2009; Anderson et al., 2008; Westerberg et al., 2006; Wolk et al., 2008). Therefore, under the assumption of the dual-process model, we originally hypothesized that patients must be able to rely on intact familiarity to support such a robust picture superiority effect. However, investigations into familiarity in patients with aMCI have been mixed, with a number showing impaired familiarity (Algarabel et al., 2009; Ally, Gold, & Budson, 2009b; Anderson et al., 2008; Hudon, Belleville, & Gauthier, 2009; Westerberg et al., 2006; Wolk et al., 2008). An important dissociation supporting our original hypothesis is that in general, studies reporting intact familiarity used picture stimuli (Anderson et al., 2008; Hudon, Belleville, & Gauthier, 2009; Westerberg et al., 2006), whereas investigations reporting impaired familiarity used verbal stimuli (Algarabel et al., 2009; Ally, Gold, & Budson, 2009b; Wolk et al., 2008). To follow-up on this hypothesis, Ally, McKeever, et al. (2009) used ERPs to investigate recollection and familiarity for pictures and words in patients with aMCI. The results for pictures revealed an intact early frontal ERP old/new effect, which is believed to be the putative correlate of familiarity (see Rugg & Curran, 2007 for review), compared to healthy older controls. In contrast, the early frontal effect was degraded for words in patients with aMCI. A substantive limitation to Ally, McKeever et al. (2009), however, was that there was no behavioral measure or estimate of recollection and familiarity. Further, recent work has challenged the notion that the early frontal ERP effect reflects familiarity, and has suggested that perhaps this effect indexes conceptual fluency (Paller, Voss, & Boehm, 2007). To fully understand whether familiarity remains intact for pictures but is degraded for words in patients with aMCI – subsequently driving the picture superiority effect in this group – the current investigation set out to obtain estimates of recollection and familiarity for pictures and words in patients and matched controls.
A variety of behavioral methodologies have been developed in an effort to measure recollection and familiarity (for a review, see Yonelinas, 2002). Two general categories of measurement techniques are task dissociation methods (e.g., response-speed method, recall/recognition method, item/associative recognition method) and process estimation methods (e.g., process dissociation method, remember/know method, receiver operating characteristic curves). Task dissociation methods typically compare performance on two tasks, at least one of which is thought to isolate either familiarity or recollection. Comparison of performance on these two tasks is used to infer how the experimental variable affects recollection and/or familiarity. While task dissociation paradigms are valuable for depicting relative reductions in performance in recollection and familiarity, they have been criticized for being unable to supply quantitative measures of these processes (Yonelinas, 2002). Alternatively, process-estimation methods utilize model equations in conjunction with performance in order to provide quantitative estimates of the contribution of recollection and familiarity (Yonelinas, 2002).
Receiver Operating Characteristics (ROC) are a process estimation method that have been utilized in research for over 50 years to estimate measurements of recognition memory (see Yonelinas & Parks, 2007 for review). Various models have been proposed to describe recognition ROCs, however most models fall into one of three general categories: threshold models, signal detection models, and hybrid models. The most widely accepted method, and the method being used in the current investigation, is a hybrid model called the dual process signal detection model (Yonelinas, 1994). It should be noted that, although other potentially feasible models have also been developed to describe ROC curves (e.g. DeCarlo, 2002; Ratcliff, Sheu, & Gronlund, 1992; Rotello, Macmillan, & Reeder, 2004; Yonelinas & Parks, 2007), these models do not provide the quantitative estimates of recollection and familiarity that are necessary for the primary aim of the current study. Further, the Yonelinas High Threshold model (YHT) is the most widely regarded method for estimating recollection and familiarity, particularly in patient populations (Ally, Gold, & Budson, 2009b; Healy, Light, & Chung, 2005; Howard, Bessette-Symons, Zhang, & Hoyer, 2006). A possible concern using ROC methodology in patients with aMCI may be the ability of these patients to assess confidence for memory decisions. However, research investigating the ability to retrieve and monitor stored general knowledge in patients with aMCI and very mild AD has shown that these patients can successfully make confidence ratings regarding the certainty of their answers (Ally, Gold, & Budson, 2009b; Dodson et al., 2011).
The current study set out to utilize ROC curves based on confidence judgments to determine whether the robust picture superiority effect in patients with aMCI is due to differentially affected familiarity for pictures and words. To accomplish this, accuracy was equated for pictures and words in a healthy older adult control group, and estimates of recollection and familiarity were derived using the YHT model. Equating performance for our control group allowed us to assess whether patients with aMCI continue to show better memory for pictures over words, and whether this enhancement for pictures was attributable to relatively intact familiarity. We predicted that estimates of recollection for patients with aMCI would be impaired for both stimulus types compared to healthy controls. In contrast, if differentially affected familiarity were contributing to the picture superiority effect in patients with aMCI, we would expect to see relatively intact estimates of familiarity for pictures but diminished estimates of familiarity for words compared to controls.
Methods
Participants
Sixteen patients with a diagnosis of probable aMCI (10 males and 6 females) and sixteen healthy controls (8 males and 8 females) participated in this experiment. Patients with aMCI were recruited from the Boston University Alzheimer’s Disease Center in Boston, Massachusetts. Healthy controls were recruited from spouses, other family members, and friends of patients as well as through IRB-approved community recruitment posters and flyers. A diagnosis of probable single or multi-domain amnestic MCI was determined using criteria proposed by Peterson (2004), which requires the presence of a subjective memory complaint, an objective memory impairment, intact functional activities, and an absence of dementia. Objective memory impairment was established by performance greater than 1.5 standard deviations below the healthy control group mean on either recall or recognition of the CERAD Word List Memory Test (Morris, et al., 1989), and an absence of functional impairment was corroborated by a companion. To help control for functional impairment, aMCI subjects were excluded if their MMSE score was below 26. Healthy controls were excluded if they scored below 28 on the MMSE, or more than 1.5 standard deviations below the healthy control group mean on any measure of standard neuropsychological functioning. Of the 16 aMCI patients, five were single domain while 11 were multi-domain. Of the multi-domain patients, six had impaired verbal fluency, 11 had impaired confrontation naming, and one had impaired executive functioning (as determined by performance greater than 1.5 standard deviations below the healthy control group).
All potential participants were excluded for a history of stroke or other focal brain injury, a significant psychiatric history, extensive alcohol or drug use, cerebrovascular disease, or if English was not their primary language. Each participant received compensation in the form of $10 per hour. Approval from the human subjects committees of Edith Nourse Rogers Memorial Veterans Hospital and the Veterans Affairs Boston Healthcare System was obtained, and written informed consent was acquired from each participant.
Procedure
The testing procedures took place in a single session lasting approximately 120 minutes and included a brief neuropsychological battery as well as the experimental recognition tests for pictures and words. The battery of standardized neuropsychological tests including the Mini Mental State Examination (Folstein, Folstein, & McHugh, 1975), the CERAD word list memory test (Morris, et al., 1989), Trail Making Tests A and B (Adjutant General’s Office, 1944), Verbal Fluency (Monsch, et al., 1992), and the Boston Naming Test – 15-item (Mack, Freed, Williams, & Henderson, 1992). Table 1 presents demographic and neuropsychological data for both groups.
Table 1.
Demographic and Neuropsychological data for all participants.
| Healthy Controls
|
aMCI
|
|||
|---|---|---|---|---|
| M | SD | M | SD | |
| Age | 74.1 | 8.1 | 77.3 | 7.3 |
| Years of Education | 15.9 | 2.7 | 16.4 | 3.1 |
| Males: Females | 8:8 | 10:6 | ||
| MMSE | 29.6 | 0.6 | 28.2 ** | 1.2 |
| CERAD | ||||
| Immediate Recall | 22.0 | 3.7 | 16.4 ** | 3.1 |
| Delayed Recall | 7.6 | 1.3 | 3.3 ** | 1.4 |
| Recognition | 9.9 | 0.3 | 7.9 ** | 1.4 |
| Phonemic Fluency | 49.5 | 12.0 | 40.3 * | 12.6 |
| Semantic Fluency | 50.4 | 12.7 | 38.1 ** | 9.5 |
| Trails A | 32.9 | 11.6 | 39.1 | 16.3 |
| Trails B | 85.1 | 41.8 | 89.1 | 36.7 |
| Boston Naming Test | 14.8 | 0.3 | 13.2 ** | 2.2 |
Note: Significant differences from the control group are indicated by
<0.05, and
<0.01.
The experiment utilized 940 unique, high-resolution photographs of single-item color pictures or their verbal referents, taken from our previous work investigating recollection and familiarity in healthy and patient populations (Ally & Budson, 2007; Ally et al., 2008; Ally, McKeever et al., 2009). At study and test, these items were displayed on a white background. Words were presented in black Arial font. Stimuli were presented using E-Prime software on a Dell Inspiron laptop computer. The stimuli were randomly divided into four groups of 235 items, where each group of items was presented in random order. The groups were counterbalanced for study status (old or new) and condition (pictures or words), resulting in four counterbalanced versions of the paradigm such that there were no overlapping stimuli across conditions (e.g. if “lamp” was presented at test for words, it was not presented for study or test for pictures).
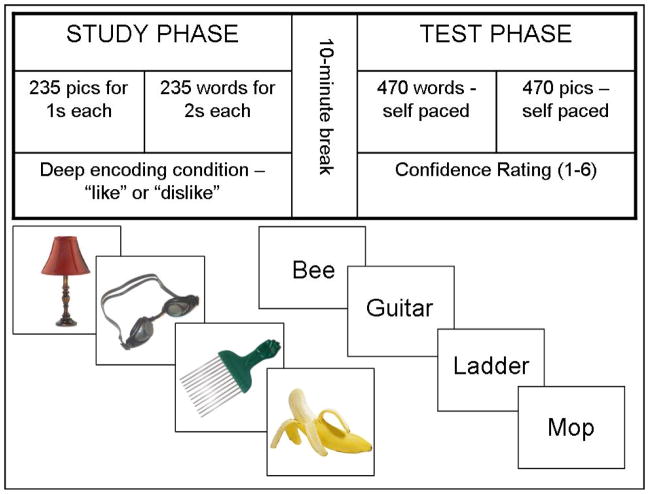
To accurately compare within-group estimates for pictures versus words, we employed different administration methods for each condition, with the goal of matching recognition memory performance for pictures and words in the healthy control group. As such, the experimental paradigm began with the study phase for pictures, followed by the study phase for words, a short (approximately 10 min) break, the test phase for words, and finally the test phase for pictures (Figure 1). This administration order was constant for all participants. Performance in Table 3 shows that we were successful at keeping both patients and controls off of ceiling and floor while keeping list length and stimulus items consistent across conditions. We were also successful in matching recognition memory performance between conditions for the healthy older adults.
Figure 1.
The top panel shows the experimental methods used for all participants. The bottom panel shows example stimuli for the picture and words conditions.
Table 3.
| Healthy Controls | aMCI | |
|---|---|---|
| Pictures | ||
| Hits | 0.81 (.10) | 0.81 (.13) |
| False Alarms | 0.18 (.14) | 0.26 (.15) |
| Pr | 0.63 (.14) | 0.55 (.21) |
| Br | 0.47 (.18) | 0.56 (.17) |
| Words | ||
| Hits | 0.85 (.08) | 0.75* (.15) |
| False Alarms | 0.23 (.19) | 0.37* (.19) |
| Pr | 0.62 (.19) | 0.38** (.15) |
| Br | 0.54 (.21) | 0.58 (.21) |
Pr = accuracy (range = 0 – 1), Br = bias (range 0 – 1; .5 = neutral.
Note: Significant differences from the control group are indicated by
<0.05, and
<0.01.
Prior to beginning the experiment, participants were informed that they would be studying a series of pictures and words then completing a subsequent memory test in which the same items plus an equal number of new items would be presented. During the study phase, participants were shown 235 pictures one at a time for one second each, followed by 235 words for two seconds each. A deep encoding condition was utilized where participants gave a verbal response based on whether they liked or disliked each item the word or picture represented, which the experimenter then entered into the computer.
During the test phase, participants were shown 470 words followed by 470 pictures (235 old and 235 new items for each stimulus type presented in random order). This phase of the paradigm was self-paced in that test stimuli remained displayed on the screen until the participant provided a response. Instructions were given to reply with one of the six confidence ratings: (6) certain the item is old; (5) sort of certain the item is old; (4) not at all certain the item is old; (3) not at all certain the item is new; (2) sort of certain the item is new; (1) certain the item is new. A display with the 6 response choices was provided for reference throughout the testing session. Participants were asked to reply as accurately as possible, but they were also instructed to distribute their answers among all six response choices as is recommended by Yonelinas et al. (1998) in an effort to avoid bimodal responses. Table 2 shows that participants in both groups generally spread out responses evenly among the six choices. Throughout the testing session, response choices were reflected back to the participant (e.g. if the participant responded “6,” the experimenter reflected “so you are certain that you saw this item during the study phase”) to confirm participants’ answers.
Table 2.
| Pictures
|
Words
|
|||
|---|---|---|---|---|
| Confidence Rating | Healthy Controls | aMCI | Healthy Controls | aMCI |
| 1 | 141 | 109 | 104 | 95 |
| 2 | 59 | 61 | 63 | 51 |
| 3 | 37 | 49 | 50 | 62 |
| 4 | 46 | 59 | 49 | 71 |
| 5 | 46 | 90 | 55 | 97 |
| 6 | 142 | 100 | 149 | 94 |
Note: Confidence ratings are as follows: (6) certain the item is old; (5) sort of certain the item is old; (4) not at all certain the item is old; (3) not at all certain the item is new; (2) sort of certain the item is new; (1) certain the item is new.
Results
Demographic and Neuropsychological Data
Demographic and neuropsychological test findings are presented in Table 1. Healthy controls and aMCI patients did not differ in age [t(30) = −1.15, p = 0.26], education [t(30) = −.55, p = 0.59], or gender [χ2 (1, N = 32) = 1.27, p = 0.72]. With regard to neuropsychological test performance, the aMCI group performed significantly worse on the MMSE [t(30) = 3.92, p < 0.01], CERAD immediate recall [t(30) = 4.62, p < 0.01], CERAD delayed recall [t(30) = 9.01, p < 0.01], CERAD recognition [t(30) = 5.25, p < 0.01], letter fluency [t(30) = 2.13, p < 0.05], semantic fluency [t(30) = 3.10, p < 0.01], and Boston Naming Test [t(30) = 2.30, p < 0.01]. Performance on Trail Making Tests A [t(30) = −1.24, p = 0.23] and B [t(30) = −.29, p = 0.78] did not significantly differ between groups.
Yonelinas High Threshold ROC Curves
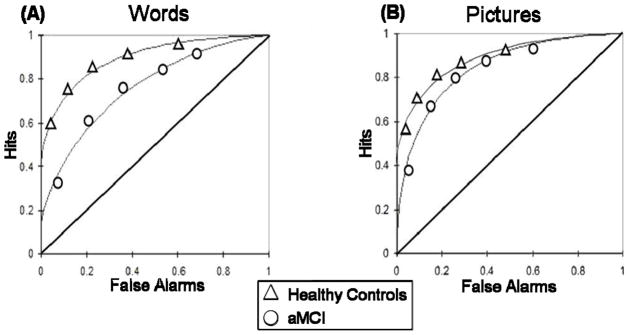
In order to examine the contribution of recollection and familiarity, ROC curves were generated for pictures and words for each participant individually as well as for the aggregate groups of aMCI and healthy controls (Yonelinas, et al., 1998). Hit rates for YHT estimates of recollection and familiarity were calculated for previously studied items endorsed as 6, 5, 4, 3, or 2 and false alarm rates were calculated for previously unstudied items endorsed with a response of 6, 5, 4, 3, or 2. Under the assumptions of ROC curves, and signal detection models in particular, for the purpose of generating ROC curves and estimates of recollection and familiarity, responses of 3 and 2 are plotted as hits or false alarms. Here, all responses other than “1” suggest that there is some detectable signal contributing to a sense of familiarity or that an item seems “old.” So for the purposes of ROC analysis, even though the subject endorses an item as “new” in responses of 2 and 3, there is enough of a detectable familiarity signal to the subject that prevented them from endorsing “1” (absolutely CERTAIN the item is NEW). These YHT parameter hit and false alarm rates were then plotted against each other on the y and x axis respectively in a cumulative fashion, such that the first and left-most point includes the hit and false alarm rates for the most confident old responses of 6, the second point includes the hit and false alarm rates for responses of 6 and 5, the third point includes the hit and false alarm rates for responses of 6, 5, and 4, etc., creating a total of 5 coordinates. Utilizing the procedure originally described by Yonelinas et al. (1998), the Yonelinas Microsoft Excel solver routine (available at http://psychology.ucdavis.edu/labs/Yonelinas) was then used to generate recollection (R) and familiarity (d′YHT) estimations for each participant. To estimate these parameters, a non-linear equation was fit to each observed participant ROC by reducing the sum of squared errors between the predicted and observed data. Recollection was estimated as the intersection of the regression line with the y-axis whereas familiarity estimates were established based on the area under the curve (Figure 2).
Figure 2.
Aggregate ROC curves for (A) words and (B) pictures. The curves for healthy older adults are represented by triangles and the curves for patients with aMCI are represented by circles.
Validity
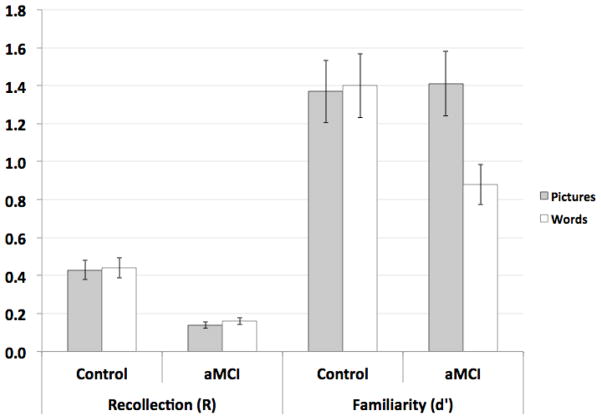
Because ROC curves are particularly sensitive to ceiling and floor effects, the parameter estimates were analyzed to establish their validity. Recollection estimates exceeding .60 have been reported to distort estimates of familiarity, thus a conservative recommendation proposed by Yonelinas (2002) is to utilize a study design that keeps recollection below .50. For the current investigation, group means for recollection are .43 and .44 for healthy controls, and .14 and .16 for aMCI patients for pictures and word respectively (Figure 3). In addition, very poor overall performance can produce a curve that approaches the chance diagonal with a slope of 1.0, resulting in familiarity estimates that are not necessarily meaningful. Thus, it has been recommended to avoid familiarity estimates below .5 (Ratcliff, et al., 1992; Yonelinas & Parks, 2007). The current investigation derived familiarity estimates of 1.37 and 1.40 for healthy controls and 1.41 and .88 for aMCI patients for pictures and words respectively.
Figure 3.
Estimates of familiarity (d′) and recollection (R) for both groups and conditions. Error bars represent the standard error of the mean.
Further, in order to assess potential group differences in rates of confidence level responding, the number of responses with high certainty (1 and 6) was collapsed and the number of lower certainty responses (2–5) was collapsed for each participant. Each participant was then labeled as having more high confidence responses or more low confidence responses. A Pearson chi-square test was conducted to examine whether the two groups differed in their high versus low response frequencies for pictures. The results revealed that there was no significant difference between the groups for pictures (χ2 = 0.51, df = 1, p = 0.48) or words (χ2 = 0.18, df = 1, p = 0.67), suggesting that the two groups used hi and low confidence responses at similar rates.
Recollection and Familiarity
In order to assess the primary hypothesis of this study, a repeated measures ANOVA was performed on the familiarity estimates (d′YHT) with the factors of Group (healthy older adults, patients with aMCI) and Condition (pictures, words). The results of the ANOVA revealed an effect of Condition [F(1, 30) = 5.73, p = 0.023], but no effect of Group [F(1, 30) = 2.08, p = 0.160]. The effect of Condition was present because estimates of familiarity were higher for pictures compared to words [t(30) = 2.20, p = 0.036]. There was also an interaction of Group and Condition [F(1, 30) = 6.83, p = 0.014]. Follow-up t-tests revealed that familiarity estimates for words were significantly greater for healthy controls compared to the patients with aMCI [t(30) = 3.39, p = 0.002]. In contrast, familiarity estimates for pictures did not differ between groups [t(30) = 0.17, p = 0.867].
To assess recollection (R), a similar mixed between- (Group) and within (Condition) subjects analysis of variance (ANOVA) was conducted. While no significance was found for the effect of Condition [F(1, 30) <1] or for the interaction of Group and Condition [F(1, 30) < 1], the effect of Group was significant [F(1, 30) = 18.68, p < 0.001], revealing a greater estimate of recollection for the healthy controls [t(30) = 4.32, p < 0.001].
Accuracy
Overall accuracy on the recognition test was also assessed (see Table 3). Hit rate was established by collapsing across confidence responses of 4, 5, and 6 for previously studied items, and false alarm rate was calculated by collapsing across confidence responses of 4, 5, and 6 for previously unstudied items. Accuracy (Pr) was computed as percent hit rate – percent false alarm rate (Snodgrass & Corwin, 1988). The Pr values were then entered into a repeated measures ANOVA with the factors of Group and Condition. The results of the ANOVA revealed effects of Group [F(1, 30) = 8.15, p = 0.008] and Condition [F(1, 30) = 14.01, p < 0.001]. The effect of group was present because older adults demonstrated significantly higher overall accuracy than patients. Interestingly, there was also an interaction of Group and Condition [F (1, 30) = 10.28, p = 0.003]. Follow-up t-tests revealed that in the word condition, healthy older adults demonstrated greater accuracy than patients [t(30) = 4.02, p < 0.001]. In contrast, there was no difference in accuracy between groups for pictures [t(30) = 1.29, p = 0.208].
As stated in the introduction, one goal of the methodological design was to equate healthy older adult performance for pictures and words. As seen in Table 3, there were no differences in condition for the healthy older adults [t(15) < 1]. In contrast, even when performance was equated for pictures and words for the healthy controls, patients with MCI continued to demonstrate a picture superiority effect [t(15) = 3.96, p = 0.001].
In addition to understanding differences in accuracy, we performed independent samples t-tests to examine differences in hit and false alarm rates for pictures and words between the two groups. For words, healthy older adults demonstrated a greater number of hits [t(30) = 2.44, p = 0.021] and fewer false alarms [t(30) = −2.12, p = 0.043] compared to patients with MCI. In contrast, there were no differences in hit rate [t(30) < 1] or false alarm rate [t(30) = 1.54, p = 0.133] between groups for pictures. For the healthy older adults, there was no difference in hit rate [t(15) = 1.77, p = .095] or false alarm rate [t(15) = 1.84, p = .086] between conditions. This was generally true for the patients with MCI. There was a marginally higher false alarm rate for words compared to pictures [t(15) = 2.09, p = .059], but no difference in hit rate [t(15) = 1.58, p = .134] between conditions.
Response Bias
In addition to accuracy, we examined response bias by calculating Br as percent false alarm rate/(1 − [percent hit rate − percent false alarm rate]) (Snodgrass & Corwin, 1988). Similar to the Pr analysis, the Br values were entered into a repeated measures ANOVA with the factors of Group and Condition (see Table 3). The results of this analysis revealed no effect of Group [F(1, 30) = 1.36, p = 0.252] or Condition [F(1, 30) = 1.37, p = 0.251]. Additionally, there was no interaction of Group and Condition [F(1, 30) < 1], suggesting no differences in response bias between groups.
Discussion
The present study set out to determine whether the robust picture superiority effect in patients with aMCI was due to intact familiarity for pictures, but impaired familiarity for words, compared to healthy older adults with no cognitive impairment. Work in healthy memory suggests that pictures improve recollection compared to words (Ally & Budson, 2007; Ally et al., 2008; Curran & Doyle, 2011; Rajaram, 1996). However, given that a substantial body of work has shown that recollection is impaired in patients with aMCI, we hypothesized that there would be no difference in the recollection estimates for pictures and words in our patient group. Further, we predicted that recollection would be diminished compared to healthy older adults for both stimulus types. These hypotheses were confirmed. Consistent with previous work, there was no difference between the recollection estimates for pictures and words in patients with aMCI, and patients demonstrated significantly impaired estimates of recollection compared to healthy controls (Ally, Gold, & Budson, 2009b; Westerberg et al., 2006). In contrast, we predicted that the robust picture superiority effect in patients with aMCI was likely driven by intact familiarity for pictures, but degraded familiarity for words. Again, our predictions were confirmed. The results showed similar estimates of familiarity for pictures between the two groups, but patients demonstrated significantly lower familiarity estimates for words compared to controls.
To fully understand why patients are able to successfully utilize familiarity for pictures but not for words, we turn to two major theories of the picture superiority effect. An initial theory of the picture superiority effect, the distinctiveness account, implicates the role of stimulus surface-sensory features in enhanced memory for pictures over words (Nelson, Reed, & Walling, 1976). Pictures provide more distinctive visual-perceptual representations at encoding, making them more memorable. At retrieval, it certainly makes sense that subjects need recollection to remember specific perceptual details from the study session (Rajaram, 1996). Given that patients with aMCI are significantly impaired in their ability to recollect specific details, perhaps the distinctive visual information works to enhance familiarity of pictures over words in this group. Indeed, it has been hypothesized that visual distinctiveness might also enhance familiarity (Ally, McKeever et al., 2009; Hamilton & Geraci, 2006; Yonelinas, 2002). Processing fluency, or the ease with which information is processed, is enhanced when a stimulus is re-processed in a subsequent encounter, regardless of whether the individual was aware of the original exposure. Fluency has long been thought to be salient in familiarity-based recognition (Jacoby & Dallas, 1981; Rajaram & Geraci, 2000; Westerman, 2001; Whittlesea, 1993; Whittlesea & Williams, 1998, 2000, 2001a, 2001b), and enhanced fluency is believed to contribute to the phenomenological experience of familiarity (Jacoby & Whitehouse, 1989; Lindsay & Kelley, 1996; Roediger & McDermott, 1995; Wolk et al., 2004). Indeed, previous work in patients with aMCI and mild AD suggest that perceptual fluency remains intact and can contribute to increased recognition performance in these patients (Ballesteros et al., 2007; Fleischman et al., 2005; Gong et al., 2010; Willems et al., 2008), likely through enhanced familiarity (Algarabel et al., 2009; O’Connor & Ally, 2010).
This hypothesis is supported by neuropathological studies investigating regions of the brain that contribute to perceptual fluency. Yonelinas et al. (2001) reported that familiarity judgments do not always require medial temporal regions, and are strongly associated with medial occipital gyri activation on functional MRI. Moreover, recent work has implicated the ventral-visual-perirhinal stream in visual familiarity, suggesting that hippocampus and surrounding regions are not critical for discrimination of simple visual stimuli, which can be made by regions posterior to the medial temporal lobe (Cowell et al., 2010). The Perceptual-Mnemonic Features Conjunction (PMFC) model posits that simple features are represented in caudal regions of the ventral-visual-perirhinal stream and progressively more complex conjunctions of these features are represented in later anterior regions, reaching the level of whole object representation in perirhinal cortex (Bussey & Saksida, 2002). Perhaps reliance on the posterior regions of the ventral-visual-perirhinal stream allows for the successful utilization of familiarity for pictures in patients with aMCI, and this reliance on posterior regions in the face of burgeoning medial temporal lobe pathology is responsible for the enhanced occipital activation seen in studies of patients with aMCI and those at genetic risk for AD (Golby et al., 2005; Koenig et al., 2008; Quiroz et al., 2011; Troller et al., 2006). Indeed, primary visual areas and the ventral visual pathway tend to be spared from AD pathology until relatively later in the disease course (Bokde et al., 2010; Corey-Bloom, 2004; Jack et al., 2008), and it has been proposed that areas in posterior brain regions might organize together in the face of hippocampal pathology to provide compensatory function in patients with AD (Scarmeas et al., 2004).
In contrast to perceptual distinctiveness, the second major theory of the picture superiority effect posits that pictures allow for deeper and more elaborate conceptual processing than words (Weldon & Roediger, 1987). Proponents of the semantic processing account hypothesize that distinctive visual information contributes to the conceptual salience and fluency of pictures (Hamilton & Geraci, 2006). Although original studies of conceptual fluency in patients with AD were mixed, more recent work has suggested that patients with aMCI and mild AD can successfully utilize conceptual fluency to enhance memory for pictures over words (Deason et al., 2012; O’Connor & Ally, 2010). Further, the neural mechanisms thought to underlie conceptual processing of pictures remains intact in patients with aMCI (Ally, McKeever et al., 2009; Wolk et al., 2005). Budson et al. (2002) suggested that pictures enhance semantic gist in patients because pictures allow them to more easily gain access to the full meaning of the words. If a patient has simply forgotten conceptual information about a word, or degraded semantic networks prevent them from elaborately processing the meaning of a word, perhaps pictures serve as this cue. For example, if a patient is presented with the word “dog” at study, he or she is left to generating an internal prototype of “dog” and spread within the semantic network may be left at “animal.” In contrast, when a patient is shown a picture of a “dog,” he or she may be more easily able to make the conceptual associations of “dalmatian,” “spotted dog,” “fire fighter dog,” and “I had a dalmatian as a child.” This more elaborate conceptual processing of the picture might allow patients with aMCI to better utilize familiarity at test.
Armed with a greater conceptual benefit from pictures along with enhanced perceptual fluency, perhaps pictures allow patients to better monitor their sense of familiarity, which in turn leads to enhanced accuracy (Ally, Gold, & Budson, 2009a; Westerberg et al., 2006) and decreased false recognition (Beth et al., 2009) compared to words. In contrast, when studying words, patients are generally left to conceptual processing with very limited perceptual information to help generate and monitor familiarity at test (O’Connor & Ally, 2010). This divergence, compounded by the fact that patients in the earliest stages of the disease have difficulty using mental imagery to enhance encoding (Hussey et al., 2011), leads to worse discrimination and higher false alarm rates for words compared to pictures (Beth et al., 2009). This difference in how patients with aMCI utilize familiarity for pictures and words likely underlies the robust picture superiority effect in this patient group. As evidenced by the current results, patients with aMCI demonstrate a significant picture superiority effect, even when discrimination is matched for pictures and words in their healthy counterparts.
We believe that the results of the current study help to resolve the ongoing question of whether familiarity is impaired in patients with aMCI. As proposed by Ally, McKeever et al. (2009), familiarity appears to be differentially affected based on stimulus type. Using ROC curves, we definitively showed in the current study that familiarity remains intact for pictures, but is significantly impaired for word in patients with aMCI. Although we currently do not fully understand how patients with aMCI and AD subjectively experience familiarity, we speculate that patients are able to better monitor familiarity for conceptual plus perceptual information, whereas they have significant difficulty relying on either type of information alone due to impaired recollection. For example, Gold et al., (2007) showed that adding a conceptual label to an ambiguous perceptual shape significantly increased accuracy and decreased false alarm rates in patients with very mild AD compared to just the perceptual shape alone. Moreover, O’Connor and Ally (2010) showed that when studying conceptual information alone, patients with aMCI and very mild AD performed far worse than when that conceptual information was accompanied by perceptual information. Data over these two studies demonstrated that when patients with aMCI or mild AD relied purely on conceptual information, false alarm rates were significantly higher than when perceptual information was added. In contrast, when relying purely on perceptual information, false alarm rates decline compared to relying on purely conceptual information, but hit rates also decline (Gold et al., 2007). It is likely that conceptual fluency initially drives the feeling of familiarity, and perceptual information may allow for post-retrieval monitoring or verification of the enhanced sense of familiarity. In support of this hypothesis, Ally, McKeever, et al. (2009) showed that the neural correlate of post-retrieval monitoring remains intact for pictures, but not for words, in patients with aMCI. The ability to successfully monitor familiarity leads to the increased hit rate and decreased false alarm rate for pictures compared to words in this patient population.
Alternatively, the combination of enhanced conceptual and perceptual fluency of the test item might drive a stronger or more robust sense of familiarity that can be accurately attributed to a studied item. In contrast to recollection, which has been proposed to be a threshold process, familiarity is described as a signal detection process (Yonelinas, 1994). Familiarity is assumed to be continuous in nature such that the more familiar an item seems (whether or not it was actually studied), the more confident the subject is that an item was in fact studied (Yonelinas et al., 1996). Future work is encouraged to investigate these two hypotheses.
The present results may also have clinical implications. A vital component of differential diagnosis and management of aMCI and early AD hinges upon the establishment of the patient’s functional status. We speculate that perhaps measures of picture memory are a more accurate indicator of everyday functioning than are measures of memory for verbal and written information. Activities such as identifying medications, people, and landmarks, which are often required for instrumental and basic activities of daily living, rely heavily on the recognition of picture stimuli. The aMCI group in the current investigation is characterized as having a subjective cognitive complaint and objective cognitive memory impairment in the absence of functional decline. Given that the present study demonstrated intact familiarity for pictures in these patients, preservation of this process may be a more ecologically valid assessment of daily functioning for these individuals. It is possible that, as the successful utilization of familiarity for pictures declines, patients’ ability to successfully complete daily functions also decline, and thus correlate with the diagnostic criteria for AD being met. Future work can examine this hypothesis more specifically with patient in the mild to moderate stages of AD. Finally, understanding exactly how memory breaks down in aMCI and AD is critical to not only diagnostic specificity, but also in identifying potential areas of intact functioning that may be targeted for pharmacologic and behavioral therapy. A very robust picture superiority effect in patients with aMCI and mild AD is now well established, and the current work helps to understand the mechanisms that likely underlie this picture superiority effect. Future development of behavioral therapies should focus on employing strategies that rely on this preserved aspect of memory function in these patients. Utilizing this type of strategy may help to delay functional decline and extend independent living.
Highlights.
We investigated the picture superiority effect in patients with aMCI and controls.
We utilized ROC curves to obtain estimates of recollection and familiarity.
Recollection is impaired for pictures and words in patients with aMCI
Familiarity remains intact for pictures but not for words in patients with aMCI.
Acknowledgments
The authors would like to thank Rebecca Deason and Erin Hussey for help with programming and running subjects. This research was supported by National Institute on Aging grants AG031925 and AG038471 to BAA and AG025815 and AG013846 to AEB. This material is also the result of work supported with resources and the use of the facilities at the VA Boston Healthcare System.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adjutant General’s Office. Army individual test battery: Manual of directions and scoring. Washington, DC: War Department; 1944. [Google Scholar]
- Algarabel S, Escudero J, Mazon JF, Pitarque A, Feuntes M, Peset V, et al. Familiarity-based recognition in the young, healthy elderly, mild cognitive impaired, and Alzheimer’s patients. Neuropsychologia. 2009;47:2056–2064. doi: 10.1016/j.neuropsychologia.2009.03.016. [DOI] [PubMed] [Google Scholar]
- Ally BA, Budson AE. The worth of pictures: Using high density event-related potentials to understand the memorial power of pictures and the dynamics of recognition memory. [doi: DOI: 10.1016/j.neuroimage.2006.11.023] NeuroImage. 2007;35(1):378–395. doi: 10.1016/j.neuroimage.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. The picture superiority effect in patients with Alzheimer’s disease and mild cognitive impairment. [doi: DOI: 10.1016/j.neuropsychologia.2008.10.010] Neuropsychologia. 2009a;47(2):595–598. doi: 10.1016/j.neuropsychologia.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Gold CA, Budson AE. An evaluation of recollection and familiarity in Alzheimer’s disease and mild cognitive impairment using receiver operating characteristics. [doi: DOI: 10.1016/j.bandc.2008.11.003] Brain and Cognition. 2009b;69(3):504–513. doi: 10.1016/j.bandc.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, McKeever JD, Waring JD, Budson AE. Preserved frontal memorial processing for pictures in patients with mild cognitive impairment. [doi: DOI: 10.1016/j.neuropsychologia.2009.03.015] Neuropsychologia. 2009;47(10):2044–2055. doi: 10.1016/j.neuropsychologia.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ally BA, Waring JD, Beth EH, McKeever JD, Milberg WP, Budson AE. Aging memory for pictures: Using high-density event-related potentials to understand the effect of aging on the picture superiority effect. [doi: DOI: 10.1016/j.neuropsychologia.2007.09.011] Neuropsychologia. 2008;46(2):679–689. doi: 10.1016/j.neuropsychologia.2007.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson ND, Ebert PL, Jennings JM, Grady CL, Cabeza R, Graham SJ. Recollection- and familiarity-based memory in healthy aging and amnestic mild cognitive impairment. Neuropsychology. 2008;22(2):177–187. doi: 10.1037/0894-4105.22.2.177. [DOI] [PubMed] [Google Scholar]
- Ballesteros S, Reales JM, Mayas J. Picture priming in normal aging and Alzheimer’s disease. Psicothema. 2007;19:239–244. [PubMed] [Google Scholar]
- Beth EH, Waring JD, Budson AE, Ally BA. Response bias for picture recognition in patients with Alzheimer’s disease. Cognitive and Behavioral Neurology. 2009;22:229–235. doi: 10.1097/WNN.0b013e3181b7f3b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokde AL, Lopez-Bayo P, Born C, Ewers M, Meindl T, Teipel SJ, et al. Alzheimer’s disease: functional abnormalities in the dorsal visual pathway. Radiology. 2010;254:219–226. doi: 10.1148/radiol.2541090558. [DOI] [PubMed] [Google Scholar]
- Budson AE, Sitarkski J, Daffner KR, Schacter DL. False recognition of pictures versus words in Alzheimer’s disease: The distinctiveness heuristic. Neuropsychology. 2002;16:163–173. doi: 10.1037//0894-4105.16.2.163. [DOI] [PubMed] [Google Scholar]
- Bussey TJ, Saksida LM. The organization of visual object representations: a connectionist model of effects of lesions in perirhinal cortex. European Journal of Neuroscience. 2002;15:355–364. doi: 10.1046/j.0953-816x.2001.01850.x. [DOI] [PubMed] [Google Scholar]
- Corey-Bloom J. Alzheimer’s disease. In: Miller AE, editor. Dementia Continuum from the American Academy of Neurology. Vol. 21. New York: Lippincott, Williams, & Wilkins; 2004. pp. 23–57. [Google Scholar]
- Cowall RA, Bussey TJ, Saksida LM. Components of recognition memory: dissociable cognitive processes or just differences in representational complexity? Hippocampus. 2010;20:1245–1262. doi: 10.1002/hipo.20865. [DOI] [PubMed] [Google Scholar]
- Curran T, Doyle J. Picture superiority doubly dissociates the ERP correlates of recollection and familiarity. Journal of Cognitive Neuroscience. 2011;23:1247–1262. doi: 10.1162/jocn.2010.21464. [DOI] [PubMed] [Google Scholar]
- Deason RG, Hussey EP, Budson AE, Ally BA. Gist-based conceptual processing of pictures remains intact in patients with amnestic mild cognitive impairment. Neuropsychology. 2012 doi: 10.1037/a0026958. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCarlo LT. Signal detection theory with finite mixture distributions: Theoretical developments with applications to recognition memory. Psychological Review. 2002;109(4):710–721. doi: 10.1037/0033-295x.109.4.710. [DOI] [PubMed] [Google Scholar]
- Dodson CS, Spaniol M, O’Connor MK, Deason RG, Ally BA, Budson AE. Alzheimer’s disease and memory-monitoring impairment: Alzheimer’s patients show a monitoring deficit that is greater than their accuracy deficit. Neuropsychologia. 2011;49:2609–2618. doi: 10.1016/j.neuropsychologia.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman DA, Wilson RS, Gabrieli JD, Schneider JA, Bienas JL, Bennett DA. Implicit memory and Alzheimer’s disease neuropathology. Brain. 2005;128:2006–2015. doi: 10.1093/brain/awh559. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”: A practical method for grading the cognitive state of patients for the clinician. [doi: DOI: 10.1016/0022-3956(75)90026-6] Journal of Psychiatric Research. 1975;12(3):189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Golby A, Silverberg G, Race E, Gabrieli S, O’Shea J, Knierim K, et al. Memory encoding in Alzheimer’s disease: an fMRI study of explicit and implicit memory. Brain. 2005;128:773–787. doi: 10.1093/brain/awh400. [DOI] [PubMed] [Google Scholar]
- Gong L, Tian Y, Cheng H, Chen Z, Yin C, Meng Y, et al. Conceptual implicit memory impaired in amnestic mild cognitive impairment patient. Neuroscience Letters. 2010;484:153–156. doi: 10.1016/j.neulet.2010.08.041. [DOI] [PubMed] [Google Scholar]
- Hamilton M, Geraci L. The Picture Superiority Effect in Conceptual Implicit Memory: A Conceptual Distinctiveness Hypothesis. The American Journal of Psychology. 2006;119(1):1–20. [PubMed] [Google Scholar]
- Healy MR, Light LL, Chung C. Dual-Process Models of Associative Recognition in Young and Older Adults: Evidence From Receiver Operating Characteristics. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2005;31(4):768–788. doi: 10.1037/0278-7393.31.4.768. [DOI] [PubMed] [Google Scholar]
- Howard MW, Bessette-Symons B, Zhang Y, Hoyer WJ. Aging selectively impairs recollection in recognition memory for pictures: Evidence from modeling and receiver operating characteristic curves. Psychology and Aging. 2006;21(1):96–106. doi: 10.1037/0882-7974.21.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudon C, Belleville S, Gauthier S. The assessment of recognition memory using the Remember/Know procedure in amnestic mild cognitive impairment and probable Alzheimer’s disease. Brain and Cognition. 2009;70:171–179. doi: 10.1016/j.bandc.2009.01.009. [DOI] [PubMed] [Google Scholar]
- Hussey EP, Smolinsky JG, Piryatinsky I, Budson AE, Ally BA. Using mental imagery to improve memory in patients with Alzheimer’s disease: Trouble generating or remembering the mind’s eye? Alzheimer’s Disease and Associated Disorders. 2011 doi: 10.1097/WAD.0b013e31822e0f73. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack CR, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL, Dallas M. On the relationship between autobiographical memory and perceptual learning. Journal of Experimental Psychology: General. 1981;110(3):306–340. doi: 10.1037//0096-3445.110.3.306. [DOI] [PubMed] [Google Scholar]
- Jacoby LL, Whitehouse K. An illusion of memory: False recognition influenced by unconscious perception. Journal of Experimental Psychology: General. 1989;118(2):126–135. [Google Scholar]
- Koenig P, Smith EE, Troiani V, Anderson C, Moore P, Grossman M. Medial temproal lobe involement in an implicit memory task: evidence of collaborating implicit and explicit memory systems from fMRI and Alzheimer’s disease. Cerebral Cortex. 2008;18:2831–2843. doi: 10.1093/cercor/bhn043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay DS, Kelley CM. Creating Illusions of Familiarity in a Cued Recall Remember/Know Paradigm. [doi: 10.1006/jmla.1996.0011] Journal of Memory and Language. 1996;35(2):197–211. [Google Scholar]
- Mack WJ, Freed DM, Williams BW, Henderson VW. Boston naming test: Shortened versions for use in Alzheimer’s disease. Journal of Gerontology. 1992;47:154–158. doi: 10.1093/geronj/47.3.p154. [DOI] [PubMed] [Google Scholar]
- Monsch AU, Bondi MW, Butters N, Salmon DP, Katzman R, Thal LJ. Comparisons of Verbal Fluency Tasks in the Detection of Dementia of the Alzheimer Type. Arch Neurol. 1992;49(12):1253–1258. doi: 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, et al. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer’s disease. Neurology. 1989;39:1159–1165. doi: 10.1212/wnl.39.9.1159. [DOI] [PubMed] [Google Scholar]
- Nelson DL, Reed VS, Walling JR. Pictorial superiority effect. Journal of Experimental Psychology: Human Learning and Memory. 1976;2(5):523–528. [PubMed] [Google Scholar]
- O’Connor MK, Ally BA. Using stimulus form change to understand memorial familiarity for pictures and words in patients with mild cognitive impairment and Alzheimer’s disease. Neuropsychologia. 2010;48:2068–2074. doi: 10.1016/j.neuropsychologia.2010.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller KA, Voss JL, Boehm SG. Validating neural correlates of familiarity. [doi: 10.1016/j.tics.2007.04.002] Trends in Cognitive Sciences. 2007;11(6):243–250. doi: 10.1016/j.tics.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Peterson RC. Mild cognitive impairment as a diagnosic entity. Journal of Internal Medicine. 2004;(256):183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Quiroz YT, Ally BA, Celone K, McKeever J, Ruiz-Rizzo AL, Lopera F, et al. Event-related potential markers of brain changes in preclinical familial Alzheimer’s disease. Neurology. 2011;77:469–475. doi: 10.1212/WNL.0b013e318227b1b0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajaram S. Perceptual effects on remembering: Recollective processes in picture recognition memory. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1996;22(2):365–377. doi: 10.1037//0278-7393.22.2.365. [DOI] [PubMed] [Google Scholar]
- Rajaram S, Geraci L. Conceptual fluency selectively influences knowing. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2000;26(4):1070–1074. doi: 10.1037//0278-7393.26.4.1070. [DOI] [PubMed] [Google Scholar]
- Ratcliff R, Sheu C-f, Gronlund SD. Testing global memory models using ROC curves. Psychological Review. 1992;99(3):518–535. doi: 10.1037/0033-295x.99.3.518. [DOI] [PubMed] [Google Scholar]
- Roediger HL, McDermott KB. Creating false memories: Remembering words not presented in lists. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1995;21(4):803–814. [Google Scholar]
- Rotello CM, Macmillan NA, Reeder JA. Sum-Difference Theory of Remembering and Knowing: A Two-Dimensional Signal-Detection Model. Psychological Review. 2004;111(3):588–616. doi: 10.1037/0033-295X.111.3.588. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Curran T. Event-related potentials and recognition memory. [doi: DOI: 10.1016/j.tics.2007.04.004] Trends in Cognitive Sciences. 2007;11(6):251–257. doi: 10.1016/j.tics.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Rugg MD, Mark RE, Walla P, Schloerscheidt AM, Birch CS, Allan K. Dissociation of the neural correlates of implicit and explicit memory. [10.1038/33396] Nature. 1998;392(6676):595–598. doi: 10.1038/33396. [DOI] [PubMed] [Google Scholar]
- Scarmeas N, Zarahn E, Andreson KE, Honig LS, Park A, Hilton J, et al. Cognitive reserve-mediated modulation of position emission tomographic activations during memory tasks in Alzheimer’s disease. Archives of Neurology. 2004;61:73–78. doi: 10.1001/archneur.61.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass JG, Corwin J. Pragmatics of measuring recognition memory: Applications to dementia and amnesia. Journal of Experimental Psychology: General. 1988;117(1):34–50. doi: 10.1037//0096-3445.117.1.34. [DOI] [PubMed] [Google Scholar]
- Troller JN, Sachdev PS, Haindl W, Brodaty H, Wen W, Walker BM. A higg-resolution single photon emission computer tomography study of verbal recognitiion memory in Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2006;21:267–274. doi: 10.1159/000091433. [DOI] [PubMed] [Google Scholar]
- Weldon MS, Roediger HL. Altering retrieval demands reverses the picture superiority effect. Memory and Cognition. 1987;15:269–280. doi: 10.3758/bf03197030. [DOI] [PubMed] [Google Scholar]
- Westerberg CE, Paller KA, Weintraub S, Mesulam MM, Holdstock JS, Mayes AR, et al. When memory does not fail: Familiarity-based recognition in mild cognitive impairment and Alzheimer’s disease. Neuropsychology. 2006;20(2):193–205. doi: 10.1037/0894-4105.20.2.193. [DOI] [PubMed] [Google Scholar]
- Westerman DL. The role of familiarity in item recognition, associative recognition, and plurality recognition on self-paced and speeded tests. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001;27(3):723–732. [PubMed] [Google Scholar]
- Whittlesea BWA. Illusions of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1993;19(6):1235–1253. [Google Scholar]
- Whittlesea BWA, Williams LD. Why do strangers feel familiar, but friends don’t? A discrepancy-attribution account of feelings of familiarity. [doi: DOI: 10.1016/S0001-6918(97)00040-1] Acta Psychologica. 1998;98(2–3):141–165. doi: 10.1016/s0001-6918(97)00040-1. [DOI] [PubMed] [Google Scholar]
- Whittlesea BWA, Williams LD. The Source of Feelings of Familiarity: The Discrepancy-Attribution Hypothesis. [Article] Journal of Experimental Psychology Learning, Memory & Cognition. 2000;26(3):547. doi: 10.1037//0278-7393.26.3.547. [DOI] [PubMed] [Google Scholar]
- Whittlesea BWA, Williams LD. The discrepancy-attribution hypothesis: I. The heuristic basis of feelings and familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001a;27(1):3–13. [PubMed] [Google Scholar]
- Whittlesea BWA, Williams LD. The discrepancy-attribution hypothesis: II. Expectation, uncertainty, surprise, and feelings of familiarity. Journal of Experimental Psychology: Learning, Memory, and Cognition. 2001b;27(1):14–33. [PubMed] [Google Scholar]
- Willems S, Salmon E, Van der Linden M. Implicit/explicit memory dissociation in Alzheimer’s disease: The consequence of inappropriate processing? Neuropsychology. 2008;22(6):710–717. doi: 10.1037/a0012986. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Berman AR, Schacter DL, Holcomb PJ, Daffner KR, Budson AE. An electrophysiological investigation of the relationship between conceptual fluency and familiarity. Neuroscience Letters. 2004;369:150–155. doi: 10.1016/j.neulet.2004.07.081. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Schacter DL, Berman AR, Holcomb PJ, Daffner KR, Budson AE. Patients with mild Alzheimer’s disease attribute conceptual fluency to prior experience. [doi: DOI: 10.1016/j.neuropsychologia.2005.01.007] Neuropsychologia. 2005;43(11):1662–1672. doi: 10.1016/j.neuropsychologia.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Wolk DA, Signoff ED, DeKosky ST. Recollection and familiarity in amnestic mild cognitive impairment: A global decline in recognition memory. [doi: DOI: 10.1016/j.neuropsychologia.2008.01.017] Neuropsychologia. 2008;46(7):1965–1978. doi: 10.1016/j.neuropsychologia.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonelinas AP. Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1994;20(6):1341–1354. doi: 10.1037//0278-7393.20.6.1341. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP. The Nature of Recollection and Familiarity: A Review of 30 Years of Research. [doi: DOI: 10.1006/jmla.2002.2864] Journal of Memory and Language. 2002;46(3):441–517. [Google Scholar]
- Yonelinas AP, Hopfinger JB, Buonocore MH, Kroll NEA, Baynes K. Hippocampal, parahippocampal, and occipital-temporal contributions to associative and item recognition memory: an fMRI study. NeuroReport. 2001;12:359–363. doi: 10.1097/00001756-200102120-00035. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Kroll NEA, Dobbins I, Lazzara M, Knight RT. Recollection and familiarity deficits in amnesia: Convergence of remember-know, process dissociation, and receiver operating characteristic data. Neuropsychology. 1998;12(3):323–339. doi: 10.1037//0894-4105.12.3.323. [DOI] [PubMed] [Google Scholar]
- Yonelinas AP, Parks CM. Receiver operating characteristics (ROCs) in recognition memory: A review. Psychological Bulletin. 2007;133(5):800–832. doi: 10.1037/0033-2909.133.5.800. [DOI] [PubMed] [Google Scholar]