Abstract
Since its discovery in the early 1990s, the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signaling pathway has been found to play key roles in regulating many key cellular processes such as survival, proliferation, and differentiation. There are seven known mammalian STAT family members: STAT1, 2, 3, 4, 5a, 5b, and 6. In the liver, activation of these STAT proteins is critical for anti-viral defense against hepatitis viral infection and for controlling injury, repair, inflammation, and tumorigenesis. The identification of functions for these STAT proteins has increased our understanding of liver disease pathophysiology and treatments, while also suggesting new therapeutic modalities for managing liver disease.
Keywords: HCV, interferon, liver injury, liver regeneration, liver tumor
Introduction
Alcohol consumption, nonalcoholic steatohepatitis, and viral hepatitis are the three major causes of chronic liver disease; each has a similar disease progression that is characterized by chronic liver inflammation, injury, cirrhosis, and hepatocellular carcinoma (HCC). Liver disease progression is controlled by a wide variety of cellular mediators, including cytokines, growth factors, hormones, and among others. Of the various downstream signaling pathways, the Janus kinase (JAK)-signal transducer and activator of transcription (STAT) pathway has been shown to play a multitude of critical roles in the pathogenesis of liver diseases.
The JAK-STAT pathway was identified in the early 1990s as a key signaling cascade mediating cytokine receptor-derived signals in mammals [1, 2]. In general, upon binding to their receptors, cytokines induce receptor dimerization and subsequent receptor-associated JAK dimerization. The JAKs then autophosphorylate one another, and receptor phosphorylation follows. Next, the phosphorylated JAK-receptor complex recruits and phosphorylates various STAT proteins. The phosphorylated STATs then form homodimers or heterodimers and translocate into the nucleus to induce the transcription of genes that regulate many cellular functions [1, 2]. To date, four JAKs (JAK1-3 and Tyk2) and seven STAT proteins (STAT1-4, 5a, 5b, and 6) have been identified. Each cytokine receptor activates its characteristic set of individual JAKs and STATs that is determined by the structure of receptor intracellular domains. In the liver, the JAK-STAT pathway is activated by growth hormone and a diverse array of cytokines [3], and to a lesser extent by other mediators such as growth factors (eg. epidermal growth factor) [4] and viral proteins (eg. HCV core protein) [5]. Fig. 1 and Fig. 2 show the simple models of JAK-STAT pathways activated by interferons (IFNs), interleukin-6 (IL-6), and IL-22. Table I and Table II list the major activators and functions of each STAT in liver parenchymal (hepatocytes) and nonparenchymal cells.
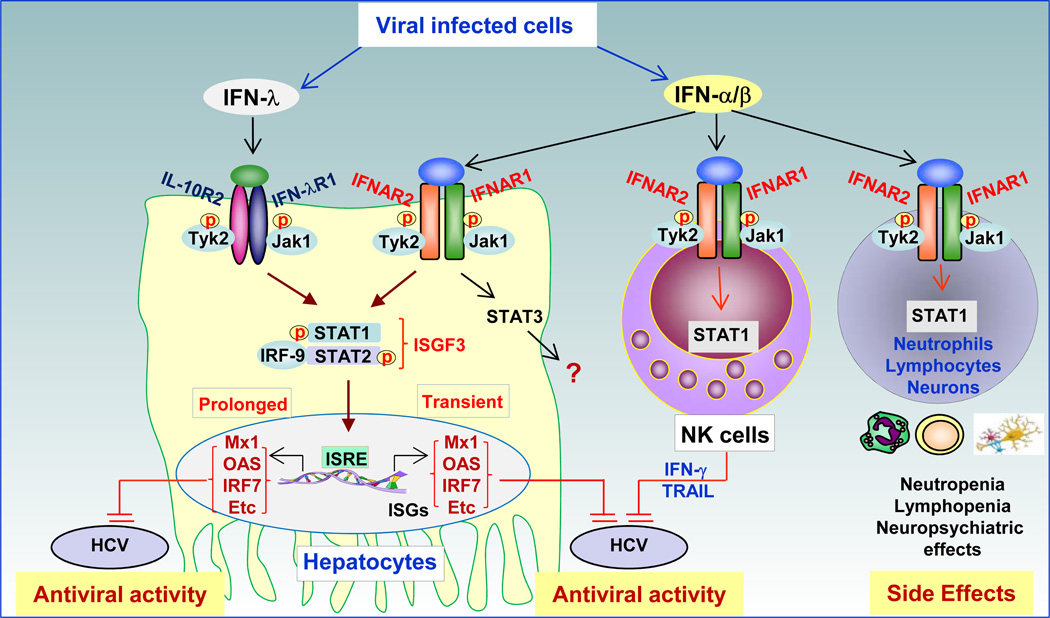
Fig. 1. Anti-viral effects of STAT1 and STAT2 in viral hepatitis.
Virus-infected cells produce both IFN-α/β and IFN-λ, which bind to their corresponding receptors and activate STAT1 and STAT2 in human hepatocytes. Activated STAT1 and STAT2 induce many anti-viral proteins (e.g., Mx1, OAS, IRF-7, etc.) that subsequently inhibit HCV replication. Although IFN-α/β stimulation also induces strong STAT3 activation in hepatocytes [39], the role of STAT3 in the anti-viral activity of IFN-α/β against HCV remains unknown. IFN-α/β usually induces transient STAT1 and STAT2 activation. In contrast, IFN-λ induces prolonged STAT activation, which may be responsible for the protective effects of IFN-λ on spontaneous and treatment-induced HCV clearance. In addition, IFN-α therapy induces STAT1 activation in NK cells and subsequent NK cell activation. The activated NK cells may also contribute to the anti-viral effects of IFN-α therapy against HCV, which is needed to be confirmed by further studies. IFN-α also activates STAT1 in hematopoietic and neuronal cells that express both IFNAR1 and IFNAR2, resulting in the various side effects associated with IFN-α therapy. IFN-λR1 (IL-28R1) is largely restricted to hepatocytes and is not expressed on immune cells. Thus, IFN-λ treatment is less likely to induce the hematopoietic and neurological side effects associated with IFN-α therapy. IRF-9: Interferon Regulatory Factor 9; ISGF3: Interferon-stimulated gene factor 3 complex; ISRE: Interferon-Sensitive Response Element. ISG: Interferon Stimulated Gene.
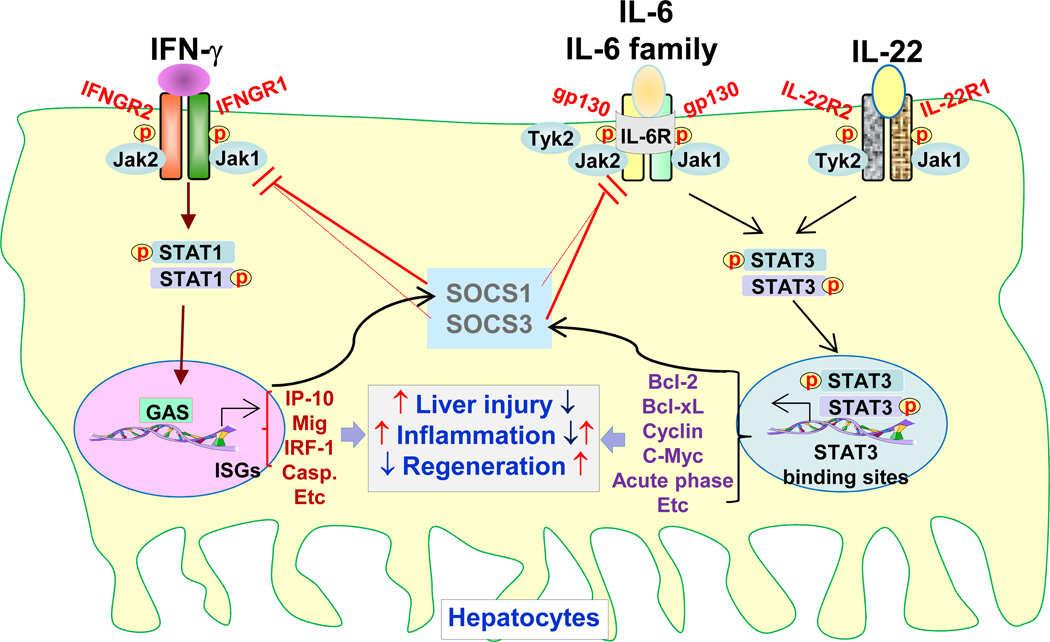
Fig. 2. Hepatocyte STAT1 and STAT3 in liver injury, inflammation, and regeneration.
While STAT1 in hepatocytes is predominately activated by IFN-γ, STAT3 in hepatocytes is predominantly activated by IL-6, IL-6 family cytokines, and IL-22. Following activation, STAT1 dimerizes and translocates into the nucleus to induce the transcription of many genes that promote liver injury and inflammation and inhibit liver regeneration. In contrast, activated STAT3 induces the expression of many genes that mitigate liver injury and promote liver regeneration. Under most conditions, activation of hepatic STAT3 blocks liver inflammation by inhibiting pro-inflammatory STAT1 signaling and protecting against liver injury. However, hepatic STAT3 may also promote liver inflammation by inducing the production of hepatocyte-derived acute phase proteins. STAT1 and STAT3 in hepatocytes also negatively regulate one another through the induction of SOCS1 and SOCS3 proteins, respectively. ISG: Interferon Stimulated Gene; GAS: Interferon-Gamma Activated Sequence.
Table I.
Major activators and functions of STAT proteins in liver parenchymal cells (hepatocytes)
| STAT proteins | Major Activators | Major Functions |
|---|---|---|
| STAT1 | IFN-α, IFN-β, IFN-γ, IFN-λ | Promotes anti-viral response Promotes anti-tumor response Induces hepatocyte apoptosis Inhibits hepatocyte proliferation Promotes liver inflammation |
| STAT2 | IFN-α, IFN-β, IFN-λ | Promotes anti-viral response |
| STAT3 | IL-6, IL-6 family cytokines, IL-22 |
Promotes hepatocyte survival Promotes hepatocyte proliferation Ameliorates steatosis Induces expression of innate immunity proteins Promotes liver tumor cell survival and growth |
| STAT4 | Unknown | Unknown |
| STAT5 | Growth hormone | Upregulates metabolism enzymes, growth factors, etc. Promotes hepatocyte survival Promotes hepatocyte proliferation Ameliorates steatosis |
| STAT6 | IL-4, IL-13, | Promotes liver injury and inflammation in T cell hepatitis Protects against ischemia/reperfusion and drug-induced liver injury |
References are cited in the text
Table II.
Major activators and functions of STAT proteins in liver nonparenchymal cells (HSCs, Kupffer cells, sinusoidal endothelial cells), and liver lymphocytes
| STATs | Cell types | Major Activators | Major Functions |
|---|---|---|---|
| STAT1 | in HSCs | IFN-α, IFN-β, IFN-γ | Inhibits liver fibrosis |
| in Kupffer cells | IFN-γ | Promotes inflammatory response | |
| In NK cells | IFN-α, IFN-β, IFN-γ | Promotes anti-viral, anti-tumor, and anti-fibrotic responses |
|
| STAT2 | in nonparenchymal cells | Unknown | Unknown |
| STAT3 | in Kupffer cells | IL-10 | Inhibits liver inflammation |
| in HSCs | Leptin, IL-6 | Promotes liver fibrogenesis | |
| in endothelial cells | Unknown | Inhibits liver inflammation | |
| STAT4 | in NK and NKT cells | IL-12, IFN-α/β | Promotes liver inflammation |
| STAT5 | In HSCs | leptin | Promotes liver fibrogenesis |
| STAT6 | In HSCs | IL-4, IL-13 | Promotes liver fibrogenesis |
References are cited in the text
After activation, the JAK-STAT pathway is usually rapidly terminated by three families of proteins, including suppressors of cytokine signaling (SOCSs), SH2-containing phosphatases (SHPs), and protein inhibitors of activated STATs (PIASs) [6, 7]. Among them, SOCS proteins, which include SOCS1-3, are cytokine-induced negative feedback-loop regulators that terminate JAK-STAT signaling by binding and inhibiting JAKs or by competing with STATs for phosphotyrosine binding sites on cytokine receptors [6, 7]. In concanavalin A (Con A)-induced T cell hepatitis model, IFN-γ activation of STAT1 is mainly responsible for SOCS1 induction, whereas IL-6 activation of STAT3 contributes to SOCS3 induction [8]. SOCS1 and SOCS3 reciprocally inhibit STAT1 and STAT3 signaling with SOCS1 preferential inhibition of IFN-γ signaling and SOCS3 preferential inhibition of IL-6 signaling in the liver [9].
In this review, we highlight the important functions of various STATs in hepatic anti-viral responses, inflammation, and tumorigenesis.
Anti-viral effects of STAT1 and STAT2 in viral hepatitis
It has been well documented that activation of both STAT1 and STAT2 plays a key role not only in host defense against HCV infection but also in IFN-α treatment-induced HCV clearance. After HCV infection, the infected hepatocytes produce IFN-β, which then activates STAT1 and STAT2 in uninfected neighboring hepatocytes via the binding of IFN-α/β receptor, and subsequently upregulates expression of various anti-viral proteins that prevent further infection [10]. The current standard therapy for chronic HCV infection is 24 or 48 weeks of treatment with pegylated IFN-α given in combination with ribavirin; this leads to viral eradication in approximately 50–60% of treated patients. The anti-HCV effects of IFN-α are believed to be mediated by signaling through a heterodimeric receptor complex composed of IFN-α receptor 1 (IFNAR1) and IFNAR2 on hepatocytes; receptor ligation results in the activation of STAT1 and STAT2 and the subsequent induction of a variety of anti-viral proteins that inhibit HCV replication (Fig. 1). Recent studies suggest that IFN-α-mediated natural killer (NK) cell activation and the subsequent elimination of HCV-infected hepatocytes by NK cells may also contribute to the anti-viral effect of IFN-α treatment against HCV infection [11–16]. NK cells can also produce IFN-γ that subsequently inhibits HCV replication in hepatocytes [17]. STAT1 protein expression and phosphorylation in NK cells are increased in HCV patients compared with healthy subjects [18, 19], and are further elevated during IFN-α therapy [19]. Elevation of STAT1 in NK cells correlates with increased NK cell cytotoxicity and the anti-viral effectiveness of IFN-α-based therapy, suggesting that STAT1 contributes to NK cell activation and the anti-HCV activity of IFN-α [19] (Fig. 1).
IFN-λ proteins are known as type III IFNs that are functionally similar to IFN-α in that they can also activate STAT1 and STAT2. To date, three IFN-λ genes that encode three distinct, yet highly related, proteins known as IFN-λ1 (also known as IL-29), IFN-λ2 (IL-28A), and IFN-λ3 (IL-28B) have been identified [20]. In this article, we use IL-29, IL-28A and IL-28B to represent the gene symbols of IFN-λs, as recommended by the Human Genome Organization Gene Nomenclature Committee, and use IFN-λs to represent the corresponding proteins to emphasize their functions. IFN-λ can initiate STAT1 and STAT2 activation by binding to a receptor complex comprised of the IL-10R2 and the unique IFN-λR1 (also known as IL-28R) chain. The subsequent upregulation of a number of anti-viral proteins leads to the inhibition of HCV replication [21–26] (Fig. 1). As the expression of IFN-λR1 is largely restricted to epithelial cells, clinical treatment with IFN-λ is less likely to induce the hematopoietic and neurologic side effects observed during IFN-α therapy [23]. Based on these exciting preclinical findings, several groups have performed phase I clinical trials with pegylated IFN-λ1. In these trials, HCV-infected patients tolerated weekly pegylated IFN-λ1 treatments with or without daily ribavirin for 4 weeks and had clear anti-viral responses [27, 28]. However, large, randomized controlled trials are needed to provide clear data regarding the safety and efficacy of pegylated IFN-λ1 for the treatment of chronic HCV infections.
In addition to the potential of IFN-λ to treat HCV, single nucleotide polymorphisms (SNPs) in the IL-28B/IFN-λ3 gene have been shown to play important roles in regulating spontaneous HCV clearance and in determining the efficacy of pegylated IFN-α plus ribavirin therapy in HCV patients. As the details of these genetic studies have been discussed in several reviews [29–31], we will only briefly summarize the findings here. First, SNPs in the IL-28B gene, such as rs12979860 or rs809917, are strongly associated with spontaneous and IFN-α treatment-induced clearance of HCV in patients infected with either HCV genotypes 1 or 4; however, the results from studies in patients with HCV genotypes 2 and 3 remain inconclusive (see reviews [29–31]). Second, the presence of IL-28B gene SNPs, in either donor or recipient tissues, has been shown to affect the responsiveness to IFN-α therapy for the treatment of recurrent HCV infection following liver transplantation [32–34]. Although the association between IL-28B SNPs and HCV infection has been extensively investigated, the results for the association of these SNPs and IFN-λ protein expression have been controversial. It was reported that patients with the IL-28B rs12979860 SNP had increased serum levels of IFN-λs that were associated with HCV clearance [35], but other reports showed that patients with the response-favorable IL-28B rs8099917 TT genotype had a lower expression of IFN-λs compared to patients with the TG or GG genotypes [36], or that IL-28B SNPs were not associated with intrahepatic IFN-λ expression in HCV patients [37]. Moreover, the mechanisms underlying the important functions of IL-28B SNPs in controlling HCV outcomes remain obscure. It may be related to IFN-λ-mediated direct inhibition of HCV replication [22, 25, 26] and IFN-λ-mediated induction of prolonged STAT1 activation and ISG expression in hepatocytes [22, 38].
In contrast to the well-documented anti-viral effects of STAT1 and STAT2, the functions of other STATs in viral infection remain largely unknown. Although activation of other STATs appears to have no direct anti-viral effects, their activation may indirectly modulate the anti-viral activity of IFNs by regulating STAT1 and STAT2 activation, modulating IFN expression, and controlling immune cell activation. For example, in addition to the activation of STAT1 and STAT2, IFN-α also induces strong STAT3 activation in primary human hepatocytes [39]. Although STAT3 activation does not induce anti-viral proteins, it may negatively regulate the anti-viral activity of IFN-α by inhibiting STAT1 and STAT2 activation through several mechanisms. First, STAT3 can heterodimerize with STAT1, thereby reducing STAT1 and STAT2 heterodimer formation. Second, STAT3 activation upregulates SOCS1 and SOCS3 expression that then inhibit IFN-α signaling. In addition, activated STAT3 is an important survival signal for hepatocytes and likely prevents HCV-infected hepatocyte cell death, thereby diminishing the anti-viral effects of IFN-α. Further studies to clarify the role of STAT3 in anti-viral IFN-α therapy may help identify novel strategies to improve the efficacy of IFN-α treatment in HCV patients.
Opposing roles of STAT1 and STAT3 in liver injury and repair
Accumulated research data from the last decade suggest that signaling through the JAK-STAT pathway plays key roles in controlling liver injury, regeneration, fibrosis, and inflammation. Interestingly, STAT1 and STAT3 activation play opposing roles in many aspects of liver pathophysiology, including liver injury and repair, which are discussed here. As the roles of the STATs in liver fibrogenesis have been summarized in a recent article [40], they are not discussed in this review.
Liver injury
Liver injury is characterized by damage to parenchymal cells, such as hepatocytes and biliary cells, and also by the sinusoidal disorganization that follows endothelial cell death. Whereas STAT1 activation in hepatocytes is a pro-apoptotic signal that leads to cell death and increased liver damage, STAT3 activation is a survival signal that protects against hepatocyte death. The opposing roles of hepatic STAT1 and STAT3 in liver injury have been extensively characterized in the Con A-induced T cell hepatitis model, where both signals are highly activated [8, 41–47]. Blockade of hepatic STAT1 activation via genetic modification of several genes prevented Con A-induced liver injury [8, 41, 42, 44, 48]; whereas inhibition of hepatic STAT3 exaggerated it [8, 43, 45]. Conversely, enhanced hepatic STAT1 activation accelerated Con A-induced hepatitis [41], while increased hepatic STAT3 activation diminished it [46, 47]. These findings suggest that STAT1 activation in hepatocytes is detrimental in Con A-induced hepatitis, whereas activation of hepatic STAT3 is protective. In addition, the detrimental effect of STAT1 has also been reported in LPS plus D-galactosamine-induced liver injury [49]; whereas the hepatoprotective function of hepatic STAT3 has been observed in many models of liver injury [50–55]. For example, conditional deletion of STAT3 in hepatocytes markedly increased mice to Fas ligand-induced hepatocyte apoptosis and liver injury [55], which is likely mediated by upregulating the expression of anti-apoptotic and antioxidant proteins (see reviews [56, 57]). Conversely, the deleterious effects of STAT1 in hepatocytes are likely mediated by the direct induction of apoptosis and the upregulation of chemokines and chemokine receptors [58, 59].
Interestingly, hepatic STAT1 and STAT3 not only functionally antagonize each other, but they also mutually inhibit each other’s activation. For example, inhibition of hepatic STAT3 mediated through deletion of either IL-6 or STAT3 resulted in enhanced STAT1 activation in Con A-induced hepatitis [8] and partial hepatectomy models [60, 61]. In contrast, deletion of STAT1 resulted in enhanced STAT3 activation in Con A-induced hepatitis model [8]. The mutual inhibition of STAT1 and STAT3 is mediated, at least in part, through the induction of SOCS1 and SOCS3, respectively, that inhibit both STAT1 and STAT3 activation in Con A-induced hepatitis models [8] (Fig. 2).
Liver regeneration
The mammalian liver has a great ability to regenerate fully after tissue loss or damage, which stimulates quiescent hepatocytes to enter the cell cycle and go through limited replication under the control of the broad spectrum of cytokines, growth factors, and hormones (see reviews [62, 63]). Among these factors, IL-6 represents the major cytokine that activates STAT3 in hepatocytes and is consequently responsible for hepatocyte proliferation following partial hepatectomy originally reported by Cressman et al. [64]; however, several follow-up studies using IL-6 knockout mice generate conflicting data on the role of IL-6 on hepatocyte proliferation and liver regeneration [65–68]. In contrast, the reports on the role of STAT3 in liver regeneration are consistent. For example, inhibition of hepatic STAT3, mediated through STAT3 or gp130 deletion, reduced liver regeneration after partial hepatectomy [60, 61, 69, 70]. Conversely, augmentation of hepatic STAT3, induced via either SOCS3 deletion or IL-22 overexpression, accelerated liver regeneration [47, 71].
Whereas STAT3 is critical for liver regeneration, STAT1 activation plays a role in inhibiting liver regeneration as STAT1 deletion accelerated liver regeneration and diminished the inhibitory effect of poly I:C treatment on liver regeneration in the partial hepatectomy model [58, 72]. Furthermore, in vitro IFN-γ treatment induced cell cycle arrest and apoptosis in wild-type but not in STAT1-deficient hepatocytes [58]. Recently, we demonstrated that hepatic STAT1 levels were highly upregulated in the double mutant mice with STAT3 deletion in myeloid cells and hepatocytes, and this STAT1 upregulation correlated with impaired liver regeneration and increased mortality in these mice following partial hepatectomy [60]. The additional deletion of STAT1 in these double mutant mice restored liver regeneration and abolished the mortality induced by partial hepatectomy, providing conclusive evidence that high STAT1 levels in the liver attenuate liver regeneration [60]. Interestingly, many viral hepatitis patients have high levels of hepatic STAT1 expression that positively correlate with liver injury but negatively correlate with hepatocyte proliferation [58, 73]. Thus, in patients with viral hepatitis, such enhanced STAT1 activation likely plays a beneficial role in eliminating HCV in the early stage of infection. However, when HCV infection fails to resolve and becomes persistent, STAT1 activation likely not only contributes to hepatocelluar damage, but also impedes liver regeneration by inhibiting hepatocyte proliferation.
Diverse functions of STAT proteins in liver inflammation
Inflammation is a key factor leading to chronic liver injury in viral hepatitis, alcoholic liver disease, and nonalcoholic steatohepatitis. The inflammatory process, which is characterized by the release of a diverse number of cytokines from immune cells, is critical for protection against infections and for triggering liver tissue repair mechanisms. However, when inflammation becomes excessive and recurrent, it can lead to chronic liver damage, which can ultimately progress to cirrhosis and HCC. Research from the last decade suggests that the activation of various STATs can act as anti- or pro-inflammatory signals in the pathogenesis of liver disease, depending on the STATs activated, the cell types in which the STATs are activated, and the type of liver disease or liver injury model being studied.
STAT1: a pro-inflammatory signal
Mice with a global deletion of STAT1 are resistant to liver injury and inflammation induced by Con A or LPS plus D-galactosamine [8, 44, 49], suggesting that STAT1 plays a pro-inflammatory role in the pathogenesis of liver disease. Accumulating evidence suggests that STAT1 activation in both liver parenchymal and nonparenchymal cells exacerbates liver inflammation and injury. In hepatocytes, STAT1 is predominantly activated by IFN-γ, and to a lesser extent by IFN-α/β and IL-27 [8, 48]. IFN-γ activation of STAT1 directly induces hepatocyte apoptosis, resulting in apoptosis-associated liver inflammation [8, 44, 49]. In addition, IFN-γ promotes liver inflammation by inducing the expression of chemokines and the adhesion molecules VCAM-1 and ICAM-1 in hepatocytes, sinusoidal endothelial cells, and Kupffer cells in an STAT1-dependent manner [59]. Finally, transgenic mice with over-expression STAT1 in T cells are more susceptible to Con A-induced hepatitis [44], suggesting that STAT1 in T cells acts as a pro-inflammatory signal to promote liver inflammation in this model.
Hepatocyte STAT3: an anti- and pro-inflammatory signal
STAT3 activation in hepatocytes occurs following stimulation with IL-22, IL-6, and IL-6 family cytokines and acts as an anti-inflammatory signal to suppress liver inflammation under most conditions [8, 45, 52, 74, 75], but could also promote liver inflammation in some models of liver injury. For example, disruption of STAT3 in hepatocytes markedly increased liver injury and inflammation after chronic CCl4 admistration [75], but decreased liver inflammation after acute CCl4 injection [50], suggesting that hepatocyte STAT3 can act as both an anti- and pro-inflammatory signal depending on the liver injury models. The anti-inflammatory effects of hepatocyte STAT3 are most likely due to the prevention of hepatocellular damage and the subsequent reduction of necrosis-associated inflammation. Moreover, hepatocyte STAT3 can suppress the pro-inflammatory functions of STAT1 in liver injury models with strong STAT1 activation, such as the Con A- and LPS-induced hepatitis models [8, 74]. The pro-inflammatory effects of hepatocyte STAT3 are thought to be mediated through the induction of acute phase proteins and chemokines in situations with weak STAT1 activation, such as the acute CCl4- and alcohol-induced liver injury models [50, 53].
Myeloid cell STAT3: an anti-inflammatory signal
STAT3 is a key downstream signaling protein of the anti-inflammatory cytokine IL-10 in macrophages [76], and accumulating evidence also confirms that STAT3 in macrophages and other myeloid cells acts as a critical anti-inflammatory signal to control liver inflammation. Myeloid-specific STAT3-deficient mice, in which STAT3 is deleted in myeloid linage cells including Kupffer cells/macrophages, are prone to a higher degree of liver inflammation in murine models of liver injury induced by a variety of hepatic toxins [42, 50, 53, 60]. Also, STAT3-deficient Kupffer cells produced higher levels of TNF-α after in vitro LPS stimulation compared with wild-type Kupffer cells [53]. These results suggest that STAT3 activation in macrophages inhibits pro-inflammatory cytokine production. At present, the mechanisms underlying the anti-inflammatory effects of STAT3 in macrophages remain largely unknown. One potential mechanism is that STAT3 mediates the inhibition of pro-inflammatory STAT1 signaling. Consistent with this, STAT1 activation is markedly upregulated in Kupffer cells/macrophages in myeloid-specific STAT3 deficient mice, the additional deletion of STAT1 in these mice reduced both hepatic and systemic inflammation in Con A-induced hepatitis and partial hepatectomy models [42, 60].
T cell STAT3: an anti- and pro-inflammatory signal
In T cells, STAT3 activation has been shown to promote or reduce liver inflammation depending on the liver injury models being studied. For example, T cell-specific STAT3-deficient mice are resistant to Con A-induced liver inflammation and exhibit reduced IL-17 production [42]. However, inhibition of STAT3 in T cells via SOCS3 overexpression accelerated acetaminophen hepatotoxicity due to the induction of IFN-γ and TNF-α production [77]. It is probable that STAT3 activation in T cells induces the expression of the RORγt and RORα transcription factors, which promote differentiation towards a Th17 phenotype. In turn, Th17 cell-derived IL-17 production could contribute to liver inflammation. However, STAT3 activation in T cells may also inhibit STAT1 signaling and prevent a polarization toward a Th1 phenotype, thus reducing IFN-γ production and inhibiting liver inflammation.
Taken together, these findings suggest that the role of STAT3 in liver inflammation is complex. While STAT1 promotes inflammation under many conditions, activation of the STAT3 signaling pathway in hepatocytes generally leads to anti-inflammatory responses by preventing hepatocellular damage and inhibiting the STAT1 signaling pathway. However, activation of STAT3 in hepatocytes may also enhance liver inflammation via the induction of acute phase proteins, chemokines, and chemokine receptors in several models. In myeloid cells, STAT3 activation is a key anti-inflammatory signal for the control of liver inflammation. Finally, in T cells, STAT3 can act as either a pro- or anti-inflammatory signal in regulating liver inflammation depending on the liver injury models being studied.
STAT4: a pro- and anti-inflammatory signal
In general, STAT4, which is activated by IL-12 and IFN-α/β in several types of immune cells, is important in generating inflammation during protective immune responses and immune-mediated diseases [78]. Overexpression of IL-12 in the liver by hydrodynamic injection of IL-12 cDNA resulted in liver injury [79]. Conversely, deletion of IL-12 suppressed liver inflammation in dominant negative TGF-β receptor transgenic mice [80] and in the Con A-induced hepatitis [81]. Also, IL-12 treatment has been shown to inhibit liver tumor growth in several animal models through the induction of a pro-inflammatory response [82, 83]. These findings suggest that IL-12 acts as a pro-inflammatory cytokine that induces liver injury and inhibits liver tumor growth by activating NK and NKT cells to produce IFN-γ [84]. Despite the fact that the functions of IL-12 in liver injury and inflammation have been extensively investigated, the role of STAT4 in the pathogenesis of liver diseases remains largely unknown. One study reported that STAT4-deficient mice were resistant to hepatic ischemia/reperfusion injury [85]; however, another study showed that STAT4-deficient and wild-type mice had equal liver injury after ischemia/reperfusion [86]. The reason for the discrepancy between these two studies is not clear and further studies are required to clarify the roles of STAT4 in liver injury and inflammation.
STAT6: a pro- and anti-inflammatory signal
Both IL-4 and IL-13 strongly induce STAT6 activation in the liver and likely play complex roles in controlling liver injury and inflammation. IL-4 has been shown to have pro-inflammatory/pathogenic effects via activation of STAT6 in a wide variety of liver injury models [87–90]. For example, IL-4- or STAT6-deficient mice were resistant to Con A-induced liver injury and inflammation [87]. Such detrimental effect of IL-4 in this model is likely mediated by upregulating eotaxins and IL-5 expression in the liver [87]. In contrast, IL-4-deficient mice were more susceptible to acetaminophen-induced liver injury, which was corrected by administration of recombinant IL-4 [91]. The hepatoprotective function of IL-4 in drug-induced injury is mediated, at least in part, via the upregulation of hepatic glutathione synthesis [91]. In addition, both IL-4 and IL-13 has also been shown to be protective against ischemia/reperfusion liver injury [92–96], which was hypothesized to be mediated through STAT6 activation and subsequent inhibition of inflammation and protection against hepatocyte and endothelial cell damage [92–96].
STATs and liver cancer
STAT1: a tumor suppressor
IFN-activated STAT1 is a well-documented tumor suppressor that induces cell cycle arrest and apoptosis in various types of tumors [97]. Consistent with this, STAT1-deficient mice are more susceptible to the development of methylcholanthrene-induced tumors and N-nitroso-N-methylurea-induced thymic tumors [98, 99]; however, they exhibit similar susceptibility to liver tumors induced by a single injection of DEN compared with wild-type mice [75]. The negligible role of STAT1 in this DEN-induced liver tumor model may be because this model is associated with minimal STAT1 activation [75]. Since STAT1 protein expression and phosphorylation are highly elevated in viral hepatitis [58, 73], STAT1 likely plays a role in preventing HCC development in patients with chronic viral hepatitis. Indeed, STAT1 gene polymorphisms with homozygous genotypes at rs867637, rs3771300, and rs2280235 in patients with viral hepatitis have been found to be associated with an increased risk for developing HCC [100]. In addition, a combination therapy of 5-fluorouracil with IFN-α, which activates STAT1 in liver cells, displayed promising results for the treatment of advanced HCC with tumor thrombi in the major portal branches [101].
STAT3: hepatoprotective versus oncogenic functions
It is generally believed that STAT3 activation contributes to the development and progression of many types of cancer, including liver cancer [102, 103]. The oncogenic effect of STAT3 in tumor cells is mediated by the upregulation of a diverse array of genes that promote tumor cell survival and proliferation, and many mediators that suppress anti-tumor immunity [102, 103]. The important role of STAT3 in promoting liver tumorigenesis has also been well documented [57, 104]. First, STAT3 protein expression and phosphorylation are elevated in human HCC tissue samples compared with surrounding non-neoplastic tissue and normal healthy liver tissue samples [105, 106]. In human HCC, the increased STAT3 activation is likely due to persistent stimulation from upstream signals such as the oncogenes and cytokines such as IL-22 [47, 107], or due to the blockade of inhibitory pathways, such as the methylation-mediated silencing of SOCS proteins [108, 109]. Second, inhibition of STAT3 activation by STAT3 inhibitors [105], miR-637 [110], or sunitinib [111] reduced liver tumor cell growth in vitro or in vivo; while activation of STAT3 by HCV core protein [5] or HBX protein [112] promoted HCC development. Third, genetic deletion of IL-6 resulted in a reduction of STAT3 activation and the prevention of diethylnitrosamine (DEN)-induced HCC development in lean [113] and obese mice [114]. In contrast, augmentation of liver STAT3 activation mediated through IL-22 overexpression or the conditional deletion of the SHP-2 or SOCS3 in hepatocytes increased DEN-induced HCC development [47, 71, 115]. Finally, conditional deletion of STAT3 in hepatocytes reduced DEN-induced HCC development in wild-type mice [75, 116] and in liver-specific SHP-2 knockout mice [115].
It is well known that more than 80% of human HCC develop following chronic liver injury, inflammation, and cirrhosis. However, the DEN model is associated with minimal liver inflammation and injury. Thus this model may not be an ideal one to investigate the molecular mechanisms of human HCC development caused by chronic liver injury and inflammation. Instead, we utilized a model of chronic liver injury induced by repeated injection of CCl4 and found that deletion of hepatic STAT3 exacerbated CCl4-induced liver inflammation and fibrosis and increased the incidence of HCC development [75]. Collectively, hepatic STAT3 accelerates liver tumor development induced by a single injection of DEN, but prevents liver tumor development in the murine model of chronic CCl4 administration [75]. These dual roles of STAT3 in liver tumorigenesis are summarized in Fig. 3. Under the conditions of persistent inflammatory stress and liver injury, STAT3 acts as a hepatoprotective signal to prevent hepatic damage and fibrosis, consequently suppressing injury- and inflammation-driven liver tumor initiation. However, once liver tumor cells have developed, STAT3 likely acts as an oncogenic factor that promotes tumorigenesis. Interestingly, both tumor suppressive and oncogenic effects of STAT3 were also recently reported in a murine model of liver tumors [117, 118]. In this model, overexpression of a constitutively active form of STAT3 promoted liver tumorigenesis in the presence of the tumor suppressor p14ARF (the human homolog of p19ARF). However, in the absence of p14ARF, constitutively active STAT3 induced tumor suppression, likely via the activation of an alternative group of STAT3-specific target genes with anti-oncogenic activity.
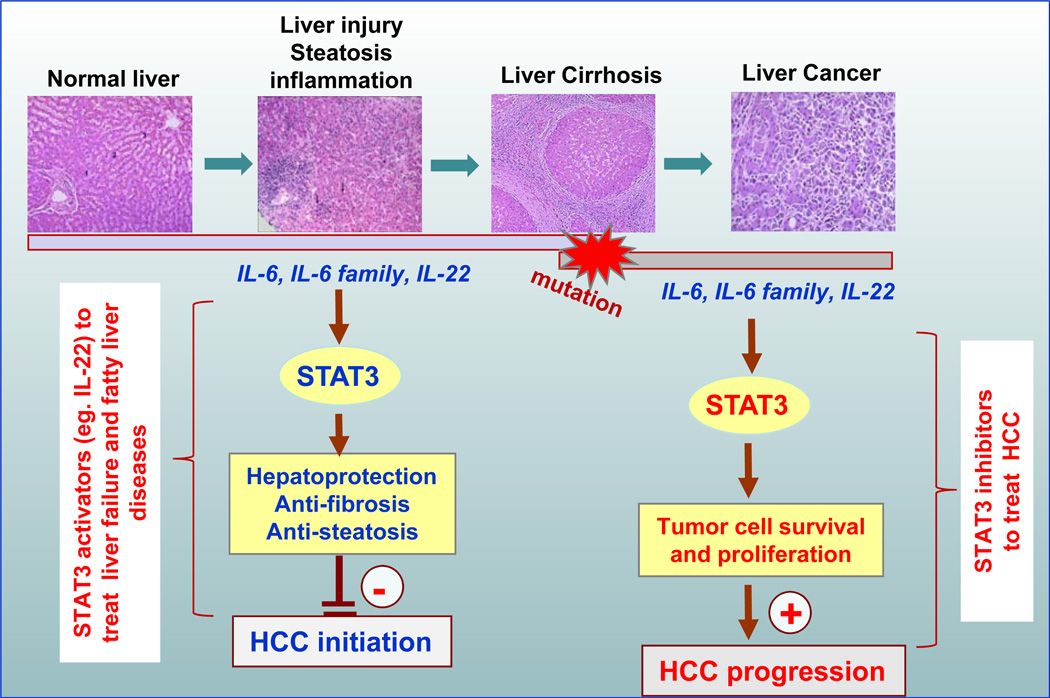
Fig. 3. Hepatoprotective versus oncogenic functions of STAT3.
During chronic liver injury, steatosis, and inflammation, IL-6, IL-6 family cytokines, and IL-22 induce STAT3 activation, leading to the upregulation of a variety of anti-apoptotic, anti-oxidative, and anti-steatogenic proteins in hepatocytes that prevent liver injury and inhibit injury-associated HCC initiation. IL-22 may have therapeutic potential in the treatment of liver failure (e.g., acute alcoholic hepatitis) and fatty liver disease. In contrast, during endstage liver cirrhosis and liver cancer, STAT3 activation promotes tumor cell survival and proliferation and therefore HCC progression. Thus, STAT3 inhibitors may have therapeutic potential in the treatment of HCC.
STAT5a/b: a tumor suppressor and hepatoprotective factor
Constitutively activated STAT5 has been observed in a wide variety of tumors, including HCC [119]. Many studies suggest that STAT5 activation plays an important role in promoting tumorigenesis via the upregulation of anti-apoptotic, cell proliferative, and invasion and metastasis-related genes [119]. However, recent studies have demonstrated that hepatic growth hormone-mediated STAT5 activation plays a hepatoprotective role in preventing the development of HCC. First, liver-specific STAT5 knockout mice are more susceptible to chronic CCl4-induced liver fibrosis and HCC development [120]. Second, the combined deletion of STAT5 and the glucocorticoid receptor in hepatocytes results in severe metabolic liver disease and spontaneous hepatic tumorigenesis [121]. Finally, the conditional deletion of hepatic STAT5 accelerated inflammatory liver cancer caused by hyperactivated growth hormone signaling despite the observed reductions in chronic inflammation [122]. These findings suggest that STAT5 acts as a tumor suppressor in liver tumorigenesis via its anti-steatogenic and hepatoprotective effects and through the upregulation of the cell cycle inhibitors Cdkn2b and Cdkn1a [121–123]. However, it is not clear whether STAT5, similar to STAT3, can also promote HCC cell proliferation once HCC cells have developed.
STATs as potential clinical targets for the treatment of liver diseases
Although STATs have been identified as the key regulators of hepatic anti-viral responses, inflammation, and tumorigenesis, the translation of them as therapeutic targets for the treatment of liver diseases has lagged behind. Here we discuss several candidates of STATs as potential therapeutic targets.
STAT1-STAT2 activators
Activation of STAT1 and STAT2 in hepatocytes plays key roles in the IFN-α-mediated anti-viral response against HCV infections. Enhancing activation of these STATs could be an attractive strategy to improve the efficiency of IFN-α therapy for the treatment of HCV. Indeed, a recent study showed that treatment with S-adenosyl methionine, which potentiates STAT1 activation, improved the early viral kinetics and increases IFN-stimulated gene induction in nonresponders treated with peg-IFN and ribavirin [124].
STAT3 inhibitors
Although STAT3 inhibitors have been actively investigated in preclinical studies for the treatment of HCC and other various types of cancer [125], they have not yet been tested in HCC patients. Sorafenib is a safe and effective drug approved for the treatment of advanced HCC [126–128]. It was originally developed as a small molecule inhibitor of the VEGFR and PDGFR tyrosine kinases and the Raf/Mek/Erk pathways [129]. However, it is now known that sorafenib also inhibits STAT3 in liver cancer cells by inducing the activation of protein tyrosine phosphatases [130, 131]. Interestingly, a recent study showed that SC-1, a sorafenib analog lacking inhibitory activity toward the VEGFR and PDGFR tyrosine kinases and the Raf/Mek/Erk pathways but retaining inhibitory activity against STAT3, was as potent as sorafenib in the induction of cell cycle arrest and apoptosis of human HCC cell lines in vitro [131]. This study suggests that STAT3 inhibition is predominately responsible for the sorafenib-mediated anti-tumor effects observed on HCC cells, whereas the inhibition of the VEGFR and PDGFR tyrosine kinases and the Raf/Mek/Erk pathways plays a minor role [131, 132]. Thus, clinical trials examining specific STAT3 inhibitors for HCC patients are warranted.
STAT3 activator
IL-22, which activates STAT3 in hepatocytes but not in immune cells, is currently under the development for the treatment of human fulminant hepatitis, liver failure, and fatty liver disease. This is based on the facts that IL-22 promotes hepatocyte survival and proliferation [47, 133], and ameliorates steatosis [54, 134] with the added benefit of antimicrobial effects and potentially few side effects. Since IL-22 also promotes liver tumor cell survival [47, 133], the application of IL-22 should not be used in patients with pre-cancerous cirrhosis or liver cancer.
Conclusions
In summary, studies from the last decade from animal models suggest that multiple STATs collectively exhibit diverse and complex biological functions in regulating hepatic anti-viral responses, inflammation, and tumorigenesis. These findings have markedly enhanced our understanding of liver disease pathophysiology and treatments, but translation of these basic research findings into new therapeutic modalities for managing human liver diseases has been modest. We hope this review article will stimulate translational and clinical research on these topics in the near future.
Key points.
STAT1 and STAT2 activated by both IFN-α and IFN-λ play a key role in inhibiting HCV replication; Enhancing activation of these STATs could be an attractive strategy to improve the efficiency of IFN-α therapy for the treatment of HCV.
Under most conditions, activated STAT1 and STAT3 play opposing roles in controlling liver injury, regeneration and inflammation.
The functions of STAT4 and STAT6 in liver injury, inflammation, and regeneration remain largely unknown.
STAT3 and STAT5 are hepatoprotective and prevent liver injury-associated HCC initiation during early stage of chronic liver injury; but STAT3 also promotes liver tumor cell survival and proliferation when tumor cells have developed during end-stage of liver cirrhosis and liver cancer.
Acknowledgments
The work described here from Dr. Bin Gao’s laboratory was supported by the intramural program of the NIAAA, NIH.
Abbreviations
- CCl4
carbon tetrachloride
- Con A
concanavalin A
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- IL
interleukin
- JAK
Janus kinase
- NK
natural killer
- SNP
single nucleotide polymorphism
- SOCS
suppressor of cytokine signaling
- STAT
signal transducer and activator of transcription
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No conflicts of interest exist for all authors.
References
- 1.Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- 2.Schindler C. Cytokines and JAK-STAT signaling. Exp Cell Res. 1999;253:7–14. doi: 10.1006/excr.1999.4670. [DOI] [PubMed] [Google Scholar]
- 3.Gao B. Cytokines, STATs and liver disease. Cell Mol Immunol. 2005;2:92–100. [PubMed] [Google Scholar]
- 4.Ruff-Jamison S, Chen K, Cohen S. Induction by EGF and interferon-gamma of tyrosine phosphorylated DNA binding proteins in mouse liver nuclei. Science. 1993;261:1733–1736. doi: 10.1126/science.8378774. [DOI] [PubMed] [Google Scholar]
- 5.Machida K, Tsukamoto H, Liu JC, Han YP, Govindarajan S, Lai MM, et al. c-Jun mediates hepatitis C virus hepatocarcinogenesis through signal transducer and activator of transcription 3 and nitric oxide-dependent impairment of oxidative DNA repair. Hepatology. 2010;52:480–492. doi: 10.1002/hep.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wormald S, Hilton DJ. Inhibitors of cytokine signal transduction. J Biol Chem. 2004;279:821–824. doi: 10.1074/jbc.R300030200. [DOI] [PubMed] [Google Scholar]
- 7.Alexander WS, Hilton DJ. The role of suppressors of cytokine signaling (SOCS) proteins in regulation of the immune response. Annu Rev Immunol. 2004;22:503–529. doi: 10.1146/annurev.immunol.22.091003.090312. [DOI] [PubMed] [Google Scholar]
- 8.Hong F, Jaruga B, Kim WH, Radaeva S, El-Assal ON, Tian Z, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Croker BA, Krebs DL, Zhang JG, Wormald S, Willson TA, Stanley EG, et al. SOCS3 negatively regulates IL-6 signaling in vivo. Nat Immunol. 2003;4:540–545. doi: 10.1038/ni931. [DOI] [PubMed] [Google Scholar]
- 10.Rehermann B. Hepatitis C virus versus innate and adaptive immune responses: a tale of coevolution and coexistence. J Clin Invest. 2009;119:1745–1754. doi: 10.1172/JCI39133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahlenstiel G, Titerence RH, Koh C, Edlich B, Feld JJ, Rotman Y, et al. Natural killer cells are polarized toward cytotoxicity in chronic hepatitis C in an interferon-alfa-dependent manner. Gastroenterology. 2010;138:325–335. e321–e322. doi: 10.1053/j.gastro.2009.08.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheent K, Khakoo SI. Natural killer cells and hepatitis C: action and reaction. Gut. 2011;60:268–278. doi: 10.1136/gut.2010.212555. [DOI] [PubMed] [Google Scholar]
- 13.Golden-Mason L, Cox AL, Randall JA, Cheng L, Rosen HR. Increased natural killer cell cytotoxicity and NKp30 expression protects against hepatitis C virus infection in high-risk individuals and inhibits replication in vitro. Hepatology. 2010;52:1581–1589. doi: 10.1002/hep.23896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amadei B, Urbani S, Cazaly A, Fisicaro P, Zerbini A, Ahmed P, et al. Activation of natural killer cells during acute infection with hepatitis C virus. Gastroenterology. 2010;138:1536–1545. doi: 10.1053/j.gastro.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stegmann KA, Bjorkstrom NK, Veber H, Ciesek S, Riese P, Wiegand J, et al. Interferon-alpha-induced TRAIL on natural killer cells is associated with control of hepatitis C virus infection. Gastroenterology. 2010;138:1885–1897. doi: 10.1053/j.gastro.2010.01.051. [DOI] [PubMed] [Google Scholar]
- 16.Ahlenstiel G, Edlich B, Hogdal LJ, Rotman Y, Noureddin M, Feld JJ, et al. Early changes in natural killer cell function indicate virologic response to interferon therapy for hepatitis C. Gastroenterology. 2011;141:1231–1239. e1232. doi: 10.1053/j.gastro.2011.06.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang SH, Huang CX, Ye L, Wang X, Song L, Wang YJ, et al. Natural killer cells suppress full cycle HCV infection of human hepatocytes. J Viral Hepat. 2008;15:855–864. doi: 10.1111/j.1365-2893.2008.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miyagi T, Takehara T, Nishio K, Shimizu S, Kohga K, Li W, et al. Altered interferon-alpha-signaling in natural killer cells from patients with chronic hepatitis C virus infection. J Hepatol. 2010;53:424–430. doi: 10.1016/j.jhep.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 19.Edlich BAG, Azpiroz AZ, Stoltzfus J, Noureddin M, Serti E, Feld JJ, Jake Liang T, Rotman Y, Rehermann B. Early changes in interferon signaling define natural killer cell response and refractoriness to interferon-based therapy of hepatitis C. Hepatology. 2011 doi: 10.1002/hep.24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Donnelly RP, Kotenko SV. Interferon-lambda: a new addition to an old family. J Interferon Cytokine Res. 2010;30:555–564. doi: 10.1089/jir.2010.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Diegelmann J, Beigel F, Zitzmann K, Kaul A, Goke B, Auernhammer CJ, et al. Comparative analysis of the lambda-interferons IL-28A and IL-29 regarding their transcriptome and their antiviral properties against hepatitis C virus. PLoS One. 2010;5:e15200. doi: 10.1371/journal.pone.0015200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marcello T, Grakoui A, Barba-Spaeth G, Machlin ES, Kotenko SV, MacDonald MR, et al. Interferons alpha and lambda inhibit hepatitis C virus replication with distinct signal transduction and gene regulation kinetics. Gastroenterology. 2006;131:1887–1898. doi: 10.1053/j.gastro.2006.09.052. [DOI] [PubMed] [Google Scholar]
- 23.Donnelly RP, Dickensheets H, O'Brien TR. Interferon-lambda and therapy for chronic hepatitis C virus infection. Trends Immunol. 2011;32:443–450. doi: 10.1016/j.it.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang L, Jilg N, Shao RX, Lin W, Fusco DN, Zhao H, et al. IL28B inhibits hepatitis C virus replication through the JAK-STAT pathway. J Hepatol. 2011;55:289–298. doi: 10.1016/j.jhep.2010.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pagliaccetti NE, Eduardo R, Kleinstein SH, Mu XJ, Bandi P, Robek MD. Interleukin-29 functions cooperatively with interferon to induce antiviral gene expression and inhibit hepatitis C virus replication. J Biol Chem. 2008;283:30079–30089. doi: 10.1074/jbc.M804296200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robek MD, Boyd BS, Chisari FV. Lambda interferon inhibits hepatitis B and C virus replication. J Virol. 2005;79:3851–3854. doi: 10.1128/JVI.79.6.3851-3854.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muir AJ, Shiffman ML, Zaman A, Yoffe B, de la Torre A, Flamm S, et al. Phase 1b study of pegylated interferon lambda 1 with or without ribavirin in patients with chronic genotype 1 hepatitis C virus infection. Hepatology. 2010;52:822–832. doi: 10.1002/hep.23743. [DOI] [PubMed] [Google Scholar]
- 28.Ramos EL. Preclinical and clinical development of pegylated interferon-lambda 1 in chronic hepatitis C. J Interferon Cytokine Res. 2010;30:591–595. doi: 10.1089/jir.2010.0066. [DOI] [PubMed] [Google Scholar]
- 29.Afdhal NH, McHutchison JG, Zeuzem S, Mangia A, Pawlotsky JM, Murray JS, et al. Hepatitis C pharmacogenetics: state of the art in 2010. Hepatology. 2011;53:336–345. doi: 10.1002/hep.24052. [DOI] [PubMed] [Google Scholar]
- 30.Balagopal A, Thomas DL, Thio CL. IL28B and the control of hepatitis C virus infection. Gastroenterology. 2010;139:1865–1876. doi: 10.1053/j.gastro.2010.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lange CM, Zeuzem S. IL28B single nucleotide polymorphisms in the treatment of hepatitis C. J Hepatol. 2011;55:692–701. doi: 10.1016/j.jhep.2011.03.006. [DOI] [PubMed] [Google Scholar]
- 32.Lange CM, Moradpour D, Doehring A, Lehr HA, Mullhaupt B, Bibert S, et al. Impact of donor and recipient IL28B rs12979860 genotypes on hepatitis C virus liver graft reinfection. J Hepatol. 2011;55:322–327. doi: 10.1016/j.jhep.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 33.Fukuhara T, Taketomi A, Motomura T, Okano S, Ninomiya A, Abe T, et al. Variants in IL28B in liver recipients and donors correlate with response to peg-interferon and ribavirin therapy for recurrent hepatitis C. Gastroenterology. 2010;139:1577–1585. doi: 10.1053/j.gastro.2010.07.058. [DOI] [PubMed] [Google Scholar]
- 34.Charlton MR, Thompson A, Veldt BJ, Watt K, Tillmann H, Poterucha JJ, et al. Interleukin-28B polymorphisms are associated with histological recurrence and treatment response following liver transplantation in patients with hepatitis C virus infection. Hepatology. 2011;53:317–324. doi: 10.1002/hep.24074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Langhans B, Kupfer B, Braunschweiger I, Arndt S, Schulte W, Nischalke HD, et al. Interferon-lambda serum levels in hepatitis C. J Hepatol. 2011;54:859–865. doi: 10.1016/j.jhep.2010.08.020. [DOI] [PubMed] [Google Scholar]
- 36.Abe H, Hayes CN, Ochi H, Maekawa T, Tsuge M, Miki D, et al. IL28 variation affects expression of interferon stimulated genes and peg-interferon and ribavirin therapy. J Hepatol. 2011;54:1094–1101. doi: 10.1016/j.jhep.2010.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Urban TJ, Thompson AJ, Bradrick SS, Fellay J, Schuppan D, Cronin KD, et al. IL28B genotype is associated with differential expression of intrahepatic interferon-stimulated genes in patients with chronic hepatitis C. Hepatology. 2010;52:1888–1896. doi: 10.1002/hep.23912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Makowska Z, Duong FH, Trincucci G, Tough DF, Heim MH. Interferon-beta and interferon-lambda signaling is not affected by interferon-induced refractoriness to interferon-alpha in vivo. Hepatology. 2011;53:1154–1163. doi: 10.1002/hep.24189. [DOI] [PubMed] [Google Scholar]
- 39.Radaeva S, Jaruga B, Hong F, Kim WH, Fan S, Cai H, et al. Interferon-alpha activates multiple STAT signals and down-regulates c- Met in primary human hepatocytes. Gastroenterology. 2002;122:1020–1034. doi: 10.1053/gast.2002.32388. [DOI] [PubMed] [Google Scholar]
- 40.Mair M, Blaas L, Osterreicher CH, Casanova E, Eferl R. JAK-STAT signaling in hepatic fibrosis. Front Biosci. 2011;17:2794–2811. doi: 10.2741/3886. [DOI] [PubMed] [Google Scholar]
- 41.Torisu T, Nakaya M, Watanabe S, Hashimoto M, Yoshida H, Chinen T, et al. Suppressor of cytokine signaling 1 protects mice against concanavalin A-induced hepatitis by inhibiting apoptosis. Hepatology. 2008;47:1644–1654. doi: 10.1002/hep.22214. [DOI] [PubMed] [Google Scholar]
- 42.Lafdil F, Wang H, Park O, Zhang W, Moritoki Y, Yin S, et al. Myeloid STAT3 inhibits T cell-mediated hepatitis by regulating T helper 1 cytokine and interleukin-17 production. Gastroenterology. 2009;137:2125–2135. e2121–e2122. doi: 10.1053/j.gastro.2009.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein C, Wustefeld T, Assmus U, Roskams T, Rose-John S, Muller M, et al. The IL-6-gp130-STAT3 pathway in hepatocytes triggers liver protection in T cell-mediated liver injury. J Clin Invest. 2005;115:860–869. doi: 10.1172/JCI200523640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Siebler J, Wirtz S, Klein S, Protschka M, Blessing M, Galle PR, et al. A key pathogenic role for the STAT1/T-bet signaling pathway in T-cell-mediated liver inflammation. Hepatology. 2003;38:1573–1580. doi: 10.1016/j.hep.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Karow M, Flavell RA. Interleukin-22 but not interleukin-17 provides protection to hepatocytes during acute liver inflammation. Immunity. 2007;27:647–659. doi: 10.1016/j.immuni.2007.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogata H, Kobayashi T, Chinen T, Takaki H, Sanada T, Minoda Y, et al. Deletion of the SOCS3 gene in liver parenchymal cells promotes hepatitis-induced hepatocarcinogenesis. Gastroenterology. 2006;131:179–193. doi: 10.1053/j.gastro.2006.04.025. [DOI] [PubMed] [Google Scholar]
- 47.Park O, Wang H, Weng H, Feigenbaum L, Li H, Yin S, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54:252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Siebler J, Wirtz S, Frenzel C, Schuchmann M, Lohse AW, Galle PR, et al. Cutting edge: a key pathogenic role of IL-27 in T cell- mediated hepatitis. J Immunol. 2008;180:30–33. doi: 10.4049/jimmunol.180.1.30. [DOI] [PubMed] [Google Scholar]
- 49.Kim WH, Hong F, Radaeva S, Jaruga B, Fan S, Gao B. STAT1 plays an essential role in LPS/D-galactosamine-induced liver apoptosis and injury. Am J Physiol Gastrointest Liver Physiol. 2003;285:G761–G768. doi: 10.1152/ajpgi.00224.2003. [DOI] [PubMed] [Google Scholar]
- 50.Horiguchi N, Lafdil F, Miller AM, Park O, Wang H, Rajesh M, et al. Dissociation between liver inflammation and hepatocellular damage induced by carbon tetrachloride in myeloid cell-specific signal transducer and activator of transcription 3 gene knockout mice. Hepatology. 2010;51:1724–1734. doi: 10.1002/hep.23532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mair M, Zollner G, Schneller D, Musteanu M, Fickert P, Gumhold J, et al. Signal transducer and activator of transcription 3 protects from liver injury and fibrosis in a mouse model of sclerosing cholangitis. Gastroenterology. 2010;138:2499–2508. doi: 10.1053/j.gastro.2010.02.049. [DOI] [PubMed] [Google Scholar]
- 52.Kroy DC, Beraza N, Tschaharganeh DF, Sander LE, Erschfeld S, Giebeler A, et al. Lack of interleukin-6/glycoprotein 130/signal transducers and activators of transcription-3 signaling in hepatocytes predisposes to liver steatosis and injury in mice. Hepatology. 2010;51:463–473. doi: 10.1002/hep.23322. [DOI] [PubMed] [Google Scholar]
- 53.Horiguchi N, Wang L, Mukhopadhyay P, Park O, Jeong WI, Lafdil F, et al. Cell type-dependent pro- and anti-inflammatory role of signal transducer and activator of transcription 3 in alcoholic liver injury. Gastroenterology. 2008;134:1148–1158. doi: 10.1053/j.gastro.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ki SH, Park O, Zheng M, Morales-Ibanez O, Kolls JK, Bataller R, et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology. 2010;52:1291–1300. doi: 10.1002/hep.23837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Haga S, Terui K, Zhang HQ, Enosawa S, Ogawa W, Inoue H, et al. Stat3 protects against Fas-induced liver injury by redox-dependent and -independent mechanisms. J Clin Invest. 2003;112:989–998. doi: 10.1172/JCI17970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taub R. Hepatoprotection via the IL-6/Stat3 pathway. J Clin Invest. 2003;112:978–980. doi: 10.1172/JCI19974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang H, Lafdil F, Kong X, Gao B. Signal transducer and activator of transcription 3 in liver diseases: a novel therapeutic target. Int J Biol Sci. 2011;7:536–550. doi: 10.7150/ijbs.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun R, Park O, Horiguchi N, Kulkarni S, Jeong WI, Sun HY, et al. STAT1 contributes to dsRNA inhibition of liver regeneration after partial hepatectomy in mice. Hepatology. 2006;44:955–966. doi: 10.1002/hep.21344. [DOI] [PubMed] [Google Scholar]
- 59.Jaruga B, Hong F, Kim WH, Gao B. IFN-{gamma}/STAT1 acts as a proinflammatory signal in T cell-mediated hepatitis via induction of multiple chemokines and adhesion molecules: a critical role of IRF-1. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1044–G1052. doi: 10.1152/ajpgi.00184.2004. [DOI] [PubMed] [Google Scholar]
- 60.Wang H, Park O, Lafdil F, Shen K, Horiguchi N, Yin S, et al. Interplay of hepatic and myeloid signal transducer and activator of transcription 3 in facilitating liver regeneration via tempering innate immunity. Hepatology. 2010;51:1354–1362. doi: 10.1002/hep.23430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Li W, Liang X, Kellendonk C, Poli V, Taub R. STAT3 contributes to the mitogenic response of hepatocytes during liver regeneration. J Biol Chem. 2002;277:28411–28417. doi: 10.1074/jbc.M202807200. [DOI] [PubMed] [Google Scholar]
- 62.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176:2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fausto N, Campbell JS, Riehle KJ. Liver regeneration. Hepatology. 2006;43:S45–S53. doi: 10.1002/hep.20969. [DOI] [PubMed] [Google Scholar]
- 64.Cressman DE, Greenbaum LE, DeAngelis RA, Ciliberto G, Furth EE, Poli V, et al. Liver failure and defective hepatocyte regeneration in interleukin-6-deficient mice. Science (New York, NY. 1996;274:1379–1383. doi: 10.1126/science.274.5291.1379. [DOI] [PubMed] [Google Scholar]
- 65.Sakamoto T, Liu Z, Murase N, Ezure T, Yokomuro S, Poli V, et al. Mitosis and apoptosis in the liver of interleukin-6-deficient mice after partial hepatectomy. Hepatology. 1999;29:403–411. doi: 10.1002/hep.510290244. [DOI] [PubMed] [Google Scholar]
- 66.Blindenbacher A, Wang X, Langer I, Savino R, Terracciano L, Heim MH. Interleukin 6 is important for survival after partial hepatectomy in mice. Hepatology (Baltimore, Md. 2003;38:674–682. doi: 10.1053/jhep.2003.50378. [DOI] [PubMed] [Google Scholar]
- 67.Sun R, Jaruga B, Kulkarni S, Sun H, Gao B. IL-6 modulates hepatocyte proliferation via induction of HGF/p21cip1: regulation by SOCS3. Biochemical and biophysical research communications. 2005;338:1943–1949. doi: 10.1016/j.bbrc.2005.10.171. [DOI] [PubMed] [Google Scholar]
- 68.Wuestefeld T, Klein C, Streetz KL, Betz U, Lauber J, Buer J, et al. Interleukin-6/glycoprotein 130-dependent pathways are protective during liver regeneration. The Journal of biological chemistry. 2003;278:11281–11288. doi: 10.1074/jbc.M208470200. [DOI] [PubMed] [Google Scholar]
- 69.Dierssen U, Beraza N, Lutz HH, Liedtke C, Ernst M, Wasmuth HE, et al. Molecular dissection of gp130-dependent pathways in hepatocytes during liver regeneration. J Biol Chem. 2008;283:9886–9895. doi: 10.1074/jbc.M705483200. [DOI] [PubMed] [Google Scholar]
- 70.Moh A, Iwamoto Y, Chai GX, Zhang SS, Kano A, Yang DD, et al. Role of STAT3 in liver regeneration: survival, DNA synthesis, inflammatory reaction and liver mass recovery. Lab Invest. 2007;87:1018–1028. doi: 10.1038/labinvest.3700630. [DOI] [PubMed] [Google Scholar]
- 71.Riehle KJ, Campbell JS, McMahan RS, Johnson MM, Beyer RP, Bammler TK, et al. Regulation of liver regeneration and hepatocarcinogenesis by suppressor of cytokine signaling 3. J Exp Med. 2008;205:91–103. doi: 10.1084/jem.20070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun R, Gao B. Negative regulation of liver regeneration by innate immunity (natural killer cells/interferon-gamma) Gastroenterology. 2004;127:1525–1539. doi: 10.1053/j.gastro.2004.08.055. [DOI] [PubMed] [Google Scholar]
- 73.Radaeva S, Jaruga B, Kim WH, Heller T, Liang TJ, Gao B. Interferon-gamma inhibits interferon-alpha signalling in hepatic cells: evidence for the involvement of STAT1 induction and hyperexpression of STAT1 in chronic hepatitis C. Biochem J. 2004;379:199–208. doi: 10.1042/BJ20031495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sakamori R, Takehara T, Ohnishi C, Tatsumi T, Ohkawa K, Takeda K, et al. Signal transducer and activator of transcription 3 signaling within hepatocytes attenuates systemic inflammatory response and lethality in septic mice. Hepatology. 2007;46:1564–1573. doi: 10.1002/hep.21837. [DOI] [PubMed] [Google Scholar]
- 75.Wang H, Lafdil F, Wang L, Park O, Yin S, Niu J, et al. Hepatoprotective versus Oncogenic Functions of STAT3 in Liver Tumorigenesis. Am J Pathol. 2011;179:714–724. doi: 10.1016/j.ajpath.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Murray PJ. Understanding and exploiting the endogenous interleukin-10/STAT3-mediated anti-inflammatory response. Curr Opin Pharmacol. 2006;6:379–386. doi: 10.1016/j.coph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 77.Numata K, Kubo M, Watanabe H, Takagi K, Mizuta H, Okada S, et al. Overexpression of suppressor of cytokine signaling-3 in T cells exacerbates acetaminophen-induced hepatotoxicity. J Immunol. 2007;178:3777–3785. doi: 10.4049/jimmunol.178.6.3777. [DOI] [PubMed] [Google Scholar]
- 78.Kaplan MH. STAT4: a critical regulator of inflammation in vivo. Immunol Res. 2005;31:231–242. doi: 10.1385/IR:31:3:231. [DOI] [PubMed] [Google Scholar]
- 79.Rodriguez-Galan MC, Reynolds D, Correa SG, Iribarren P, Watanabe M, Young HA. Coexpression of IL-18 strongly attenuates IL-12-induced systemic toxicity through a rapid induction of IL-10 without affecting its antitumor capacity. J Immunol. 2009;183:740–748. doi: 10.4049/jimmunol.0804166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yoshida K, Yang GX, Zhang W, Tsuda M, Tsuneyama K, Moritoki Y, et al. Deletion of interleukin-12p40 suppresses autoimmune cholangitis in dominant negative transforming growth factor beta receptor type II mice. Hepatology. 2009;50:1494–1500. doi: 10.1002/hep.23132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhu R, Diem S, Araujo LM, Aumeunier A, Denizeau J, Philadelphe E, et al. The Pro-Th1 cytokine IL-12 enhances IL-4 production by invariant NKT cells: relevance for T cell-mediated hepatitis. J Immunol. 2007;178:5435–5442. doi: 10.4049/jimmunol.178.9.5435. [DOI] [PubMed] [Google Scholar]
- 82.Chang CJ, Chen YH, Huang KW, Cheng HW, Chan SF, Tai KF, et al. Combined GM-CSF and IL-12 gene therapy synergistically suppresses the growth of orthotopic liver tumors. Hepatology. 2007;45:746–754. doi: 10.1002/hep.21560. [DOI] [PubMed] [Google Scholar]
- 83.Harada N, Shimada M, Okano S, Suehiro T, Soejima Y, Tomita Y, et al. IL-12 gene therapy is an effective therapeutic strategy for hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2004;173:6635–6644. doi: 10.4049/jimmunol.173.11.6635. [DOI] [PubMed] [Google Scholar]
- 84.Subleski JJ, Hall VL, Back TC, Ortaldo JR, Wiltrout RH. Enhanced antitumor response by divergent modulation of natural killer and natural killer T cells in the liver. Cancer Res. 2006;66:11005–11012. doi: 10.1158/0008-5472.CAN-06-0811. [DOI] [PubMed] [Google Scholar]
- 85.Shen XD, Ke B, Zhai Y, Gao F, Anselmo D, Lassman CR, et al. Stat4 and Stat6 signaling in hepatic ischemia/reperfusion injury in mice: HO-1 dependence of Stat4 disruption-mediated cytoprotection. Hepatology. 2003;37:296–303. doi: 10.1053/jhep.2003.50066. [DOI] [PubMed] [Google Scholar]
- 86.Kato A, Graul-Layman A, Edwards MJ, Lentsch AB. Promotion of hepatic ischemia/reperfusion injury by IL-12 is independent of STAT4. Transplantation. 2002;73:1142–1145. doi: 10.1097/00007890-200204150-00023. [DOI] [PubMed] [Google Scholar]
- 87.Jaruga B, Hong F, Sun R, Radaeva S, Gao B. Crucial Role of IL-4/STAT6 in T Cell-Mediated Hepatitis: Up-Regulating Eotaxins and IL-5 and Recruiting Leukocytes. J Immunol. 2003;171:3233–3244. doi: 10.4049/jimmunol.171.6.3233. [DOI] [PubMed] [Google Scholar]
- 88.Higuchi S, Kobayashi M, Yoshikawa Y, Tsuneyama K, Fukami T, Nakajima M, et al. IL-4 mediates dicloxacillin-induced liver injury in mice. Toxicol Lett. 2011;200:139–145. doi: 10.1016/j.toxlet.2010.11.006. [DOI] [PubMed] [Google Scholar]
- 89.Njoku DB, Li Z, Washington ND, Mellerson JL, Talor MV, Sharma R, et al. Suppressive and pro-inflammatory roles for IL-4 in the pathogenesis of experimental drug-induced liver injury. Eur J Immunol. 2009;39:1652–1663. doi: 10.1002/eji.200838135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Douglas DB, Beiting DP, Loftus JP, Appleton JA, Bliss SK. Combinatorial effects of interleukin 10 and interleukin 4 determine the progression of hepatic inflammation following murine enteric parasitic infection. Hepatology. 2010;51:2162–2171. doi: 10.1002/hep.23576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ryan PBM, Korrapati MC, Proctor WR, Vasquez RV, Yee SB, Quinn TD, Chakraborty M, Pohl L. Endogenous interleukin-4 regulates glutathione synthesis following acetaminophen-induced liver injury in mice. Chem Res Toxicol. 2012 doi: 10.1021/tx2003992. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kato A, Yoshidome H, Edwards MJ, Lentsch AB. Reduced hepatic ischemia/reperfusion injury by IL-4: potential anti- inflammatory role of STAT6. Inflamm Res. 2000;49:275–279. doi: 10.1007/PL00000207. [DOI] [PubMed] [Google Scholar]
- 93.Kato A, Okaya T, Lentsch AB. Endogenous IL-13 protects hepatocytes and vascular endothelial cells during ischemia/reperfusion injury. Hepatology. 2003;37:304–312. doi: 10.1053/jhep.2003.50075. [DOI] [PubMed] [Google Scholar]
- 94.Yoshidome H, Kato A, Miyazaki M, Edwards MJ, Lentsch AB. IL-13 activates STAT6 and inhibits liver injury induced by ischemia/reperfusion. Am J Pathol. 1999;155:1059–1064. doi: 10.1016/S0002-9440(10)65208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ke B, Shen XD, Gao F, Busuttil RW, Kupiec-Weglinski JW. Interleukin 13 gene transfer in liver ischemia and reperfusion injury: role of Stat6 and TLR4 pathways in cytoprotection. Hum Gene Ther. 2004;15:691–698. doi: 10.1089/1043034041361244. [DOI] [PubMed] [Google Scholar]
- 96.Cao Z, Yuan Y, Jeyabalan G, Du Q, Tsung A, Geller DA, et al. Preactivation of NKT cells with alpha-GalCer protects against hepatic ischemia-reperfusion injury in mouse by a mechanism involving IL-13 and adenosine A2A receptor. Am J Physiol Gastrointest Liver Physiol. 2009;297:G249–G258. doi: 10.1152/ajpgi.00041.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Adámková LSK, Kovarík J. Transcription protein STAT1: biology and relation to cancer. Folia Biol (Praha) 2007;53:1–6. [PubMed] [Google Scholar]
- 98.Kaplan DH, Shankaran V, Dighe AS, Stockert E, Aguet M, Old LJ, et al. Demonstration of an interferon gamma-dependent tumor surveillance system in immunocompetent mice. Proc Natl Acad Sci U S A. 1998;95:7556–7561. doi: 10.1073/pnas.95.13.7556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lee CK, Smith E, Gimeno R, Gertner R, Levy DE. STAT1 affects lymphocyte survival and proliferation partially independent of its role downstream of IFN-gamma. J Immunol. 2000;164:1286–1292. doi: 10.4049/jimmunol.164.3.1286. [DOI] [PubMed] [Google Scholar]
- 100.Zhu ZZ, Di JZ, Gu WY, Cong WM, Gawron A, Wang Y, et al. Association of genetic polymorphisms in STAT1 gene with increased risk of hepatocellular carcinoma. Oncology. 2010;78:382–388. doi: 10.1159/000320521. [DOI] [PubMed] [Google Scholar]
- 101.Nagano H. Treatment of advanced hepatocellular carcinoma: intraarterial infusion chemotherapy combined with interferon. Oncology. 2010;78(Suppl 1):142–147. doi: 10.1159/000315243. [DOI] [PubMed] [Google Scholar]
- 102.Haura EB, Turkson J, Jove R. Mechanisms of disease: Insights into the emerging role of signal transducers and activators of transcription in cancer. Nat Clin Pract Oncol. 2005;2:315–324. doi: 10.1038/ncponc0195. [DOI] [PubMed] [Google Scholar]
- 103.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 104.He G, Karin M. NF-kappaB and STAT3 - key players in liver inflammation and cancer. Cell Res. 2011;21:159–168. doi: 10.1038/cr.2010.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Lin L, Amin R, Gallicano GI, Glasgow E, Jogunoori W, Jessup JM, et al. The STAT3 inhibitor NSC 74859 is effective in hepatocellular cancers with disrupted TGF-beta signaling. Oncogene. 2009;28:961–972. doi: 10.1038/onc.2008.448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calvisi DF, Ladu S, Gorden A, Farina M, Conner EA, Lee JS, et al. Ubiquitous activation of Ras and Jak/Stat pathways in human HCC. Gastroenterology. 2006;130:1117–1128. doi: 10.1053/j.gastro.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 107.Jiang RTZ, Deng L, Chen Y, Xia Y, Gao Y, Wang X, Sun B. Interleukin-22 promotes human hepatocellular carcinoma by activation of STAT3. Hepatology. 2011;54:900–909. doi: 10.1002/hep.24486. [DOI] [PubMed] [Google Scholar]
- 108.Yoshikawa H, Matsubara K, Qian GS, Jackson P, Groopman JD, Manning JE, et al. SOCS-1, a negative regulator of the JAK/STAT pathway, is silenced by methylation in human hepatocellular carcinoma and shows growth-suppression activity. Nat Genet. 2001;28:29–35. doi: 10.1038/ng0501-29. [DOI] [PubMed] [Google Scholar]
- 109.Niwa Y, Kanda H, Shikauchi Y, Saiura A, Matsubara K, Kitagawa T, et al. Methylation silencing of SOCS-3 promotes cell growth and migration by enhancing JAK/STAT and FAK signalings in human hepatocellular carcinoma. Oncogene. 2005;24:6406–6417. doi: 10.1038/sj.onc.1208788. [DOI] [PubMed] [Google Scholar]
- 110.Zhang JFHM, Fu WM, Wang H, Chen LZ, Zhu X, Chen Y, Xie D, Lai P, Chen G, Lu G, Lin MC, Kung HF. Primate-specific miRNA-637 inhibits tumorigenesis in hepatocellular carcinoma by disrupting stat3 signaling. Hepatology. 2011 doi: 10.1002/hep.24595. [DOI] [PubMed] [Google Scholar]
- 111.Avella DMLG, Schell TD, Liu D, Shao-Min Zhang S, Lou X, Berg A, Kimchi ET, Tagaram HR, Yang Q, Shereef S, Garcia LS, Kester M, Isom HC, Bart Rountree C, Staveley-O'Carroll KF. Regression of established hepatocellular carcinoma is induced by chemo-immunotherapy in an orthotopic murine model. Hepatology. 2011 doi: 10.1002/hep.24652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wang CYW, Yan HX, Luo T, Zhang J, Tang L, Wu FQ, Zhang HL, Yu LX, Zheng LY, Li YQ, Dong W, He YQ, Liu Q, Zou SS, Lin Y, Hu L, Li Z, Wu MC, Wang HY. HBx induces tumorigenicity of hepatic progenitor cells in 3,5-diethoxycarbonyl-1,4-dihydrocollidine (DDC) Treated HBx transgenic mice. Hepatology. 2011 doi: 10.1002/hep.24675. [DOI] [PubMed] [Google Scholar]
- 113.Naugler WE, Sakurai T, Kim S, Maeda S, Kim K, Elsharkawy AM, et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science. 2007;317:121–124. doi: 10.1126/science.1140485. [DOI] [PubMed] [Google Scholar]
- 114.Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, et al. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Bard-Chapeau EA, Li S, Ding J, Zhang SS, Zhu HH, Princen F, et al. Ptpn11/Shp2 acts as a tumor suppressor in hepatocellular carcinogenesis. Cancer Cell. 2011;19:629–639. doi: 10.1016/j.ccr.2011.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.He G, Yu GY, Temkin V, Ogata H, Kuntzen C, Sakurai T, et al. Hepatocyte IKKbeta/NF-kappaB inhibits tumor promotion and progression by preventing oxidative stress-driven STAT3 activation. Cancer Cell. 2010;17:286–297. doi: 10.1016/j.ccr.2009.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schneller D, Machat G, Sousek A, Proell V, van Zijl F, Zulehner G, et al. p19(ARF) /p14(ARF) controls oncogenic functions of signal transducer and activator of transcription 3 in hepatocellular carcinoma. Hepatology. 2011;54:164–172. doi: 10.1002/hep.24329. [DOI] [PubMed] [Google Scholar]
- 118.Calvisi DF. Dr. Jekyll and Mr. Hyde: a paradoxical oncogenic and tumor suppressive role of signal transducer and activator of transcription 3 in liver cancer. Hepatology. 2011;54:9–12. doi: 10.1002/hep.24435. [DOI] [PubMed] [Google Scholar]
- 119.Ferbeyre G, Moriggl R. The role of Stat5 transcription factors as tumor suppressors or oncogenes. Biochim Biophys Acta. 2011;1815:104–114. doi: 10.1016/j.bbcan.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 120.Hosui A, Kimura A, Yamaji D, Zhu BM, Na R, Hennighausen L. Loss of STAT5 causes liver fibrosis and cancer development through increased TGF-{beta} and STAT3 activation. J Exp Med. 2009;206:819–831. doi: 10.1084/jem.20080003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Mueller KM, Kornfeld JW, Friedbichler K, Blaas L, Egger G, Esterbauer H, et al. Impairment of hepatic growth hormone and glucocorticoid receptor signaling causes steatosis and hepatocellular carcinoma in mice. Hepatology. 2011;54:1398–1409. doi: 10.1002/hep.24509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Friedbichler KTM, Mueller KM, Schlederer M, Kornfeld JW, Terracciano LM, Kozlov AV, Haindl S, Kenner L, Kolbe T, Mueller M, Snibson KJ, Heim MH, Moriggl R. Growth hormone-induced STAT5 signaling causes gigantism, inflammation and premature death but protects mice from aggressive liver cancer. Hepatology. 2011 Oct 26; doi: 10.1002/hep.24765. [DOI] [PubMed] [Google Scholar]
- 123.Yu JH, Zhu BM, Wickre M, Riedlinger G, Chen W, Hosui A, et al. The transcription factors signal transducer and activator of transcription 5A (STAT5A) and STAT5B negatively regulate cell proliferation through the activation of cyclin-dependent kinase inhibitor 2b (Cdkn2b) and Cdkn1a expression. Hepatology. 2010;52:1808–1818. doi: 10.1002/hep.23882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Feld JJ, Modi AA, El-Diwany R, Rotman Y, Thomas E, Ahlenstiel G, et al. S-adenosyl methionine improves early viral responses and interferon-stimulated gene induction in hepatitis C nonresponders. Gastroenterology. 2011;140:830–839. doi: 10.1053/j.gastro.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Page BD, Ball DP, Gunning PT. Signal transducer and activator of transcription 3 inhibitors: a patent review. Expert Opin Ther Pat. 2011;21:65–83. doi: 10.1517/13543776.2011.539205. [DOI] [PubMed] [Google Scholar]
- 126.Iavarone M, Cabibbo G, Piscaglia F, Zavaglia C, Grieco A, Villa E, et al. Field-practice study of sorafenib therapy for hepatocellular carcinoma: A prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. doi: 10.1002/hep.24644. [DOI] [PubMed] [Google Scholar]
- 127.Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. doi: 10.1056/NEJMoa0708857. [DOI] [PubMed] [Google Scholar]
- 128.Villanueva A, Llovet JM. Targeted therapies for hepatocellular carcinoma. Gastroenterology. 2011;140:1410–1426. doi: 10.1053/j.gastro.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Siegel AB, Olsen SK, Magun A, Brown RS., Jr Sorafenib: where do we go from here? Hepatology. 2010;52:360–369. doi: 10.1002/hep.23633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Blechacz BR, Smoot RL, Bronk SF, Werneburg NW, Sirica AE, Gores GJ. Sorafenib inhibits signal transducer and activator of transcription-3 signaling in cholangiocarcinoma cells by activating the phosphatase shatterproof 2. Hepatology. 2009;50:1861–1870. doi: 10.1002/hep.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Tai WT, Cheng AL, Shiau CW, Huang HP, Huang JW, Chen PJ, et al. Signal transducer and activator of transcription 3 is a major kinase-independent target of sorafenib in hepatocellular carcinoma. J Hepatol. 2011;55:1041–1048. doi: 10.1016/j.jhep.2011.01.047. [DOI] [PubMed] [Google Scholar]
- 132.Rosmorduc O, Desbois-Mouthon C. Targeting STAT3 in hepatocellular carcinoma: Sorafenib again. J Hepatol. 2011;55:957–959. doi: 10.1016/j.jhep.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 133.Radaeva S, Sun R, Pan HN, Hong F, Gao B. Interleukin 22 (IL-22) plays a protective role in T cell-mediated murine hepatitis: IL-22 is a survival factor for hepatocytes via STAT3 activation. Hepatology. 2004;39:1332–1342. doi: 10.1002/hep.20184. [DOI] [PubMed] [Google Scholar]
- 134.Yang L, Zhang Y, Wang L, Fan F, Zhu L, Li Z, et al. Amelioration of high fat diet induced liver lipogenesis and hepatic steatosis by interleukin-22. J Hepatol. 2010;53:339–347. doi: 10.1016/j.jhep.2010.03.004. [DOI] [PubMed] [Google Scholar]