Figure 2.
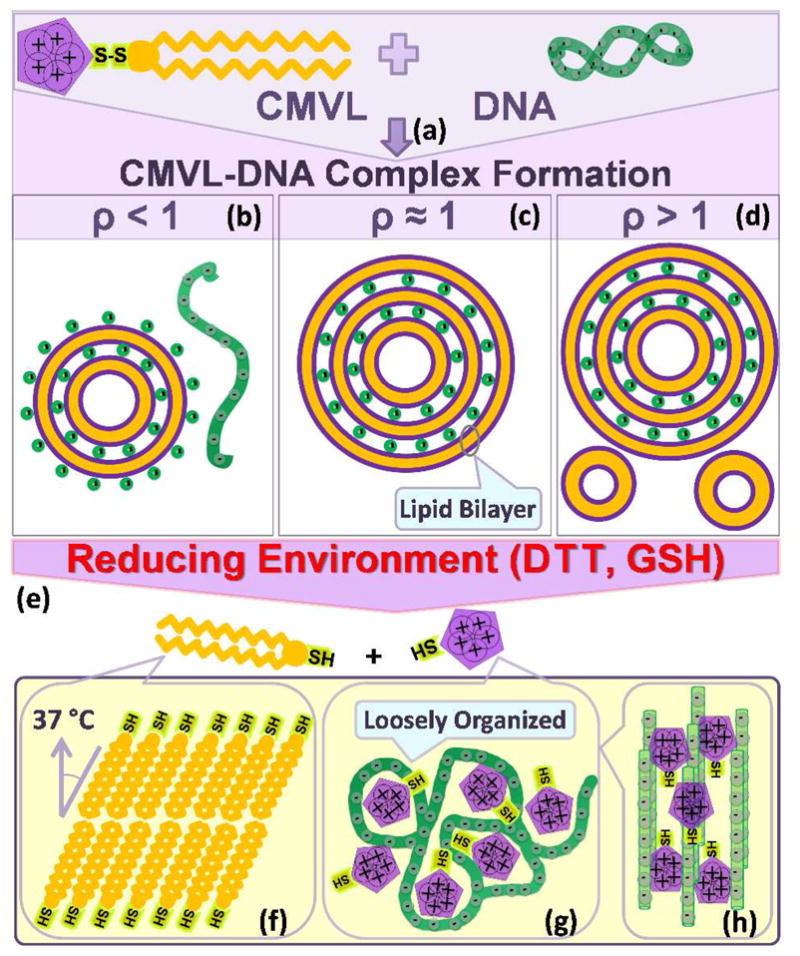
Schematic depiction of the formation of CMVL–DNA complexes and the evolution of their structure in response to a reducing environment. Mixing aqueous solutions of DNA and cationic liposomes containing the CMVL leads to spontaneous formation of CMVL–DNA complexes with a multilamellar structure (a). The entropy gained from the release of the small counterions of the DNA and the CMVL liposomes is the main driving force for complex formation. At CMVL/DNA charge ratios (ρ) below one, anionic complexes (b) form, which coexist with free DNA at small ρ. At the isoelectric point (ρ = 1), where the charges on the DNA and the CMVL match, neutral complexes are formed (c). For values of ρ near the isoelectric point, all DNA and liposomes are incorporated into the complexes, resulting in charged complexes.47 At large ρ (excess CMVL) positively charged complexes coexist with CMVL liposomes (d). Reducing agents cleave the disulfide linker between the headgroup and the hydrophobic tails of the CMVL (e), resulting in disassembly of the lamellar complexes. The cleaved parts of the CMVLs become the building blocks for new phases that form as a function of headgroup charge, time and temperature. The cationic headgroups condense the released DNA into a “loosely organized” phase (g) with a characteristic broad SAXS correlation profile. This phase can evolve into a phase of tightly condensed hexagonal bundles of DNA for CMVLs with high headgroup charge (h). In addition, incubation at 37 °C for 24 hours (after cleavage with the reducing agent DTT) induces self-assembly of the cleaved hydrocarbon into chain-ordered lamellar phases with tilted chains (f).
