Abstract
Sepsis is the leading cause of mortality in intensive care units. Early detection and intervention are critical to prevent death. The acute radiation syndrome is characterized by damage of the gastrointestinal and hematopoietic systems. Translocation of intestinal microflora combined with immune system compromise may lead to septicemia and death. This work examined the utility of procalcitonin, a clinical sepsis biomarker, in a mouse model of radiation toxicity.
C57/BL6 mice were exposed to total body irradiation (TBI). Intestinal mucosal permeability was measured in vivo, and liver bacterial load and plasma levels of procalcitonin (PCT), lipopolysaccharide (LPS), and lipopolysaccharide binding protein (LBP) were measured at baseline and 3.5, 7, and 10 days after TBI. The value of early PCT in predicting subsequent lethality was determined by receiver operator characteristics (ROC) analysis.
Four days after TBI a dose-dependent increase in permeability of the intestinal mucosa was observed, while bacterial translocation was present from day 7 onward. There was a high positive correlation between bacterial translocation and all sepsis biomarkers, with PCT exhibiting the strongest correlation. Moreover, plasma PCT levels were elevated already from day 3.5 onwards, whereas, LPS was elevated from day 7 and LBP only 10 days after TBI. ROC analysis revealed that PCT levels measured 3.5 days after TBI predicted lethality at 10 days.
These data demonstrate the value of PCT as an early biomarker in radiation-induced bacteremia for mouse studies and suggest that clinical results from other septic conditions may apply to post-radiation septicemia in humans.
Keywords: radiation, acute radiation syndromes, procalcitonin, biomarker, endotoxin, bacteremia, lipopolysaccharide
Introduction
Radiation exposure in association with accidents, terror strikes, and war is a small, but serious threat to the general public, as well as to first responders, cleanup and rescue workers, and military personnel. In a radiological emergency scenario, a large number of casualties would be expected, thus requiring triage and optimized management in intensive care departments (1).
Acute radiation sickness is characterized by damage to the hematopoietic system and gastrointestinal tract. Translocation of intestinal microflora by the lymphatic or hematogenous routes with resulting septic complications is a major cause of morbidity and mortality after exposure to moderate to high doses of total or near-total body irradiation (TBI) (1). Similarly, in most animal species, systemic microbial infection due to dysfunction of the intestinal mucosal barrier plays a major role in development of radiation-induced sepsis and lethality (2, 3).
There are obvious interactions between the hematopoietic and gastrointestinal systems after exposure to TBI. Low doses of TBI (3–7.5 Gy in the mouse model) result in temporary injury to the tight junction between epithelial cells of the intestinal mucosal lining. Higher doses of radiation (8–15 Gy) also lead to crypt stem cell death or arrest of mitotic activity, thus resulting in inadequate replacement of the villus epithelium. Mice with a germfree gut were observed to be more resistant to acute lethality after exposure to moderate to high radiation doses (4). Conversely, mice receiving bone marrow transplantation or irradiation with partial bone marrow shielding have significantly increased LD50/10 (the time point generally viewed as “intestinal”), although the number of surviving crypts at a given dose remains the same (5). Analogously, the benefits of selective decontamination of the digestive tract (SDD) with poorly absorbable antimicrobials have been well documented in several preclinical studies, and SDD is recommended in clinical radiological emergency scenarios under certain conditions (1).
Bacteremia-associated endotoxin accumulation in the circulation is the primary cause of inflammation, multiple organ failure, and mortality in septic conditions (6). The early detection of sepsis has received increased attention in recent years due to the high rate of mortality in intensive care units and because timely intervention with antimicrobial and supportive therapy has proven effective in reducing mortality in critically ill patients (7). To that end, the utility of specific and sensitive sepsis biomarkers, i.e., early markers that demonstrate high correlation and predictive value with subsequent development of systemic infection and/or lethality have been explored (8–10). Several early onset markers have been proposed and evaluated in animal models and clinically in various conditions associated with septicemia (reviewed in 11). The pro-hormone, procalcitonin (PCT), is produced only by the thyroid during normal conditions, but is produced and secreted by other parenchymal cells during conditions associated with bacteremia. PCT shows particular promise as an early marker of bacteremia and has thus been proposed as a method for timely detection and intervention in systemic infection (12–14).
Thus far, little is known about the utility of PCT and other markers in radiation-induced bacterial translocation and lethality. While late progression of acute radiation sickness is quite similar to classical sepsis, the early onset point and cascade of events are poorly understood. The present study assessed the utility of PCT as a biomarker of radiation-induced endotoxemia and bacterial translocation and evaluated the predictive ability of PCT in post-TBI lethality in a mouse model. The results may have implications for the preclinical development of radiation protectors and mitigators, and may be relevant to clinical radiological emergency scenarios.
Materials and methods
Animals
The animal experimental protocols used in this study were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Central Arkansas Veterans Healthcare System (CAVHS) and University of Arkansas for Medical Sciences (UAMS), Little Rock, AR. Male C57/BL6 mice (Charles River, Wilmington, MA) were used in this study. Mice were housed in conventional cages under standardized conditions with controlled temperature and humidity and a 12–12-h day-night light cycle. Mice were allowed free access to water and chow (Harlan Teklad laboratory diet 7012, Purina Mills, St. Louis, MO) during the entire period of handling.
A total of 177 mice, obtained at 6–7 weeks of age and weighing 22–25 g at the time of experimentation were used for the studies reported here. The mice were observed twice daily during the experimental period and those appearing moribund (exhibiting excessive weight loss, lethargy, huddling, shivering, hunched posture or vocalization) were euthanized immediately by CO2 asphyxiation followed by cervical dislocation, in accordance with American Veterinary Medical Association (AVMA) Guidelines on Euthanasia.
Irradiation studies
Irradiation was performed in a Shepherd Mark I model 25 137Cs irradiator (J. L. Shepherd & Associates, San Fernando, CA). Un-anesthetized mice were held in a well-ventilated custom-made Plexiglas restrainer on a turntable rotating at five revolutions per minute. The mice were exposed to uniform TBI at a dose rate of 1.35 Gy per minute.
Initial survival studies to determine the LD50/10 were carried out in 40 mice exposed to a TBI dose gradient of 8, 8.5, 9, 9.5 and 10 Gy (8 mice per group). The mice were monitored for 30 days after TBI and the number of dead/moribund mice recorded. Survival data at day 10 was used to estimate the LD50/10 dose for subsequent experiments. The doses TBI doses chosen for the subsequent experiment were chosen so as to straddle the LD50 as closely as possible in order to achieve the maximum statistical power for the receiver operating characteristics (ROC) analysis (see below).
In vivo intestinal permeability assay
Intestinal permeability was measured in 17 mice (4–5 per group) as described by Li et al. (15). Briefly, 4 days after exposure to 0, 9, 10, or 12 Gy TBI, the mice were anesthetized with isoflurane inhalation, a midline laparotomy was performed, and the renal artery and vein were ligated bilaterally. A 10 cm small intestinal segment, located 5 cm distal to the ligament of Treitz was isolated and tied off. One hundred microliters of 4-kDa fluorescein isothiocyanateconjugated dextran (FITC-dextran 25mg/ml in phosphate–buffered saline) was injected into the isolated intestine using a 30 Gauge needle and the abdominal incision was closed. After 90 min, blood was collected from the retro-orbital sinus and plasma was separated by centrifuging at 4°C, 8000 rpm for 10 min. The concentration of FITC-dextran was determined with a fluorescence spectrophotometer (Synergy HT, Bio-Tek Instruments, Winooski, VT) at an excitation wavelength of 480 nm and an emission wavelength of 520 nm. Standard curves to calculate FITC-dextran concentration in the plasma samples were prepared from dilutions of FITC-dextran in PBS.
Evaluation of parameters of systemic infection
First, the post-TBI time course of plasma PCT levels was established. Seventy-two mice were exposed to 9 or 10 Gy (i.e., closely straddling the LD50/10 estimate) and euthanized (6 per group) at baseline and 4, 6, 8, and 10 days after TBI. Plasma PCT was measured as described below.
In order to determine the correlation among the various parameters of systemic infection, analysis of bacterial translocation, PCT, LPS, and LBP in the same animals (see description of methods below) was performed on 36 mice (4 per group), euthanized on schedule at baseline or on days 3.5, 7 and 10 after exposure to 9 and 10 Gy TBI. Peripheral blood counts were measured on days 0, 3.5, and 10 by collecting whole blood by retro-orbital bleeding using heparinized capillary tubes (Fisher Scientific) into sodium-EDTA coated microtubes (Fisher Scientific). Hematology profiles were measured using a HEMAVET 950 instrument (Drew Scientific, Oxford, CA).
Finally, the value of early plasma PCT levels in predicting post-TBI lethality was assessed with ROC analysis in a separate experiment, performed on 12 mice exposed to 9 Gy TBI.
Bacterial translocation
Bacterial translocation was determined as bacterial load in liver tissue and was quantified by real time PCR using the 16S rRNA gene consensus sequence. The gene sequence of rRNA is highly conserved in bacteria throughout evolution, owing to their central role in protein synthesis (16). Several studies have established the use of the 16S rRNA gene as a standard method for identification and classification of prokaryotes (17). The total load of bacteria in the liver was determined using primer sequences to amplify the highly conserved sequence for a broad species consensus as reported elsewhere (18, 19).
Livers were removed aseptically and homogenized immediately. Bacterial translocation was quantified by real-time PCR (20). Briefly, DNA was isolated from sterile livers harvested at baseline and at 3.5, 7, and 10 days after exposure to TBI (9 Gy or 10 Gy) using a DNA purification kit (Promega, Madison, WI). Real-time PCR was performed using Power SYBR green PCR master mix (Applied Biosystems, Foster City, CA) and 16S rRNA gene targeted primers, forward (5′-AAC GCG AAG AAC CTT AC-3′) and reverse (5′-CGG TGT GTA CAA GAC CC-3′). Serially diluted bacterial genomic DNA was used to generate the standard curve. PCR-derived bacterial counts were expressed as nanogram bacterial DNA per gram mouse liver tissue.
PCT assay
Plasma PCT levels were measured using the mouse PCT ELISA kit (CSB-E10371m) from Cusabio Biotech, Carlsbad, CA. Briefly, blood collected in EDTA coated tubes was centrifuged at 1500×g at 4°C for 15 min and plasma used immediately for assay. The 96 well pre-coated plates were loaded with 100μl of standard and plasma samples. After incubation for 1 hr at 37°C with biotinylated tracer antibody, samples were developed against a horseradish peroxidase-avidin conjugate and absorbance measured at 450 nm. The concentration of PCT in each sample was quantified against the standard curve. Values are expressed as nanogram PCT protein per ml plasma.
LPS assay
Plasma levels of LPS were measure at baseline and 3.5, 7, and 10 days after TBI (9 or 10 Gy) by the PyroGene recombinant factor C endotoxin detection assay kit (50-658U) from Lonza, Walkersville, MD. The assay is based on the activation of recombinant Factor C by endotoxin binding whereby the activated moiety cleaves a synthetic substrate to generate a fluorogenic compound with excitation/emission wavelength of 380/440. Blood was obtained and centrifuged as described above, collected into endotoxin free tubes, and used immediately. Serially diluted E. coli endotoxin standard and samples were loaded on a pre-coated 96 well plate and incubated for 1 hr at 37°C. The difference in fluorescence between time zero and 1 hr was used to calculate endotoxin concentration from the standard curve. Values are expressed as endotoxin units (EU) per ml plasma.
LBP assay
Plasma LBP level was measured using the mouse LBP ELISA kit (HK205) from Hycult Biotech, Uden, Netherlands. The assay is based on a sandwich ELISA method with biotinylated anti-LBP antibody. Blood was collected and centrifuged as described above and plasma was used immediately for assay. One hundred μl of standard and plasma samples were loaded onto pre-coated 96 well plates, incubated for 2 hrs at room temperature. Biotinylated tracer antibody was incubated for 1 hr at room temperature, developed against a streptavidin-peroxidase conjugate, and absorbance was measured at 450 nm. The concentration of LBP was determined against the standard curve. Values are expressed as nanogram LBP protein per ml plasma.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Statistical analysis was performed with NCSS 2007 (NCSS, Kaysville, UT). The LD50/10 was estimated with probit analysis, comparisons of bacterial translocation, hematological parameters, LPS, LBP, and PCT among groups according to radiation dose and time point were performed with variance (ANOVA) and two-sided t-test as appropriate, and predictive value was assessed with binormal ROC analysis. Correlation between two variables was estimated by Pearson correlation coefficient. Two-tailed p-values <0.05 were considered statistically significant.
Results
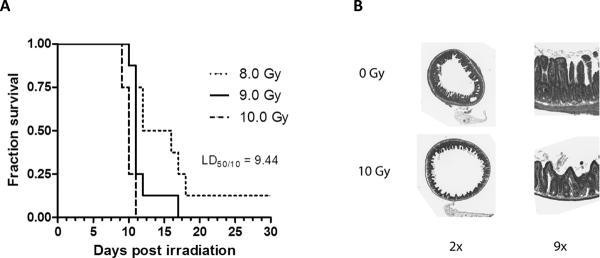
The estimated LD50/10 in mice exposed to 8–10 Gy with 0.5 Gy intervals was 9.44 Gy ± 0.24 Gy (Figure 1A–B). Therefore, the doses selected for investigation of parameters of systemic infection were 9 Gy and 10 Gy, i.e., doses that, because of the steepness of the radiation dose response curve, straddle the LD50/10 closely.
Figure 1. Post-irradiation survival.
-
A)Kaplan-Meier survival curves for mice (8 per radiation dose group) were exposed to 8–10 Gy total body irradiation (TBI) with increments of 0.5 Gy (only 8, 9, and 10 Gy are shown on this graph for clarity). The LD50/10 (radiation dose associated with 50% lethality at 10 days) was estimated by probit analysis to be 9.44 Gy.
-
B)Cross sections and detail of mouse proximal jejunum procured 3.5 days after exposure to sham irradiation (0 Gy) and 10 Gy total body irradiation. Original magnification as indicated on the figure.
In vivo assessment of intestinal permeability 4 days after irradiation revealed a highly significant dose dependent (p<0.0001) increase when all exposure groups were considered (Figure 2A). Compared to sham-irradiated controls permeability in mice exposed to 10 Gy was increased 5-fold (p=0.01), while 12 Gy TBI was associated with a 15-fold increase (p=0.004). Analyzed as a separate group, permeability in the 9 Gy exposure group was elevated 3-fold, but the difference did not reach statistical significance.
Figure 2. Post-irradiation increase in intestinal permeability and time course of PCT levels.
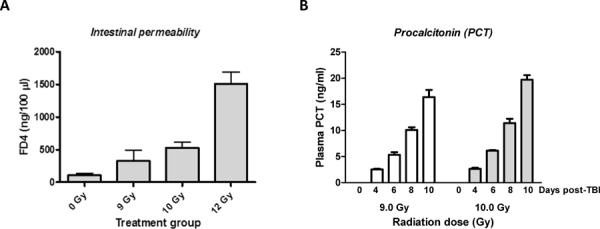
-
C)Intestinal permeability was measured in vivo, 4 days after sham-irradiation or exposure to 9, 10, or 12 Gy total body irradiation (TBI).
- Compared to sham-irradiated mice, the increase in permeability was highly statistically significant both after 10 Gy (p=0.01) and after 12 Gy (p=0.004).
- Mean ± SEM, 4–5 mice per group.
-
D)Plasma procalcitonin (PCT) levels were measured by a specific ELISA assay at baseline and 4, 6, 8, or 10 days after TBI (9 Gy or 10 Gy).
- The increase in plasma PCT was highly time-dependent (p<10−6) and radiation dose dependent (p=0.002).
- Mean ± SEM, 6 mice per group.
Measurement of plasma PCT levels 4, 6, 8, and 10 days after exposure to 9 or 10 Gy TBI showed a significant increase on day 4 with a highly statistically significant post-exposure time-dependence (p<10−6) and radiation dose dependence (p=0.002) (Figure 2B).
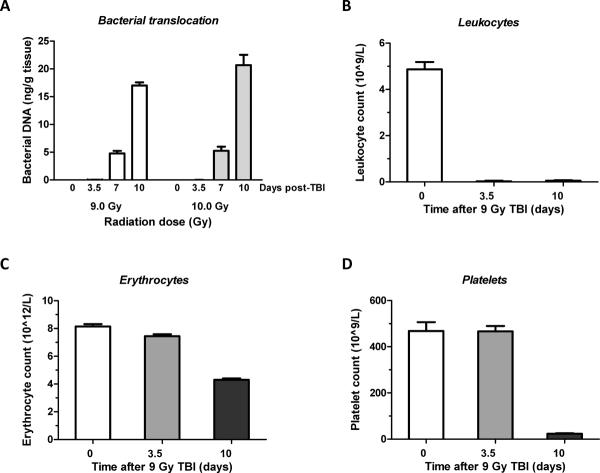
Radiation-induced bacterial translocation is known to start around day 7 and peaks around 2 weeks after TBI (21, 22). Bacterial translocation was determined on day 0, 3.5, 7 and 10 after exposure to 9 Gy or 10 Gy TBI, as shown in Figure 3A. No bacterial translocation was observed at baseline and 3.5 days after TBI. In contrast, significant bacterial loads were detected on day 7 (p=0.0006 compared to 3.5 days) and this increased further between day 7 and day 10 (p=0.000005). Translocation was more pronounced after 10 Gy than after 9 Gy TBI (ANOVA p=0.01).
Figure 3. Bacterial translocation to the liver and peripheral blood cell counts after exposure to TBI.
-
A)Bacterial translocation was determined as bacterial load in liver tissue at baseline and 3.5, 7, and 10 days after exposure to 9 Gy or 10 Gy TBI.
- Significant amounts of bacterial DNA observed on day 7 (p=0.0006) and on day 10 (p=0.000005). Translocation was significantly greater after 10 Gy than after 9 Gy (p=0.01).
- Mean ± SEM, 4 mice per group (2 mice died before day 10 in the 10 Gy exposure group).
-
B–D)Changes in the level of blood cells were assesses at day 0, 3.5 and 10 after exposure to 9.0 Gy TBI.
- Leukocytes exhibited a significant decrease at 3.5 days and the erythrocyte count was borderline significantly decreased. At 10 days after TBI, all peripheral blood count parameters were highly significantly reduced.
- Mean ± SEM, 4–6 mice per group.
Changes in peripheral blood count were measured to reflect immune system dysfunction on day 0, 3.5 and 10 (Figure 3B–D). White blood cells (WBC) were significantly diminished on both day 3.5 and 10 (P<0.0001) compared to baseline. The red blood cell counts also showed a significant decline on day 3.5 (P=0.02) and 10 (P<0.0001). Platelet counts did not show a significant difference on day 3.5 but were significantly reduced (P<0.0001) on day 10.
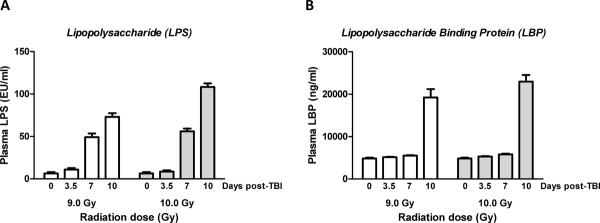
Plasma endotoxin (LPS) levels and plasma LBP levels, measured by the recombinant factor-C LAL method and an ELISA method, respectively, are shown in Figure 4A and Figure 4B.
Figure 4. Changes in lipopolysaccharide (LPS) and LPS binding protein (LBP) after exposure to TBI.
-
A)Endotoxin level indicating bacteremia was determined as lipopolysaccharide (LPS) level in plasma.
- LPS levels increased significantly from 3.5 to 7 days (p=0.001) and from 7 to 10 days (p=0.009) and the increase was significantly greater after exposure to 10 Gy than after 9 Gy (p=0.0003).
- Mean ± SEM, 4 mice per group (2 mice died before day 10 in the 10 Gy exposure group).
-
B)Levels of lipopolysaccharide binding protein (LBP) in plasma as a function of time after TBI.
- There was no significant elevation on days 3.5 and 7, while the increase in LBP levels 10 days after exposure to TBI was highly statistically significant (p=0.006). Mean ± SEM, 4 mice per group (2 mice died before day 10 in the 10 Gy exposure group).
LPS is shed from the cell wall of gram negative bacteria and generally correspond with the systemic bacterial load. Similar to bacterial translocation, while there was no increase in LPS levels after exposure to radiation between baseline and 3.5 days, the LPS levels increased significantly from 3.5 to 7 days (p=0.001) with a further increase from 7 to 10 days (p=0.009). A significant difference in LPS level was observed as a function of radiation dose (ANOVA p<0.0003), mainly due to a difference at 10 days (p=0.008).
After exposure to 9 Gy TBI, the LBP levels exhibited no increase from baseline to 3.5 days, a borderline significant increase (p=0.03) from 3.5 to 7 days, but a highly significant increase between days 7 and 10 (p=0.006). ANOVA revealed no difference in LBP levels between the two radiation doses.
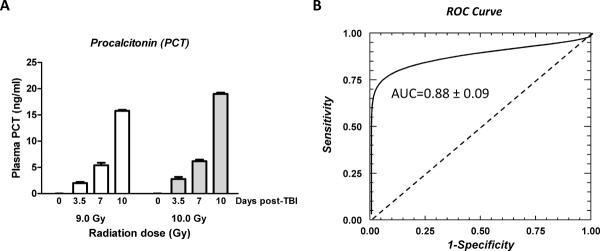
Plasma PCT levels, measured by an ELISA method, and the results of the ROC analysis of PCT level at day 3.5 as a predictor of 10-day lethality after 9 Gy TBI are shown in Figure 5A. After exposure to 9 Gy, plasma PCT levels were significantly elevated already on day 3.5 (p=0.002 compared to baseline) and increased further from 3.5 to 7 days (p=0.003) and from 7 to 10 days (p=0.00005). Moreover, the difference between the two TBI doses was highly statistically significant (ANOVA p=0.00005) again mainly because of a difference at 10 days (0.003).
Figure 5. Procalcitonin levels and receiver operating characteristic (ROC) curve to assess predictive ability of procalcitonin level for post-TBI lethality.
-
A)Changes in plasma procalcitonin (PCT) after exposure to 9 Gy or 10 Gy radiation. PCT levels were significantly elevated already after 3.5 days (p=0.002), increased further to 7 days (p=0.003) and even further up to 10 days after TBI (p=0.0005).
- There was also a highly significant difference between the two TBI doses (p=0.0005).
- Mean ± SEM, 4 mice per group (2 mice died before day 10 in the 10 Gy exposure group).
-
B)Value of early procalcitonin (PCT) levels (3.5 day post TBI) as a predictor of later (up to 10 days) lethality after exposure to 9.0 Gy TBI.
- The ROC curve evaluates the diagnostic value of a potential predictor that cannot be dichotomized because a) a cut-off value between normal and pathological values is not known, or b) dichotomizing the predictor would not be appropriate. It displays, for all possible values of the predictor, 1-specificity (along the abscissa) versus sensitivity (along the ordinate). A random predictor (with no predictive value) follows the diagonal, while the area under the curve (AUC) for the predictor being evaluated represents its estimated predictive value.
- The AUC for the binormal ROC curve was 0.88 +/−0.09 for PCT as a predictor of 10-day lethality.
- Twelve mice, 9 survivors and 3 deaths.
ROC analysis revealed that the area under the curve (AUC) was 0.88±0.09 (i.e., significantly better than 0.5 as for a random predictor), thus confirming that PCT levels at 3.5 days can serve as an early indicator of post-TBI lethality (Figure 5B).
The correlation among the various sepsis parameters and bacterial translocation was calculated for the pooled data set. Significant positive correlations were observed between bacterial translocation and LPS (0.91), LBP (0.95), and PCT (0.98), thus providing an r2-value of 0.83 for LPS, 0.90 for LBP, and 0.96 for PCT.
Discussion
Extensive research and data are available from laboratory models and clinical studies on the progression of sepsis. However, there is a paucity of predictive biomarkers for use in preclinical radiation models.
Ionizing radiation, rather than causing massive tissue necrosis, affects primarily actively and rapidly proliferating cells in the bone marrow and the intestinal crypts. The killing of intestinal progenitor cells in the crypts elicits an increase in mucosal permeability through a combination of disruption of epithelial tight junctions and insufficient replacement of the villus epithelium. Intestinal bacteria that penetrate the defective mucosal barrier are an important source of bacteremia after radiation exposure and the main cause of lethality in radiological or nuclear emergencies. Exposure to ionizing radiation causes enhanced intestinal permeability, leading to bacterial and LPS translocation, development of systemic inflammation/ sepsis, a so-called “radiation induced multiple organ dysfunction syndrome”. The present study aimed at determining whether PCT can be used as an early predictive marker of lethality in a mouse model of TBI.
An extensive body of literature exists regarding the importance of gut bacteria for the outcome after exposure to TBI, the types of bacteria encountered, and the recovery of bacteria from the systemic circulation and various organs (21, 23). The kinetics of radiation-induced bacteremia is significantly different from that of general sepsis models. For example, studies using the cecal ligation puncture (CLP) model have documented translocation of gut microflora and the rapid progression of sepsis in animal models, with death generally occurring within 48 hrs of CLP (24). Although the sequence of events for TBI exposure is similar, the TBI model progresses more gradually, with most deaths occurring between 7 and 21 days. Notably, while mucosal injury peaks 3.5–4 days after exposure to radiation, bacterial translocation is generally first present at 6–7 days and peaks about 2 weeks after TBI, thus highlighting the importance of concomitant mucosal barrier dysfunction and immune system compromise (22). While irradiated mice have reduced oral intake compared to controls and frequently exhibit a substantial loss of body weight, compensating for poor intake of water and/or food has been shown to adversely impact outcomes in radiation studies (25). Therefore, because pair-feeding would likely have had an adverse effect on the outcome in this study, it was not used.
Endotoxins are complex polysaccharides found on the outer membrane of bacteria and are shed naturally into their surroundings. The predominant endotoxin of gram negative bacteria is lipopolysaccharide (LPS), while that of gram positive bacteria are lipoteichoic acid. A gradual buildup of endotoxin level in circulation predates the onset of detectable bacteremia and hence, can be used as a reasonably early marker. Clinically, LPS levels of up to 25 pg/ml (≈0.25 EU) is normal, levels of 25–700 pg/ml (≈0.25–7 EU) indicate early onset of bacteremia, and values above 10ng/ml (≈100 EU) are associated with severe septicemia (26). In our study, elevated levels of LPS were not observed before 7 days after TBI, thus at an intermediate time that roughly corresponded to the onset of detectable bacterial translocation to the liver. Hence, while LPS can serve as a minimally invasive indicator of bacterial translocation, it is not suitable as a predictor of septicemia.
The high affinity interaction between LBP, a 65 KDa protein, and LPS forms the stimulatory complex for macrophages via CD14 leading to the production of cytokines (27). Hence, LBP-LPS-sCD14 interaction forms the basis for immune cell activation and immune response against bacteremia. Elevated levels of serum LBP were found in critically ill neonates with both gram-positive and gram-negative infections and were reported to be on par with PCT and a significantly better diagnostic marker for bacterial sepsis than interleukin 6 (IL-6) and C reactive protein (CRP) (28, 29). In contrast, our study revealed that LBP levels were elevated only 10 days after TBI, at a time when there is already significant lethality. Thus, LBP does not appear to have value as an early predictor of radiation-induced bacterial translocation or lethality.
PCT has generated substantial interest as a sepsis biomarker since its first use in 1993 to differentiate bacterial infection from viral meningitis (30). PCT is produced as a pro-hormone of calcitonin in the C-cells of thyroid and is rarely, if not never, secreted into the circulation under normal physiological conditions. During conditions of bacteremia, on the other hand, almost every parenchymal cell in the body has been reported to produce and secrete PCT (31). Hence, circulating PCT is primarily extra-thyroid in origin and associated with bacteremia. The unrestrained production of cytokines acts as the primary stimulatory factor of PCT production (32). Plasma PCT levels are normally below 0.1 ng/ml, increase within 4 hrs of onset of bacteremia, and often reach 100–1000 fold normal levels in severe sepsis (33). In the clinic, a concentration of >1.2 ng/ml is generally considered as a cutoff for bacteremia confirmation in critically ill patients (33). PCT is also a fairly specific marker of bacteremia. PCT levels in plasma are not significantly altered by viral infection and in non-septic systemic inflammatory response syndrome (SIRS) and PCT has been proposed as an early biomarker of late mortality in clinical septicemia (34). While PCT can also be elevated in severe bacterial infection as well as in severe sterile inflammation, these conditions would be unlikely to occur in the early phase after exposure to radiation in the absence of combined injury (35).
In the present study, bacterial translocation to liver tissue was virtually undetectable on day 3.5 after TBI, significantly elevated by day 7, and very high at day 10. An increase in plasma PCT level was evident already at the earliest time point (3.5–4 days) and exhibited a high positive correlation with increase in bacterial load and endotoxin accumulation post-irradiation. Moreover, the predictive value of PCT as an early biomarker, as estimated by ROC analysis, showed an area under the curve of 0.88 ± 0.9, significantly better than the 0.5 value of a random “predictor”. Because antimicrobial therapy is of documented benefit in radiation-induced sepsis, if PCT were to be validated as a predictor of radiation-induced bacterial translocation, appropriate antibiotics could be instituted and thus help rescue some individuals exposed to a potentially lethal dose of TBI. Further studies are needed to establish a cutoff number for various animal models, and a larger number of subjects are needed to construct empirical, rather than binormal ROC curves.
In conclusion, an increase in intestinal mucosal permeability and bacterial translocation with a more or less parallel accumulation of endotoxin in plasma was observed after exposure to TBI. LBP, contradictory to expectation, did not exhibit consistent early changes post irradiation, and LPS coincided with, but did not precede, the onset of bacterial translocation. Elevated plasma PCT was observed from day 3.5 onward, i.e., before appreciable bacterial translocation or endotoxemia, but coinciding with the increase in permeability of the intestinal mucosa. While these findings will have to be extended to animal models and with a wider range of TBI doses, alone and in combination with other injuries, correlation and ROC analysis of the present data suggest that PCT can be used as an early onset biomarker for TBI-induced lethality. These findings may have implications for the preclinical development of radiation protectors and mitigators, and may also prove relevant to clinical radiological emergency scenarios.
Acknowledgements
This work was supported by the National Institutes of Health U19 AI67798 (MH-J), R01 AA015731 (MAC), and R33 AI080528 (EJK); the Marian C. Falk Research Trust (EJK); and the Veterans Administration (MH-J).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dainiak N, Gent RN, Carr Z, Schneider R, Bader J, Buglova E, Chao N, Coleman N, Ganser A, Gorin C, Hauer-Jensen M, Huff LA, Lillis-Hearne P, Maekawa K, Nemhauser J, Powles R, Schunemann H, Shapiro A, Stenke L, Valverde N, Weinstock D, White D, Albanese J, Meineke V. Literature review and global consensus on management of acute radiation syndrome affecting non-hematopoietic organ systems. Disaster Med Public Health Prep. 2011;5:183–201. doi: 10.1001/dmp.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon LE, Ruml D, Hahne HJ, Miller CP. Studies on susceptibility to infection following ionizing radiation. IV. The pathogenesis of the endogenous bacteremias in mice. J Exp Med. 1955;102:413–424. doi: 10.1084/jem.102.4.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller CP, Hammond CW, Tompkins M. The role of infection in radiation injury. J Lab Clin Med. 1951;38:331–343. [PubMed] [Google Scholar]
- 4.Crawford PA, Gordon JI. Microbial regulation of intestinal radiosensitivity. Proc Natl Acad Sci U S A. 2005;102:13254–13259. doi: 10.1073/pnas.0504830102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Terry NHA, Travis EL. The influence of bone marrow depletion on intestinal radiation damage. Int J Radiat Oncol Biol Phys. 1989;17:569–573. doi: 10.1016/0360-3016(89)90108-9. [DOI] [PubMed] [Google Scholar]
- 6.Tsiotou AG, Sakorafas GH, Anagnostopoulos G, Bramis J. Septic shock; current pathogenetic concepts from a clinical perspective. Med Sci Monit. 2005;11:RA76–RA85. [PubMed] [Google Scholar]
- 7.de Oliveira CF. Early goal-directed therapy in treatment of pediatric septic shock. Shock. 2010;34(Suppl 1):44–47. doi: 10.1097/SHK.0b013e3181e7e6d5. [DOI] [PubMed] [Google Scholar]
- 8.Lissauer ME, Johnson SB, Bochicchio GV, Feild CJ, Cross AS, Hasday JD, Whiteford CC, Nussbaumer WA, Towns M, Scalea TM. Differential expression of toll-like receptor genes: sepsis compared with sterile inflammation 1 day before sepsis diagnosis. Shock. 2009;31:238–244. doi: 10.1097/SHK.0b013e3181834991. [DOI] [PubMed] [Google Scholar]
- 9.Schulte J, Struck J, Kohrle J, Muller B. Circulating levels of peroxiredoxin 4 as a novel biomarker of oxidative stress in patients with sepsis. Shock. 2011;35:460–465. doi: 10.1097/SHK.0b013e3182115f40. [DOI] [PubMed] [Google Scholar]
- 10.Ware LB, Fessel JP, May AK, Roberts LJ. Plasma biomarkers of oxidant stress and development of organ failure in severe sepsis. Shock. 2011;36:12–17. doi: 10.1097/SHK.0b013e318217025a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kibe S, Adams K, Barlow G. Diagnostic and prognostic biomarkers of sepsis in critical care. J Antimicrob Chemother. 2011;66(Suppl 2):ii33–ii40. doi: 10.1093/jac/dkq523. [DOI] [PubMed] [Google Scholar]
- 12.Uzzan B, Cohen R, Nicolas P, Cucherat M, Perret GY. Procalcitonin as a diagnostic test for sepsis in critically ill adults and after surgery or trauma: a systematic review and meta-analysis. Crit Care Med. 2006;34:1996–2003. doi: 10.1097/01.CCM.0000226413.54364.36. [DOI] [PubMed] [Google Scholar]
- 13.Nakamura A, Wada H, Ikejiri M, Hatada T, Sakurai H, Matsushima Y, Nishioka J, Maruyama K, Isaji S, Takeda T, Nobori T. Efficacy of procalcitonin in the early diagnosis of bacterial infections in a critical care unit. Shock. 2009;31:586–591. doi: 10.1097/SHK.0b013e31819716fa. [DOI] [PubMed] [Google Scholar]
- 14.Schneider CP, Yilmaz Y, Kleespies A, Jauch KW, Hartl WH. Accuracy of procalcitonin for outcome prediction in unselected postoperative critically ill patients. Shock. 2009;31:568–573. doi: 10.1097/SHK.0b013e318193cb52. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Schwacha MG, Chaudry IH, Choudhry MA. Heme oxygenase-1 protects against neutrophil-mediated intestinal damage by down-regulation of neutrophil p47phox and 67phox activity and O2-production in a two-hit model of alcohol intoxication and burn injury. J Immunol. 2008;180:6933–6940. doi: 10.4049/jimmunol.180.10.6933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hillis DM, Moritz C, Porter CA, Baker RJ. Evidence for biased gene conversion in concerted evolution of ribosomal DNA. Science. 1991;251:308–310. doi: 10.1126/science.1987647. [DOI] [PubMed] [Google Scholar]
- 17.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev. 1995;59:143–169. doi: 10.1128/mr.59.1.143-169.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berbee M, Fu Q, Boerma M, Wang J, Kumar KS, Hauer-Jensen M. Gamma-tocotrienol ameliorates intestinal radiation injury and reduces vascular oxidative stress after total body irradiation by an HMG-CoA reductase-dependent mechanism. Radiat Res. 2009;171:596–605. doi: 10.1667/RR1632.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fu Q, Berbee M, Boerma M, Wang J, Schmid HA, Hauer-Jensen M. The somatostatin analog SOM230 (pasireotide) ameliorates injury of the intestinal mucosa and increases survival after total body irradiation by inhibiting exocrine pancreatic secretion. Radiat Res. 2009;171:698–707. doi: 10.1667/RR1685.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Minnen LP, Timmerman HM, Lutgendorff F, Verheem A, Harmsen W, Konstantinov SR, Smidt H, Visser MR, Rijkers GT, Gooszen HG, Akkermans LM. Modification of intestinal flora with multispecies probiotics reduces bacterial translocation and improves clinical course in a rat model of acute pancreatitis. Surgery. 2007;141:470–480. doi: 10.1016/j.surg.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Brook I, MacVittie TJ, Walker RI. Recovery of aerobic and anaerobic bacteria from irradiated mice. Infect Immun. 1984;46:270–271. doi: 10.1128/iai.46.1.270-271.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kobayashi T, Ohmori T, Yanai M, Kawanishi G, Mitsuyama M, Nomoto K. The analysis of the defense mechanism against indigenous bacterial translocation in X-irradiated mice. Microbiol Immunol. 1991;35:315–324. doi: 10.1111/j.1348-0421.1991.tb01560.x. [DOI] [PubMed] [Google Scholar]
- 23.Brook I, Elliott TS, Ledney GD, Shoemaker MO, Knudson GB. Management of postirradiation infection: lessons learned from animal models. Mil Med. 2004;169:194–197. doi: 10.7205/milmed.169.3.194. [DOI] [PubMed] [Google Scholar]
- 24.Hubbard WJ, Choudhry M, Schwacha MG, Kerby JD, Rue LW, Bland KI, Chaudry IH. Cecal ligation and puncture. Shock. 2005;24(S1):52–57. doi: 10.1097/01.shk.0000191414.94461.7e. [DOI] [PubMed] [Google Scholar]
- 25.Smith WW, Ackerman IB, Smith F. Body wieght, fasting and forced feeding after whole body X-irradiation. Am J Physiol. 1952;168:382–390. doi: 10.1152/ajplegacy.1952.168.2.382. [DOI] [PubMed] [Google Scholar]
- 26.Shenep JL, Flynn PM, Barrett FF, Stidham GL, Westenkirchner DF. Serial quantitation of endotoxemia and bacteremia during therapy for gram-negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 27.Schumann RR, Leong SR, Flaggs GW, Gray PW, Wright SD, Mathison JC, Tobias PS, Ulevitch RJ. Structure and function of lipopolysaccharide binding protein. Science. 1990;249:1429–1431. doi: 10.1126/science.2402637. [DOI] [PubMed] [Google Scholar]
- 28.Berner R, Furll B, Stelter F, Drose J, Muller HP, Schutt C. Elevated levels of lipopolysaccharide-binding protein and soluble CD14 in plasma in neonatal early-onset sepsis. Clin Diagn Lab Immunol. 2002;9:440–445. doi: 10.1128/CDLI.9.2.440-445.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavcnik-Arnol M, Hojker S, Derganc M. Lipopolysaccharide-binding protein in critically ill neonates and children with suspected infection: comparison with procalcitonin, interleukin-6, and C-reactive protein. Intensive Care Med. 2004;30:1454–1460. doi: 10.1007/s00134-004-2307-4. [DOI] [PubMed] [Google Scholar]
- 30.Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet. 1993;341:515–518. doi: 10.1016/0140-6736(93)90277-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meisner M, Muller V, Khakpour Z, Toegel E, Redl H. Induction of procalcitonin and proinflammatory cytokines in an anhepatic baboon endotoxin shock model. Shock. 2003;19:187–190. doi: 10.1097/00024382-200302000-00017. [DOI] [PubMed] [Google Scholar]
- 32.Preas HL, Nylen ES, Snider RH, Becker KL, White JC, Agosti JM, Suffredini AF. Effects of anti-inflammatory agents on serum levels of calcitonin precursors during human experimental endotoxemia. J Infect Dis. 2001;184:373–376. doi: 10.1086/322031. [DOI] [PubMed] [Google Scholar]
- 33.Guven H, Altintop L, Baydin A, Esen S, Aygun D, Hokelek M, Doganay Z, Bek Y. Diagnostic value of procalcitonin levels as an early indicator of sepsis. Am J Emerg Med. 2002;20:202–206. doi: 10.1053/ajem.2002.33005. [DOI] [PubMed] [Google Scholar]
- 34.Phua J, Koay ES, Lee KH. Lactate, procalcitonin, and amino-terminal pro-B-type natriuretic peptide versus cytokine measurements and clinical severity scores for prognostication in septic shock. Shock. 2008;29:328–333. doi: 10.1097/SHK.0b013e318150716b. [DOI] [PubMed] [Google Scholar]
- 35.Hauer-Jensen M, Kumar KS, Wang J, Berbee M, Fu Q, Boerma M. Intestinal toxicity in radiation- and combined injury: significance, mechanisms, and countermeasures. In: Larche RA, editor. Global Terrorism Issues and Developments. Nova Science Publishers; New York, NY: 2008. pp. 61–100. [Google Scholar]