Abstract
The LOTUS or OST-HTH domain is a recently discovered motif of about 80 amino acids and is found in several germline-specific proteins including the Tudor domain-containing proteins TDRD5 and TDRD7, which are important for germ cell development. The LOTUS domain is an RNA binding domain but its exact function is unknown. Here, we report the 1H, 13C and 15N resonance assignments for the three LOTUS domains present in mouse TDRD7. These assignments will allow three-dimensional structure determination of the LOTUS domains and mapping of their interaction with RNA, steps toward deciphering the function of TDRD7.
Keywords: TDRD7, LOTUS domain, OST-HTH domain, Tudor domain, RNA binding, NMR assignments
Biological context
Germ cells are specialized cells that form early during embryo development and eventually become sperm or egg. Present in the germ cells are cytoplasmic organelles called germinal granules or nuage that are electron-dense, amorphous and membrane-less structures (Eddy 1975). The granules are enriched in RNAs and proteins and are closely associated with mitochondria or nuclei. Among the several identified proteins in the nuage, the Piwi proteins and the Tudor proteins are particularly abundant.
Piwi proteins belong to a subclass of the Argonaute protein family whose members bind to small non-coding Piwi-interacting RNAs (piRNAs) (Thomson and Lin 2009). Argonaute proteins contain four domains including the PAZ and PIWI domains. The PAZ domain has been implicated in the binding to piRNAs, which base-pair with complementary target RNAs, while the PIWI domain is involved in the cleavage of target RNAs. Piwi proteins and piRNAs repress activation of transposons and thus protect the germline DNA from deleterious mutations caused by transposon mobilization.
Tudor proteins possess the Tudor domain which is a ~ 60 amino acid module made up of five anti-parallel β-strands, forming a β-barrel structure that encases a pocket lined with aromatic residues (Botuyan et al. 2006; Chen et al. 2009; Côté and Richard 2005; Selenko et al. 2001). This aromatic cage has been shown in some proteins to specifically bind methylated lysines while in other proteins it binds symmetrically dimethylated or asymmetrically dimethylated arginines.
Tudor domains exist singly or in multiple copies, in the absence or in conjunction with other types of domains. In the TDRD group (TDRD1-12) of methylarginine or methyllysine binding Tudor proteins, most of which are enriched or selectively expressed in the germ line and associated with RNA metabolism, the Tudor domain/s is/are associated with various RNA binding domains. Examples of RNA binding motifs are the KH domain of TDRD2, the RNA helicase domain of TDRD9, the RNA recognition motif of TDRD10 and the recently discovered LOTUS domain (also known as OST-HTH domain) of TDRD5 and TDRD7. The LOTUS domain is a conserved protein fold of about 80 amino acids and predicted to adopt a winged helix-turn-helix conformation (Callebaut and Mornon 2010; Anantharaman et al. 2010). Three copies of the LOTUS domain are found at the N-termini of TDRD5 and TDRD7.
Germ cell-specific Piwi proteins that are arginine-methylated and germline Tudor proteins interact with each other. Additionally, they bind other proteins and RNAs, contributing to the formation of functional ribonucleoprotein (RNP) assemblies that are required for germline development. In mice harboring Tdrd1 mutations, the Piwi protein MILI fails to localize to nuage during spermatogenesis while a mutation in Mili causes TDRD1 to lose nuage localization in pro-spermatogonia (Vagin et al. 2009). Similarly, TDRD5 and TDRD9 associate with the MIWI2 Piwi protein and mutations in TDRD5/9 cause male-specific sterility in mice (Shoji et al. 2009; Yabuta et al. 2011). Male sterility is also observed in mice with Tdrd7 mutations (Tanaka et al. 2011). In humans, mutations in Tdrd7 lead to cataract and glaucoma (Lachke et al. 2011). TDRD7 has been shown to co-immunoprecipitate with specific mRNAs in the lens and to interact with the STAU1-RNPs. While these findings point to the importance of RNP substituents, it remains to be understood how RNP components come together in the germinal granules and interact with one another.
The LOTUS domain, being an RNA binding motif, may have a role in the assemblage and function of RNPs, specifically in the TDRD-Piwi-piRNA pathway. Knowing the structure and interactions of the LOTUS domain could help clarify its exact role. As a step toward structure determination, we report here the resonance assignments of the three LOTUS domains of mouse TDRD7.
Methods and experiments
Protein expression and purification
LOTUS domain 1 (LOTUS 1, amino acids 34–109) and LOTUS domains 2 and 3 (LOTUS 2–3, amino acids 256–433) of mouse TDRD7 (GenBank entry CAM17034.1) were cloned in a modified pET15b expression vector (Novagen) conferring to the proteins an N-terminal (His)6 tag cleavable by the tobacco etch virus (TEV) protease. Each plasmid was transformed in Rosetta(DE3)pLysS competent cells (EMD Chemicals) which were cultured at 37 °C in 1 L of M9 media enriched with either 1 g of 15NH4Cl or 1 g 15NH4Cl and 2 g of 13C6-glucose (Sigma-Aldrich) to produce 15N-labeled or 15N/13C-labeled proteins, respectively. Upon reaching D600 nm of ~0.6, the cells were transferred to a 15 °C shaker where after 1 h they were induced with 1 mM final concentration of IPTG. The cells were grown for another 16–20 h before they were harvested, resuspended in 50 mL of buffer A (50 mM sodium phosphate, pH 7.5, 300 mM NaCl) and lyzed using an EmulsiFlex-C5 high-pressure homogenizer (Avestin). After centrifugation, the lysate was loaded onto a Ni–NTA column (Qiagen) and the column was washed extensively with buffer A containing 20 mM imidazole. Afterwards, the protein was eluted with buffer A containing 250 mM imidazole. Protein fractions were pooled and concentrated to 5 mL using a 3,000 Da cutoff concentrator (Millipore) before TEV protease was added. Digestion proceeded at room temperature overnight and once the (His)6 tag was cleaved, the protein sample was further purified by FPLC using Superdex 75 column (GE Healthcare) and 50 mM sodium phosphate, pH 7.0, 20 mM NaCl, 20 mM DTT as running buffer. The purity of the protein samples was>95 % as judged by SDS-PAGE.
NMR spectroscopy
LOTUS 1 was concentrated to 1.5 mM in a buffer containing 100 mM sodium acetate, pH 4.3, 10 mM NaCl, 50 μM DSS, 90 % H2O and 10 % D2O; while 1 mM of LOTUS 2–3 was prepared in 50 mM sodium phosphate, pH 7.0, 20 mM NaCl, 50 μM DSS, 90 % H2O and 10 % D2O. All NMR spectra were collected at 298 K on a Bruker Avance (III) 700 MHz spectrometer equipped with a 5 mm Z-gradient TCI (H/C/N) cryogenic probe. DSS was used to reference chemical shifts. 2D 1H–15N HSQC and 3D HNCACB, CBCA(CO)NH, HNCO and HN(CA)CO were used for backbone assignment. 2D 1H–13C HSQC (aliphatic region) and 3D HBHA(CO)NH, (H)CC(CO)NH, HCC(CO)NH and (H)CCH-TOCSY were used for side chain assignment. 2D 1H–13C HSQC (aromatic region) and 3D 15N-edited NOESY-HSQC and 13C-edited NOESY-HSQC (aliphatic and aromatic regions) were used for assignment of aromatic resonances. The amide side chains of Asn and Gln, as well as Arg (Hε–Nε) were assigned from analysis of the 3D 15N-edited NOESY-HSQC spectra. The secondary structures of LOTUS 1 and LOTUS 2–3 were identified from CSI analysis using Hα, Cα, Cβ and C′ chemical shifts (Wishart et al. 1992). All NMR data were processed using NMRPipe (Delaglio et al. 1995) and analyzed using NMRView (Johnson and Blevins 1994).
Extent of assignments and data deposition
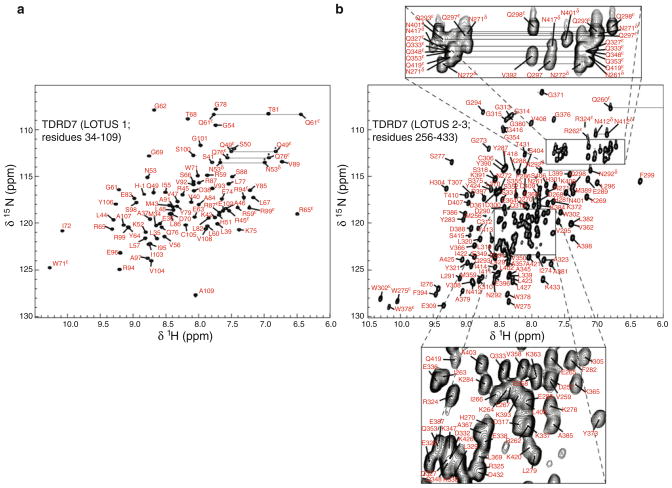
Figure 1a shows the 1H–15N HSQC spectrum of LOTUS 1 of TDRD7. The backbone atoms (HN, N, Hα, Cα, C′) of non-proline residues except Ser66 HN–N were assigned (98.4 % completion). The signals in the 1H–13C HSQC aliphatic and aromatic spectra were assigned with 84.8 % of the side chain resonances determined. The assignment of the side chain amide groups of all Asn (Hδ–Nδ), Gln (Hε–Nε) and Arg (Hε–Nε) residues, the H–C pairs and H–N pairs of aromatic moieties, as well as the Hδ2–Cδ2 and Hε1–Cε1 of His residues has been completed. None of the exchangeable side chain protons of Lys (Hζ), Arg (Hη) and His (Hδ1 and Hε2) were assigned as their signals were missing from the NMR spectra collected.
Fig. 1.
Spectra of TDRD7 LOTUS domains. a 1H–15N HSQC spectrum of 1.5 mM LOTUS 1 of TDRD7 in 100 mM sodium acetate, pH 4.3, 10 mM NaCl, 50 μM DSS, 90 % H2O and 10 % D2O. Spectrum was acquired at 298 K on a Bruker Avance (III) 700 MHz spectrometer. b 1H–15N HSQC spectrum of 1 mM LOTUS 2–3 of TDRD7 in 50 mM sodium phosphate, pH 7.0, 20 mM NaCl, 50 μM DSS, 90 % H2O and 10 % D2O. Acquisition conditions were the same as in a
Figure 1b shows the 1H–15N HSQC spectra of LOTUS 2–3 of TDRD7. The backbone atoms of non-proline residues, with the exception of Ser374 HN–N and Asn417 HN–N, were assigned at 96.9 % completion. The C′ assignment for Pro340, Pro341, Pro342 and Pro343 were not determined. The assignment of signals in the aliphatic and aromatic 1H–13C HSQC spectra was accomplished with the side chain assignment reaching 87.9 % completion. All the side chain H–N and H–C pairs except exchangeable side chain protons of Lys, Arg and His were assigned.
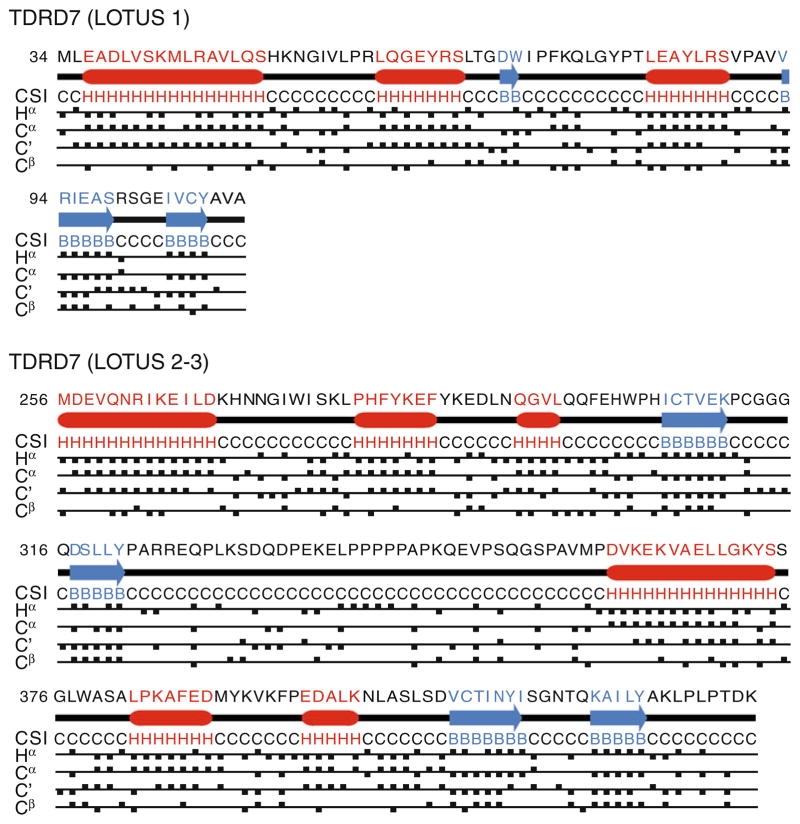
Figure 2 summarizes the CSI analysis done for LOTUS 1 and LOTUS 2–3. Based on the Hα, Cα, Cβ and C′ chemical shifts, the secondary structure elements found are consistent with three α-helices followed by two β-strands previously predicted for the LOTUS domain (Callebaut and Mornon 2010; Anantharaman et al. 2010). Based on these chemical shifts and additional nuclear Overhauser enhancement (NOE) measurements (data not shown), the 36-residue linker region tethering the LOTUS 2 and LOTUS 3 domains (residues 324–359) is disordered.
Fig. 2.
Chemical shift index (CSI) analysis for the LOTUS 1 and LOTUS 2–3 domains of TDRD7 using Hα, Cα, Cβ and C′ chemical shifts. H stands for α-helix, B for β-strand and C for coil. Numbering follows GenBank entry CAM17034.1
The chemical shift assignments for LOTUS 1 and LOTUS 2–3 of TDRD7 have been deposited in the Biological Magnetic Resonance Data Bank under accession numbers 17835 and 18211, respectively.
Acknowledgments
We are very grateful to Dr. Shinichiro Chuma, Kyoto University, Japan, for providing the cDNA for mouse TDRD7. This research was supported by the National Institutes of Health, grant R01 CA132878.
Abbreviations
- DSS
4,4-Dimethyl-4-silapentane-1-sulfonic acid
- IPTG
Isopropyl β-D-1-thiogalactopyranoside
- TDRD
Tudor domain-containing
- CSI
Chemical shift index
Footnotes
Conflict of interest The authors declare that they have no conflict of interest.
Ethical standards The experiments carried out in this work comply with the current laws of the United States of America.
References
- Anantharaman V, Zhang D, Aravind L. OST-HTH: a novel predicted RNA-binding domain. Biol Direct. 2010;5:1–8. doi: 10.1186/1745-6150-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botuyan MV, Lee J, Ward IM, Kim JE, Thompson JR, Chen J, Mer G. Structural basis for the methylation state-specific recognition of histone H4–K20 by 53BP1 and Crb2 in DNA repair. Cell. 2006;127:1361–1373. doi: 10.1016/j.cell.2006.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callebaut I, Mornon JP. LOTUS, a new domain associated with small RNA pathways in the germline. Bioinformatics. 2010;26:1140–1144. doi: 10.1093/bioinformatics/btq122. [DOI] [PubMed] [Google Scholar]
- Chen C, Jin J, James DA, Adams-Cioaba MA, Park JG, Guo Y, Tenaglia E, Xu C, Gish G, Min J, Pawson T. Mouse Piwi interactome identifies binding mechanism of Tdrkh Tudor domain to arginine methylated Miwi. Proc Natl Acad Sci USA. 2009;106:20336–20341. doi: 10.1073/pnas.0911640106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Côté J, Richard S. Tudor domains bind symmetrical dimethylated arginines. J Biol Chem. 2005;280:28476–28483. doi: 10.1074/jbc.M414328200. [DOI] [PubMed] [Google Scholar]
- Delaglio F, Grzesiek S, Vuister G, Zhu G, Pfeifer J, Bax A. NMRPipe: a multidimensional spectral processing system based on UNIX pipes. J Biomol NMR. 1995;6:277–293. doi: 10.1007/BF00197809. [DOI] [PubMed] [Google Scholar]
- Eddy M. Germ plasm and the differentiation of the germ cell line. Int Rev Cytol. 1975;43:229–280. doi: 10.1016/s0074-7696(08)60070-4. [DOI] [PubMed] [Google Scholar]
- Johnson BA, Blevins RA. NMRView: a computer program for the visualization and analysis of NMR data. J Biomol NMR. 1994;4:603–614. doi: 10.1007/BF00404272. [DOI] [PubMed] [Google Scholar]
- Lachke SA, Alkuraya FS, Kneeland SC, Ohn T, Aboukhalil A, Howell GR, Saadi I, Cavallesco R, Yue Y, Tsai AC, Nair KS, Cosma MI, Smith RS, Hodges E, Alfadhli SM, Al-Hajeri A, Shamseldin HE, Behbehani A, Hannon GJ, Bulyk ML, Drack AV, Anderson PJ, John SW, Maas RL. Mutations in the RNA granule component TDRD7 cause cataract and glaucoma. Science. 2011;331:1571–1576. doi: 10.1126/science.1195970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selenko P, Sprangers R, Stier G, Bülher D, Fischer U, Sattler M. SMN tudor domain structure and its interaction the Sm proteins. Nat Struct Biol. 2001;8:27–31. doi: 10.1038/83014. [DOI] [PubMed] [Google Scholar]
- Shoji M, Tanaka T, Hosokawa M, Reuter M, Stark A, Kato Y, Kondoh G, Okawa K, Chujo T, Suzuki T, Hata K, Martin SL, Noce T, Kuramochi-Miyagawa S, Nakano T, Sasaki H, Pillai RS, Nakatsuji N, Chuma S. The TDRD9-MIWI2 complex is essential for piRNA-mediated retrotransposon silencing in the mouse male germline. Dev Cell. 2009;17:775–787. doi: 10.1016/j.devcel.2009.10.012. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Hosokawa M, Vagin VV, Reuter M, Hayashi E, Mochizuki AL, Kitamura K, Yamanaka H, Kondoh G, Okawa K, Kuramochi-Miyagawa S, Nakano T, Sachidanandam R, Hannon GJ, Pillai RS, Nakatsuji N, Chuma S. Tudor domain containing 7 (Tdrd7) is essential for dynamic ribonucleoprotein (RNP) remodeling of chromatoid bodies during spermatogenesis. Proc Natl Acad Sci USA. 2011;108:10579–10584. doi: 10.1073/pnas.1015447108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson T, Lin H. The biogenesis and function of PIWI proteins and piRNAs: progress and prospect. Ann Rev Cell Dev Biol. 2009;25:355–376. doi: 10.1146/annurev.cellbio.24.110707.175327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagin VV, Wohlschlegel J, Qu J, Jonsson Z, Huang X, Chuma S, Girard A, Sachidanandam R, Hannon GJ, Aravin AA. Proteomic analysis of murine Piwi proteins reveals a role for arginine methylation in specifying interaction with Tudor family members. Genes Dev. 2009;23:1749–1762. doi: 10.1101/gad.1814809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart DS, Sykes BD, Richards FM. The chemical shift index: a fast and simple method for the assignment of protein secondary structure through NMR spectroscopy. Biochemistry. 1992;31:1647–1651. doi: 10.1021/bi00121a010. [DOI] [PubMed] [Google Scholar]
- Yabuta Y, Ohta H, Abe T, Kurimoto K, Chuma S, Saitou M. TDRD5 is required for retrotransposon silencing, chromatoid body assembly, and spermiogenesis in mice. J Cell Biol. 2011;192:781–795. doi: 10.1083/jcb.201009043. [DOI] [PMC free article] [PubMed] [Google Scholar]