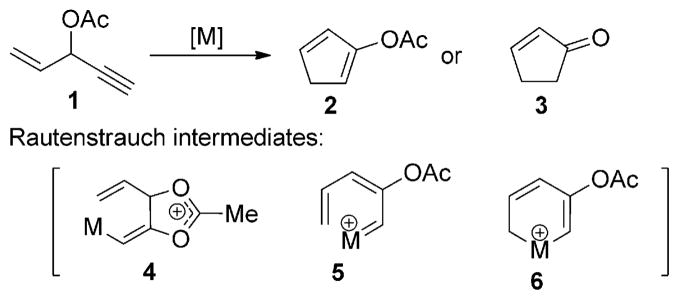
In 1984, Rautenstrauch reported that the 3-acyloxy-1,4-enyne 1 could undergo cyclization to form cyclopentadiene 2 and cyclopentenone 3 in the presence of a palladium catalyst through 1,2-acyloxy migration (Scheme 1).[1] The vinyl metal complex 4, metal carbene 5, and metallacyclohexadiene 6 were proposed as intermediates in this transformation.[1,2] The scope of this rearrangement reaction has been expanded significantly by the use of π-acidic metals,[3] such as gold- and platinum-based catalysts, for the synthesis of functionalized five-membered rings.[4] The 1,2-acyloxy migration of propargyl esters has also been employed in other synthetically useful transformations catalyzed by gold,[5,6] platinum,[6,7] ruthenium,[8,9] copper,[6] and more recently rhodium.[10]
Scheme 1.
Rautenstrauch rearrangement.
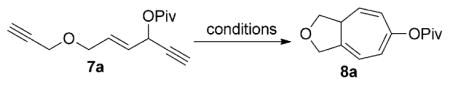
We recently found that [{Rh(CO)2Cl}2] was able to catalyze the 1,3-acyloxy migration of propargyl esters in the synthesis of functionalized cyclohexenones.[11] The combination of this novel reactivity of RhI in promoting acyloxy migration and its well-known capability to undergo facile oxidative addition, migratory insertion, and reductive elimination may offer many opportunities for the design of new reactions. We envisioned that a conceptually new approach to seven-membered rings was possible if intermediate 6 in the Rautenstrauch rearrangement could be intercepted by a tethered alkyne in a [5+2] cycloaddition under rhodium catalysis.[12–16] We herein report a new atom-economical[17] synthesis of a bicyclo[5.3.0]decatriene 8 through a rhodium(I)-catalyzed cycloisomerization[18] of a 3-acyloxy-4-ene-1,9-diyne 7 [Eq. (1)]. The net result of this reaction is an intramolecular [5+2] cycloaddition[14–16] with concomitant 1,2-acyloxy migration. The resulting complex bicyclo-[5.3.0]decane skeletons are present in many natural products.[19]
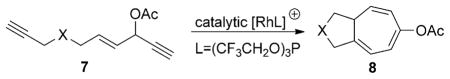 |
(1) |
Besides the Rautenstrauch rearrangement to form five-membered rings, a number of other pathways may also compete with the desired cycloisomerization of enyne 7 to the bicyclic compound 8. For example, if a carbene intermediate similar to 5 is generated, it may undergo cyclopropanation or cyclopropenation with alkenes or alkynes in the system. However, when substrate 7 a, available in four steps from 2-butene-1,4-diol,[20] was treated with a catalytic amount of [{Rh(CO)2Cl}2], cycloisomerization occurred to give the bicyclic product 8 a in 19 and 48% yield in toluene and dichloroethane (DCE), respectively (Table 1, entries 1 and 2). Several other RhI catalysts also promoted this reaction (Table 1, entries 4–6). The cationic RhI catalyst [Rh-(cod)2]BF4 promoted the tandem cycloisomerization even at room temperature (Table 1, entry 6). The reaction is solvent-dependent (Table 1, entries 7 and 8), and higher yields were generally observed with chlorinated solvents (entries 9 and 10). A complex 5,7-fused bicyclic compound can thus be prepared in a single step from a readily available linear 3-acyloxy-4-ene-1,9-diyne under rhodium catalysis. AuI, PtII, or Brønsted acid catalysts did not provide any of the desired product (Table 1, entries 11–13).
Table 1.
Screening of catalysts and conditions for rhodium(I)-catalyzed cycloisomerization.
| ||
|---|---|---|
| Entry | Conditions | Yield [%][a] |
| 1 | [{Rh(CO)2Cl}2] (5 mol%), toluene, 90 °C, 8 h | 19 |
| 2 | [{Rh(CO)2Cl}2] (5 mol%), DCE, 90 °C, 8 h | 48 |
| 3 | [{Rh(CO)2Cl}2] (5 mol%), TCE, 90 °C, 1.5 h | 43 |
| 4 | [{Rh(cod)Cl}2] (5 mol%), TCE, 90 °C, 8 h | 21 |
| 5 | [Rh(PPh3)3Cl] (5 mol%), TCE, 90 °C, 8 h | 77 |
| 6 | [Rh(cod)2]BF4 (5 mol%), DCE, RT, 8 h | 70 |
| 7 | [Rh(cod)2]BF4 (5 mol%), toluene, RT, 8 h | n.r. |
| 8 | [Rh(cod)2]BF4 (5 mol%), dioxane, RT, 8 h | n.r. |
| 9 | [Rh(cod)2]BF4 (5 mol%), TCE, 50 °C, 20 h | 81 |
| 10 | [Rh(COD)2]BF4 (5 mol%), CH2Cl2, RT, 8 h | 83 |
| 11 | [AuCl(PPh3)] (5 mol%), AgOTf (5 mol%), MeCN, RT, 20 h | 0 |
| 12 | PtCl2 (10 mol%), DCE, 80 °C, 20 h | 0 |
| 13 | HNTf2 (10 mol%), CH2Cl2, RT, 20 h | 0 |
The yield was calculated on the basis of 1H NMR spectroscopy with an internal standard.
cod = 1,5-cyclooctadiene, n.r. = no reaction, Piv = pivaloyl, TCE = tetrachloroethane, Tf = trifluoromethanesulfonyl.
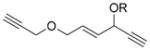
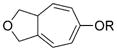
We next examined the scope of this tandem cycloisomerization under conditions A (Table 2). The reaction remained efficient when the ester was changed from a pivalate to an acetate or benzoate (Table 2, entries 1–3). Substrates with a nitrogen or a gem-diester linker in the 1,6-enyne yielded bicyclic compounds 8 d and 8 e successfully (Table 2, entries 4 and 5). The structure of bicyclic product 8 d was assigned unambiguously by X-ray crystallographic analysis.[21]
Table 2.
Scope of the rhodium(I)-catalyzed cycloisomerization.
| Entry | Substrate | Product | Yield [%][a] (cond.[b]) |
|---|---|---|---|
|
|
||
| 1 | 7 a, R = Piv | 8 a, R = Piv | 85 (A) |
| 2 | 7 b, R = Ac | 8 b, R = Ac | 81 (A) |
| 3 | 7 c, R = Bz | 8 c, R = Bz | 83 (A) |
| 4 |
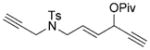 7 d |
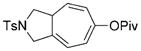 8 d |
96 (A) |
| 5 |
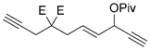 7 e |
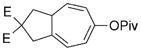 8 e |
75 (A) |
| 6 |
7 f |
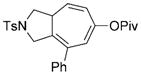 8 f |
88 (B) |
| 7 |
7 g |
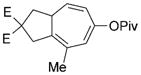 8 g |
82 (B) |
| 8 |
7 h |
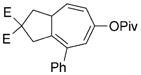 8 h |
70 (B) |
| 9 |
7 i |
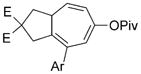 8 i |
60 (B) |
| 10 |
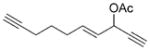 7 j |
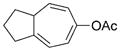 8 j |
76 (B) |
| 11 |
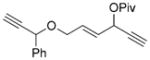 7 k[c] |
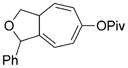 8 k[c] |
90 (A) |
| 12 |
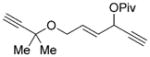 7 l |
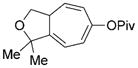 8 l |
90 (A) |
| 13 |
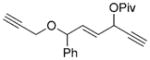 7 m[c] |
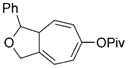 8 m[c] |
76 (B) |
| 14 |
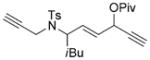 7 n[c] |
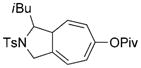 8 n[c] |
80 (B) |
| 15 |
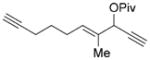 7 o |
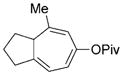 8 o |
80 (B) |
| 16 |
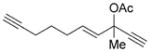 7 p |
complex mixture | (A or B) |
Yield of the isolated product.
Conditions A: [Rh(cod)2]BF4 (3–5 mol%), CH2Cl2 (0.05 M), RT or 50 °C, 8–48 h; conditions B: [Rh-(cod)2]BF4 (5–10 mol%), (CF3CH2O)3P (10–20 mol%), CH2Cl2 (0.025–0.05M), 50°C, 8–24 h.
The diastereomeric ratio is 1:1. Bz = benzoyl, Ts = p-toluenesulfonyl.
We systematically examined the scope of this rhodium(I)-catalyzed cycloisomerization by placing substituents at different positions on the 1,9-diyne. For substrates 7 f–7 i with an internal alkyne on the left-hand side, either no reaction or only a trace amount of the product was observed under conditions A. We then explored the effect of ligands on the cycloisomerization of substrate 7 f with the [Rh(cod)2]BF4 catalyst. The addition of PPh3, iBu3P, or 1,2-bis(diphenyl-phosphanyl)ethane (dppe) had no effect. Triethyl phosphite improved the conversion of substrate 7 f to 21% according to 1H NMR spectroscopy. Similar conversion was also observed with the electron-poor phosphine ligands (C6F5)3P and (p-CF3C6H4)3P. The electron-poor phosphite ligand (CF3CH2O)3P significantly improved the conversion: substrate 7 f was completely consumed within 8 hours, and product 8 f was isolated in 88% yield (Table 2, entry 6). A novel catalytic system composed of cationic RhI and tris(2,2,2-trifluoroethyl) phosphite was thus developed (conditions B).
Dramatic improvements were also observed for other substrates with internal alkynes when a combination of the catalyst [Rh(cod)2]BF4 and the ligand (CF3CH2O)3P was used (Table 2, entries 7–9). The all-carbon tether was not limited to substrates with gem-diester substituents. Moderate conversion (40–50%) was observed for substrate 7 j when the catalyst [Rh(cod)2]BF4 (3–10 mol%) was used alone. Again, the addition of the ligand (CF3CH2O)3P improved the yield of product 8 j (Table 2, entry 10).
We then examined the effects of substituents in the tether region. Substituents adjacent to the left-hand alkyne had no apparent effect, and the cycloisomerization proceeded efficiently under conditions A (Table 2, entries 11 and 12). We were very pleased to find that the reaction even tolerated the quaternary carbon center adjacent to the reacting alkyne in substrate 7 l. Substituents adjacent to the alkene, however, lowered the conversion, and the addition of (CF3CH2O)3P as a ligand was necessary for the formation of the product in good yield (Table 2, entries 13 and 14). A trisubstituted olefin was also tolerated: the bicyclic product 8 o was obtained in good yield (Table 2, entry 15). However, when a substrate with a tertiary ester was subjected to conditions A or B, a complex mixture was formed (Table 2, entry 16).
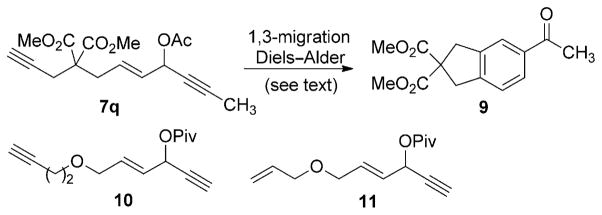
For substrates with an internal alkyne at the right-hand end (e.g. 7 q, Scheme 2), the formation of benzene derivatives (e.g. 9) in the presence of a PtCl2 catalyst has been reported.[22] A 1,3-acyloxy migration followed by a Diels–Alder-type reaction was proposed for this transformation. When we treated 7 q with the cationic RhI catalyst, a trace amount of product 9 was observed, and the starting material was mainly recovered. We have previously shown that [{Rh(CO)2Cl}2] is an efficient catalyst for the 1,3-acyloxy migration of propargyl esters.[11] Indeed, our preliminary study showed that product 9 could be obtained in 30–40% yield with the catalyst [{Rh(CO)2Cl}2]. Since this transformation has been carried out with the catalyst PtCl2, no further optimization was conducted. These results, however, did show that the 1,2- and 1,3-acyloxy migration of propargyl esters is dependent on the nature of the substrate and the RhI catalyst, and are thus consistent with observations made with other metal catalysts.[3]
Scheme 2.
Attempted cycloisomerization of other substrates.
Substrates with six-atom or longer tethers between the two reactive π systems are often challenging in transition-metal-catalyzed intramolecular cycloaddition and cycloisomerization reactions.[18] Substrate 10 (Scheme 2) was prepared to test the limits of the present cycloisomerization. Under standard conditions A or B, no reaction occurred, and the starting material was recovered. A tethered alkene also failed to intercept the Rautenstrauch intermediate: when the 3-acyloxy-substituted dienyne 11 was subjected to conditions A or B, the starting material was recovered.
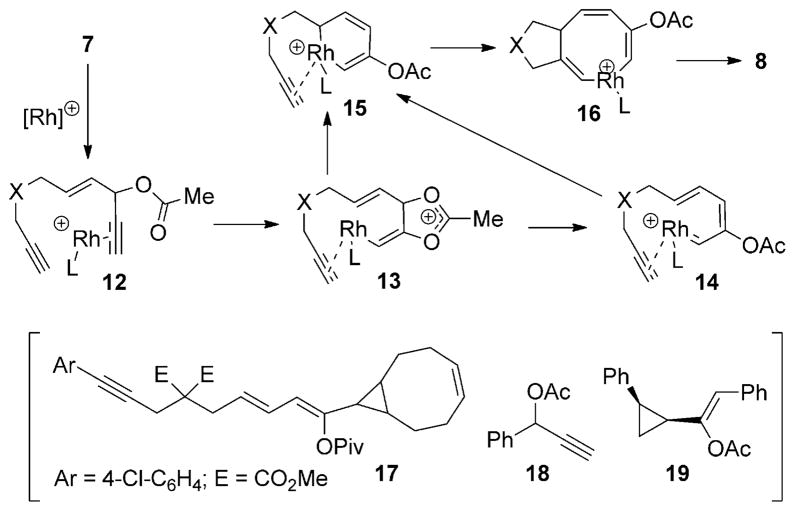
We propose a mechanism involving a Rautenstrauch intermediate for the formation of products 8 from enediynes 7 (Scheme 3): A rhodium(I)-promoted 1,2-acyloxy migration of the propargyl ester in complex 12 provides a vinyl metal species 13. The metallacyclohexadiene 15 may be formed through the direct cyclization of intermediate 13, or via carbene 14 through a 6 π electrocyclization. Insertion of the tethered alkyne into the metallacycle 15, followed by reductive elimination of the metallacyclooctatriene 16, then produces product 8 with a seven-membered ring.[18] As the yield for the transformation of substrate 7 i into product 8 i was the lowest observed for the successful reactions in this study (Table 2, entry 9), we carefully analyzed the byproducts of this reaction. We isolated a small amount of cyclopropane 17 (Scheme 3), which was presumably derived from the reaction between a RhI carbene and one of the cyclooctadiene ligands in the catalyst. Compound 17 became the major product when excess external cyclooctadiene (2.0 equiv) was added to the reaction mixture. However, when the external cyclooctadiene was replaced by the same amount of styrene, no cyclopropanation product derived from styrene was observed. This difference may be attributed to the bidentate nature of cyclooctadiene. When we treated propargyl ester 18 with the different RhI catalysts in Table 1 in the presence of styrene, the known cyclopropane 19[8] was isolated in several cases. This outcome again suggested the formation of a RhI carbene from the propargyl ester. Although there are other potential mechanisms, the above results are consistent with the mechanism proposed in Scheme 3 based on the interception of a Rautenstrauch intermediate by an alkyne.
Scheme 3.
Proposed mechanism for the rhodium(I)-catalyzed cycloisomerization and evidence for the involvement of a rhodium(I) carbene.
In summary, we have developed a conceptually novel intramolecular [5+2] cycloaddition with concomitant 1,2-acyloxy migration for the synthesis of highly functionalized seven-membered rings. Various substituted bicyclo-[5.3.0]decatrienes were synthesized in this way from readily available linear starting materials. The cycloheptatriene in the resulting bicyclic system has three well-differentiated double bonds ready for further functionalization.[19] Cycloheptatrienes themselves are also widely present in polycyclic natural products and pharmaceutical agents.[23] Further studies to uncover the details of the mechanism, expand the scope of the reaction, and apply this novel cycloisomerization to the synthesis of natural products and pharmaceutical agents are currently in progress.
Supplementary Material
Footnotes
We thank the NIH (R01GM088285) and the University of Wisconsin for funding. S.H. was partially supported by a fellowship from the Chinese Scholarship Council.
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/anie.201103136.
Contributor Information
Dr. Xing-zhong Shu, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA)
Suyu Huang, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA).
Dongxu Shu, Department of Chemistry, University of Wisconsin (USA).
Ilia A. Guzei, Department of Chemistry, University of Wisconsin (USA)
Prof. Dr. Weiping Tang, Email: wtang@pharmacy.wisc.edu, The School of Pharmacy, University of Wisconsin, Madison, WI 53705-2222 (USA).
References
- 1.Rautenstrauch V. J Org Chem. 1984;49:950. [Google Scholar]
- 2.Rautenstrauch V. Tetrahedron Lett. 1984;25:3845. [Google Scholar]
- 3.For reviews on π-acidic-metal-catalyzed reactions, see: Miki K, Uemura S, Ohe K. Chem Lett. 2005;34:1068.Marion N, Nolan SP. Angew Chem. 2007;119:2806. doi: 10.1002/anie.200604773.Angew Chem Int Ed. 2007;46:2750.Fürstner A, Davies PW. Angew Chem. 2007;119:3478. doi: 10.1002/anie.200604335.Angew Chem Int Ed. 2007;46:3410.Hashmi ASK. Chem Rev. 2007;107:3180. doi: 10.1021/cr000436x.Hashmi ASK. Angew Chem. 2008;120:6856.Angew Chem Int Ed. 2008;47:6754.Jiménez-Núñez E, Echavarren AM. Chem Rev. 2008;108:3326. doi: 10.1021/cr0684319.Gorin DJ, Sherry BD, Toste FD. Chem Rev. 2008;108:3351. doi: 10.1021/cr068430g.
- 4.Shi XD, Gorin DJ, Toste FD. J Am Chem Soc. 2005;127:5802. doi: 10.1021/ja051689g.Bhanu Prasad BA, Yoshimoto FK, Sarpong R. J Am Chem Soc. 2005;127:12468. doi: 10.1021/ja053192c.Nakanishi Y, Miki K, Ohe K. Tetrahedron. 2007;63:12138.DeKorver KA, Hsung RP, Lohse AG, Zhang Y. Org Lett. 2010;12:1840. doi: 10.1021/ol100446p.for a computational study, see: Nieto Faza O, Silva López C, Álvarez R, de Lera AR. J Am Chem Soc. 2006;128:2434. doi: 10.1021/ja057127e.
- 5.a) Johansson MJ, Gorin DJ, Staben ST, Toste FD. J Am Chem Soc. 2005;127:18002. doi: 10.1021/ja0552500. [DOI] [PubMed] [Google Scholar]; b) Gorin DJ, Dube P, Toste FD. J Am Chem Soc. 2006;128:14480. doi: 10.1021/ja066694e. [DOI] [PubMed] [Google Scholar]; c) Gorin DJ, Watson IDG, Toste FD. J Am Chem Soc. 2008;130:3736. doi: 10.1021/ja710990d. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Li G, Zhang G, Zhang L. J Am Chem Soc. 2008;130:3740. doi: 10.1021/ja800001h. [DOI] [PubMed] [Google Scholar]; e) Moreau X, Goddard JP, Bernard M, Lemière G, López-Romero JM, Mainetti E, Marion N, Mouriès V, Thorimbert S, Fensterbank L, Malacria M. Adv Synth Catal. 2008;350:43. [Google Scholar]; f) Watson IDG, Ritter S, Toste FD. J Am Chem Soc. 2009;131:2056. doi: 10.1021/ja8085005. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Harrak Y, Makhlouf M, Azzaro S, Mainetti E, Romero JML, Cariou K, Gandon V, Goddard JP, Malacria M, Fensterbank L. J Organomet Chem. 2011;696:388. [Google Scholar]
- 6.a) Fehr C, Galindo J. Angew Chem. 2006;118:2967. [Google Scholar]; Angew Chem Int Ed. 2006;45:2901. [Google Scholar]; b) Fehr C, Winter B, Magpantay I. Chem Eur J. 2009;15:9773. doi: 10.1002/chem.200901292. [DOI] [PubMed] [Google Scholar]
- 7.a) Mainetti E, Mouriès V, Fensterbank L, Malacria M, Marco-Contelles J. Angew Chem. 2002;114:2236. [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:2132. [Google Scholar]; b) Harrak Y, Blaszykowski C, Bernard M, Cariou K, Mainetti E, Mouriès V, Dhimane AL, Fensterbank L, Malacria M. J Am Chem Soc. 2004;126:8656. doi: 10.1021/ja0474695. [DOI] [PubMed] [Google Scholar]; c) Pujanauski BG, Prasad BAB, Sarpong R. J Am Chem Soc. 2006;128:6786. doi: 10.1021/ja061549m. [DOI] [PubMed] [Google Scholar]; d) Ji K, Shu X, Chen J, Zhao S, Zheng Z, Lu L, Liu X, Liang Y. Org Lett. 2008;10:3919. doi: 10.1021/ol8015463. [DOI] [PubMed] [Google Scholar]
- 8.a) Miki K, Ohe K, Uemura S. J Org Chem. 2003;68:8505. doi: 10.1021/jo034841a. [DOI] [PubMed] [Google Scholar]; b) Miki K, Ohe K, Uemura S. Tetrahedron Lett. 2003;44:2019. [Google Scholar]
- 9.Tenaglia A, Marc S. J Org Chem. 2006;71:3569. doi: 10.1021/jo060276a. [DOI] [PubMed] [Google Scholar]
- 10.a) Shibata Y, Noguchi K, Tanaka K. J Am Chem Soc. 2010;132:7896. doi: 10.1021/ja102418h. [DOI] [PubMed] [Google Scholar]; b) Brancour C, Fukuyama T, Ohta Y, Ryu I, Dhimane AL, Fensterbank L, Malacria M. Chem Commun. 2010;46:5470. doi: 10.1039/c0cc00747a. [DOI] [PubMed] [Google Scholar]
- 11.Shu D, Li X, Zhang M, Robichaux PJ, Tang W. Angew Chem. 2011;123:1382. doi: 10.1002/anie.201006881. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2011;50:1346. [Google Scholar]
- 12.For selected reviews on the synthesis of seven-membered rings, see: Battiste MA, Pelphrey PM, Wright DL. Chem Eur J. 2006;12:3438. doi: 10.1002/chem.200501083.Butenschön H. Angew Chem. 2008;120:5367.Angew Chem Int Ed. 2008;47:5287.Harmata M. Chem Commun. 2010;46:8886. doi: 10.1039/c0cc03620j.Harmata M. Chem Commun. 2010;46:8904. doi: 10.1039/c0cc03621h.Pellissier H. Adv Synth Catal. 2011;353:189.
- 13.For selected examples of the transition-metal-mediated synthesis of seven-membered rings, see: Noyori R. Acc Chem Res. 1979;12:61. doi: 10.1021/ar7000809.Trost BM, Macpherson DT. J Am Chem Soc. 1987;109:3483.Trost BM, Matelich MC. J Am Chem Soc. 1991;113:9007.Schwiebert KE, Stryker JM. J Am Chem Soc. 1995;117:8275.Evans PA, Inglesby PA. J Am Chem Soc. 2008;130:12838. doi: 10.1021/ja803691p.Trillo B, López F, Gulías M, Castedo L, Mascareñas JL. Angew Chem. 2008;120:965. doi: 10.1002/anie.200704566.Angew Chem Int Ed. 2008;47:951.Bhargava G, Trillo B, Araya M, López F, Castedo L, Mascareñas JL. Chem Commun. 2010;46:270. doi: 10.1039/b919258a.
- 14.For representative examples of transition-metal-mediated [5+2] cycloaddition reactions, see: Wender PA, Takahashi H, Witulski B. J Am Chem Soc. 1995;117:4720.Wender PA, Husfeld CO, Langkopf E, Love JA. J Am Chem Soc. 1998;120:1940.Wender PA, Rieck H, Fuji M. J Am Chem Soc. 1998;120:10976.Wender PA, Glorius F, Husfeld CO, Langkopf E, Love JA. J Am Chem Soc. 1999;121:5348.Dzwiniel TL, Etkin N, Stryker JM. J Am Chem Soc. 1999;121:10640.Trost BM, Toste FD, Shen H. J Am Chem Soc. 2000;122:2379.Tanino K, Shimizu T, Miyama M, Kuwajima I. J Am Chem Soc. 2000;122:6116.Trost BM, Shen HC. Angew Chem. 2001;113:2375.Angew Chem Int Ed. 2001;40:2313.Wender PA, Pedersen TM, Scanio MJC. J Am Chem Soc. 2002;124:15154. doi: 10.1021/ja0285013.Zuo G, Louie J. J Am Chem Soc. 2005;127:5798. doi: 10.1021/ja043253r.Wegner HA, de Meijere A, Wender PA. J Am Chem Soc. 2005;127:6530. doi: 10.1021/ja043671w.Fürstner A, Majima K, Martin R, Krause H, Kattnig E, Goddard R, Lehmann CW. J Am Chem Soc. 2008;130:1992. doi: 10.1021/ja0777180.Jiao L, Ye S, Yu Z. J Am Chem Soc. 2008;130:7178. doi: 10.1021/ja8008715.Inagaki F, Sugikubo K, Miyashita Y, Mukai C. Angew Chem. 2010;122:2252. doi: 10.1002/anie.200906994.Angew Chem Int Ed. 2010;49:2206.Feng JJ, Zhang J. J Am Chem Soc. 2011;133:7304. doi: 10.1021/ja2014604.
- 15.For selected applications of [5+2] cycloaddition reactions in natural product synthesis, see: Wender PA, Fuji M, Husfeld CO, Love JA. Org Lett. 1999;1:137.Wender PA, Zhang L. Org Lett. 2000;2:2323. doi: 10.1021/ol006085q.Ashfeld BL, Martin SF. Org Lett. 2005;7:4535. doi: 10.1021/ol051945u.Trost BM, Hu Y, Horne DB. J Am Chem Soc. 2007;129:11781. doi: 10.1021/ja073272b.Trost BM, Waser J, Meyer A. J Am Chem Soc. 2008;130:16424. doi: 10.1021/ja806724x.Jiao L, Yuan C, Yu Z. J Am Chem Soc. 2008;130:4421. doi: 10.1021/ja7100449.
- 16.For computational studies on [5+2] cycloaddition reactions, see: Yu Z, Wender PA, Houk KN. J Am Chem Soc. 2004;126:9154. doi: 10.1021/ja048739m.Wang Y, Wang J, Su JC, Huang F, Jiao L, Liang Y, Yang D, Zhang S, Wender PA, Yu Z. J Am Chem Soc. 2007;129:10060. doi: 10.1021/ja072505w.Yu Z, Cheong PHY, Liu P, Legault CY, Wender PA, Houk KN. J Am Chem Soc. 2008;130:2378. doi: 10.1021/ja076444d.Liu P, Cheong PHY, Yu Z, Wender PA, Houk KN. Angew Chem. 2008;120:4003. doi: 10.1002/anie.200800420.Angew Chem Int Ed. 2008;47:3939.Liu P, Sirois LE, Cheong PHY, Yu Z, Hartung IV, Rieck H, Wender PA, Houk KN. J Am Chem Soc. 2010;132:10127. doi: 10.1021/ja103253d.
- 17.Trost BM. Science. 1991;254:1471. doi: 10.1126/science.1962206. [DOI] [PubMed] [Google Scholar]
- 18.For selected reviews on transition-metal-catalyzed cycloisomerization and cycloaddition, see: Lautens M, Klute W, Tam W. Chem Rev. 1996;96:49. doi: 10.1021/cr950016l.Ojima I, Tzamarioudaki M, Li ZY, Donovan RJ. Chem Rev. 1996;96:635. doi: 10.1021/cr950065y.Frühauf HW. Chem Rev. 1997;97:523. doi: 10.1021/cr941164z.Trost BM, Krische MJ. Synlett. 1998:1.Yet L. Chem Rev. 2000;100:2963. doi: 10.1021/cr990407q.Aubert C, Buisine O, Malacria M. Chem Rev. 2002;102:813. doi: 10.1021/cr980054f.Evans PA. Modern Rhodium-Catalyzed Organic Reactions. Wiley-VCH; Weinheim: 2005. Michelet V, Toullec PY, Genêt J-P. Angew Chem. 2008;120:4338. doi: 10.1002/anie.200701589.Angew Chem Int Ed. 2008;47:4268.Yu ZX, Wang Y, Wang Y. Chem Asian J. 2010;5:1072. doi: 10.1002/asia.200900712.Aubert C, Fensterbank L, Garcia P, Malacria M, Simonneau A. Chem Rev. 2011;111:1954. doi: 10.1021/cr100376w.
- 19.For a recent review on natural products with bicyclo-[5.3.0]decane skeletons, see: Foley DA, Maguire AR. Tetrahedron. 2010;66:1131.
- 20.See the Supporting Information for details.
- 21.CCDC 823148 (8 d) contains the supplementary crystallographic data for this paper. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
- 22.Lu L, Liu X, Shu X, Yang K, Ji K, Liang Y. J Org Chem. 2009;74:474. doi: 10.1021/jo802043z. [DOI] [PubMed] [Google Scholar]
- 23.For recent reviews on cycloheptatriene-containing compounds, see: Zhao J. Curr Med Chem. 2007;14:2597. doi: 10.2174/092986707782023253.Bentley R. Nat Prod Rep. 2008;25:118. doi: 10.1039/b711474e.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.