Abstract
Regulatory T cells (Tregs) have become an important player in regulating anti-cancer immune responses. In fact, published studies describe a correlation between tumor infiltrating Tregs and poor prognosis. Once called “suppressor T cells”, these T cells evaded isolation due to a lack of known markers that distinguished them from other T cells. However, the biology of these T cells is currently a major focus of immunologic research. Markers have since been discovered that identify these T cells and provide insights into how these T cells are regulated. Despite these advances, much needs to be learned about the sub-sets of Tregs and their specific roles in regulating immune responses. In addition, specific agents that target Tregs are currently unavailable. Cyclophosphamide (CY) has emerged as a clinically feasible agent that can suppress Tregs and allow more effective induction of antitumor immune responses. This review will focus on the use of CY in targeting Tregs to augment cancer vaccine approaches. However, these principles can also be applied to other immunotherapy strategies.
Introduction
Cancer vaccines have come of age with the first vaccine approved for prostate cancer treatment. Yet, the survival benefit as single agent is modest. Accumulating evidence now support multiple mechanisms of immune tolerance that inhibit the most potent antitumor immune responses, and reinforce employing a multi-pronged approach which combines agents that prime and expand the best tumor specific T cells with agents that target immune suppressive factors. Combinatorial vaccination strategies are under development testing immune adjuvants that recruit and activate antigen presenting cells (APCs), and agents that provide additional activating signals to APCs and T cells. Also under development are antagonist antibodies that target inhibitory signaling pathways, promoting checkpoint blockade of signals T cells receive from APCs, tumors, and Tregs. However, currently there are no agents that specifically inhibit Tregs. CY is the agent used most extensively to inhibit Tregs not only because it is widely available and inexpensive, but increasing evidence suggests it has multiple immune modifying properties. Entire reviews have focused on this multifaceted aspect of CY and have enumerated the vast number of proposed mechanisms responsible for its immune properties. This review will focus specifically on the use of CY to inhibit Tregs in the context of cancer vaccines.
Historical perspective
CY has many purported immune modulatory mechanisms. Sistigu and colleagues recently reviewed many mechanisms which include TH2/TH1–TH17 shifts in cytokine production, induction of TH17 cells, inhibition of Tregs, enhancement of T cell proliferation and survival, and resetting of dendritic cell homeostasis(1)(2). However, the earliest mechanism proposed was the inhibition of a population of suppressor T cells. These T cells were difficult to isolate due to the lack of a marker specific for this population until the discovery of FOXP3, a transcriptional regulator of what are now known as Tregs. Multiple subsets of Tregs, constitutive and inducible, CD4+ and CD8+, FoxP3+ and FoxP3-, have since been described in the context of malignancy. Most studies associate the presence of CD4+CD25+FoxP3+ Tregs in tumors with poor prognosis. This has been demonstrated in a number of cancers including breast, ovarian, and pancreatic cancers(3-5). In some cancers, such as Hodgkin’s lymphoma, Tregs in the tumor microenvironment have been associated with improved clinical outcomes (6). More recent studies suggest that it is T effector (Teff)/Treg ratios that correlate with effective anti-tumor responses(7). In fact, a natural response to vaccination is the concurrent induction of Teffs and Tregs. Thus, it is likely that the balance of these T cell subsets influence outcomes.
As early as 1974, Polak and Turk postulated that CY reversed immune tolerance in a guinea pig sensitization model through inhibition of a yet to be identified suppressor T cell population(8). Throughout the 1980s, experiments performed by North’s group suggested that CY augmented adoptive immunotherapy by inhibiting suppressor T cells. In 1988, North published on the effects of CY (150mg/kg intravenously (i.v.)) in combination with passively transferred tumor-sensitized T cells in a CY-resistant tumor model(9). Adoptively transferred T cells were administered 1 hour after CY. Since the tumor was resistant to CY, this model suggested that CY’s effects were through the inhibition of suppressor T cells rather than a direct effect on tumor burden. That same year, Berd and Mastrangelo used a regimen of low-dose CY (300mg/m2 i.v.) given 3 days prior to vaccination with autologous melanoma cells admixed with bacille Calmette-Guerin (BCG) to treat patients with melanoma(10). CY plus vaccine resulted in a decrease in the proportion of CD4+ T cells expressing 2H4 (CD45), which they stated was an identifier of “inducers of suppression”. The reduction did not become apparent until day 28 and became statistically significant on day 49. Interestingly, they did not see an effect of CY on the suppressor population expressing the IL2 receptor (CD25).
Jaffee and colleagues (2001) evaluated the potential of several chemotherapies, including CY, doxorubicin (DOX) and paclitaxel, to potentiate the effects of GM-CSF-secreting whole cell vaccines in the HER-2/neu mouse model of mammary cancers (11). They determined that both the dose and sequence of drug administration in relation to vaccine delivery was important in mediating enhancement of vaccine effects. Again, the mechanism for CY’s effect was not due to direct cytolytic effect on cancer cells but through CY’s influence on immunity. When CY was given at a dose range between 50 and 150mg/kg one day prior to vaccine, the combination controlled tumors more effectively than either agent alone. The same treatment 7 days later was ineffective. Higher doses of CY did not enhance and often abrogated vaccine efficacy. The improved efficacy of lower doses of CY supported that the anti-tumor effects were not mediated through CY’s cytolytic capacity. In these studies, CY was shown to amplify the T helper 1 neu-specific T cell response.
In 2004, Ghiringhelli and colleagues showed that a single administration of CY at 25-30mg/kg in rats depleted CD4+CD25+ T cells and delayed the growth of colon carcinomas (12). In addition, CY given prior to tumor cells mixed with BCG, resulted in complete regression of tumors. Furthermore, CY induced a decrease in the CD4+CD25+/CD4+ splenic T cell ratio in the spleen resected 7 days after a single dose. This decrease was also seen with methotrexate and anti-CD25 mAb. In this model the CD4+CD25+/CD4+ ratio reached its nadir at 7 days.
After Sakaguchi identified (2003) the transcription factor FoxP3 as a key regulator in Treg development, reports followed demonstrating CY-mediated reductions in FoxP3+ Tregs(13). Subsequent studies demonstrated that in addition to CY’s effect on decreasing Treg number, low-dose CY decreased the functionality of Tregs(14). Lutsiak and colleagues isolated Tregs from untreated and CY-treated (2mg intraperitoneal, i.p.) mice, 2 and 10 days after CY treatment, and evaluated the Tregs in suppression assays. CY-treated Tregs had significant impairment in their suppressive capacity, which returned by day 10 after treatment. CY also interfered with homeostatic proliferation of Tregs, increased their susceptibility to apoptosis, and decreased their expression of suppression markers including glucocorticoid-induced TNFR-related protein (GITR) and FoxP3.
Low- versus high-dose cyclophosphamide
Depending on the dose administered, CY’s anti-tumor effects are either through immune potentiation or direct cytolytic activity. In the HER2/neu mouse model, CY was most effective in enhancing vaccine effects given at a dose range between 50 and 150mg/kg(11). Higher doses hampered vaccine induced immunity by causing bone marrow suppression. This was further supported by Motoyoshi et al who showed that low (20mg/kg) but not high-dose (200mg/kg) CY selectively suppressed CD4+CD25+ T cell numbers, sparing conventional CD4+ and CD8+ T cells, and preventing murine hepatoma growth (15). In the low-dose group, the decline in CD4+CD25+ T cells was more profound and recovered more slowly than CD4+ T cells resulting in lower ratios of CD4+CD25+/CD4+ T cells for longer periods of time. In contrast, in the high-dose group, all T cell subsets and the ratio were severely decreased. Low and high doses of CY were also compared in immuncompetent and nude mice. While low doses were effective in treating tumors only in immunocompetent mice, the high doses worked in immunocompetent and nude mice. This suggested that low-dose CY contributes to anti-tumor immunity whereas high-dose CY worked solely through its cytotoxic effects. Low-dose CY also resulted in higher intratumoral lymphocyte infiltration. Repletion of CD4+CD25+ T cells abolished the anti-tumor effect of low-dose CY.
Emens and colleagues conducted a trial in breast cancer patients to address the question of dosing(16). In this study, an allogeneic, HER2-positive GM-CSF-secreting breast tumor vaccine was given alone or in sequence with low-dose CY and DOX. The study used a factorial design to identify the CY and DOX dose combination that maximized vaccine-induced immune responses. The range of CY doses tested were 200mg/m2 to 450mg/m2. Based on the HER2/neu mouse model, CY was given 1 day prior to vaccine at the time of T cell priming and DOX was given on day 7 at the time of T cell expansion (11). Immune readouts included assessment of delayed-type hypersensitivity (DTH) responses to HER2 HLA-class II restricted peptides and measurement of HER2 antibodies. The addition of 200mg/m2 CY had no impact on the rate of DTH development, but CY doses higher than 200mg/m2 suppressed vaccine induced DTH responses compared to vaccine alone. Furthermore, induction of HER2-specific humoral immunity was optimally enhanced at the 200mg/m2 dose and dropped off with higher CY doses. While this study assessed the combination of CY and DOX and not CY alone, the results suggest that CY doses above 200mg/m2 may abrogate immune responses induced by vaccination, and that the optimal CY dose for enhancing vaccine induced immunity in humans is 200mg/m2 or lower. Lower doses were not tested in this study. However, Greten et al evaluated single-agent CY doses of 150, 250, and 350mg/m2 in hepatocellular carcinoma (HCC) patients and reported that the 2 lower doses induced a decrease in the absolute and relative frequency of Tregs in the blood of HCC patients and the 250mg/m2 dose impaired suppressor function and demonstrated decreased Treg frequency out to day 71. AFP-specific T cell responses were also induced in the lower treatment arms(17). On the contrary, a previous report testing an allogeneic melanoma cell vaccine in patients identified 300mg/m2 given 3 days prior to vaccine as the optimal dose (18). The other doses tested were 150mg/m2 and 75mg/m2. However, the immune readout was the reduction in peripheral CD8+CD11B+ suppressor cells. A second melanoma study evaluating the addition of melanoma-associated helper peptides and CY 300mg/m2 i.v. 3 days prior to a melanoma vaccine concluded that CY did not augment T cell responses to that vaccine(19).
Given the findings in murine studies and the clinical trials assessing actual effector responses, future studies should focus on studying the lower range of CY doses typically used to inhibit Tregs (range 150-1000mg/m2 i.v.). Additional studies are needed to better understand the effects of CY at lower doses.
Metronomic oral cyclophosphamide
Metronomic oral CY is administered in an iterative low-dose fashion. Historically, low doses of chemotherapeutic agents have been given in this manner to inhibit angiogenesis. The potential benefit of an alternative way to administer CY is that a lower, more continuous dosing schedule may allow for more effective and prolonged inhibition of Tregs as most studies suggest that Treg levels recover 7-10 days after i.v. administration. Ghiringhelli and colleagues first evaluated the effects of metronomic CY in patients with advanced solid tumors(20). Patients received CY 50mg orally given twice a day, 1 week on and 1 week off for 1 month or more. The number of circulating CD4+CD25high Tregs in the 9 patients studied was higher at baseline compared to healthy volunteers. After 1 month of CY treatment CD4+CD25high T cells were decreased both in percentage (7.9 to 3.1%) and absolute numbers (28.7 to 6.4 cells/mm3). The decrease occurred in all patients. Of the 4 patients with adequate samples for evaluation, the number of FoxP3+ cells also decreased. This decrease was selective and did not occur in other T or NK cell subsets.
CY’s effect on T cell and NK cell function were also evaluated. In addition to Teff inhibition, Tregs inhibit innate immunity by downregulating NK cell proliferation and function. NK cell lytic activity was tested after one month of metronomic CY by determining the capacity of patient NK cells to kill NKG2D ligand-expressing K562 cells. NK activity in patients receiving CY was enhanced and restored to healthy volunteer levels. T cell proliferation was also tested using CFSE-labeled PBMC either untreated or depleted of CD25+ T cells, and then cultured with anti-CD3 and anti-CD28 for stimulation. CY treatment also restored T cell proliferation.
Metronomic CY was also evaluated in breast cancer patients where it has historically been used for its anti-angiogenic properties. Breast cancer patients treated with continuous low-dose CY had a transient reduction in Tregs lasting 4-6 weeks(21). Patients received CY 50mg orally daily for 3 months. Tregs were reduced within 14 days (3.0 vs 5.1%), remained decreased until day 42, and returned to pretreatment levels by day 84. Interestingly, endogenous breast tumor-reactive T cells were detected in 27% of patients before CY treatment, and increased to 73% on day 14, 80% on day 42, and 88% on day 84. Fifty-eight percent of patients had stable disease (SD). An increase in breast tumor-reactive T cells was associated with both SD and overall survival (OS). Although Ghiringhelli observed diminished functionality of Tregs at 30 days, suppressive function changes were only tested at 84 days and were not seen in this study. Despite a transient and minimal effect on Treg numbers and function, metronomic CY stably increased breast tumor-reactive T cell responses.
The use of metronomic CY combined with active immunotherapy has recently been reported(22). Patients with advanced solid tumors were treated with three different regimens of low-dose CY in combination with an oncolytic adenovirus. CY was given either as oral metronomic (50mg/day), a single i.v. injection (1000mg), or both. Metronomic CY was given starting 1 week before the adenovirus and i.v. CY was given 1 hour prior to the adenovirus. The adenovirus was injected intratumorally. Metronomic CY (oral and oral + i.v.) decreased Tregs and induced antitumor or antiviral responses. All CY regimens resulted in higher rates of disease control when compared with the adenovirus vaccine only. The metronomic groups were most effective in decreasing Treg numbers. However, prior studies with i.v. CY would have predicted recovery of Treg numbers at the 30 day time point evaluated. In addition the dose of 1000mg (approximately 600mg/m2) is higher than what is used in many studies. The i.v. CY was administered only 1 hour prior to adenovirus administration. This may be appropriate as the kinetics of the immune response induced by virus-induced tumor lysis differs from that of peripherally administered vaccines. While it may be difficult to directly extrapolate these data to other vaccine strategies, it is important to note that all CY groups performed better than adenovirus vaccine alone. While numerically, the best progression-free survival (PFS) and OS were seen in the oral + i.v. group, the study was not powered to compare the clinical outcomes between the different groups. Numerous studies are in progress combining metronomic CY with active vaccination strategies (clinicaltrials.gov) for a variety of cancers. These studies are incorporating a range of immune analyses. Results from these studies will influence future trial designs.
Downstream effects of Treg inhibition
In parallel to studying the optimal CY dose, schedule, and route of administration required to optimally modify Tregs, studies are exploring the mechanisms by which CY modulates anti-tumor immunity. Enhancement of NK and T cell lytic activity and proliferation were described above. To further elaborate on the downstream effects on the anti-tumor T cell response, Jaffee and colleagues (2005) reported on another mechanism of CY-mediated vaccine enhancement(23). Using HER2/neu mice, which are tolerized to neu-expressing tumors, they found that CY inhibited Tregs by selectively depleting the cycling population of CD4+CD25+ T cells. Tetramer-binding studies demonstrated that CY pretreatment allowed activation of high-avidity HER-2/neu-specific CD8+ T cells comparable to those generated in the parental strain in which HER-2/neu is immunogenic. The discovery that latent pools of high-avidity tumor specific T cells can exist in a tolerized host gives further credence to the potential for active immunization in cancer.
The concept that Treg inhibition may recruit higher avidity effector T cells was further evaluated in a clinical trial in advanced pancreatic cancer patients testing an allogeneic, GM-CSF-pancreatic cancer vaccine given alone or in sequence with low-dose CY(24). The GM-CSF vaccine given one day after CY resulted in higher rates of mesothelin-specific T cell responses that were also of higher avidity than observed in patients treated with vaccine alone. In addition, higher avidity T cell responses were associated with prolonged PFS and OS in a heavily treated patient population (4.3 vs 2.3 months OS).
In subsequent studies in HER2/neu mice, CY was shown to exert its effects specifically by depleting a CD25low Treg effector/memory subpopulation, which reside in the tumor microenvironment and preferentially suppress high avidity HER-2/neu-specific T cells(25). Effector/memory-like CD4+FoxP3+ Tregs preferentially hone to non-lymphoid and inflamed tissues and are the predominant cells that traffic into tumors. CD25low Tregs express an activated phenotype with higher levels of ICOS, CD44, CTLA-4, GITR, β1 integrin, LFA1, CXCR3 and lower levels of CD62L. In contrast, CD25high Tregs are predominantly a lymph-node residing population. As a result of CY’s effects on the CD25low Treg subset, adoptively transferred high avidity HER-/2neu-specific T cells from vaccine plus CY-treated HER2/neu mice expressed higher tumor trafficking integrins and CXCR3 levels than the T cells from the no CY group. This effect was not seen on low avidity T cells. Specific targeting of the most relevant Treg subsets which are both present in the tumor microenvironment and capable of suppressing high avidity tumor-specific T cells is extremely relevant in cancer-bearing hosts. Ongoing research dissecting the roles of Treg subpopulations will allow further refinement in approaches targeting these suppressor subsets (Figure 1).
Figure 1. Regulatory T Cell Modulation Using Cyclophosphamide.
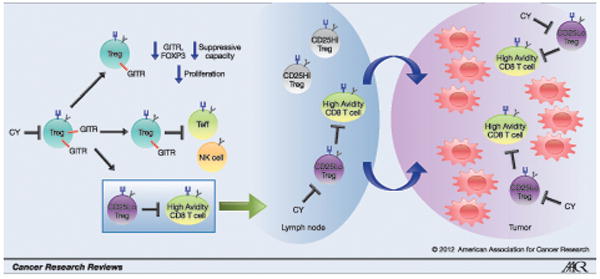
CY decreases Treg number and function. The use of CY to preferentially inhibit Treg subsets that suppress high avidity tumor-specific T cells has implications for cancer immunotherapy.
Key findings.
Low dose CY (i.v.) results in transiently decreased Treg frequencies.
Metronomic CY results in prolonged Treg supporession that returns to baseline with continued administration within 4-6 weeks.
Tumor-specific immune responses are enhanced despite only transient reductions in Treg numbers.
CY mediated alterations in Treg function may contribute to CY’s efficacy.
Differences in dose, schedule, and routes of CY administration contribute to variable outcomes between studies.
CY depletion of Tregs can uncover high avidity tumor-specific T cells.
Future directions.
The results from reported studies are already informing the design of future studies. In addition, preclinical studies are showing efficacy of low-dose CY in combination with other immunotherapeutic agents, such as OX40 receptor ligands and PD-1 antagonists. As these agents make it to the clinics, combinations with CY are likely to follow. Future studies should evaluate CY’s effects on Treg to T cell ratios in tumors and on downstream immune responses. These may be more accurate indicators of clinical outcomes than observing changes in systemic Treg numbers. Finally, the identification of specific Treg subsets responsible for effector T cell suppression should lead to the development of more specific drugs that alter these populations, leaving in place populations that suppress autoimmunity.
References
- 1.Sistigu A, Viaud S, Chaput N, Bracci L, Proietti E, Zitvogel L. Immunomodulatory effects of cyclophosphamide and implementations for vaccine design. Semin Immunopathol. 2011;33(4):369–83. doi: 10.1007/s00281-011-0245-0. [DOI] [PubMed] [Google Scholar]
- 2.Viaud S, Flament C, Zoubir M, Pautier P, LeCesne A, Ribrag V, Soria JC, Marty V, Vielh P, Robert C, Chaput N, Zitvogel L. Cyclophosphamide induces differentiation of Th17 cells in cancer patients. Cancer research. 2011;71(3):661–5. doi: 10.1158/0008-5472.CAN-10-1259. [DOI] [PubMed] [Google Scholar]
- 3.Bates GJ, Fox SB, Han C, Leek RD, Garcia JF, Harris AL, Banham AH. Quantification of regulatory T cells enables the identification of high-risk breast cancer patients and those at risk of late relapse. J Clin Oncol. 2006;24(34):5373–80. doi: 10.1200/JCO.2006.05.9584. [DOI] [PubMed] [Google Scholar]
- 4.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10(9):942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 5.Liyanage UK, Moore TT, Joo HG, Tanaka Y, Herrmann V, Doherty G, Drebin JA, Strasberg SM, Eberlein TJ, Goedegebuure PS, Linehan DC. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol. 2002;169(5):2756–61. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 6.Alvaro T, Lejeune M, Salvado MT, Bosch R, Garcia JF, Jaen J, Banham AH, Roncador G, Montalban C, Piris MA. Outcome in Hodgkin’s lymphoma can be predicted from the presence of accompanying cytotoxic and regulatory T cells. Clin Cancer Res. 2005;11(4):1467–73. doi: 10.1158/1078-0432.CCR-04-1869. [DOI] [PubMed] [Google Scholar]
- 7.Quezada SA, Peggs KS, Curran MA, Allison JP. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J Clin Invest. 2006;116(7):1935–45. doi: 10.1172/JCI27745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polak L, Turk JL. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974;249(458):654–6. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- 9.Awwad M, North RJ. Cyclophosphamide (Cy)-facilitated adoptive immunotherapy of a Cy-resistant tumour. Evidence that Cy permits the expression of adoptive T-cell mediated immunity by removing suppressor T cells rather than by reducing tumour burden. Immunology. 1988;65(1):87–92. [PMC free article] [PubMed] [Google Scholar]
- 10.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: depletion of CD4+, 2H4+ suppressor-inducer T-cells. Cancer Res. 1988;48(6):1671–5. [PubMed] [Google Scholar]
- 11.Machiels JP, Reilly RT, Emens LA, Ercolini AM, Lei RY, Weintraub D, Okoye FI, Jaffee EM. Cyclophosphamide, doxorubicin, and paclitaxel enhance the antitumor immune response of granulocyte/macrophage-colony stimulating factor-secreting whole-cell vaccines in HER-2/neu tolerized mice. Cancer Res. 2001;61(9):3689–97. [PubMed] [Google Scholar]
- 12.Ghiringhelli F, Larmonier N, Schmitt E, Parcellier A, Cathelin D, Garrido C, Chauffert B, Solary E, Bonnotte B, Martin F. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34(2):336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 13.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–61. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 14.Lutsiak ME, Semnani RT, De Pascalis R, Kashmiri SV, Schlom J, Sabzevari H. Inhibition of CD4(+)25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood. 2005;105(7):2862–8. doi: 10.1182/blood-2004-06-2410. [DOI] [PubMed] [Google Scholar]
- 15.Motoyoshi Y, Kaminoda K, Saitoh O, Hamasaki K, Nakao K, Ishii N, Nagayama Y, Eguchi K. Different mechanisms for anti-tumor effects of low- and high-dose cyclophosphamide. Oncol Rep. 2006;16(1):141–6. [PubMed] [Google Scholar]
- 16.Emens LA, Asquith JM, Leatherman JM, Kobrin BJ, Petrik S, Laiko M, Levi J, Daphtary MM, Biedrzycki B, Wolff AC, Stearns V, Disis ML, Ye X, Piantadosi S, Fetting JH, Davidson NE, Jaffee EM. Timed sequential treatment with cyclophosphamide, doxorubicin, and an allogeneic granulocyte-macrophage colony-stimulating factor-secreting breast tumor vaccine: a chemotherapy dose-ranging factorial study of safety and immune activation. J Clin Oncol. 2009;27(35):5911–8. doi: 10.1200/JCO.2009.23.3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greten TF, Ormandy LA, Fikuart A, Hochst B, Henschen S, Horning M, Manns MP, Korangy F. Low-dose cyclophosphamide treatment impairs regulatory T cells and unmasks AFP-specific CD4+ T-cell responses in patients with advanced HCC. J Immunother. 2010;33(2):211–8. doi: 10.1097/CJI.0b013e3181bb499f. [DOI] [PubMed] [Google Scholar]
- 18.Hoon DS, Foshag LJ, Nizze AS, Bohman R, Morton DL. Suppressor cell activity in a randomized trial of patients receiving active specific immunotherapy with melanoma cell vaccine and low dosages of cyclophosphamide. Cancer Res. 1990;50(17):5358–64. [PubMed] [Google Scholar]
- 19.Slingluff CL, Jr, Petroni GR, Chianese-Bullock KA, Smolkin ME, Ross MI, Haas NB, von Mehren M, Grosh WW. Randomized multicenter trial of the effects of melanoma-associated helper peptides and cyclophosphamide on the immunogenicity of a multipeptide melanoma vaccine. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(21):2924–32. doi: 10.1200/JCO.2010.33.8053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghiringhelli F, Menard C, Puig PE, Ladoire S, Roux S, Martin F, Solary E, Le Cesne A, Zitvogel L, Chauffert B. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end stage cancer patients. Cancer Immunol Immunother. 2007;56(5):641–8. doi: 10.1007/s00262-006-0225-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ge Y, Domschke C, Stoiber N, Schott S, Heil J, Rom J, Blumenstein M, Thum J, Sohn C, Schneeweiss A, Beckhove P, Schuetz F. Metronomic cyclophosphamide treatment in metastasized breast cancer patients: immunological effects and clinical outcome. Cancer Immunol Immunother. 2011 doi: 10.1007/s00262-011-1106-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cerullo V, Diaconu I, Kangasniemi L, Rajecki M, Escutenaire S, Koski A, Romano V, Rouvinen N, Tuuminen T, Laasonen L, Partanen K, Kauppinen S, Joensuu T, Oksanen M, Holm SL, Haavisto E, Karioja-Kallio A, Kanerva A, Pesonen S, Arstila PT, Hemminki A. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol Ther. 2011;19(9):1737–46. doi: 10.1038/mt.2011.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, Bieler JG, Emens LA, Reilly RT, Jaffee EM. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. 2005;201(10):1591–602. doi: 10.1084/jem.20042167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Laheru D, Lutz E, Burke J, Biedrzycki B, Solt S, Onners B, Tartakovsky I, Nemunaitis J, Le D, Sugar E, Hege K, Jaffee E. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: a pilot study of safety, feasibility, and immune activation. Clin Cancer Res. 2008;14(5):1455–63. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss V, L T, Song H, Kouo T, Black C, Sgouros G, Jaffee E, Armstrong T. Trafficking of High Avidity HER-2/neu-specific T Cells into HER-2/neu-Expressing Tumors after Depletion of Effector/Memory-Like Regulatory T Cells. PLoS ONE. 2012;7(2):16. doi: 10.1371/journal.pone.0031962. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


