Abstract
Purpose
Dopamine and cAMP-regulated phosphoprotein, Mr 32000 (DARPP-32), is overexpressed during the gastric carcinogenesis cascade. Here, we investigated the role of DARPP-32 in promoting resistance to treatment with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL).
Experimental Design
In vitro cell models including stable expression and knockdown of DARPP-32 were used. The role of DARPP-32 in regulating TRAIL-dependent apoptosis was evaluated by clonogenic survival assay, Annexin V staining, immunofluorescence, qRT-PCR, western blot, and luciferase reporter assays.
Results
Stable expression of DARPP-32 in MKN28 cells enhanced cell survival and suppressed TRAIL-induced cytochrome c release and activation of caspases 8, 9 and 3. Conversely, shRNA-mediated knockdown of endogenous DARPP-32 sensitized the resistant MKN-45 cells to TRAIL-induced apoptosis and enhanced TRAIL-mediated activation of caspases 8, 9 and 3. DARPP-32 induced BCL-xL expression through activation of Src/STAT3 signaling, and treatment with the Src-specific inhibitor PP1 abrogated DARPP-32-dependent BCL-xL up-regulation and cell survival in MKN-28 cells. The TRAIL treatment induced caspase-dependent cleavage of NF-kBp65 protein; this cleavage was prevented by DARPP-32, thus maintaining NF-kB activity and the expression of its target; FLIP(S) protein. This suggests that up-regulation of BCL-xL could play a possible role in blocking the mitochondria intrinsic apoptosis pathway whereas the DARPP-32 effect on the NF-kB/FLIP(S) axis could serve as an additional negative feedback loop that blocks TRAIL-induced activation of caspase 8.
Conclusion
Our findings uncover a novel mechanism of TRAIL resistance mediated by DARPP-32, whereby it inhibits the intrinsic apoptosis pathway through up-regulation of BCL-xL, and the extrinsic apoptosis pathway through the NF-kB/FLIP(S) axis.
Keywords: STAT3, Src, NF-kB, p65, BCL-xL, FLIP, cytochrome c, caspases
Introduction
Gastric cancer is one of the most frequent malignancies worldwide (1, 2). Although various chemotherapeutic drugs have been utilized, drug resistance has significantly hampered the effectiveness of chemotherapy leading to poor survival rates in patients with gastric cancer (3, 4).
Death receptor-mediated cell death is one of the major apoptosis pathways. Death receptors including DR4 and DR5, relay a death signal upon binding with tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) (5, 6), which shares protein homology with Fas ligand and TNFα (7). Upon treatment with TRAIL, the initiator procaspase 8 is recruited to death receptors through the adaptor protein FADD, forming the death-inducing signaling complex (DISC). This results in autocatalytic activation of caspase 8 that directly activates caspase 3 and apoptosis in type I cells. However, in type II cells, caspase 8 through cleavage of Bid activates the intrinsic mitochondrial apoptosis pathway, which is mediated by cytochrome c release and activation of caspases 9 and 3 (8–10). TRAIL selectively induces apoptosis in a variety of tumor cells, whereas several normal cells remain unresponsive to its effects (11, 12). This unique capacity has placed TRAIL in the forefront as a promising anti-cancer agent. Unfortunately, resistance to TRAIL is not uncommon as TRAIL resistance can be developed by several mechanisms; for instance, overexpression of BCL-xL in type II cells has been shown to protect against TRAIL-mediated apoptosis through inhibition of the intrinsic pathway (13, 14). Several reports have indicated that transcription factors such as NF-kB and STAT3 could up-regulate BCL-xL in cancer cells (reviewed by Grad et al. (15)). FLIP, which resembles caspase 8 but lacks the enzymatic activity, competes with caspase 8 to bind to FADD, thereby inhibiting TRAIL-induced death receptor signaling (16). Increased expression of FLIP has been shown to promote resistance of cancer cells to death receptor-mediated apoptosis (17). In addition, expression of FLIP(S) completely inhibited TRAIL-induced apoptosis through blocking activation of caspase 8 in gastric cancer cells, and AKT activity promoted cancer cell survival through up-regulation of FLIP(S) (18). The FLIP gene is regulated by several anti-apoptotic pathways including the AKT, MAPK, and NF-kB pathways (19, 20).
We have previously reported that dopamine and cAMP-regulated phosphoprotein, Mr 32000 (DARPP-32) is amplified and overexpressed in about two-thirds of upper gastrointestinal adenocarcinomas (21). We demonstrated that DARPP-32 expression was associated with the multistep gastric carcinogenesis cascade involving the transition to intestinal metaplasia and the progression to neoplasia (22). Little is known about the mechanisms of TRAIL resistance in gastric cancer. In the current study, we uncovered a novel mechanism by which DARPP-32 blocks TRAIL-induced apoptosis in gastric cancer cells.
Materials and methods
Cell lines and reagents
The human gastric cancer cell lines, MKN-28 and MKN-45, were maintained in culture using Dulbecco’s modified Eagle’s medium (GIBCO, Carlsbad, CA) supplemented with 10% fetal bovine serum (Invitrogen Life Technologies, Carlsbad, CA) and 1% penicillin/streptomycin (GIBCO). Recombinant human TRAIL/Apo2 ligand was purchased from BioVision Research Products (Mountain View, CA). 4-Amino-5-(4-methylphenyl)-7-(t-butyl)pyrazolo[3,4-d]- pyrimidine (PP1), a specific Src inhibitor, was purchased from Enzo Life Sciences (Farmingdale, PA). Rabbit anti-DARPP-32 antibody was obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal anti-cytochrome c antibody was purchased from BD Pharmingen (Chicago, IL). A secondary anti-mouse antibody conjugated to TRITC and anti-rabbit antibody conjugated to FITC were obtained from Invitrogen Life Technologies. Horseradish peroxidase-conjugated rabbit secondary antibody; NF-kBp65, FLIP, BCL-xL, caspase 3, cleaved caspase 3, caspase 9, caspase 8, p-Src (Y416), Src, and β-actin antibodies were purchased from Cell Signaling Technology (Danvers, MA). STAT3 and p-STAT3(Y705) antibodies were obtained from GenScript (Piscataway, NJ).
DARPP-32 expression and shRNA vectors
The mammalian expression plasmid for DARPP-32 was produced by PCR amplification of the full-length coding sequence of DARPP-32 and cloned in-frame into pcDNA3 (Invitrogen). MKN-28 cells stably expressing DARPP-32 or pcDNA3 empty vector were generated following standard protocols as previously described (21). After selection with 500 µg/ml neomycin (G418) (Invitrogen), DARPP-32 protein expression was evaluated by Western blot analysis and clones were selected. Lentivirus particles expressing DARPP-32 shRNA or control shRNA were produced by GeneCopoeia (Rockville, MD) and then utilized to transduce cells.
Clonogenic survival assay
Cells were first washed with PBS, trypsinized, and harvested in single cell suspension. Cells (1000 cells per well) were seeded onto 6-well plates. The next day, cells were treated for 24h with TRAIL or vehicle. After incubation for two weeks, colonies were fixed with 4% paraformaldehyde and stained with 0.05% crystal violet. Colonies with ≥50 cells were counted. Alternatively, the long-term cell viability was determined using the Cell Titer-Glo Luminescent Cell Viability Assay Kit (Promega) following the supplier’s instructions.
Apoptosis assay
MKN-28 stably expressing DARPP-32 or pcDNA3 empty vector, and MKN-45 cells transduced with lentivirus particles expressing DARPP-32 shRNA or control shRNA were seeded onto 60 mm culture plates. The next day, cells were treated with TRAIL (200 ng/ml) or vehicle overnight. Cells were then collected and stained with Annexin V-FITC and propidium iodide (PI) (R&D Systems), or Annexin V-PE (BioVision, Exton, PA). The samples were washed with PBS and resuspended in binding buffer for subsequent fluorescence-activated cell sorting (FACS) analysis. Apoptotic cell death was evaluated by counting the number of cells that stained positive for Annexin V-FITC and positive for PI (MKN-28 cells), or positive for Annexin V-PE (MKN-45 cells).
Immunofluorescence assay
To evaluate cytochrome c release, MKN-28 cells stably expressing DARPP-32 or pcDNA3 empty vector were treated with 200 ng/ml TRAIL or vehicle for 24h. Immunofluorescence was performed with anti-cytochrome c antibody (1:200) and a secondary antibody conjugated to TRITC (1:800), as described previously (23). The cells were examined by fluorescence microscopy (Olympus America Inc., Center Valley, PA). In healthy cells, cytochrome c was depicted by punctuated red fluorescence. In apoptotic cells, cytochrome c release was indicated by diffused red staining. 4’, 6-Diamidino-2-phenylindole (DAPI) was used as a nuclear counterstain (blue fluorescence). The ratio of cells that presented diffused cytochrome c staining to the total number of cells was averaged from six random microscopic fields for more than 100 cells in each category.
To assess NF-kBp65 nuclear localization, MKN-28 cells stably expressing DARPP-32 or pcDNA3 empty vector, were treated with 200 ng/ml TRAIL or vehicle for 24h. Cells were subjected to immunofluorescence assay with anti-NF-kBp65 antibody (1:200) and a secondary antibody conjugated to FITC (1:800). DAPI was used as a nuclear counterstain. The cells were visualized by fluorescence microscopy. The ratio of cells that presented nuclear NF-kBp65 staining (indicating activation of NF-kB) to the total number of cells was averaged from five random microscopic fields for more than 100 cells in each category.
Quantitative real-time RT-PCR
Quantitative real-time RT-PCR was performed in triplicate using an iCycler (Bio-Rad) with a threshold cycle number determined by use of iCycler software version 3.0. Total RNA was isolated from cells using an RNeasy Mini Kit (Qiagen, Valencia, CA). Single-stranded cDNA was synthesized from a total RNA amount of 1 µg by an iScript cDNA synthesis kit (Bio-Rad, Hercules, CA). mRNA specific primers for FLIP(L), FLIP(S), BCL-xL, and HPRT1 were designed, and the results were normalized to HPRT1 as a stable reference gene for quantitative real-time RT-PCR. All primer sequences are available upon request. The mRNA fold expression levels were calculated according to the formula 2(RT-ET)/2(Rn-En), as described previously (24).
Western blot analysis
Cell lysates were prepared in 0.5% Triton X-100, 150 mM NaCl, 5 mM Tris supplemented with 1 × Halt protease inhibitor cocktail and 1 × Halt phosphatase inhibitor cocktail (Pierce, Rockford, IL), centrifuged at 3500 r.p.m. for 10 min at 4°C. Protein concentration was measured using a Bio-Rad protein assay (Bio-Rad Laboratories, Hercules, CA). Proteins (10–20 µg) from each sample were separated on 10% SDS-PAGE and transferred to Immobilon PVDF membrane (Millipore, Billerica, MA). Membranes were probed with specific antibodies, and proteins were visualized by using horseradish peroxidase (HRP)-conjugated secondary antibodies and Immobilon Western Chemiluminescent HRP Substrate detection reagent (Millipore). Gel loading was normalized for equal β-actin.
Luciferase reporter assay
To assess the activity of the NF-kB signaling pathway, we used the NF-kB-Luc reporter vector that contains multiple copies of the consensus sequence (Clontech). After endogenous NF-kB proteins bind to the kappa enhancer element, transcription is induced and the reporter gene is activated. MKN-28 cells stably expressing DARPP-32 or pcDNA3 empty vector, and MKN-45 cells transduced with lentivirus particles expressing DARPP-32 shRNA or control shRNA were seeded in 96-well plates (104 cells per well). Cells were transiently co-transfected with 60 ng of the NF-kB-Luc and 6 ng of an ubiquitin promoter, Renilla luciferase control plasmid, using Fugene according to the manufacturer’s instructions. The next day, cells were treated with 200 ng/ml TRAIL for 24h. Luciferase activity was measured using the dual-luciferase reporter assay kit (Promega, Madison, WI) according to the manufacturer’s instructions. Firefly luciferase activities were normalized to Renilla luciferase levels. Results are the average of three independent experiments and expressed as mean values ± standard deviation.
Statistical analysis
The results were expressed as the mean ± SEM of three independent experiments. Statistical significance of the studies was evaluated by the parametric unpaired Student’s t test. Differences with p values ≤0.05 are considered significant.
Results
DARPP-32 enhances cell survival of gastric cancer cells
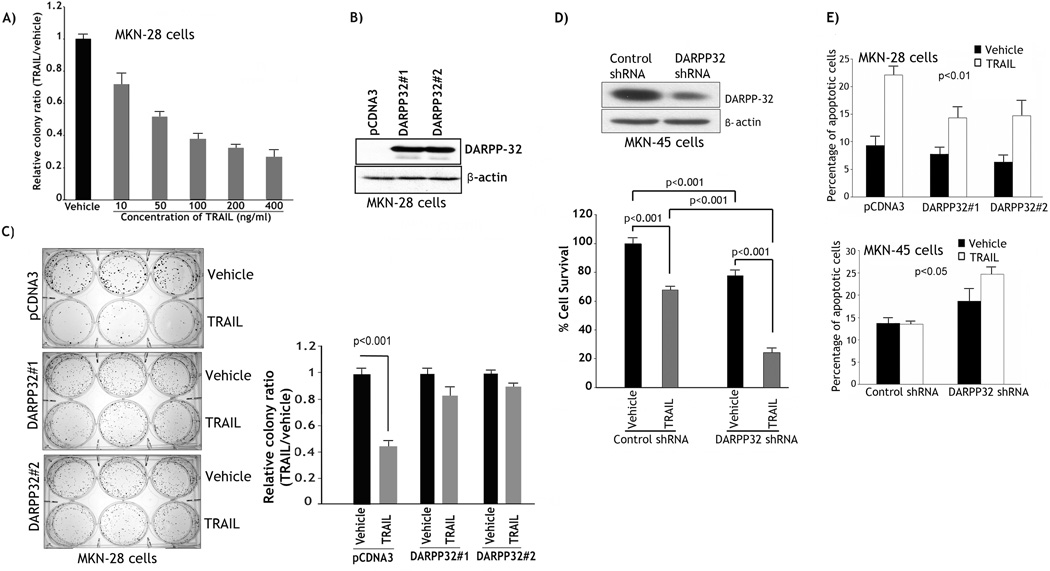
To determine the sensitivity of MKN-28 cells to TRAIL, we employed the clonogenic survival assay for long-term assessment of cell viability. The results showed a TRAIL dose-dependent decrease in cell survival (Figure 1A). MKN-28 cells, which are negative for DARPP-32 expression, were stably transfected with DARPP-32 expression plasmid or pcDNA3 empty vector (Figure 1B). Based on the survival data (Figure 1A), we selected the TRAIL concentration 200 ng/ml as an approximate IC50 in MKN-28 cells to assess the role of DARPP-32 in regulating long-term cell survival by clonogenic survival assay (2- week-long assay). As expected, following TRAIL treatment, cell survival of control cells decreased by approximately 60% relative to vehicle (p<0.001) (Figure 1C). In contrast, in response to TRAIL, cell survival of two clones of MKN-28/DARPP-32 cells decreased by only 10–15% relative to vehicle (Figure 1C). Interestingly, the size of colonies was relatively larger in pcDNA3 control cells than DARPP-32-expressing cells. This suggests that DARPP-32 may regulate cell aggregation and or migration. These results clearly demonstrated that stable DARPP-32 expression promoted cell survival in response to TRAIL in gastric cancer cells. To confirm this finding, we utilized the resistant MKN-45 cells to knockdown endogenous DARPP-32 by shRNA and subject the cells to clonogenic survival assay after treatment with vehicle or 200 ng/ml TRAIL (Figure 1D). The data clearly indicated that knocking down of endogenous DARPP-32 alone was sufficient to decrease cell survival by 20% (p<0.001), implying the important role of DARPP-32 in cell survival (Figure 1D). In addition, knockdown of DARPP-32 in combination with TRAIL induced approximately 70% decrease in cell survival relative to treatment with TRAIL alone (p<0.001) (Figure 1D). The results demonstrated that knocking down of endogenous DARPP-32 sensitized MKN-45 cells to TRAIL.
Figure 1. DARPP-32 promotes survival of gastric cancer cells.
A) MKN-28 cells were treated with vehicle or with the indicated concentrations of recombinant TRAIL for 24h and subjected to clonogenic survival assay as described in Methods and Materials. A TRAIL dose-dependent decrease in cell survival was observed. B) Western blot; MKN-28 cells stably transfected with DARPP-32 or pcDNA3 control plasmids. C) Two clones of MKN-28/DARPP-32 cells and MKN-28/pcDNA3 cells were treated with vehicle or 200 ng/ml TRAIL for 24h and subjected to clonogenic survival assay. The data indicated that DARPP-32 expression counteracted TRAIL-induced cell death. D) Upper panel, Western blot of MKN-45 cells stably transfected with DARPP32-shRNA or control-shRNA plasmids. Lower panel, Cells were treated with vehicle or TRAIL (200 ng/ml) for 24h and then subjected to clonogenic survival assay. The survival of the treated cells was assessed by Cell Titer-Glo Luminescent Cell Viability Assay Kit as described in Methods and Materials. The results indicated that knocking down of endogenous DARPP-32 was sufficient to reduce cell viability and sensitize cells to TRAIL. E) Upper panel, MKN-28/pcDNA3 and MKN-28/DARPP-32 cells were treated with vehicle or TRAIL (200 ng/ml) for 24h. Annexin V staining and flow cytometry indicated that DARPP-32 overexpression counteracted TRAIL-induced apoptosis. Lower panel, MKN-45/control-shRNA and MKN-45/DARPP-32-shRNA cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Annexin V staining and flow cytometry showed that knocking down endogenous DARPP-32 sensitized cells to TRAIL. Results are representative of at least three experiments.
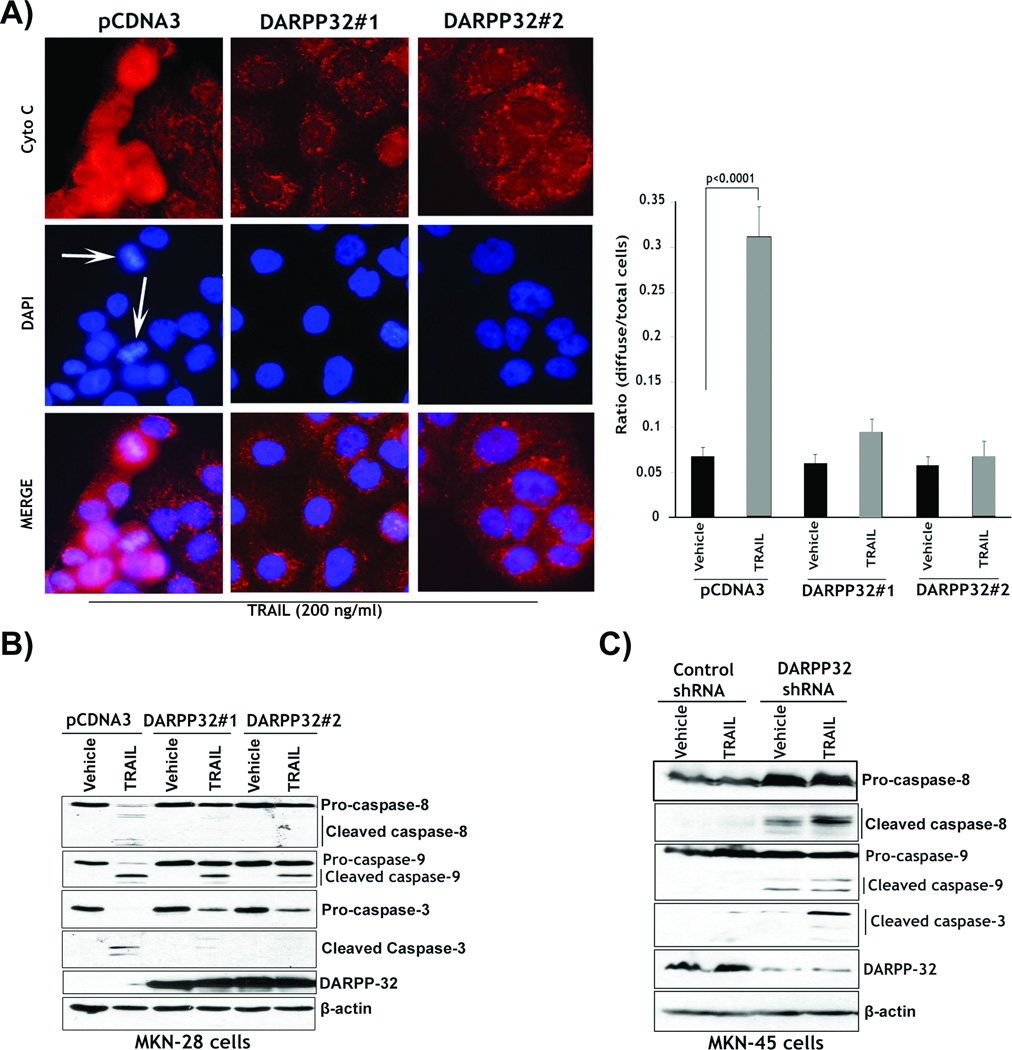
DARPP-32 counteracts TRAIL-induced apoptosis by blocking cytochrome c release and activation of caspases in gastric cancer cells
In addition to long-term clonogenic cell survival assays (Figure 1C and 1D), we next utilized the Annexin V apoptosis assay to evaluate the role of DARPP-32 in regulating TRAIL-induced apoptosis following short-term treatments (24h-long assay). The Annexin V staining and FACS analysis results showed that treatment with TRAIL resulted in as twice as high apoptotic cells in the MKN-28 control cells as compared to MKN-28/DARPP-32 cells (Figure 1E, upper). This indicated that ectopic expression of DARPP-32 significantly blocked TRAIL-induced apoptosis. Concordant with these findings, the knocking down of endogenous DARPP-32 in MKN-45 cells and treatment with TRAIL induced twice the number of apoptotic cells as compared to control cells (Figure 1E, lower). These results showed that knocking down endogenous DARPP-32 sensitized resistant cells to TRAIL. In addition, knocking down endogenous DARPP-32 alone in MKN-45 cells induced approximately 25% increase in apoptotic cells relative to control cells (Figure 1E, lower). This evidently underscored the pro-survival properties of DARPP-32 in gastric cancer. To investigate whether DARPP-32 regulated the mitochondrial intrinsic apoptosis pathway, we assessed cytochrome c release in response to TRAIL in MKN-28 cells by immunofluorescence assay. In healthy cells, cytochrome c was punctuated. In contrast, in apoptotic cells, cytochrome c release was indicated by diffused staining, which depicted the onset of mitochondria-dependent apoptosis. The results showed that treatment with 200 ng/ml TRAIL led to diffused cytochrome c staining of approximately a 6-fold increase in pcDNA3 control cells (p<0.0001) as opposed to less than a 2-fold increase in two clones of MKN-28/DARPP-32 cells relative to vehicle (Figure 2A). The results clearly indicated that DARPP-32 blocked TRAIL-induced cytochrome c release. We next investigated the role of DARPP-32 in blocking TRAIL-induced activation of caspases associated with the extrinsic and intrinsic apoptosis pathways in MKN-28 and MKN-45 cell models. Western blot analysis showed that treatment with 200 ng/ml TRAIL led to a significant activation of caspases 8, 9, and 3 in MKN-28/pcDNA3 control cells (Figure 2B). In contrast, TRAIL had no significant effect on the activation of caspases 8, 9, and 3 in two clones of MKN-28/DARPP-32 cells (Figure 2B). The results indicated that TRAIL induced more significant activation of caspases 8, 9, and 3 in MKN-45/DARPP-32-shRNA cells than MKN-45/control-shRNA cells (Figure 2C). Interestingly, knockdown of DARPP-32 alone and without TRAIL treatment led to some activation of caspases 8 and 9 as opposed to control cells (Figure 2C). Together, these results showed that ectopic expression of DARPP-32 blocked TRAIL-induced activation of caspases in MKN-28 cells. Conversely, knocking down endogenous DARPP-32 enhanced TRAIL-induced activation of caspases in MKN-45 cells.
Figure 2. DARPP-32 blocks cytochrome c release and activation of caspases.
A) MKN-28/pcDNA3 and MKN-28/DARPP-32 cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Immunofluorescence was performed with anti-cytochrome c antibody and a secondary antibody conjugated to TRITC. 4’,6-Diamidino-2-phenylindole (DAPI) was used as a nuclear counterstain. White arrows indicate TRAIL-induced chromatin condensation and nuclear fragmentation. In response to TRAIL, approximately 10% of DARPP-32-expressing cells released cytochrome c as opposed to 30% of control cells. Results are representative of three experiments and shown as the mean ± SEM. Significance of difference was calculated using Student’s t test analysis. B) MKN-28/DARPP-32 and MKN-28/pcDNA3 cells were treated with vehicle or TRAIL, as in panel A. Western blotting data showed that treatment with TRAIL led to a more activation of caspases 8, 9 and 3 in control cells than DARPP-32-expressing cells. C) MKN-45/control-shRNA and MKN-45/DARPP-32-shRNA cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Western blotting results showed that knocking down endogenous DARPP-32 in combination with TRAIL led to activation of caspases 8, 9 and 3. Gel loading was normalized for equal β-actin.
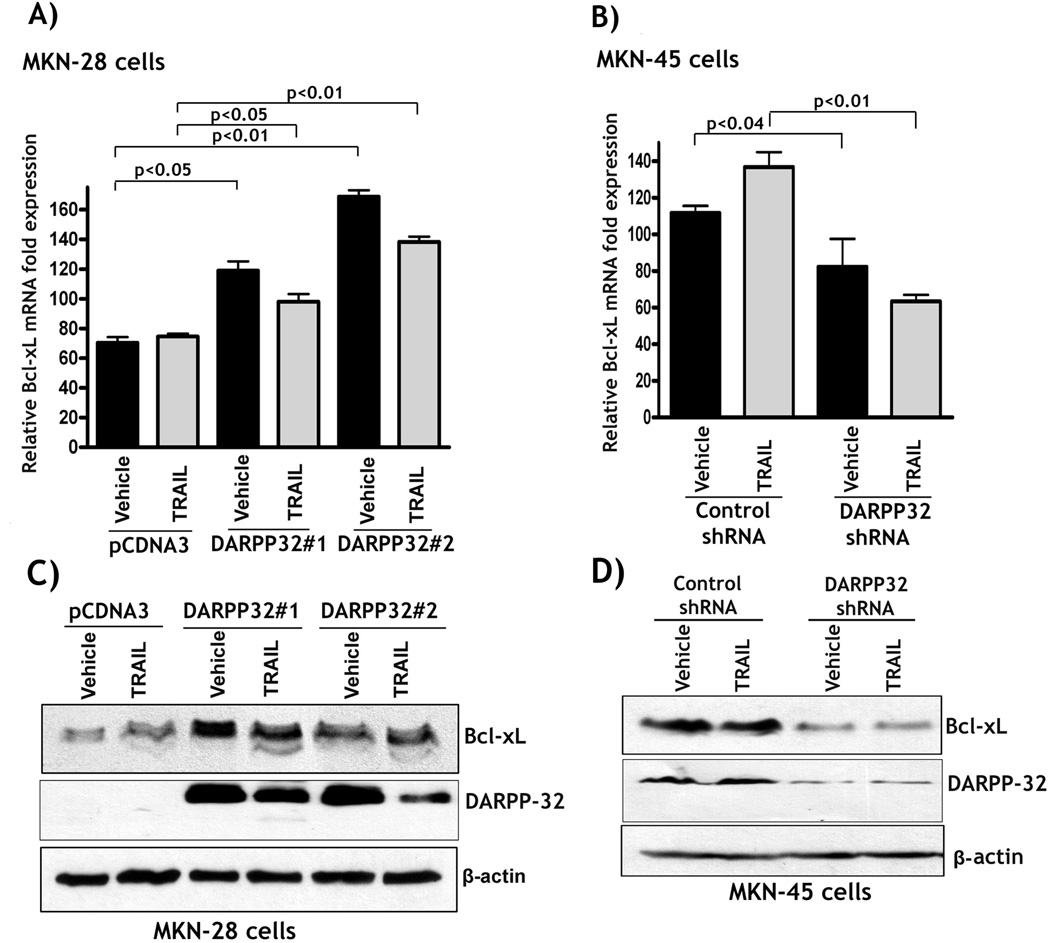
DARPP-32 promotes the pro-survival BCL-xL expression through activation of Src/STAT3 signaling
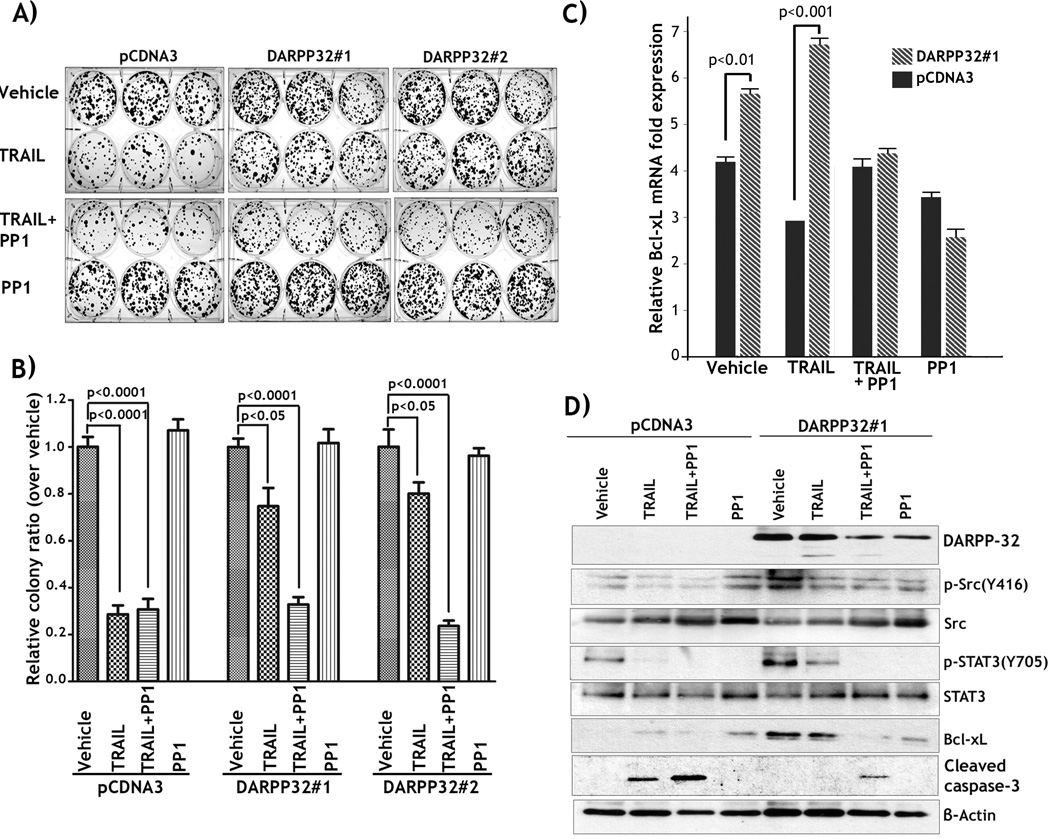
As the pro-survival BCL2 family members are mainly mitochondrial membrane proteins, and their expression inhibits cytochrome c release and activation of caspase 9 (reviewed by Gross et al. (25)), we evaluated BCL-xL mRNA and protein expression in the two cell models described above. The qRT-PCR results indicated that the BCL-xL mRNA level was higher in two clones of MKN-28/DARPP-32 cells than control cells (p<0.01) (Figure 3A). In agreement with these findings, the knocking down of endogenous DARPP-32 with shRNA alone or in combination with TRAIL treatment led to a significant reduction (p=0.01) in BCL-xL mRNA levels in MKN-45 cells (Figure 3B). We also noticed that the increase in the levels of BCL-xL in DARPP-32 cells was more robust at the protein level. The Western blot results showed higher BCL-xL protein levels in the two clones of MKN-28/DARPP-32 cells than control cells (Figure 3C). Similarly, Western blot data showed that the BCL-xL protein level was significantly lower in MKN-45/DARPP-32-shRNA cells than MKN-45/control-shRNA cells (Figure 3D). Of note, treatment with TRAIL had no effect on BCL-xL expression levels in MKN-45 cells (Figure 3D). Therefore, these results established the role of DARPP-32 in regulating BCL-xL expression, thus potentially suppressing the intrinsic apoptosis pathway and promoting cell survival; a finding supported by the effect of DARPP-32 on cytochrome c release as shown in Figure 2A. In an attempt to identify the mechanism by which DARPP-32 regulates BCL-xL expression, we investigated whether DARPP-32 activated NF-kB signaling in MKN-28 cell model. Immunofluorescence assay data indicated that stable expression of DARPP-32 had no significant effect on NF-kB activation as depicted by nuclear NF-kBp65 staining (Supplementary Figure 1). Based on this observation, we examined the possibility of DARPP-32 regulation of BCL-xL and cell survival through activation of Src/STAT3 signaling in MKN-28 cells. We first confirmed the role of this pathway in DARPP-32-mediated TRAIL resistance by treating cells with PP1, a specific Src inhibitor, alone or in combination with TRAIL. Indeed, clonogenic survival assay data indicated that following TRAIL treatment cell survival of control cells decreased by 70% relative to vehicle (p<0.0001, Figures 4A&4B). In contrast, in response to TRAIL, cell survival of two clones of MKN-28/DARPP-32 cells decreased by approximately 20% relative to vehicle (p<0.05, Figures 4A&4B). However, the protective effect of DARPP-32 was completely reversed when the cells were treated with TRAIL in combination with PP1, as cell survival was reduced to the level of TRAIL-treated control cells (p<0.0001, Figures 4A&4B). Of note, combining PP1 with TRAIL had no significant effect on survival as compared to treatment with TRAIL alone in control cells (Figures 4A&4B). These data indicated that the Src tyrosine kinase activity contributes to the pro-survival effect of DARPP-32 in MKN-28 cells.
Figure 3. DARPP-32 up-regulates BCL-xL expression in gastric cancer cells.
A) MKN-28/pcDNA3 and MKN-28/DARPP-32 cells were treated with vehicle or 200 ng/ml TRAIL for 24h. qRT-PCR results showed that BCL-xL mRNA level was significantly higher in DARPP-32-expressing cells than in control cells (p<0.01). B) MKN-45/DARPP-32-shRNA and MKN-45/control-shRNA cells were treated with vehicle or 200 ng/ml TRAIL for 24h. qRT-PCR results indicated that knocking down endogenous DARPP-32 led to a significant decrease in BCL-xL mRNA level relative to control cells (p<0.01). Results are representative of three experiments and shown as the mean ± SEM. C) Western blot analysis showed that BCL-xL protein levels were higher in DARPP-32-expressing cells than control cells. D) Western blot analysis indicated that knockdown of endogenous DARPP-32 led to down-regulation of BCL-xL protein levels irrespective of treatment with TRAIL.
Figure 4. DARPP-32 promotes TRAIL resistance by up-regulating BCL-xL through activation of Src/STAT3 signaling.
A) Two clones of MKN-28/DARPP-32 and MKN-28/pcDNA3 cells were treated with vehicle, 200 ng/ml TRAIL, TRAIL in combination with 5 µM PP1, a specific Src inhibitor, or PP1 alone for 24h and subjected to clonogenic survival assay. B) The quantification of surviving colonies indicated that DARPP-32 significantly promoted cell survival in response to TRAIL, as compared to control. However, as shown, TRAIL in combination with PP1 completely reversed the effect of DARPP-32 on cell survival. C) MKN-28/DARPP-32 and MKN-28/pcDNA3 cells were treated with vehicle, TRAIL, TRAIL in combination with PP1, or PP1 as described in panel A. qRT-PCR results showed that BCL-xL mRNA level was significantly higher in DARPP-32-expressing cells than in control cells, following treatment with vehicle (p<0.01) or TRAIL (p<0.001), respectively. However, treatment with PP1 alone or in combination with TRAIL abrogated DARPP-32-induced increase in BCL-xL mRNA level. D) Western blot analysis demonstrated DARPP-32-dependent increase in BCL-xL protein expression that was associated with increased levels of p-Src(Y416) and p-STAT3(Y705) proteins. Treatment with TRAIL in combination with PP1 reversed the pro-survival effect of DARPP-32 as shown by a notable decrease in protein levels of p-Src(Y416), p-STAT3(Y705), and BCL-xL; and evidenced by cleavage of caspase-3.
In parallel, we examined BCL-xL mRNA and protein expression following treatment with vehicle, TRAIL, TRAIL in combination with PP1, or PP1 in MKN-28/DARPP32#1 cells or MKN-28/pCDNA3 control cells. Real-time qPCR and western blot data showed that BCL-xL mRNA (p<0.01, Figure 4C) and protein (Figure 4D) levels were significantly higher in DARPP-32-expressing cells than control cells. In addition, treatment with TRAIL sustained DARPP-32-induced increase in BCL-xL mRNA and protein levels relative to control cells (Figures 4C&4D). In contrast, TRAIL in combination with PP1 abrogated the effect of DARPP-32 and decreased BCL-xL expression to approximately the levels of TRAIL-treated control cells (Figures 4C&4D). BCL-xL mRNA and protein expression levels directly correlated with the cell survival data shown in Figures 4A&4B. In fact, Western blot data indicated that cleavage of caspase-3 was induced by TRAIL in control cells but not in DARPP-32-expressing cells (Figure 4D). Confirming the pro-survival role of BCL-xL in DARPP-32-expressing cells, TRAIL in combination with PP1 induced cleavage of caspase-3 (Figure 4C). Interestingly, while treatment with PP1 alone induced a significant decrease in BCL-xL mRNA and protein levels relative to vehicle in DARPP-32-expressing cells (Figures 4C&4D), cell survival was not affected as the cells were not challenged by TRAIL (Figures 4A&4B). In line with these results, Western blot analysis showed that treatment with PP1 alone failed to induce cleavage of caspase-3 (Figure 4C).
We next examined the role of DARPP-32 in regulating Src/STAT3 signaling pathway. Western blot data indicated that DARPP-32-induced up-regulation of BCL-xL expression was associated with a significant increase in p-Src(Y416) and p-STAT3(Y705) protein levels as compared to control (Figure 4D). In addition, we showed that treatment with PP1 reversed the effects of DARPP-32 on Src/STAT3 signaling and BCL-xL expression (Figure 4D). Together, these data demonstrated that the upregulation of BCL-xL via the Src/STAT3 signaling mediates the pro-survival function of DARPP-32 in MKN-28 cells.
DARPP-32 suppresses TRAIL-induced down-regulation of NF-kB activity and FLIP(S) expression
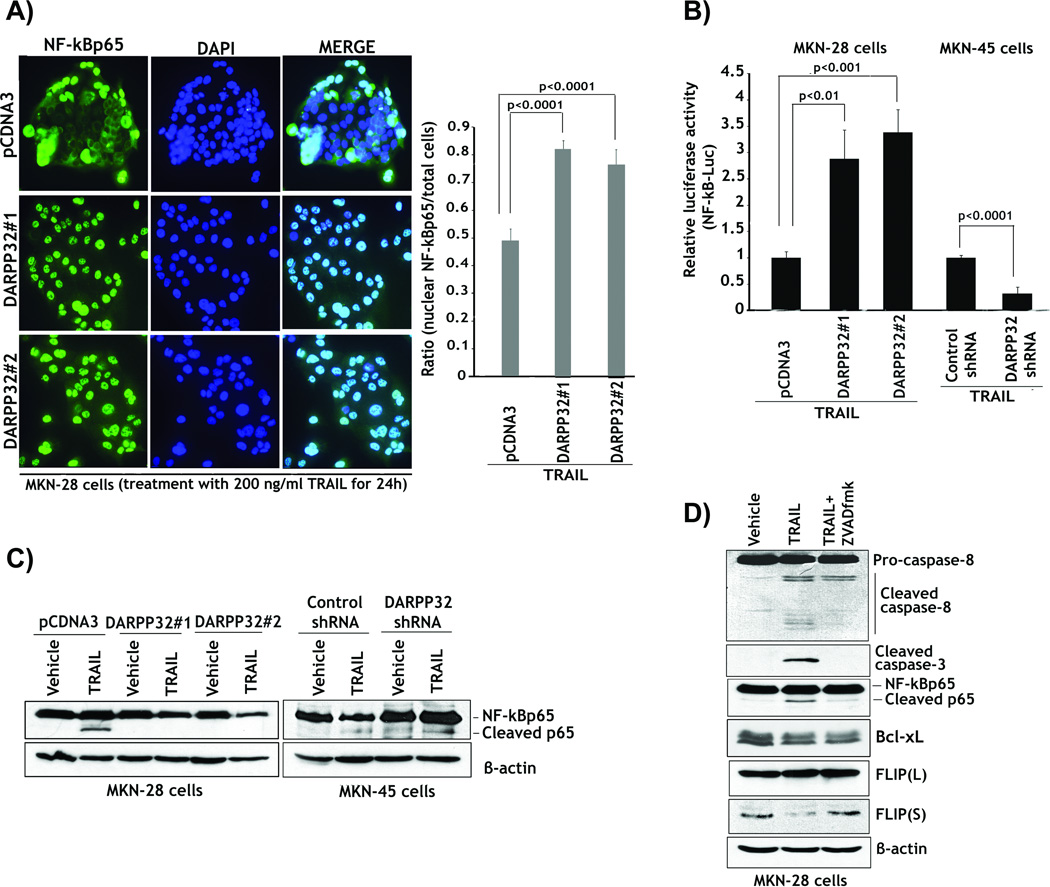
We have demonstrated that DARPP-32 up-regulated the pro-survival BCL-xL expression and blocked TRAIL-induced activation of caspases through regulation of Src/STAT3 signaling in gastric cancer cells. It is well established that BCL-xL expression inhibits the intrinsic apoptosis pathway through blocking activation of caspase 9 in response to multiple apoptotic stimuli. Interestingly, our cell models indicated that DARPP-32 blocked TRAIL-induced activation of caspase 8 in addition to caspases 9 and 3. Therefore, we next tested our hypothesis that DARPP-32 may regulate the NF-kB following TRAIL treatment, thus explaining the suppression of TRAIL-induced activation of caspase 8 (Figure 2B). We could not detect any significant effect on nuclear localization of NF-kBp65 in absence of TRAIL treatment (Supplementary Figure 1). However, the results were in agreement with our assumption and TRAIL treatment induced a decrease of approximately 50% in nuclear NF-kBp65 in MKN-28/pcDNA3 control cells as compared to MKN-28/DARPP-32 cells (p<0.001) (Figure 5A). Accordingly, DARPP-32 expression was required to maintain the nuclear NF-kBp65 protein levels in response to TRAIL.
Figure 5. DARPP-32 abrogation of TRAIL-induced inhibition of NF-kB is associated with blocking caspases-dependent cleavage of NF-kBp65 protein.
A) MKN-28/DARPP-32 and MKN-28/pcDNA3 cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Cells were subjected to immunofluorescence with anti-NF-kBp65 antibody and a secondary antibody conjugated to FITC. DAPI was used as a nuclear counterstain. Left panel, representative immunofluorescence images of cells following treatment with TRAIL showing localization of NF-kBp65. Cells that presented nuclear NF-kBp65 staining indicated activation of NF-kB. Right panel, the quantitative results showed that treatment with TRAIL led to a decrease of approximately 50% in nuclear NF-kBp65 in control cells relative to DARPP-32-expressing cells (p<0.0001). B) The luciferase reporter assay using a pNF-kB-Luc plasmid in MKN-28/pcDNA3, MKN-28/DARPP-32, MKN-45/control-shRNA, and MKN-45/DARPP-32-shRNA cells. After treatment with 200 ng/ml TRAIL for 24h, luciferase activity was 2.5 to 3-fold lower in MKN-28 control cells than DARPP-32-expressing MKN-28 cells (p<0.01). Knocking down endogenous DARPP-32 in MKN-45 cells and treatment with TRAIL resulted in a 3-fold decrease in luciferase activity relative to control cells (p<0.0001). C) Left panel, MKN-28/pcDNA3 and MKN-28/DARPP-32 cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Western blot analysis of NF-kBp65 expression indicated that, unlike DARPP-32-expressing cells, treatment of control cells with TRAIL induced cleavage of p65 protein. Right panel, MKN-45/control shRNA and MKN-45/DARPP-32 shRNA cells were treated with vehicle or TRAIL, as in panel C. Immunoblotting data showed that knocking down endogenous DARPP-32 significantly enhanced TRAIL-induced cleavage of p65 protein as opposed to control cells. D) MKN-28 cells were treated with vehicle, 200 ng/ml TRAIL alone or in combination with 10 µM z-VAD-fmk inhibitor for 24h. Western blot results indicated that treatment with TRAIL alone induced activation of caspases and cleavage of p65. However, TRAIL in combination with z-VAD-fmk failed to activate caspases and cleave p65 protein. Down-regulation of FLIP(S) was correlated with TRAIL-induced activation of caspases and cleavage of p65. Gel loading was normalized for equal β-actin. Results are representative of three experiments and shown as the mean ± SEM. Significance of difference was calculated using Student’s t test analysis.
To further confirm the role of DARPP-32 in blocking TRAIL-induced inhibition of NF-kB, we utilized the luciferase reporter assay using a pNF-kB-Luc plasmid with TRAIL treatment in MKN-28 and MKN-45 cell models. The results indicated that NF-kB luciferase activity was 2.5 to 3-fold lower in MKN-28/pcDNA3 control cells than in MKN-28/DARPP-32 cells (p<0.01) (Figure 5B). In addition, knocking down endogenous DARPP-32 in MKN-45 cells and treatment with TRAIL led to a 3-fold decrease in NF-kB luciferase activity relative to control cells (p<0.0001) (Figure 5B). In an attempt to identify the mechanism by which TRAIL inhibited NF-kB activity and how DARPP-32 abrogated this effect, we evaluated NF-kBp65 protein expression in MKN-28 and MKN-45 cells. Surprisingly, the Western blot results showed that TRAIL induced cleavage of p65 protein producing a lower molecular weight protein, which was prominent in MKN-28/pcDNA3 control cells (Figure 5C). In contrast, there was no apparent cleavage of NF-kBp65 protein induced by TRAIL in two clones of MKN-28/DARPP-32 cells (Figure 5C). Knocking down endogenous DARPP-32 with shRNA in MKN-45 cells significantly enhanced TRAIL-induced cleavage of p65 protein relative to control cells (Figure 5C). These results suggest a direct correlation between TRAIL-induced cleavage of p65 protein and NF-kB activity shown in Figure 5B, and DARPP-32 clearly blocked the effects of TRAIL. Taken together, the data indicated that DARPP-32 sustained nuclear NF-kBp65 protein level, and inhibited its cleavage in response to TRAIL.
Next, we investigated whether cleavage of the p65 protein was dependent on the activation of caspases and the onset of apoptosis. Western blot results showed, as expected, that TRAIL significantly activated caspases 8 and 3, and induced cleavage of the p65 protein (Figure 5D). In contrast, z-VAD-fmk inhibitor blocked TRAIL-induced activation of caspases and cleavage of p65 protein in MKN-28 cells (Figure 5D). These results demonstrated that TRAIL-induced cleavage of the p65 protein was dependent on activation of caspases.
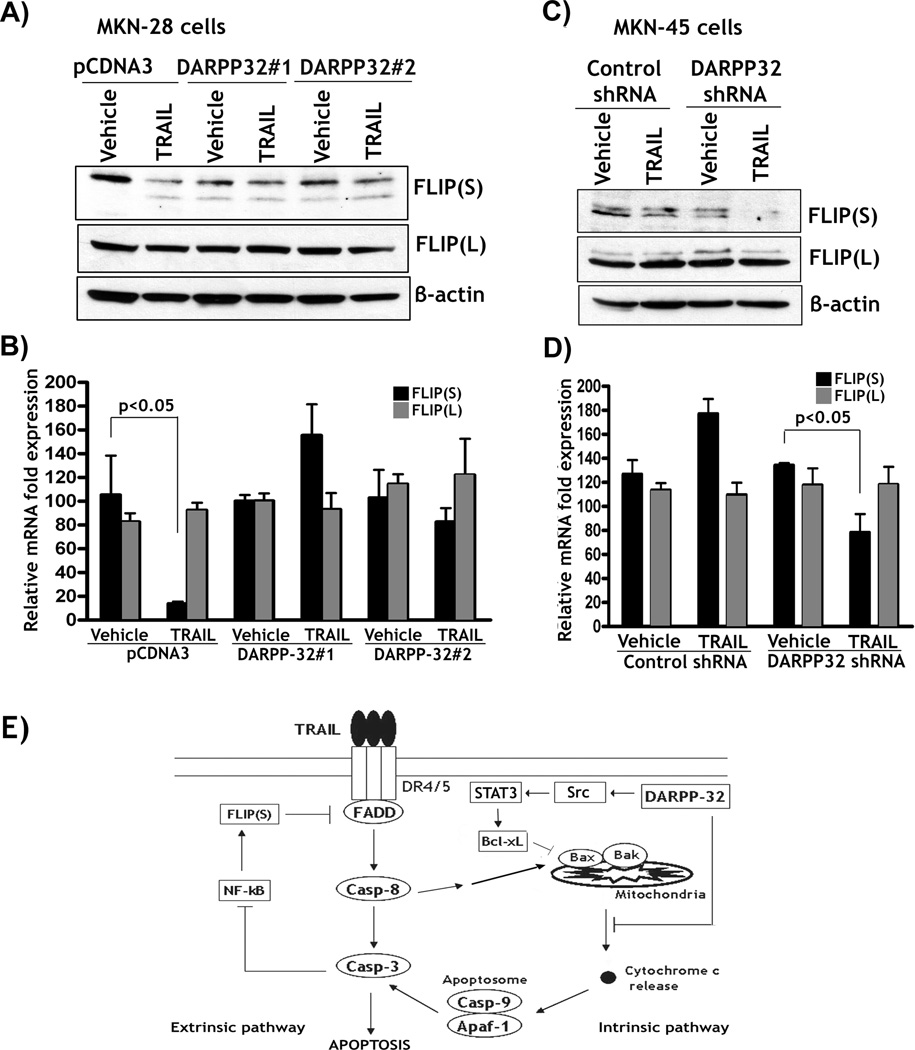
We also found that TRAIL-induced cleavage of the p65 protein was dependent on the activation of caspases and was associated with a significant decrease in the protein expression of FLIP(S), a transcriptional target of NF-kB, in MKN-28 cells (Figure 5D). Of note, TRAIL had no significant effect on the expression of BCL-xL, another transcriptional target of NF-kB, in MKN-28 cells (Figure 5D). We investigated whether DARPP-32 could block TRAIL-induced down-regulation of FLIP expression in MKN-28 and MKN-45 cells. The Western blot results indicated that TRAIL induced a significant decrease in protein expression in FLIP(S) but not FLIP(L) in MKN-28 cells (Figure 6A). Conversely, TRAIL failed to affect the protein levels of both FLIP(S) and FLIP(L) in MKN-28/DARPP-32 cells (Figure 6A). The qRT-PCR results revealed that TRAIL significantly down-regulated FLIP(S) mRNA expression in MKN-28/pcDNA3 control cells, but had no significant effects on both FLIP(S) and FLIP(L) mRNA levels in MKN-28/DARPP-32 cells (Figure 6B). We next investigated whether TRAIL-induced down-regulation of FLIP(S) expression was time-dependent in MKN-28 cells. The qRT-PCR data demonstrated that the effect of TRAIL was evident as early as two hours after treatment (Supplementary Figure 2). In MKN-45 cell model, we demonstrated that knocking down endogenous DARPP-32 significantly enhanced TRAIL-induced down-regulation of protein expression of FLIP(S) without affecting FLIP(L) protein level (Figure 6C). Similarly, the qRT-PCR results demonstrated that knocking down endogenous DARPP-32 significantly decreased the mRNA level of FLIP(S) (p<0.05), but not FLIP(L) in response to TRAIL in MKN-45 cells (Figure 6D). Together, these results demonstrated that DARPP-32 blocked TRAIL-induced cleavage and inhibition of NF-kB activity, which was associated with caspase-dependent cleavage of the p65 protein. Furthermore, DARPP-32 suppressed TRAIL-induced down-regulation of FLIP(S), thereby counteracting TRAIL-mediated activation of caspase 8 in gastric cancer cells.
Figure 6. DARPP-32 blocks TRAIL-induced down-regulation of FLIP expression.
A) MKN-28/pcDNA3 and MKN-28/DARPP-32 cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Western blot results showed that treatment with TRAIL induced a significant decrease in protein expression of FLIP(S) but not FLIP(L) in control cells. In contrast, TRAIL failed to affect both FLIP(S) and FLIP(L) protein levels in DARPP-32-expressing cells. B) qRT-PCR results indicated that TRAIL significantly decreased mRNA level of FLIP(S) in control cells as opposed to DARPP-32-expressing cells (p<0.05). Overall, TRAIL had no significant effect on FLIP(L) mRNA expression in all cells. C) MKN-45/control shRNA and MKN-45/DARPP-32 shRNA cells were treated with vehicle or 200 ng/ml TRAIL for 24h. Western blot data indicated that knocking down endogenous DARPP-32 enhanced TRAIL-induced down-regulation of FLIP(S) without affecting FLIP(L) protein expression. D, qRT-PCR results showed that knockdown of endogenous DARPP-32 in combination with TRAIL led to a 2-fold decrease in FLIP(S) mRNA level as compared to control cells (p<0.05). FLIP(L) mRNA levels were not affected in all cells. E) A schematic representation; DARPP-32 inhibits TRAIL-induced apoptosis through regulating both the intrinsic and extrinsic apoptosis pathways in gastric cancer cells.
Discussion
Recombinant TRAIL proteins and TRAIL receptor agonistic antibodies have been tested in clinical trials, showing promising anti-tumor activities with mild side effects (reviewed by Kruyt (26)). Although TRAIL could specifically induce tumor cell death, there are many cancer cells that are TRAIL resistant (27). Resistance to TRAIL is a major clinical challenge that leads to failure of treatment, poor prognosis, and reduced cancer patient survival. Little is known about the mechanisms of TRAIL resistance in gastric cancer. In the current study, we demonstrate a novel signaling mechanism for TRAIL resistance mediated by DARPP-32, which is overexpressed in more than two-thirds of adenocarcinomas of the stomach and esophagus (21, 22). Given the fact that DARPP-32 is also overexpressed in several other malignancies such as breast and colon cancers, findings from the current study provide novel insight in understanding the complexity of TRAIL resistance which can guide the development of new approaches to circumvent apoptotic blockades or suppress survival signals.
The findings in the present study demonstrated that DARPP-32 promoted cell survival and blocked apoptosis in response to treatment with TRAIL. In an attempt to uncover DARPP-32-mediated signaling mechanism, we found that overexpression of DARPP-32 blocked TRAIL-induced release of cytochrome c and activation of caspases 8, 9, and 3. Conversely, knockdown of endogenous DARPP-32 enhanced TRAIL-induced activation of caspases 8, 9, and 3. These results suggested that DARPP-32 regulates both the death receptors-mediated extrinsic and the mitochondrial intrinsic apoptosis pathways. Cytochrome c release occurs in response to several apoptotic stimuli and it is a prerequisite for caspase 9 activation (8, 28). This implies that DARPP-32-mediated suppression of TRAIL-induced activation of the intrinsic apoptosis pathway might involve up-regulated expression of the pro-survival BCL2 family members. Overexpression of BCL-xL and BCL2 has been shown to protect type II cells against TRAIL-mediated apoptosis (13). The fact that DARPP-32 up-regulated BCL-xL expression at the protein and mRNA levels through activation of Src/STAT3 signaling, provides a plausible explanation for suppression of the intrinsic apoptosis. Accordingly, DARPP-32-induced up-regulation of BCL-xL may be responsible for blocking TRAIL-mediated cytochrome c release, activation of caspase 9, and subsequently caspase-3. Our finding is in agreement with previous reports that have shown that BCL-xL promotes TRAIL resistance through inhibition of the intrinsic mitochondrial apoptosis pathway in cancer cells (13, 14). Several reports show that transcription factors such as NF-kB and STAT3 could up-regulate BCL-xL in cancer cells (reviewed by Grad et al. (15)). In our MKN-28 cell model, we demonstrated that DARPP-32-induced BCL-xL expression involved activation of STAT3 transcription factor, and excluded a role for NF-kB as a mediator.
Caspase 8 is normally associated with the extrinsic apoptosis pathway, whereby its activation is regulated by the pro-apoptotic molecules (TRAIL, FasL, TNFα) or by a negative feedback loop mediated by NF-kB and FLIP (16, 29). NF-kB activation prevents apoptosis by induction of anti-apoptotic proteins such as BCL-xL, cIAP1&2, xIAP and FLIP (29–33). We hypothesized that TRAIL inhibits NF-kB/FLIP signaling, thereby amplifying apoptosis through increased activation of caspase-8. To uncover the signaling mechanism by which DARPP-32 blocks TRAIL-induced activation of caspase 8, we investigated whether the NF-kB/FLIP negative feedback loop was regulated by DARPP-32 in response to TRAIL.
The results indicated that TRAIL-induced apoptosis was accompanied by the inhibition of NF-kB activity, cleavage of NF-kBp65 protein, and down-regulation of FLIP(S) expression. We further confirmed that TRAIL-induced cleavage of the p65 protein and down-regulation of FLIP(S) was dependent on active caspases. This is consistent with the notion that activation of caspases can block NF-kB activity and downstream signaling by inducing cleavage of NF-kBp65 protein (34, 35). Caspase-mediated cleavage of NF-kBp65 does not simply lead to its inactivation but converts it into a dominant-negative variant of its non-cleaved p65 protein (35). Although DARPP-32 did not significantly activate NF-kB in basal conditions, it suppressed activation of caspases and maintained NF-kB activity and FLIP(S) expression by blocking the cleavage of the NF-kBp65 protein in response to TRAIL treatment. FLIP proteins consist of the short form (FLIP(S)), which contains two death effector domains, and the long form (FLIP(L)), contains in addition the pseudo-caspase domain. Both FLIP(S) and FLIP(L) can interfere with the FADD-caspase-8-interaction, thus blocking the recruitment and activation of caspase 8 during TRAIL-mediated apoptosis (36). Our results, showing TRAIL-dependent down-regulation of FLIP(S) but not FLIP(L), were consistent with the study on the role of FLIP(S) in modulating TRAIL-induced apoptosis in cancer (18). Although we cannot rule out other potential mechanism(s) by which DARPP-32 suppresses TRAIL-induced activation of caspase 8, our findings strongly suggest that DARPP-32 blocked activation of caspase 8 and promoted TRAIL resistance through the inhibition of the mitochondrial apoptosis pathway and maintaining activation of the NF-kB-FLIP feedback loop based on the following lines of evidence: 1) DARPP-32 up-regulated the pro-survival BCL-xL expression through activation of Src/STAT3 signaling; 2) DARPP-32 inhibited TRAIL-mediated cytochrome c release; 3) DARPP-32 blocked TRAIL-induced activation of caspases 8, 9&3; 4) DARPP-32 suppressed TRAIL-mediated inhibition of NF-kB and caspases-dependent degradation of the p65 protein; 5) DARPP-32 inhibited TRAIL-induced down-regulation of FLIP(S) expression.
In conclusion, our findings indicate that DARPP-32 promotes TRAIL resistance and suggest that the frequent overexpression of DARPP-32 underscores a clinical challenge in gastric cancers. The capacity of DARPP-32 to block the mitochondrial apoptosis pathway by up-regulating BCL-xL expression, and suppress TRAIL-induced activation of caspase 8 through maintaining the activity of the NF-kB-FLIP feedback loop can modulate the response to TRAIL and possibly other chemotherapeutics.
Supplementary Material
Translational Relevance.
TRAIL selectively induces apoptosis in a variety of tumor cells, whereas majority of normal cells remain unresponsive to its effect. This unique capacity has placed TRAIL in the forefront as a promising anti-cancer agent. Unfortunately, resistance to TRAIL is not uncommon as TRAIL resistance can be developed by several mechanisms. DARPP-32 is frequently overexpressed in a number of human malignancies including adenocarcinomas of the colon, pancreas, breast, esophageal, and gastric. In this study, we used gastric cancer models and identified DARPP-32 as a major mediator of TRAIL resistance. Our findings provide novel insight in understanding the complexity of TRAIL resistance which could be applicable to other cancers. These findings can guide the development of therapeutic management approaches to circumvent apoptotic blockades or suppress survival signals.
Acknowledgments
Grant Support: This study was supported by grants from the National Institute of Health; R01CA93999 (WER), Vanderbilt SPORE in Gastrointestinal Cancer (P50 CA95103), Vanderbilt Ingram Cancer Center (P30CA68485) and the Vanderbilt Digestive Disease Research Center (DK058404).
Footnotes
Conflict of interest
The authors declare no conflict of interest.
The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Cancer Institute or Vanderbilt University.
Contributor Information
Abbes Belkhiri, Email: abbes.belkhiri@vanderbilt.edu.
Shoumin Zhu, Email: shoumin.zhu@vanderbilt.edu.
Zheng Chen, Email: zheng.chen@vanderbilt.edu.
Mohammed Soutto, Email: mohammed.soutto@vanderbilt.edu.
Wael El-Rifai, Email: wael.el-rifa@vanderbilt.edu.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Roder DM. The epidemiology of gastric cancer. Gastric Cancer. 2002;5(Suppl 1):5–11. doi: 10.1007/s10120-002-0203-6. [DOI] [PubMed] [Google Scholar]
- 3.Becker JC, Muller-Tidow C, Serve H, Domschke W, Pohle T. Role of receptor tyrosine kinases in gastric cancer: new targets for a selective therapy. World J Gastroenterol. 2006;12:3297–3305. doi: 10.3748/wjg.v12.i21.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee SH, Kim HS, Kim SY, Lee YS, Park WS, Kim SH, et al. Increased expression of FLIP, an inhibitor of Fas-mediated apoptosis, in stomach cancer. APMIS. 2003;111:309–314. doi: 10.1034/j.1600-0463.2003.1110203.x. [DOI] [PubMed] [Google Scholar]
- 5.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 6.Pan G, O'Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–113. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 7.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and characterization of a new member of the TNF family that induces apoptosis. Immunity. 1995;3:673–682. doi: 10.1016/1074-7613(95)90057-8. [DOI] [PubMed] [Google Scholar]
- 8.Yamada H, Tada-Oikawa S, Uchida A, Kawanishi S. TRAIL causes cleavage of bid by caspase-8 and loss of mitochondrial membrane potential resulting in apoptosis in BJAB cells. Biochem Biophys Res Commun. 1999;265:130–133. doi: 10.1006/bbrc.1999.1641. [DOI] [PubMed] [Google Scholar]
- 9.Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME. Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem. 1999;274:22532–22538. doi: 10.1074/jbc.274.32.22532. [DOI] [PubMed] [Google Scholar]
- 11.Walczak H, Miller RE, Ariail K, Gliniak B, Griffith TS, Kubin M, et al. Tumoricidal activity of tumor necrosis factor-related apoptosis-inducing ligand in vivo. Nat Med. 1999;5:157–163. doi: 10.1038/5517. [DOI] [PubMed] [Google Scholar]
- 12.Almasan A, Ashkenazi A. Apo2L/TRAIL: apoptosis signaling, biology, and potential for cancer therapy. Cytokine Growth Factor Rev. 2003;14:337–348. doi: 10.1016/s1359-6101(03)00029-7. [DOI] [PubMed] [Google Scholar]
- 13.Hinz S, Trauzold A, Boenicke L, Sandberg C, Beckmann S, Bayer E, et al. Bcl-XL protects pancreatic adenocarcinoma cells against CD95- and TRAIL-receptor-mediated apoptosis. Oncogene. 2000;19:5477–5486. doi: 10.1038/sj.onc.1203936. [DOI] [PubMed] [Google Scholar]
- 14.Kim K, Nakagawa H, Fei P, Rustgi AK, El-Deiry WS. Targeting Bcl-xL in esophageal squamous cancer to sensitize to chemotherapy plus TRAIL-induced apoptosis while normal epithelial cells are protected by blockade of caspase 9. Cell Death Differ. 2004;11:583–587. doi: 10.1038/sj.cdd.4401388. [DOI] [PubMed] [Google Scholar]
- 15.Grad JM, Zeng XR, Boise LH. Regulation of Bcl-xL: a little bit of this and a little bit of STAT. Curr Opin Oncol. 2000;12:543–549. doi: 10.1097/00001622-200011000-00006. [DOI] [PubMed] [Google Scholar]
- 16.Tschopp J, Irmler M, Thome M. Inhibition of fas death signals by FLIPs. Curr Opin Immunol. 1998;10:552–558. doi: 10.1016/s0952-7915(98)80223-9. [DOI] [PubMed] [Google Scholar]
- 17.Griffith TS, Chin WA, Jackson GC, Lynch DH, Kubin MZ. Intracellular regulation of TRAIL-induced apoptosis in human melanoma cells. J Immunol. 1998;161:2833–2840. [PubMed] [Google Scholar]
- 18.Nam SY, Jung GA, Hur GC, Chung HY, Kim WH, Seol DW, et al. Upregulation of FLIP(S) by Akt, a possible inhibition mechanism of TRAIL-induced apoptosis in human gastric cancers. Cancer Sci. 2003;94:1066–1073. doi: 10.1111/j.1349-7006.2003.tb01402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krueger A, Baumann S, Krammer PH, Kirchhoff S. FLICE-inhibitory proteins: regulators of death receptor-mediated apoptosis. Mol Cell Biol. 2001;21:8247–8254. doi: 10.1128/MCB.21.24.8247-8254.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thome M, Tschopp J. Regulation of lymphocyte proliferation and death by FLIP. Nat Rev Immunol. 2001;1:50–58. doi: 10.1038/35095508. [DOI] [PubMed] [Google Scholar]
- 21.Belkhiri A, Zaika A, Pidkovka N, Knuutila S, Moskaluk C, El-Rifai W. Darpp-32: a novel antiapoptotic gene in upper gastrointestinal carcinomas. Cancer Res. 2005;65:6583–6592. doi: 10.1158/0008-5472.CAN-05-1433. [DOI] [PubMed] [Google Scholar]
- 22.Mukherjee K, Peng D, Brifkani Z, Belkhiri A, Pera M, Koyama T, et al. Dopamine and cAMP regulated phosphoprotein MW 32 kDa is overexpressed in early stages of gastric tumorigenesis. Surgery. 148:354–363. doi: 10.1016/j.surg.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Belkhiri A, Dar AA, Zaika A, Kelley M, El-Rifai W. t-Darpp promotes cancer cell survival by up-regulation of Bcl2 through Akt-dependent mechanism. Cancer Res. 2008;68:395–403. doi: 10.1158/0008-5472.CAN-07-1580. [DOI] [PubMed] [Google Scholar]
- 24.El-Rifai W, Moskaluk CA, Abdrabbo MK, Harper J, Yoshida C, Riggins GJ, et al. Gastric cancers overexpress S100A calcium-binding proteins. Cancer Res. 2002;62:6823–6826. [PubMed] [Google Scholar]
- 25.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13:1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 26.Kruyt FA. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 27.Kim K, Fisher MJ, Xu SQ, el-Deiry WS. Molecular determinants of response to TRAIL in killing of normal and cancer cells. Clin Cancer Res. 2000;6:335–346. [PubMed] [Google Scholar]
- 28.Arnoult D, Parone P, Martinou JC, Antonsson B, Estaquier J, Ameisen JC. Mitochondrial release of apoptosis-inducing factor occurs downstream of cytochrome c release in response to several proapoptotic stimuli. J Cell Biol. 2002;159:923–929. doi: 10.1083/jcb.200207071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Micheau O, Lens S, Gaide O, Alevizopoulos K, Tschopp J. NF-kappaB signals induce the expression of c-FLIP. Mol Cell Biol. 2001;21:5299–5305. doi: 10.1128/MCB.21.16.5299-5305.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stehlik C, de Martin R, Kumabashiri I, Schmid JA, Binder BR, Lipp J. Nuclear factor (NF)-kappaB-regulated X-chromosome-linked iap gene expression protects endothelial cells from tumor necrosis factor alpha-induced apoptosis. J Exp Med. 1998;188:211–216. doi: 10.1084/jem.188.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 32.Chu ZL, McKinsey TA, Liu L, Gentry JJ, Malim MH, Ballard DW. Suppression of tumor necrosis factor-induced cell death by inhibitor of apoptosis c-IAP2 is under NF-kappaB control. Proc Natl Acad Sci U S A. 1997;94:10057–10062. doi: 10.1073/pnas.94.19.10057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kreuz S, Siegmund D, Scheurich P, Wajant H. NF-kappaB inducers upregulate cFLIP, a cycloheximide-sensitive inhibitor of death receptor signaling. Mol Cell Biol. 2001;21:3964–3973. doi: 10.1128/MCB.21.12.3964-3973.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim HS, Chang I, Kim JY, Choi KH, Lee MS. Caspase-mediated p65 cleavage promotes TRAIL-induced apoptosis. Cancer Res. 2005;65:6111–6119. doi: 10.1158/0008-5472.CAN-05-0472. [DOI] [PubMed] [Google Scholar]
- 35.Levkau B, Scatena M, Giachelli CM, Ross R, Raines EW. Apoptosis overrides survival signals through a caspase-mediated dominant-negative NF-kappa B loop. Nat Cell Biol. 1999;1:227–233. doi: 10.1038/12050. [DOI] [PubMed] [Google Scholar]
- 36.Irmler M, Thome M, Hahne M, Schneider P, Hofmann K, Steiner V, et al. Inhibition of death receptor signals by cellular FLIP. Nature. 1997;388:190–195. doi: 10.1038/40657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.