Abstract
The extracellular promastigote stage of Leishmania spp. is transmitted to mammals by a sand fly vector. Leishmania promastigotes ligate host macrophage receptors, triggering phagocytosis and subsequent internalization, a critical step for survival. Parasites transform intracellularly to the amastigote stage. Many studies document different receptors detecting promastigotes and amastigotes, but the relative importance of each interaction is ill-defined. Recent studies suggest the macrophage receptors utilized during phagocytosis impact the intracellular fate of the parasite. This review summarizes the receptors implicated in Leishmania phagocytosis over the past 30 years. It then proceeds to weigh the evidence for or against their potential roles in intracellular parasite trafficking.
Keywords: Leishmania, promastigote, amastigote, metacyclic promastigote, macrophage, phagocytosis, parasitophorous vacuole, receptor, CR1, CR3, DC-SIGN, Fc receptor, fibronectin receptor, mannose receptor
Macrophage receptors implicated in Leishmania phagocytosis
More than 20 species of Leishmania cause symptomatic leishmaniasis in over 12 million people, with disease severity ranging from cutaneous ulcerations to fatal visceral infections [1]. Leishmania are found in several mammalian cells, but the majority of parasites reside in macrophages [2, 3]. The promastigote stage of Leishmania ligates macrophage surface receptors that trigger phagocytosis. This is followed by parasite transformation to the obligate intracellular amastigote stage. Receptors reported to facilitate Leishmania internalization include the third complement receptor (CR3), first complement receptor (CR1) mannose receptor (MR), Fc gamma receptors (FcγRs, in particular FcγRII-B2) and fibronectin receptors (FnRs) (Table 1) [4–8]. A definitive understanding of the roles of various receptors in parasite survival during natural infection has remained elusive [9, 10]. The mechanism of macrophage entry reflects, in part, the dynamic nature of the parasite surface. The most abundant surface membrane components differ between the extracellular promastigote form found in insects and the intracellular amastigote form found in mammals [11]. Metacyclogenesis, the developmental process leading promastigotes to transform into a virulent form in the sand fly gut, is characterized by further modifications in surface proteins and other glycoconjugates [12, 13]. Different sources of macrophages and different Leishmania spp. vary in their surface molecule composition, leading to unique parasite-host receptor interactions for each species-cell pair [2, 14].
Table 1.
Receptors for Leishmania phagocytosis
| Receptor, host cell typea | Cooperationb | Life stagesc | Binding mechanismd | Functional consequencese |
|---|---|---|---|---|
|
CR3[6,11,31,34–35,42]
(CD11b/CD18) Primary MΦ: BMM, MDM, MPM Cell lines: J774A.1, THP-1, COS+CR3, 293+CR3 Other: PMN |
CR1[44] L. major MR[10,34] L. donovani L. i. chagasi FnRs[49,55–56] L. amazonensis FcγRs[6] L. mexicana |
Avirulent PM: L. amazonensis L. i. chagasi L. donovani, L. infantum L. major L. mexicana Metacyclic PM: L. i. chagasi L. infantum L. major Amastigote: L. amazonensis L. donovani L. major L. mexicana |
Direct: Unknown ligand binds alternate lectin-like binding site of CR3 (L. infantum)[4,7,34] PSA-2 binds CD11b subunit (L. infantum)[35] iC3b-mediated: Opsonized C3b from serum is converted by CR1 and factor I into iC3b, a CR3 α-chain ligand. (All spp.)[4,31,33–34,44] |
CR3-mediated phagocytosis recruits Rho GTPase for cytoskeletal rearrangement[67] Ligation of CR3 by iC3b inhibits IFN-γ mediated pro-inflammatory signaling, and IL-12 down-regulating H2O2 production[69] PVs formed after CR3-mediated uptake are tight fitting[2] Phagolysosome maturation is delayed. LAMP-1 and cathepsin-D accumulation is impaired[10, 28] |
|
CR1[5,44]
(CD35) Primary MΦ: MDM Cell lines: CHO+CR1 |
CR3[44] L. major |
Avirulent PM: L. amazonensis L. major Metacyclic PM: L. major |
C3b-mediated: Opsonized C3 from serum is cleaved by GP63 into C3b, the natural ligand for CR1 (L. major)[24] |
CR1 is likely functions to enhance CR3 ligation[44,71] CR1 signaling by Leishmania spp. may not trigger respiratory burst[5] |
|
MR[4,9,10,34]
(CD206) Primary MΦ: BMM, MDM, MPM |
CR3[10,34] L. donovani |
Avirulent PM: L. i. chagasi L. donovani |
Direct: Mannan-capped LPG backbone and side chains possibly bind the CRD4 and CRD5 domains of MR (L. chagasi, L. donovani)[26–27,78] Virulent parasites avoid MR ligation (L. chagasi, L. donovani, L. major)[9,10,48] |
Signaling depends on MΦ activation state[78–79,83] MR can trigger synthesis of TNF-α, O2− and lysosomal enzymes[80–82] Lysosomal fusion can be prevented by inhibiting tyrosine kinase Hck recruitment[84] MR inhibits IL-12 production[85] |
|
FnRs[8,49,50]
(CD49d.CD29, CD49e/CD29, CD41/CD61) Primary MΦ: MDM, MPM, Human monocytes Cell lines: CHO+α4 subunit |
CR3[49,55–56] L. amazonensis L. major L. infantum |
Avirulent PM: L. amazonensis L. major L. infantum Amastigotes: L. amazonensis |
Direct: α4 subunit of “fibroblast-origin” FnR binds to a non-RGDS motif of GP63 (L. amazonensis)[49,52] Fibronectin-mediated: Non-specific opsonization by serum fibronectin allows FnRs to detect RGDS and EILDV motifs (L. amazonensis)[49,87] |
Internalization via FnRs requires intact β1 subunit[49] Activation of CR3 by FnR is required for CR3-bound particle ingestion[38] FnR-mediated phagocytosis generates O2−[51] PM can shed membrane bound to fibronectin to evade intracellular lysis[50] |
|
FcγRs[6,11,60–62]
(CD64, CD32, CD16) Primary MΦ: BMM Dendritic Cells: MDDC Cell lines: COS+ FcγRII-B2 |
CR3[6] L. mexicana |
Amastigotes: L. amazonensis L. major L. mexicana L. pifanoi |
IgG-mediated: Circulating IgG following initial infection bind to amastigotes (L. major)[6,11,60] FcγRI, II, and III bind to amastigotes opsonized by IgG (L. amazonensis, L. mexicana, L. pifanoi)[11,61] |
FcγR-mediated phagocytosis recruits Rac GTPase for cytoskeletal rearrangement Rac activates NADPH oxidase PVs formed after FcγR-mediated uptake are spacious[67] |
|
DC-SIGN[64–65]
(CD209) Dendritic Cells: MDDC Cell lines: K562+DC-SIGN |
Unknown |
Avirulent PM: L. donovani L. infantum L. pifanoi Metacyclic PM: L. infantum L. pifanoi Amastigotes: L. infantum L. pifanoi |
Direct: Unknown ligand binds to an unknown region of DC- SIGN[64] Binding is independent of LPG. (L. donovani, L. infantum, L. pifanoi)[65] |
DC-SIGN mediated uptake into immature MDDCs does not stimulate maturation[66] |
Surface receptors that ligate Leishmania are written in bold. The host cell type on which the receptors are expressed are divided in to primary macrophages (Primary MΦ), neutrophils (PMNs), dendritic cells, and cell lines. Cell lines that have undergone transfection to express receptors are designated cell line name + receptor name’.
Receptors that have been shown to cooperate with the receptor mentioned in the left column in ‘co-ligating’ Leishmania are written in bold. Parasite species demonstrated to co-ligate these receptors are listed below.
Leishmania life stages that ligate each receptor are separated into non-metacyclic promastigotes (Avirulent PM), metacyclic promastigotes (Metacyclic PM), and amastigotes.
Direct or opsonin-mediated mechanisms of binding to receptors are briefly outlined, with the parasite species studied listed in parentheses.
Immunological consequences of the host cell entry via each receptor are briefly outlined.
Abbreviations: AM, amastigote; BMM, bone marrow macrophage; CR1, first complement receptor; CR3, third complement receptor; CRD, carbohydrate recognition domain; DC-SIGN, dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin; FcγR, Fc gamma receptors; FnR, fibronectin receptors; IgG, immunoglobulin G; LAMP-1, lysosome associated membrane protein-1; LPG, lipophosphoglycan; MDDC, monocyte-derived dendritic cell; MDM, monocyte-derived macrophage; MPM, murine peritoneal macrophage; MR, mannose receptor; MΦ, macrophage; PM, promastigote; PMN, neutrophil; PV, parasitophorous vacuole.
Variations in promastigote surface antigen composition
The ability to differentiate from avirulent promastigotes to metacyclic forms has led scientists to appreciate the tremendous molecular complexity in the Leishmania spp. stages. Amastigotes in the blood meal develop first to procyclic promastigotes, which differentiate into motile nectomonad promastigotes and traverse to the anterior midgut of the sand fly, where they attach to the epithelial wall. At the stomodeal valve, the parasites develop into leptomonad, haptomonad, and finally metacyclic promastigotes, the highly infectious form regurgitated into host skin during feeding [15].
In liquid culture, promastigotes undergo developmental changes that mimic metacyclogenesis. Logarithmically growing promastigotes have been used as a model of procyclic promastigotes, and stationary phase promastigotes as a model of an unpurified metacyclic-containing population. Purification of the metacyclic subpopulation can be accomplished according to their low buoyant density or the glycosylation of lipophosphoglycan (LPG) [16, 17]. BALB/c mice injected with in vitro-isolated metacyclic Leishmania major or Leishmania infantum chagasi promastigotes develop larger lesions or harbor more parasites in the visceral organs, respectively, than those inoculated with unpurified stationary phase cultures [18, 19].
Characteristics contributing to increased virulence of metacyclics include resistance to lysis by serum complement due to upregulation of critical virulence factors [19]. The third complement protein C3 is associated with increased promastigote virulence [20]. Several reports document covalent binding of C3b to the surface metalloprotease, GP63 [also called MSP (major surface protease), leishmanolysin, or PSP (promastigote surface protease)], and to LPG [21, 22]. GP63 is expressed more abundantly in metacyclic than procyclic promastigotes [13, 23], and serves a special role in that it converts complement component C3b to the cleavage product iC3b, which in turn mediates the opsonic recognition of promastigotes by macrophage CR3 [24].
Metacyclogenesis is also marked by doubling of the phosphorylated disaccharide backbone repeats and addition of terminal arabinose to LPG oligosaccharide side chains [25]. This could serve to bypass broad-range pattern recognition receptors such as MR. Direct binding of either the high-mannose oligosaccharides on GP63 or procyclic LPG to MR has not been reported. Nonetheless, there are exposed mannose residues on the terminus of LPG of procyclic L. major and Leishmania donovani [26], and purified LPG from L. major and Leishmania mexicana can be targeted by serum mannan-binding protein [27].
The LPG of L. major, L. donovani, L. mexicana, and Leishmania amazonensis differ in structure [26]. As such, promastigotes from different growth phases and also of different species vary in the abundance of GP63 versus LPG, and cannot be considered identical when determining the mechanism of host cell association.
Differential receptor ligation by promastigote stages
L. i. chagasi promastigotes isolated from different growth phases ligate different receptors on human monocyte-derived macrophages (MDMs). Avirulent promastigotes in logarithmic growth enter parasitophorous vacuoles (PVs) lined with CR3 and MR, whereas PVs surrounding density-purified metacyclics contained only CR3 [10]. CR3, but not MR, clusters in cholesterol- and caveolin-containing microdomains, which were previously characterized as entry portals that direct L. i. chagasi promastigotes into a pathway that leads to a 24 to 48 hr delay in lysosomal fusion and promotes intracellular survival [28]. Consistently, metacyclics that enter via PVs lined with caveolin and CR3 replicate better than their avirulent counterparts for up to 96 hr following entry. This leads us to hypothesize that PVs containing CR3 but excluding MR may constitute a ‘safe’ route of phagocytosis, associated with delayed lysosomal fusion.
As an added variable to all studies of receptor utilization, parasites enter macrophages from different sources via dissimilar mechanisms. For instance, metacyclic L. donovani or L. amazonensis promastigotes first contact the surface of bone marrow macrophages (BMMs) via the flagellar tip on the anterior end of the parasite, and then reorient to enter via the posterior end as macrophage pseudopods encircle the cell body [29, 30]. Anchoring via the flagellum has been recapitulated in L. i. chagasi promastigotes, which attach to the MDM surface via their anterior ends and become surrounded by symmetrical pseudopod extensions [2]. By contrast, attachment to the human monocytic U937 cells is not directional, and parasites are taken up through ‘coiling’ layers of pseudopods. These observations correlated with differences in surface receptor expression (i.e. U937 cells were negative for surface CR3), and intracellular survival. Although U937 cells are not as representative of a natural infection as primary macrophages, these data illustrate a connection between differential surface receptor ligation between cell types and the subsequent intracellular fate of the parasite [2].
Receptors mediating binding and phagocytosis of Leishmania promastigotes
The third complement receptor
Long-standing evidence suggests that Leishmania promastigotes ligate CR3 both directly and through opsonized iC3b. This comes from the observation that L. major or L. donovani promastigote entry into murine peritoneal macrophages (MPMs) is inhibited by the M1/70 blocking monoclonal antibody (mAb) to the CR3 α-chain [7] or by 5C6 mAb, which hinders binding to the iC3b recognition site of CR3 [31]. M1/70 was observed to inhibit macrophage binding by either promastigotes that were opsonized with fresh serum, or promastigotes in the absence of added serum opsonins [4]. This and other reports of serum-independent binding of Leishmania promastigotes to CR3 seem to indicate that parasites can ligate the lectin-binding site on CR3. However, macrophages in culture synthesize small amounts of complement proteins and opsonize nearby particles or cells. Illustrating this, C3 has been visualized on the surface of L. donovani promastigotes or amastigotes incubated with MPMs without serum pretreatment [32]. Consistently, anti-C3 Fab blocked the attachment of non-opsonized L. donovani to macrophages by 80% [33]. Thus, ‘non-opsonized’ promastigotes are never truly free of complement during co-culture with macrophages.
There is, nonetheless, evidence that Leishmania binds both the complement-binding and the lectin-binding sites of CR3 in MDMs. In one study, mAb clone OKM1, which blocks the CR3 iC3b binding site, reduced the serum-free attachment of stationary phase L. donovani promastigotes to MDMs by over 60%. mAb clone OKM10, specific for the lectin-like binding site on CR3, demonstrated an inhibitory effect of 18% [34]. Furthermore, the L. infantum recombinant surface glycoprotein PSA-2 may attach directly to the CD11b subunit of CR3 on the human monocytic THP-1 cell line, and on human kidney 293 cells transfected with CR3 [35].
In both nature and in vitro culture, the proportion of metacyclics increases as promastigotes grow from logarithmic to stationary phase. Promastigote growth also influences interactions with macrophage receptors. Using confocal microscopy, both CR3 and MR were documented in the PVs of MDMs after phagocytosis of logarithmic promastigotes, which contain a low proportion of metacyclics. By contrast, vacuoles surrounding serum-opsonized purified metacyclic L. i. chagasi promastigotes contained CR3 but excluded MR[ 10]. The amount of C3 deposition onto the surface of L. donovani promastigotes increases as parasites develop to stationary phase in culture, presumably due to the increased metacyclic content [36]. Indeed, stationary phase L. major, L. amazonensis, and L. donovani promastigotes all bind immobilized purified human CR3 following incubation in exogenous serum [37]. GP63 is the major C3 acceptor molecule on L. mexicana promastigotes according to co-immunoprecipitation studies [22]. Consistent with the increased C3 binding, there is increasing expression of GP63 on the surface of several Leishmania spp. during growth to stationary phase in culture [13, 23]. The relative roles of the CR3 lectin-like domain and locally secreted opsonizing C3 in natural infection remain ill-defined, but the preponderance of evidence suggests that CR3 is a common pathway for the entry of several Leishmania spp. into macrophages.
During natural infection it is likely that Leishmania simultaneously ligate more than one host cell receptor and that all receptors contribute to subsequent intracellular events. CR3 alone is incapable of triggering phagocytosis of iC3b-coated sheep erythrocytes and requires the assistance of a second receptor such as the FnR to initiate phagocytosis [38]. Both CR3 and MR are required for optimal binding of unpurified L. donovani promastigotes to macrophages, which implies co-recognition by more than one receptor [4, 34]. Due to this, the independent effect of CR3 ligation on parasite survival is difficult to prove. Nonetheless, there is evidence that CR3 ligation channels promastigotes toward a pathway leading to enhanced survival in the macrophage. Leishmania spp. promastigotes are susceptible to the microbicidal activity of O2- and H2O2 [39]. At least some promastigotes must enter through a route that offers protection against the macrophage microbicidal machinery, and it is possible that CR3 ligation itself could provide this survival advantage (Box 1). Consistent with a non-inflammatory effect of CR3, serum-opsonized L. major promastigotes stimulate a less vigorous MPM respiratory burst than non-opsonized promastigotes and survive better intracellularly [20].
Box 1. The third Complement Receptor (CR3) and the first Complement Receptor (CR1).
CR3 (CD18/CD11b) is an integrin expressed on the surface of neutrophils (PMNs) and mononuclear phagocytes that plays significant roles in immune defense. In addition to detecting iC3b and pathogen ligands, it is an adhesion factor during phagocyte migration, a co-receptor for actin cytoskeletal rearrangement, and an initiator of kinase signaling cascades [67]. CR3 has two binding sites: one for particles opsonized with the inactivated form of C3b (iC3b) and a complement-independent lectin-binding domain. iC3b-coated sheep erythrocytes binding to human monocyte and PMN CR3 does not induce H2O2 formation, whereas FcγR ligation by IgG-coated erythrocytes does [68]. Furthermore, CR3 activation either with an agonistic mAb recognizing the functional domain of human CD11b, or with iC3b-coated erythrocytes downregulated the IFN-γ mediated release of IL-12 [69]. These observations suggest CR3 does not induce inflammatory signals.
Different members of the Rho family of GTPases are activated by phagocytic receptors, with consequences for subsequent oxidant generation and cytoskeletal rearrangement. Phagocytosis via FcγR leads to activation of Rac GTPases and Cdc42 which in turn activate the phagocyte NADPH oxidase, whereas CR3-mediated phagocytosis leads to activation of Rho [67]. Particle phagocytosis via FcγRs creates spacious phagosomes, whereas entry via CR3 leads to tight-fitting phagosomes. The avidity of Leishmaniamexicana and L. amazonensis for FcγR over CR3 could partially explain their residence in large, communal vacuoles [11, 29, 70]. By contrast, promastigotes of L. i. chagasi and other species that prefer CR3 reside in tight, individual compartments in human and murine macrophages [2].
CR1 on the surface of monocytes, macrophages, and PMNs primarily recognizes C3b and C4b [38]. CR1 also binds iC3b, but at a 100-fold weaker affinity than C3b. CR1 is one of several factor I cofactors, binding C3b and facilitating its cleavage to iC3b through factor I serine protease activity [71]. CR1 and CR3 can cooperate and mediate stable rosetting of C3b coated sheep erythrocytes, a finding that can be explained by initial interaction of C3b with CR1, factor I-mediated cleavage to iC3b, and then high affinity interaction between iC3b and CR3 on the erythrocyte surface [71].
Recently, neutrophils (PMNs) have been found to be a predominant, possibly the most predominant cell type that become parasitized by Leishmania immediately following inoculation into mammalian skin [3]. Neutrophils housing parasites are then quiescently phagocytosed into either macrophages or dendritic cells (DCs) via PMNs serving astheir ‘Trojan horse’ (Box 2) [40, 41]. CR3 also plays an important role in this pathway, as Leishmania undergo an opsonin-dependent uptake into PMNs via this receptor. mAb M1/70, but not mannan-binding lectin, has been shown to significantly reduce internalization of L. major promastigotes in vitro [42].
Box 2. Intracellular trafficking of Leishmania following phagocytosis.
Phagocytosis of virulent L. donovani promastigotes proceeds through an endocytic pathway, and phagolysosome maturation is delayed for several hours after phagocytosis. Incoming phagosomes surrounding any particle undergo sequential interactions with endocytic organelles during maturation, in which a series of transient fusion (kiss) and fission (run) events deposits membrane proteins and delivers degradative enzymes into the phagosome lumen. This process was dubbed the ‘kiss and run’ hypothesis [72]. Phagocytosis of Leishmania into Rab5-positive early endosomes occurs readily, but shedding of Rab5 and acquisition of the later markers Rab7 and LAMP-1 is delayed in parasites that express the full length LPG on their surface [73]. A longer delay for lysosome fusion was observed, i.e. 24 to 48 hr, after phagocytosis of L. i. chagasi [10, 28], coinciding with the conversion to amastigotes [74].
The kinetics of phagolysosome maturation differs between parasite species and host cell type. Metacyclic L. amazonensis promastigotes, unlike their L. donovani counterpart, resides in spacious, communal PVs that fuse with Rab7 and cathepsin D, but not EEA-1, as early as 0.5 hr in BMMs. This process was even faster in L. amazonensis amastigotes, implicating stage- and species-specific discrepancies in PV biogenesis [29]. Real time microscopy also visualized the rapid accumulation of lysosomes to PVs containing metacyclic L. donovani promastigotes. During phagocytosis, the flagellae ‘wounded’ the host cell and released lysosomes from the damaged plasma membrane. The PV then underwent a refractory period, during which there was an absence of further recruitment of these organelles [30]. These authors’ observations challenge the aforementioned model for phagolysosome maturation delay, although they concur the inconsistencies could be due to differences in experimentation.
Developments in the last 10 years have focused on the exploitation of PMNs as a ‘Trojan horse’ for L. major entry into macrophages. Up to 90% of metacyclic L. major promastigotes were initially phagocytosed by PMNs at the bite wound site in B57BL/6 mice, with few instances of entering macrophages or DCs directly [3]. Virulent inoculums of L. major contain phosphatidylserine-positive apoptotic populations, presumably silencing the inflammatory functions of PMNs as parasites are taken up by means for apoptotic clearance [75]. Entry into macrophages occurs while parasites still reside in PMNs or following PMN apoptosis, which can become accelerated following Leishmania infection [40]. PMNs harboring Leishmania secrete MIP-1β, a chemo-attractant for macrophages [41], and furthermore, demonstrate a higher propensity to be taken up by DCs than uninfected cells [76].
The trafficking of metacyclic L. donovani promastigotes inside PMNs was shown to occur via traditional phagolysosomes, as well as alternative lysosome-independent compartments. The latter PVs stained positive for glucose-6-phosphate and calnexin, components of the host endoplasmic reticulum, and encircled tightly around the parasites. Virulent promastigotes followed this route and were protected from degradation, whereas LPG-deficient avirulent promastigotes were more likely to encounter a lytic environment [77]. The use of short-lived granulocytes as a vector presents a novel means by which Leishmania ensures quiescent entry into their final host cell. Receptors for triggering ‘safe’ uptake into PMNs have yet to be identified.
The first complement receptor
L. major has long been the only species in which a prominent role for binding to CR1 has been documented. However, L. amazonensis stationary phase promastigotes were recently characterized to bind to MDMs in part via CR1 [43]. Attachment of serum-opsonized metacyclic L. major promastigotes to MDMs was reduced by 65% in the presence of 1B4, a blocking mAb specific for CR1, but not by two antibodies to CR3 [5]. Similar to CR3 studies, blocking CR1 never eliminated parasite attachment, underscoring the presence of a co-receptor. Cooperation between CR1 and CR3 was clarified in a study using mAbs to block attachment to CHO cells expressing CR1, CR3 or both. The authors showed that, in the absence of factor I, serum-opsonized metacyclic L. major promastigotes bind CR1 but not CR3. By contrast, the addition of factor I allows parasites to preferentially attach via CR3. CR1 is a cofactor allowing the factor I serine protease to generate iC3b from C3b [44]. These studies delineate a cooperative model in which CR1 is required for cofactor I to generate iC3b from C3b, leading to high affinity binding with CR3. Signaling events initiated by CR1 or CR3 ligation do not trigger a respiratory burst [5]. Thus, L. major appears to be ultimately guided into the cell via a CR3-mediated pathway, evading the generation of oxidant radicals as described above.
Mannose receptor
There is evidence that Leishmania spp. ligateMR on MPMs and on MDMs. The soluble MR ligand mannan inhibits binding of L. donovani promastigotes by 60% or 70% in different respective reports [4, 45]. Soluble mannan and blocking anti-CR3 mAb synergistically inhibit promastigote binding to MDMs but not MPMs, suggesting receptor cooperation. Both attachment and phagocytosis by MDMs is inhibited with mAb to CR3 and mannosylated BSA (bovine serum albumin), a more defined MR inhibitor [34]. Amastigote binding to MPMs was affected to a lesser degree than promastigotes (30%) [4], but the role of MR in amastigote binding to MDMs was not examined. These data suggest that both MR and CR3 coordinate attachment of L. donovani promastigotes to murine and human macrophages, although the importance of the receptors may differ between the two cell types (Box 3).
Box 3. Mannose receptor.
MR is a well-studied lectin which serves as a phagocytic receptor in primary macrophages and myeloid DCs but not in monocytes. It is a C-type lectin that has eight tandem carbohydrate recognition domains (CRDs), of which CRD4 and CRD5 demonstrate sugar binding ability [78]. MR is a microbial pattern recognition receptor capable of MR binding the terminal sugar residue of microbial surface oligosaccharides with the affinity: L-fucose > D-mannose > D-N-acetyl-glucosamine ⋙ D-galactose [79]. Because of its preferential detection of branched oligo-mannoses and the fact that Leishmania spp. display abundant high-mannose type N-linked oligosaccharides but not complex N-linked oligosaccharides [34], MR is a logical candidate receptor for Leishmania promastigotes.
Several reports suggest that MR ligation leads to proinflammatory gene expression in macrophages: (i) control MPMs generate O2− generation after phagocytosis of serum-opsonized zymosan; however, MPMs depleted of MR utilize CR3 for opsonized zymosan phagocytosis, eliminating O2− generation [80]. (ii) Ligation of MR on rabbit alveolar macrophages with the synthetic neoglycoprotein mannosylated-BSA leads to the release of lysosomal enzymes [81]. (iii) Exposure of MR to mannan isolated from Candida albicans increases TNF-α production by murine alveolar macrophages [82]. Considering these pro-microbicidal properties of the MR, it could be hypothesized that highly infectious metacyclic population of Leishmania promastigotes evolved a sophisticated form of LPG-masking MR ligands in part to avoid ligation of MR. Conflicting with the above evidence, however, MR ligation has also been associated with non-inflammatory phagocyte polarization. Expression of MR is up-regulated during M2a (alternative) macrophage activation [83]. Phagocytosis of zymosan by MDMs, a particle that ligates the MR, does not stimulate the release of O2− or phagosome-lysosome fusion, possibly due to a failure to recruit the tyrosine kinase, hemopoietic cell kinase (Hck) [84]. In human MDDCs, mannose-capped lipoarabinomannans purified from Mycobacterium bovis or Mycobacterium tuberculosis inhibit LPS-induced IL-12 generation [85]. The dual-edged nature of MR signaling could be influenced by not only ligand structure, which determines access to a particular CRD, but also cooperation with another receptor.
Given the above findings, studies of leishmaniasis in MR-knockout (KO) mice led to surprising results. Introduction of metacyclic L. major promastigotes into either wild type or MR-KO C56BL/6 mice resulted in lesions that did not differ in the rates of formation or resolution, with nearly identical TNF-α and IL-12 production from splenocytes. Together with the observation that mannan had no effect on the internalization of L. donovani or L. major by murine BMMs from wild type or control mice, these results challenged the necessity of MR for successfully establishing murine leishmaniasis [9].
A potential explanation for this contradiction is that early experiments were performed with either logarithmic or unpurified stationary phase promastigote cultures, whereas recent studies utilized purified metacyclics. One likely L. i. chagasi ligand for MR is GP63, a high mannose glycoconjugate [46]. In the case of L. major, GP63 is masked by the metacyclic form of LPG, leaving other surface molecules inaccessible [47]. Thus, GP63 might only interact with MR in non-metacyclic L. major promastigotes. Indeed, confocal studies of CR3 and MR showed that avirulent logarithmic L. i. chagasi promastigotes were three times more likely to engage MR on MDMs than metacyclics during the first 60 minutes after phagocytosis, indicating MR is important only for the phagocytosis of non-metacyclic promastigotes [10]; similar observations were reported for MPMs, i.e. internalization of L. donovani promastigotes that were attenuated by repeated passage in liquid culture was inhibited by mannosylated BSA twice as efficiently as virulent promastigotes that were freshly isolated from BALB/c mice. Down- or up-regulation of MR expression on MPMs by H2O2 or dexamethasone, respectively, affected the uptake of attenuated promastigotes more than that of virulent L. donovani [48].
The entry pathway of virulent metacyclic L. i. chagasi promastigotes into MDMs is characterized by exclusion of MR from the phagocytic cup, followed by a delay in phagolysosome maturation (Box 2). Indeed, lysosome-associated membrane protein-1 (LAMP-1) and cathepsin D, markers for macrophage late endosomes and lysosomes, respectively, are recruited to PVs containing logarithmic, but not metacyclic, L. i. chagasi promastigotes two hr following phagocytosis [10]. It is not known how MR modifies the signaling pathways that are activated following logarithmic promastigote phagocytosis (Box 3).
Receptors for fibronectin
Opsonization with fibronectin enhances attachment of Leishmania spp. to host phagocytes (Box 4) [8, 49, 50]. Fibronectin binding to the L. amazonensis promastigote surface is saturable, and either cultivation in fibronectin-depleted serum or incubation in anti-fibronectin antibodies decreases promastigote or amastigote attachment to human monocytes [8]. Treatment of MDMs with mAb to the integrin β1, a subunit of the predominant macrophage FnR, impairs their ability to internalize serum-opsonized L. amazonensis promastigotes [49]. Ironically, the downstream effect of FnR ligation leads to diminished, rather than enhanced intracellular survival compared to parasites that do not ligate FnR [50]. In PMNs, fibronectin-mediated phagocytosis of Staphylococcusaureus triggers O2- dependent and independent killing [51]. Parenthetically, a mAb raised against fibronectin could be used to immunoprecipitate a protein of corresponding molecular weight to GP63 from L. i. chagasi lysate [52]. Whether this means that GP63 can directly ligate the FnR is not established. FnR more likely detects promastigotes using fibronectin as a bridge to parasite antigens. mAbs that recognize the RGDS epitope on fibronectin can be used to immunoprecipitate GP63 from L.i. chagasi promastigotes [52]. This fibronectin-mimicking sequence was later revealed to be a tetrapeptide, SRYD, in L. major GP63, and pre-treatment of MPMs with a synthetic octapeptide containing SRYD was shown to inhibit L. major attachment [53]. These findings are strengthened by evidence that L. major or L. donovani promastigotes, and L. amazonensis promastigotes and amastigotes degrade fibronectin in a GP63-dependent manner [54].
Box 4. Fibronectin receptor.
Fibronectin is abundant in connective tissue and is required for phagocytes to leave the vasculature and migrate through the endothelium and subendothelium in response to an inflammatory stimulus [86]. Receptors for fibronectin (FnRs) are integrins that are expressed in fibroblasts, monocytes and PMNs, with designations CD49d/CD29 (α4/β1), CD49e/CD29 (α5/β1) and CD41/CD61 (α2b/β3). The major receptor for fibronectin on human monocytes is integrin α5/β1 (CD49e/CD29) [86]. α2b/β3 is a second type of FnR expressed on monocytes, which is similar to FnRs found on platelets. Integrins α4/β1and α 5/β1 are structurally similar, and interact with the Arg-Gly-Asp-Ser (RGDS) or the Glu-Ile-Leu-Asp-Val (EILDV) regions of fibronectin, respectively [87]. Integrin α2b/β3 recognizes the RGDS motif expressed by fibrinogen, collagen and vitronectin. PMNs exclusively express integrin α2b/β3 but not other FnRs [88].
Despite the potential adverse effects of ligating FnRs alone, there is evidence that FnR cooperates with complement receptors CR1 and CR3 to facilitate internalization of iC3b or C3b coated particles [55, 56]. Cooperation of FnRs with CR3 facilitates the phagocytosis of L. amazonensis promastigotes. A study of the GP63-null versus GP63-overexpressing parasites provided evidence that promastigote GP63 ligates both receptors on macrophages, or on CHO cells transfected with the α4 integrin subunit, using blocking antibodies to each receptor [49]. A predicted model for co-ligation of FnRs and either CR3 or CR1 implicates initial promastigote binding to the complement receptor, with actin remodeling and phagocytosis stimulated by ligation of FnRs. The FnRs or CR3 would either bind directly to Leishmania GP63 or through opsonized fibronectin or iC3b, respectively.
Receptor-mediated phagocytosis of amastigotes
Whereas promastigotes encounter host macrophages only at the onset of host infection, amastigotes must continually exit highly infected macrophages and enter uninfected macrophages throughout a progressive infection. Given their lack of surface LPG and paucity of surface-exposed GP63 [57–59], it is not surprising that amastigotes employ different strategies for macrophage entry than promastigotes. L. major amastigotes were found to be coated with IgG1 immediately after isolation from the footpads of BALB/c mice, raising the possibility that FcγRs could constitute an entry portal into macrophages [6]. Several groups have confirmed that L. major, L. mexicana, or L. amazonensis amastigotes opsonized with specific antibodies will adhere primarily to FcγRs on the surface of macrophages and monocyte-derived DCs (MDDCs) [6, 11, 60]. Consistently, the African green monkey kidney COS cell line transfected with FcγRII-B2 bound dramatically larger numbers of L. mexicana lesion-derived amastigotes than MR-transfected COS cells after 30 minutes of incubation [11]. Whether FcγR binding is an artifact occurring only in recently isolated amastigotes or whether it participates in the propagation of natural infection was addressed in vivo in KO mice. Compared to wild type BALB/c mice, either JHD mice lacking circulating antibody, or FcγR−/− mice lacking the common γ chain of FcγRI, FcγRIII and FcεRI formed smaller cutaneous lesions than wild type control mice following injection with L. amazonensis or Leishmania pifanoi [61]. This suggests that parasite-specific IgG produced during leishmaniasis facilitates amastigote opsonization and FcγR ligation. Ligation of FcγR on BMMs results in IL-10 expression, and this has been shown to facilitate parasite survival and replication [62].
Similar to promastigotes, amastigotes can undergo phagocytosis via several avenues. Binding to FnRs or the heparin binding protein was observed to facilitate phagocytosis of L. amazonensis [8, 60]. Several groups have also reported that blocking antibodies to CR3 decreased uptake of Leishmania spp. amastigotes [6, 32]. By contrast, the MR ligand mannan did not affect amastigote entry [4, 6, 11, 60]. Confirming the importance of CR3, COS cells transfected with CR3 expedited the attachment of serum-opsonized L. mexicana amastigotes but had no effect on the binding kinetics of non-opsonized amastigotes [11]. A newly established line of axenic L. amazonensis amastigotes that was shown to cause footpad swelling in mice comparable to injections with metacyclic promastigotes also entered MDMs via CR3, inducing little pro-inflammatory cytokines TNFα and CCL3/4 [43]. It is unknown whether amastigotes are opsonized with C3, and neither is a putative C3 acceptor molecule(s) on amastigotes. The amastigote surface composition displays fewer proteins than promastigotes and lacks the thick LPG layer (visualized by transmission electron micrography in L. major) [12]. Amastigotes instead display free glycosylphosphatidylinositol lipids that lack the disaccharide repeats of LPG [63] and exhibit diminished expression of surface GP63 [57, 62]. The implications of the various possible amastigote binding and entry routes into macrophages are not yet defined.
Axenic L. pifanoi amastigotes devoid of serum-opsonins were found to directly bind to DC-specific intercellular adhesion molecule-3-grabbing non-integrin (DC-SIGN; CD209), an ICAM2/3-binding attachment protein [64]. DC-SIGN is a C-type lectin on macrophages and DCs which recognizes mannosylated glycoconjugates. The detection of parasites seems to be independent of LPG [65]. Competitive inhibition with the anti-DC-SIGN mAb MR-1, but not LPG, decreased the attachment of L. pifanoi and L. infantum amastigotes to MDDCs. Furthermore, LPG-deficient L. donovani stationary phase promastigotes bound to CD209- transfected K562 cells with higher avidity than wild type parasites [65]. This supports the hypothesis that DC-SIGN ligates a common molecule on the surface of both promastigotes and amastigotes, which becomes masked by the complex LPG structure, as seen with GP63 on promastigotes [47].
Because mAb to DC-SIGN completely ablated amastigote binding to CD209-expressing K562 cells but partially inhibited binding to MDDCs, it is likely that DC-SIGN is only one of several naturally expressed surface receptors initiating amastigote interaction with DCs [64]. The specific surface ligand on amastigotes that binds to DC-SIGN has not been identified. L. major promastigotes could not bind to DC-SIGN, whereas L. pifanoi, L. infantum, and L. donovani could [65]. This may reflect either a lack of expression of the DC-SIGN ligand, or masking of the ligand by LPG. DC-SIGN-mediated uptake of L.infantum amastigotes into immature MDDCs does not stimulate the cell surface expression of CD83, CD86, and major histocompatibility complex Class II. The absence of such markers for DC maturation during Leishmania infection may be the result of a strategy by the parasite for avoiding immunosurveillance [66].
Concluding remarks
The literature documents multiple macrophage receptors that interact with Leishmania (Figure 1). Although reports have claimed these routes are redundant, we have emphasized in this review the fact that ligation of specific receptors elicits different downstream functions in the macrophage, and that each interaction occurs as a consequence of factors expressed by the parasite itself. Leishmania virulence factors are tightly regulated during the life cycle, often granting the ability to select quiescent routes for invasion. Remarkably, the parasite exploits host serum opsonins that facilitate phagocytosis of infective stages of Leishmania spp For example,. metacyclic binding to CR3 due to the protease action of GP63 results in opsonization with ligands for CR3 and/or CR1. In some cases this interaction is further strengthened by interactions with MR and FnRs, either directly through mannose-terminal surface glycoconjugates or indirectly through bound fibrinogen. Contrastingly, amastigotes become opsonized by IgG and enter via FcγRs. Amastigotes also bind to CR3 on macrophages or DC-SIGN on DCs. Virulent parasites employ selected anti-inflammatory pathways that are advantageous for subsequent survival, leading to phagocytosis through a path that avoids the respiratory burst and delays phagolysosomal maturation. Thus, Leishmania can be viewed as carefully selecting the optimal phagocyte receptors to promote its survival in the host. Further understanding of these receptors could shed light on therapeutic strategies that could guide the mechanism of phagocytosis toward a microbicidal pathway, as a novel approach to treating the manifestations of disease.
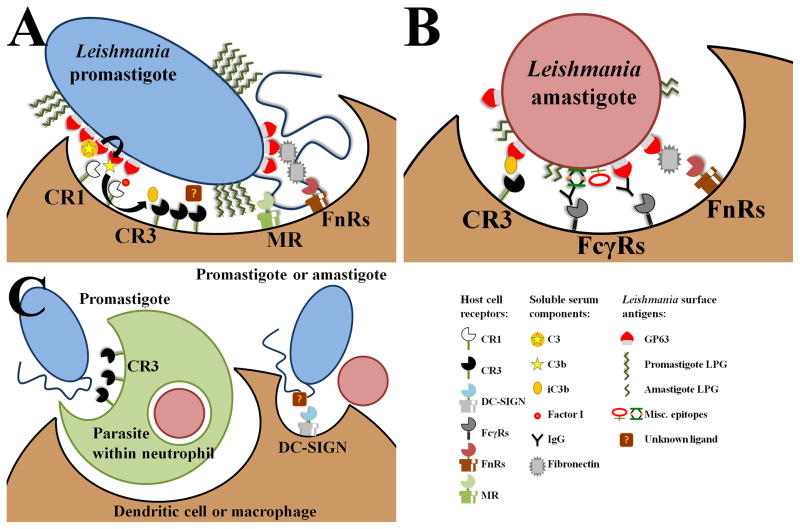
Figure 1. Receptors for Leishmania entry into host phagocytes.
Leishmania employ several receptor-mediated entry pathways into host macrophages, PMNs, or DCs. Promastigotes are depicted in blue, amastigotes in red, macrophages or DCs in brown, and PMNs in green. (a) GP63, which is highly expressed in promastigotes, convert C3 opsonins into C3b, the natural ligand for CR1. CR1, with factor I, cleave C3b into iC3b, facilitating binding to CR3. CR3 may also mediate direct binding to promastigotes via a yet unknown surface epitope on promastigotes. The terminal sugar residues on LPG could be recognized by MR, though this has not been proven. MR is not present in phagocytic compartments that have uptaken virulent metacyclic promastigotes. GP63 also binds fibronectin, which then bridges the parasite to FnRs. (b) LPG expression on amastigotes is less robust than on promastigotes, allowing the scarcely expressed GP63 to access C3 protein, and subsequently CR3. Antibody and fibronectin detection of amastigotes leads to ligation of FcγRs and FnRs, respectively. (c) Immediately following inoculation by the sand fly, promastigotes parasitize predominantly PMNs. Promastigotes enter PMNs via CR3, and then enter macrophages or DCs while contained in the short-lived granulocyte. Promastigotes and amastigotes may also directly enter DCs via DC-SIGN, but the ligand on the parasite surface has yet to be identified.
Acknowledgments
This work was supported in part by National Institutes of Health (NIH) grants (R01 AI045540, R01 AI067874, R01 AI07623) and a Merit Review and Operation Enduring Freedom/Operation Iraqi Freedom grants from the Department of Veterans’ Affairs.
Glossary
- Amastigote
the aflagellated form of Leishmania spp. parasites that resides intracellularly within vertebrate host cells
- COS cells
an African Green Monkey kidney cell line, with the name derived from ‘being CV-1 in Origin’, carrying portions of the SV40 genome
- DC-SIGN (Dendritic cell-specific intercellular adhesion molecule-3-grabbing non-integrin)
a receptor for mannosylated glycoconjugates that is found on macrophages and mature dendritic cells. DC-SIGN has been implicated in pathogen recognition as well as leukocyte adhesion via attachment to intercellular adhesion molecules (ICAM)-2 and -3
- Fc gamma receptors
receptors on the surface of phagocytes, including macrophages and neutrophils, that bind to the Fc fragment of immunoglobulin G and stimulate the phagocytosis of particles attached to the antibodies
- Fibronectin receptors
integrins on the surface of a variety of cell types, such as phagocytes and fibroblasts, which bind to fibronectin, an abundant glycoprotein in the extracellular matrix that mediates tissue connectivity
- CR1 (First complement receptor)
a receptor on the surface of phagocytes, including macrophages and neutrophils, that bind predominantly to C3b and C4b proteins of the complement cascade, facilitating clearance of particles opsonized by these proteins via phagocytosis
- GP63 (Glycoprotein 63)
the major metalloprotease that is abundantly expressed on the surface of virulent Leishmania promastigotes. When promastigotes are opsonized by C3b, GP63 cleaves this to iC3b, which facilitates promastigote recognition by CR3. GP63 is also called leishmanolysin, PSP (promastigote surface protease) or MSP (major surface protease)
- Ligate/ligation
specific binding of receptors on the host cell with their appropriate antigen on the surface of pathogens
- Lipophosphoglycan (LPG)
a Leishmania surface antigen that densely coats the surface of promastigotes. LPG is attached to the parasite surface by a glycoinositide anchor. A phosphodiester bond links to the anchor an extended polysaccharide backbone, ‘capped’ by terminal sugar residues that vary between different Leishmania spp
- Mannose receptor (MR)
a C-type lectin carbohydrate binding protein that serves as a broad range pattern recognition receptor via detection of mannose on the surface of pathogens. MR is predominantly expressed in macrophages but are also found on neutrophils and dendritic cells
- Metacyclic promastigotes
the virulent form of Leishmania promastigotes that arise following metacyclogenesis, a developmental process that occurs as the parasites move progressively from the midgut to the foregut and proboscis of the sand fly insect vector
- Opsonin
proteins or protein complexes that bind to antigens on the surface of foreign particles. Opsonins form part of the host’s innate immune response. Receptors on immune cells then bind to opsonins, resulting in indirect detection of pathogens
- Phagolysosome
a pathogen-containing compartment that develops within phagosomes following a series of fusion events with endosomal and lysosomal organelles. A fully mature phagolysosome is a hostile environment for intracellular organisms that are unfit to evade the parasitophorous vacuole
- Promastigote
the flagellated form of Leishmania that exists extracellularly in the sand fly insect vector
- Pseudopod
protrusions of the macrophage membrane that is the result of extensions of actin filaments near the edges of the cell
- Third complement receptor (CR3)
an integrin on the surface of phagocytes, including macrophages and neutrophils that primarily detect iC3b during pathogen recognition. The role of CR3 is varied, and encompasses particle clearance, leukocyte adherence, and actin reassembly
- U937 cells
an immortalized monocyte-like cell line of human origin. U937 cells can be stimulated to differentiate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Pearson RD, Sousa AQ. Clinical spectrum of Leishmaniasis. Clin Infect Dis. 1996;22:1–13. doi: 10.1093/clinids/22.1.1. [DOI] [PubMed] [Google Scholar]
- 2.Hsiao CH, et al. The effects of macrophage source on the mechanism of phagocytosis and intracellular survival of Leishmania. Microbes and infection/Institut Pasteur. 2011;13:1033–1044. doi: 10.1016/j.micinf.2011.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Peters NC, et al. In vivo imaging reveals an essential role for neutrophils in leishmaniasis transmitted by sand flies. Science (New York, N Y. 2008;321:970–974. doi: 10.1126/science.1159194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blackwell JM, et al. Macrophage complement and lectin-like receptors bind Leishmania in the absence of serum. The Journal of experimental medicine. 1985;162:324–331. doi: 10.1084/jem.162.1.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Da Silva RP, et al. CR1, the C3b receptor, mediates binding of infective Leishmania major metacyclic promastigotes to human macrophages. J Immunol. 1989;143:617–622. [PubMed] [Google Scholar]
- 6.Guy RA, Belosevic M. Comparison of receptors required for entry of Leishmania major amastigotes into macrophages. Infection and immunity. 1993;61:1553–1558. doi: 10.1128/iai.61.4.1553-1558.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mosser DM, Edelson PJ. The mouse macrophage receptor for C3bi (CR3) is a major mechanism in the phagocytosis of Leishmania promastigotes. J Immunol. 1985;135:2785–2789. [PubMed] [Google Scholar]
- 8.Wyler DJ, et al. In vitro parasite-monocyte interactions in human leishmaniasis: possible role of fibronectin in parasite attachment. Infection and immunity. 1985;49:305–311. doi: 10.1128/iai.49.2.305-311.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akilov OE, et al. The role of mannose receptor during experimental leishmaniasis. Journal of leukocyte biology. 2007;81:1188–1196. doi: 10.1189/jlb.0706439. [DOI] [PubMed] [Google Scholar]
- 10.Ueno N, et al. Differences in human macrophage receptor usage, lysosomal fusion kinetics and survival between logarithmic and metacyclic Leishmania infantum chagasi promastigotes. Cellular microbiology. 2009;11:1827–1841. doi: 10.1111/j.1462-5822.2009.01374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters C, et al. The role of macrophage receptors in adhesion and uptake of Leishmania mexicana amastigotes. Journal of cell science. 1995;108 ( Pt 12):3715–3724. doi: 10.1242/jcs.108.12.3715. [DOI] [PubMed] [Google Scholar]
- 12.Pimenta PF, et al. The comparative fine structure and surface glycoconjugate expression of three life stages of Leishmania major. Experimental parasitology. 1991;72:191–204. doi: 10.1016/0014-4894(91)90137-l. [DOI] [PubMed] [Google Scholar]
- 13.Ramamoorthy R, et al. Three distinct RNAs for the surface protease gp63 are differentially expressed during development of Leishmania donovani chagasi promastigotes to an infectious form. The Journal of biological chemistry. 1992;267:1888–1895. [PubMed] [Google Scholar]
- 14.Maia C, et al. Infectivity of five different types of macrophages by Leishmania infantum. Acta tropica. 2007;103:150–155. doi: 10.1016/j.actatropica.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Bates PA. Transmission of Leishmania metacyclic promastigotes by phlebotomine sand flies. International journal for parasitology. 2007;37:1097–1106. doi: 10.1016/j.ijpara.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacks DL, Perkins PV. Identification of an infective stage of Leishmania promastigotes. Science (New York, N Y. 1984;223:1417–1419. doi: 10.1126/science.6701528. [DOI] [PubMed] [Google Scholar]
- 17.Spath GF, Beverley SM. A lipophosphoglycan-independent method for isolation of infective Leishmania metacyclic promastigotes by density gradient centrifugation. Experimental parasitology. 2001;99:97–103. doi: 10.1006/expr.2001.4656. [DOI] [PubMed] [Google Scholar]
- 18.da Silva R, Sacks DL. Metacyclogenesis is a major determinant of Leishmania promastigote virulence and attenuation. Infection and immunity. 1987;55:2802–2806. doi: 10.1128/iai.55.11.2802-2806.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao C, et al. Leishmania chagasi: homogenous metacyclic promastigotes isolated by buoyant density are highly virulent in a mouse model. Experimental parasitology. 2008;118:129–133. doi: 10.1016/j.exppara.2007.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosser DM, Edelson PJ. The third component of complement (C3) is responsible for the intracellular survival of Leishmania major. Nature. 1987;327:329–331. doi: 10.1038/327329b0. [DOI] [PubMed] [Google Scholar]
- 21.Puentes SM, et al. Complement binding by two developmental stages of Leishmania major promastigotes varying in expression of a surface lipophosphoglycan. The Journal of experimental medicine. 1988;167:887–902. doi: 10.1084/jem.167.3.887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russell DG. The macrophage-attachment glycoprotein gp63 is the predominant C3-acceptor site on Leishmania mexicana promastigotes. European journal of biochemistry/FEBS. 1987;164:213–221. doi: 10.1111/j.1432-1033.1987.tb11013.x. [DOI] [PubMed] [Google Scholar]
- 23.Kweider M, et al. Development of metacyclic Leishmania promastigotes is associated with the increasing expression of GP65, the major surface antigen. Parasite immunology. 1989;11:197–209. doi: 10.1111/j.1365-3024.1989.tb00659.x. [DOI] [PubMed] [Google Scholar]
- 24.Brittingham A, et al. Role of the Leishmania surface protease gp63 in complement fixation, cell adhesion, and resistance to complement-mediated lysis. J Immunol. 1995;155:3102–3111. [PubMed] [Google Scholar]
- 25.McConville MJ, et al. Developmental modification of lipophosphoglycan during the differentiation of Leishmania major promastigotes to an infectious stage. The EMBO journal. 1992;11:3593–3600. doi: 10.1002/j.1460-2075.1992.tb05443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pimenta PF, et al. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:9155–9159. doi: 10.1073/pnas.91.19.9155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Green PJ, et al. Recognition of the major cell surface glycoconjugates of Leishmania parasites by the human serum mannan-binding protein. Molecular and biochemical parasitology. 1994;66:319–328. doi: 10.1016/0166-6851(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 28.Rodriguez NE, et al. Role of caveolae in Leishmania chagasi phagocytosis and intracellular survival in macrophages. Cellular microbiology. 2006;8:1106–1120. doi: 10.1111/j.1462-5822.2006.00695.x. [DOI] [PubMed] [Google Scholar]
- 29.Courret N, et al. Biogenesis of Leishmania-harbouring parasitophorous vacuoles following phagocytosis of the metacyclic promastigote or amastigote stages of the parasites. Journal of cell science. 2002;115:2303–2316. doi: 10.1242/jcs.115.11.2303. [DOI] [PubMed] [Google Scholar]
- 30.Forestier CL, et al. Imaging host cell-leishmania interaction dynamics implicates parasite motility, lysosome recruitment, and host cell wounding in the infection process. Cell Host Microbe. 2011;9:319–330. doi: 10.1016/j.chom.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Cooper A, et al. Monoclonal antibodies that recognize distinct epitopes of the macrophage type three complement receptor differ in their ability to inhibit binding of Leishmania promastigotes harvested at different phases of their growth cycle. Immunology. 1988;65:511–514. [PMC free article] [PubMed] [Google Scholar]
- 32.Wozencraft AO, et al. Macrophage type 3 complement receptors mediate serum-independent binding of Leishmania donovani. Detection of macrophage-derived complement on the parasite surface by immunoelectron microscopy. The Journal of experimental medicine. 1986;164:1332–1337. doi: 10.1084/jem.164.4.1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blackwell JM. Role of macrophage complement and lectin-like receptors in binding Leishmania parasites to host macrophages. Immunology letters. 1985;11:227–232. doi: 10.1016/0165-2478(85)90172-5. [DOI] [PubMed] [Google Scholar]
- 34.Wilson ME, Pearson RD. Roles of CR3 and mannose receptors in the attachment and ingestion of Leishmania donovani by human mononuclear phagocytes. Infection and immunity. 1988;56:363–369. doi: 10.1128/iai.56.2.363-369.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kedzierski L, et al. A leucine-rich repeat motif of Leishmania parasite surface antigen 2 binds to macrophages through the complement receptor 3. J Immunol. 2004;172:4902–4906. doi: 10.4049/jimmunol.172.8.4902. [DOI] [PubMed] [Google Scholar]
- 36.Wozencraft AO, Blackwell JM. Increased infectivity of stationary-phase promastigotes of Leishmania donovani: correlation with enhanced C3 binding capacity and CR3-mediated attachment to host macrophages. Immunology. 1987;60:559–563. [PMC free article] [PubMed] [Google Scholar]
- 37.Mosser DM, et al. Leishmania promastigotes require opsonic complement to bind to the human leukocyte integrin Mac-1 (CD11b/CD18) The Journal of cell biology. 1992;116:511–520. doi: 10.1083/jcb.116.2.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aderem A, Underhill DM. Mechanisms of phagocytosis in macrophages. Annual review of immunology. 1999;17:593–623. doi: 10.1146/annurev.immunol.17.1.593. [DOI] [PubMed] [Google Scholar]
- 39.Gantt KR, et al. Oxidative responses of human and murine macrophages during phagocytosis of Leishmania chagasi. J Immunol. 2001;167:893–901. doi: 10.4049/jimmunol.167.2.893. [DOI] [PubMed] [Google Scholar]
- 40.Peters NC, Sacks DL. The impact of vector-mediated neutrophil recruitment on cutaneous leishmaniasis. Cellular microbiology. 2009;11:1290–1296. doi: 10.1111/j.1462-5822.2009.01348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ritter U, et al. Are neutrophils important host cells for Leishmania parasites? Trends Parasitol. 2009;25:505–510. doi: 10.1016/j.pt.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 42.Laufs H, et al. Intracellular survival of Leishmania major in neutrophil granulocytes after uptake in the absence of heat-labile serum factors. Infection and immunity. 2002;70:826–835. doi: 10.1128/iai.70.2.826-835.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wenzel UA, et al. Leishmania major parasite stage-dependent host cell invasion and immune evasion. Faseb J. 2012;26:29–39. doi: 10.1096/fj.11-184895. [DOI] [PubMed] [Google Scholar]
- 44.Rosenthal LA, et al. Leishmania major-human macrophage interactions: cooperation between Mac-1 (CD11b/CD18) and complement receptor type 1 (CD35) in promastigote adhesion. Infection and immunity. 1996;64:2206–2215. doi: 10.1128/iai.64.6.2206-2215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wilson ME, Pearson RD. Evidence that Leishmania donovani utilizes a mannose receptor on human mononuclear phagocytes to establish intracellular parasitism. J Immunol. 1986;136:4681–4688. [PubMed] [Google Scholar]
- 46.Wilson ME, Hardin KK. The major concanavalin A-binding surface glycoprotein of Leishmania donovani chagasi promastigotes is involved in attachment to human macrophages. Journal of immunology. 1988;141:265–272. [PubMed] [Google Scholar]
- 47.Karp CL, et al. Lipophosphoglycan masks recognition of the Leishmania donovani promastigote surface by human kala-azar serum. Journal of immunology. 1991;147:680–684. [PubMed] [Google Scholar]
- 48.Chakraborty P, et al. Modulation of macrophage mannose receptor affects the uptake of virulent and avirulent Leishmania donovani promastigotes. The Journal of parasitology. 2001;87:1023–1027. doi: 10.1645/0022-3395(2001)087[1023:MOMMRA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 49.Brittingham A, et al. Interaction of Leishmania gp63 with cellular receptors for fibronectin. Infection and immunity. 1999;67:4477–4484. doi: 10.1128/iai.67.9.4477-4484.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vannier-Santos MA, et al. Fibronectin shedding by Leishmania may influence the parasite-macrophage interaction. European journal of cell biology. 1992;59:389–397. [PubMed] [Google Scholar]
- 51.Hermann M, et al. Neutrophil bactericidal activity against Staphylococcus aureus adherent on biological surfaces. Surface-bound extracellular matrix proteins activate intracellular killing by oxygen-dependent and -independent mechanisms. The Journal of clinical investigation. 1990;86:942–951. doi: 10.1172/JCI114796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rizvi FS, et al. The major surface protein of Leishmania promastigotes is a fibronectin-like molecule. European journal of immunology. 1988;18:473–476. doi: 10.1002/eji.1830180323. [DOI] [PubMed] [Google Scholar]
- 53.Soteriadou KP, et al. The Ser-Arg-Tyr-Asp region of the major surface glycoprotein of Leishmania mimics the Arg-Gly-Asp-Ser cell attachment region of fibronectin. The Journal of biological chemistry. 1992;267:13980–13985. [PubMed] [Google Scholar]
- 54.Kulkarni MM, et al. Fibronectin binding and proteolytic degradation by Leishmania and effects on macrophage activation. Infection and immunity. 2008;76:1738–1747. doi: 10.1128/IAI.01274-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wright SD, et al. Fibronectin and serum amyloid P component stimulate C3b- and C3bi-mediated phagocytosis in cultured human monocytes. The Journal of experimental medicine. 1983;158:1338–1343. doi: 10.1084/jem.158.4.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wright SD, et al. Communication between receptors for different ligands on a single cell: ligation of fibronectin receptors induces a reversible alteration in the function of complement receptors on cultured human monocytes. The Journal of cell biology. 1984;99:336–339. doi: 10.1083/jcb.99.1.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hsiao CH, et al. The major surface protease (MSP or GP63) in the intracellular amastigote stage of Leishmania chagasi. Molecular and biochemical parasitology. 2008;157:148–159. doi: 10.1016/j.molbiopara.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.McConville MJ, Blackwell JM. Developmental changes in the glycosylated phosphatidylinositols of Leishmania donovani. Characterization of the promastigote and amastigote glycolipids. The Journal of biological chemistry. 1991;266:15170–15179. [PubMed] [Google Scholar]
- 59.Medina-Acosta E, et al. The promastigote surface protease (gp63) of Leishmaniais expressed but differentially processed and localized in the amastigote stage. Molecular and biochemical parasitology. 1989;37:263–273. doi: 10.1016/0166-6851(89)90158-8. [DOI] [PubMed] [Google Scholar]
- 60.Bosetto MC, Giorgio S. Leishmania amazonensis: multiple receptor-ligand interactions are involved in amastigote infection of human dendritic cells. Experimental parasitology. 2007;116:306–310. doi: 10.1016/j.exppara.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 61.Kima PE, et al. Internalization of Leishmania mexicana complex amastigotes via the Fc receptor is required to sustain infection in murine cutaneous leishmaniasis. The Journal of experimental medicine. 2000;191:1063–1068. doi: 10.1084/jem.191.6.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gallo P, et al. The influence of IgG density and macrophage Fc (gamma) receptor cross-linking on phagocytosis and IL-10 production. Immunology letters. 2010;133:70–77. doi: 10.1016/j.imlet.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turco SJ, Sacks DL. Expression of a stage-specific lipophosphoglycan in Leishmania major amastigotes. Molecular and biochemical parasitology. 1991;45:91–99. doi: 10.1016/0166-6851(91)90030-a. [DOI] [PubMed] [Google Scholar]
- 64.Colmenares M, et al. Dendritic cell (DC)-specific intercellular adhesion molecule 3 (ICAM-3)-grabbing nonintegrin (DC-SIGN, CD209), a C-type surface lectin in human DCs, is a receptor for Leishmania amastigotes. The Journal of biological chemistry. 2002;277:36766–36769. doi: 10.1074/jbc.M205270200. [DOI] [PubMed] [Google Scholar]
- 65.Colmenares M, et al. The dendritic cell receptor DC-SIGN discriminates among species and life cycle forms of Leishmania. J Immunol. 2004;172:1186–1190. doi: 10.4049/jimmunol.172.2.1186. [DOI] [PubMed] [Google Scholar]
- 66.Caparros E, et al. Role of the C-type lectins DC-SIGN and L-SIGN in Leishmania interaction with host phagocytes. Immunobiology. 2005;210:185–193. doi: 10.1016/j.imbio.2005.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ehlers MR. CR3: a general purpose adhesion-recognition receptor essential for innate immunity. Microbes and infection/Institut Pasteur. 2000;2:289–294. doi: 10.1016/s1286-4579(00)00299-9. [DOI] [PubMed] [Google Scholar]
- 68.Wright SD, Silverstein SC. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. The Journal of experimental medicine. 1983;158:2016–2023. doi: 10.1084/jem.158.6.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Marth T, Kelsall BL. Regulation of interleukin-12 by complement receptor 3 signaling. The Journal of experimental medicine. 1997;185:1987–1995. doi: 10.1084/jem.185.11.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Real F, et al. Leishmania (L.) amazonensis: fusion between parasitophorous vacuoles in infected bone-marrow derived mouse macrophages. Experimental parasitology. 2008;119:15–23. doi: 10.1016/j.exppara.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 71.Sutterwala FS, et al. Cooperation between CR1 (CD35) and CR3 (CD 11b/CD18) in the binding of complement-opsonized particles. Journal of leukocyte biology. 1996;59:883–890. doi: 10.1002/jlb.59.6.883. [DOI] [PubMed] [Google Scholar]
- 72.Desjardins M. Biogenesis of phagolysosomes: the ‘kiss and run’ hypothesis. Trends in cell biology. 1995;5:183–186. doi: 10.1016/s0962-8924(00)88989-8. [DOI] [PubMed] [Google Scholar]
- 73.Duclos S, Desjardins M. Subversion of a young phagosome: the survival strategies of intracellular pathogens. Cellular microbiology. 2000;2:365–377. doi: 10.1046/j.1462-5822.2000.00066.x. [DOI] [PubMed] [Google Scholar]
- 74.Streit JA, et al. Developmental changes in the expression of Leishmania chagasi gp63 and heat shock protein in a human macrophage cell line. Infection and immunity. 1996;64:1810–1818. doi: 10.1128/iai.64.5.1810-1818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.van Zandbergen G, et al. Leishmania disease development depends on the presence of apoptotic promastigotes in the virulent inoculum. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:13837–13842. doi: 10.1073/pnas.0600843103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ribeiro-Gomes FL, et al. Efficient capture of infected neutrophils by dendritic cells in the skin inhibits the early anti-leishmania response. PLoS Pathog. 2012;8:e1002536. doi: 10.1371/journal.ppat.1002536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gueirard P, et al. Trafficking of Leishmania donovani promastigotes in non-lytic compartments in neutrophils enables the subsequent transfer of parasites to macrophages. Cellular microbiology. 2008;10:100–111. doi: 10.1111/j.1462-5822.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 78.Linehan SA, et al. Macrophage lectins in host defence. Microbes and infection/Institut Pasteur. 2000;2:279–288. doi: 10.1016/s1286-4579(00)00300-2. [DOI] [PubMed] [Google Scholar]
- 79.Allavena P, et al. From pattern recognition receptor to regulator of homeostasis: the double-faced macrophage mannose receptor. Critical reviews in immunology. 2004;24:179–192. doi: 10.1615/critrevimmunol.v24.i3.20. [DOI] [PubMed] [Google Scholar]
- 80.Berton G, Gordon S. Modulation of macrophage mannosyl-specific receptors by cultivation on immobilized zymosan. Effects on superoxide-anion release and phagocytosis. Immunology. 1983;49:705–715. [PMC free article] [PubMed] [Google Scholar]
- 81.Ohsumi Y, Lee YC. Mannose-receptor ligands stimulate secretion of lysosomal enzymes from rabbit alveolar macrophages. The Journal of biological chemistry. 1987;262:7955–7962. [PubMed] [Google Scholar]
- 82.Garner RE, et al. Secretion of TNF-alpha by alveolar macrophages in response to Candida albicans mannan. Journal of leukocyte biology. 1994;55:161–168. doi: 10.1002/jlb.55.2.161. [DOI] [PubMed] [Google Scholar]
- 83.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Astarie-Dequeker C, et al. The mannose receptor mediates uptake of pathogenic and nonpathogenic mycobacteria and bypasses bactericidal responses in human macrophages. Infection and immunity. 1999;67:469–477. doi: 10.1128/iai.67.2.469-477.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Nigou J, et al. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J Immunol. 2001;166:7477–7485. doi: 10.4049/jimmunol.166.12.7477. [DOI] [PubMed] [Google Scholar]
- 86.Brown DL, et al. Synthesis and expression of the fibroblast fibronectin receptor in human monocytes. The Journal of clinical investigation. 1989;84:366–370. doi: 10.1172/JCI114166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Brittingham J, et al. Identification of distinct molecular phenotypes in cultured gastrointestinal smooth muscle cells. Gastroenterology. 1998;115:605–617. doi: 10.1016/s0016-5085(98)70140-4. [DOI] [PubMed] [Google Scholar]
- 88.Brown EJ, Goodwin JL. Fibronectin receptors of phagocytes. Characterization of the Arg-Gly-Asp binding proteins of human monocytes and polymorphonuclear leukocytes. The Journal of experimental medicine. 1988;167:777–793. doi: 10.1084/jem.167.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]