Abstract
The decreased internal knee extensor moment is a significant gait asymmetry among patients with anterior cruciate ligament (ACL) deficiency, yet the muscular strategy driving this altered moment for the injured limb is unclear.
Purpose
To determine whether patients with ACL deficiency and characteristic knee instability would demonstrate normal extensor and increased flexor muscle force to generate a decreased internal extensor moment (i.e. employ a hamstring facilitation strategy).
Methods
Gait analysis was performed on 31 athletes with acute ACL rupture who exhibited characteristic knee instability after injury. Peak internal knee extensor moment was calculated using inverse dynamics and muscle forces were estimated using an EMG-driven modeling approach. Comparisons were made between the injured and contralateral limbs.
Results
As expected, patients demonstrated decreased peak knee flexion (p=0.028) and internal knee extensor moment (p=0.0004) for their injured limb, but exhibited neither an isolated decrease in extensor force (quadriceps avoidance), nor an isolated increase in flexor force (hamstring facilitation) at peak knee moment. Instead, they exhibited decreased muscle force from both flexor (p=0.0001) and extensor (p=0.0103) groups. This strategy of decreased muscle force may be explained in part by muscle weakness which frequently accompanies ACL injury, or by apprehension, low confidence and fear of further injury.
Conclusions
This is the first study to estimate muscle forces in the ACL-deficient knee using an EMG-driven approach. These results affirm the existence of neuromuscular asymmetries in the individuals with ACL deficiency and characteristic knee instability.
Keywords: Knee Moment, Muscle Force, Quadriceps Avoidance, Hamstring Facilitation, Instability
INTRODUCTION
Rupture of the anterior cruciate ligament (ACL) is a frequent, traumatic knee injury (27) that often results in knee instability with daily activity. Most patients with ACL deficiency lack dynamic knee stability (21) and experience episodes of instability with daily activities. After injury, these patients adopt an asymmetrical gait pattern which includes less knee flexion and a decreased internal knee extensor moment for the injured limb (22, 30, 31). The decreased knee moment is an important gait asymmetry among patients with ACL deficiency (18); however the muscular strategy driving it is unclear. Numerous studies report the persistence of aberrant gait patterns following reconstructive surgery (5, 8, 13, 15, 17, 19); which casts importance on understanding their causative muscle actions. The key to restoring normal movement lies with re-training and restoring strength in the muscles affected by injury. A better understanding of the muscular strategy which these patients adopt after injury may promote more successful rehabilitation programs to resolve their gait asymmetries.
Several muscular strategies which could produce the decreased knee extensor moment have been proposed. One such strategy is a “quadriceps avoidance” strategy (2). The quadriceps muscles are responsible for generating the knee extensor moment early in stance, and an isolated reduction in quadriceps force would produce a smaller knee extensor moment. Alternatively, a “hamstring facilitation” strategy (1) may also accomplish the smaller peak knee moment seen in ACL-deficient gait. The hamstrings oppose the knee extensors and an isolated increase in hamstring muscle force would also produce a smaller knee extensor moment.
The net joint moment has limited usefulness in determining which muscular stabilization strategy patients with ACL-deficiency employ because muscle activity patterns are altered after injury (1, 3, 22, 30). Muscle force contributions from the flexors and extensors cannot be resolved using inverse dynamics, especially in cases where co-activation of opposing muscles occurs. Muscle co-activation has been observed in ACL-deficient knees using electromyography (EMG) (1, 3, 22, 30). While EMG is a useful measure of neural command to muscle, it does not directly represent the timing and magnitude of muscle force. Muscle force contributions from antagonistic groups cannot be evaluated with either the net joint moment or from EMG alone.
Estimation of muscle forces in a population which exhibits neuromuscular compensation for injury requires a computational model which takes into account individual muscle activation patterns. Use of an EMG-driven musculoskeletal model (4) provides estimates of muscle force from EMG. Subsequently, the net joint moment can be partitioned into flexor and extensor components and the relative contributions to the net joint moment can be examined. This modeling approach has been used to estimate muscle forces during dynamic movements for healthy individuals (26, 38), patients with osteoarthritis (23) and individuals who have sustained a stroke (32). Muscle forces have not been estimated in individuals with ACL deficiency using this approach, and estimates of muscle forces will enhance our understanding of the neuromuscular strategy adopted by individuals with ACL-deficiency and characteristic knee instability.
The purpose of this study was to 1) to confirm that our group of acutely-injured patients with ACL-deficiency exhibited decreased knee flexion and peak knee moments during gait as has been previously described, and 2) to partition flexor and extensor muscle forces for each limb in order to determine the cause of the asymmetrical knee moment. Noncopers (those who lack dynamic knee stability (14)) were chosen because this group constitutes the majority of individuals with ACL injury. We hypothesized that these patients would demonstrate less knee flexion and a decreased internal knee extensor moment for their injured limb, as has been described in patients following ACL injury. We further hypothesized that patients would demonstrate similar extensor force and increased flexor force for their injured limbs (i.e. hamstrings facilitation strategy) to generate a decreased internal knee extensor moment.
METHODS
Subjects
Thirty-one individuals (18 men and 13 women) with unilateral ACL-deficiency and who were functionally classified noncopers (14) participated in this study (mean ± sd; age 29.0 ± 10.1 years, body mass 82.3 ± 15.7 kg, height 1.74 ± 0.10 m, time from injury 7.9 ± 7.4 weeks). The acute phase was operationally defined as the first 7 months following injury. All had sustained complete, acute, unilateral ACL rupture (confirmed with MRI and through clinical examination with KT 1000 arthrometer side-to-side difference more than 3 mm) and were regular participants in jumping, cutting and pivoting activities (20) prior to injury. Exclusion criteria were bilateral knee involvement, concomitant or symptomatic grade III injury to other knee ligaments, repairable meniscus tear or full-thickness articular cartilage defect greater than 1cm2. Knee range of motion, effusion, pain and obvious gait impairments were treated and resolved prior to testing in accordance with the impairment treatment protocol described by Hurd et al. (21). The screening exam described in detail by Fitzgerald et al. (14) was used to classify patients for this study. The exam consisted of the following tests (cutoff score for noncoper classification): Single limb timed hop test (<80% limb symmetry index), global rating of knee function (<60%), the Knee Outcome Survey of Activities of Daily Living Scale (<80%) and reported of the number of knee giving-way episodes from the time of injury to the time of testing (>1). This work was approved by the University of Delaware Human Subjects Review Board and all patients provided written informed consent prior to testing.
Testing
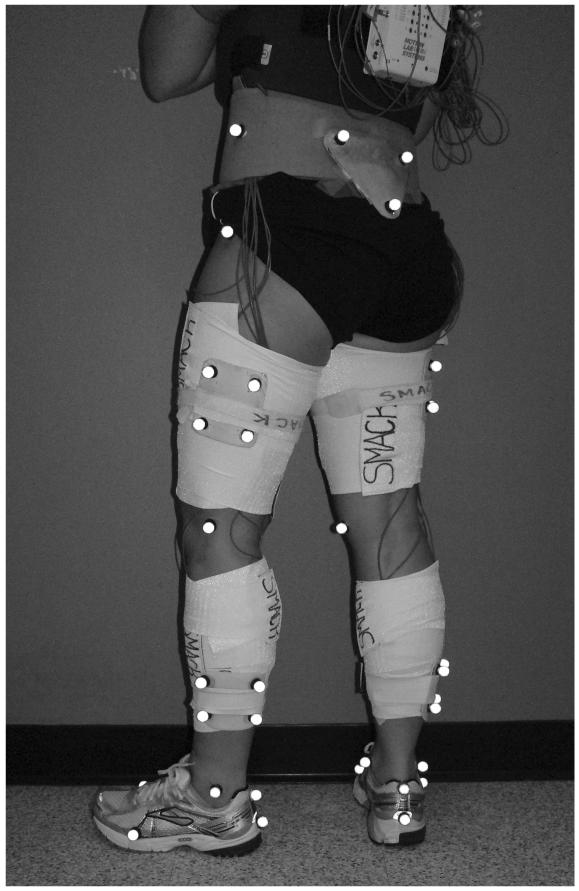
Motion analysis was performed using an eight-camera video system (VICON, Oxford Metrics Ltd., London, UK) with a sampling rate of 120 Hz and force platform (Bertec Corporation, Worthington, OH) with a sampling rate of 1080 Hz. Retro-reflective markers were applied to anatomical landmarks to capture segment alignment and identify joint centers, with additional clusters fixed to rigid thermoplastic shells for tracking motion during dynamic trials according to Figure 1. Several practice trials were used to determine each patient’s self-selected walking speed, and only subsequent walking trials within ± 5% of that speed were accepted. The mean walking speed was 1.53 m/s. Joint angles and moments were calculated using Visual3D software (C-motion, Germantown, MD). For analysis, knee joint angles and moments were time-normalized to 100 percent of stance phase and moments were normalized to body mass × height.
Figure 1.
Subject with anatomical and tracking markers applied to the lower extremity.
EMG was recorded at 1080 Hz using an MA-300 EMG system (Motion Lab Systems, Baton Rouge, LA) from 7 lower extremity muscles, bilaterally: vastus medialis and lateralis (VM and VL), rectus femoris (RF), semimembranosus (SM), biceps femoris (BFL) and medial and lateral gastrocnemius (MG and LG). Patients performed isolated maximum voluntary isometric contractions and a unilateral squat trial to elicit maximal EMG. Gastrocnemius was tested isometrically during standing with the patient’s hands gripped underneath the countertop for self-resistance; quadriceps were tested with patient seated and knee secured in 60° of flexion; hamstrings were tested with the patient lying prone with the knee secured in 30° of flexion. EMG data were high-pass filtered (cutoff 30 Hz), rectified and low-pass filtered (cutoff 6 Hz) to create a linear envelope. Linear envelopes for each muscle were normalized to the maximum EMG found during isometric, unilateral squat or walking trials. Surface EMG was not available for all muscles in the musculoskeletal model (described in a later section) and therefore, activity for semitendinosus (ST) and short head of biceps femoris (BFS) were assumed to be equal to activity recorded from SM and BFL, respectively; activity for vastus intermedius (VI) was assumed equal to the average of activities recorded from VM and VL (26).
Estimation of Muscle Forces
Muscle forces were estimated using an EMG-driven musculoskeletal model, which has been described in detail previously (4, 26) and will be summarized briefly here. The EMG-driven model contained three components (anatomical model, activation dynamics model and contraction dynamics model) and was calibrated on a subject-specific basis prior to the prediction of muscle forces during walking trials.
The lower extremity anatomical model (SIMM 4.0.2, Musculographics, Inc. Chicago, IL (7)) included 10 musculotendon actuators crossing the knee (VM, VL, VI, RF, SM, ST, BFL, BFS, MG and LG). The anatomical model was scaled according to subject-specific anatomical dimensions captured by the camera system during standing. Stance phase kinematics were input to the scaled model in order to obtain subject-specific muscle-tendon lengths, contraction velocities and muscle moment arms for each recorded walking trial.
The activation dynamics model included a recursive filter (34) in which muscle excitation was estimated from the EMG linear envelope and up to 70 ms of electromechanical delay was allowed. This recursive filter was characterized by 3 parameters which were determined for each subject during model calibration: γ1, γ2, d (Table). Additionally, the non-linear EMG-to-force relationships observed at low activation levels was modeled using a piecewise logarithmic function (28), characterized by a single parameter (Alen) which was also determined during calibration (Table).
Table.
Adjustable model parameters during calibration. Ge and Gf are global parameters, and the remaining parameters are specified for each muscle.
| Parameter | Description | Limits Used | |
|---|---|---|---|
| ℓ o m | optimal fiber length | sarcomere length at which optimal actin-myosin overlap is achieved |
± 20% * |
| ℓ s t | tendon slack length | the length at which the viscoelastic tendon is slack; below it the tendon carries no load |
± 20% * |
| d | electromechanical delay |
represents the time delay between EMG signal and the start of the resulting force |
0 - 70 ms |
| Alen | non-linear shape factor |
characterizes the potential for a non-linear EMG- to-force relationship, particularly at low forces (<30% MVC) |
0.01 - 0.12 |
| γ1, γ2 | recursive filter coefficients |
characterizes the observation that EMG signal is shorter in duration than resulting force |
−0.9 < γ1,2< 0.9 |
|
| |||
| Ge | extensor strength coefficient |
characterizes varying degrees of extensor strength among individuals |
50 - 250% * |
| Gf | flexor strength coefficient |
characterizes varying degrees of flexor strength among individuals |
|
indicate limits which vary by the given percentage from published (7) mean values.
The contraction dynamics model was represented by a modified Hill-type muscle model which consisted of a muscle fiber in series with a tendon(39). The tendon was modeled according to a non-linear function normalized to tendon slack length (ℓst) (39). The muscle fiber consisted of a contractile element in parallel with elastic and damping elements which characterized the passive force-length and the viscoelastic properties of muscle, respectively. The force produced by the contractile element was governed by a generic force-length curve which was normalized to maximal isometric muscle force, optimal fiber length (ℓom) and maximum contraction velocity as described by Lloyd and Buchanan (26).
During model calibration, the muscle parameters that define the relationship of EMG to muscle force were allowed to vary within physiological bounds for each subject. In addition to 2 strength multipliers which were used to scale the maximum isometric force values for each subject (Gf for knee flexors and Ge for knee extensors), there were 6 parameters per muscle that uniquely characterized the EMG-to-force relationship. (Parameters are listed in Table.) During model calibration, the governing assumption was that the net internal flexor/extensor muscle moment should equal the net sagittal plane inverse dynamics joint moment (i.e. that the knee capsule and ligaments do not contribute to the net sagittal plane inverse dynamics moment). In accordance with this assumption, the muscle parameters were iteratively adjusted so that the sagittal plane moments from forward and inverse methods were in good agreement. Using a simulated annealing search strategy (16), an optimal solution was obtained in which the squared difference between the two moment curves was minimized. The degree of convergence was assessed by calculating the coefficient of determination (R2) and the relative root mean squared error (RMSE) between the inverse dynamics knee moment and the moment predicted by the calibrated EMG-driven model. Relative RMSE was computed by normalizing RMSE to the range of the inverse dynamics moment curve.
After calibrating the model and defining a subject-specific EMG-to-force relationship using one gait trial, muscle forces were predicted for three novel gait trials. For each novel trial, muscle tendon lengths and moment arms (computed from the measured kinematics) and recorded EMG were input to the calibrated model to compute muscle force.
Muscle forces were normalized to subject body weight (BW) and time-normalized to 100 percent of stance phase. Three trials per subject were averaged for each limb (in 10 limbs, only 2 trials were averaged for analysis). Muscle forces were grouped as knee extensors (EXT = RF+VL+VM+VI) or flexors (FLEX = SM, ST, BFS, BFL, MG, LG) or comparison of force at the peak internal knee extensor moment.
Data Analysis
Paired t-tests were performed (PASSW 18.0, SPSS Inc., Chicago, IL) to compare the following measures between limbs: peak knee flexion angle during the first half of stance, measured peak knee extensor moment, EXT force at the point of peak knee extensor moment and FLEX force at the point of peak knee extensor moment. Significance was set at 0.05.
RESULTS
The model was calibrated for each limb separately, resulting in a total of 62 model calibrations. The average coefficient of determination (R2) between the inverse dynamics knee moment and the moment from the calibrated EMG-driven model was 0.836. The mean relative RMSE of the calibrated model moment curve from the inverse dynamics moment curve was 9.9%. The convergence of the calibrated EMG-driven model knee moment to the inverse dynamics moment validated our tuned model’s predictions of joint moments.
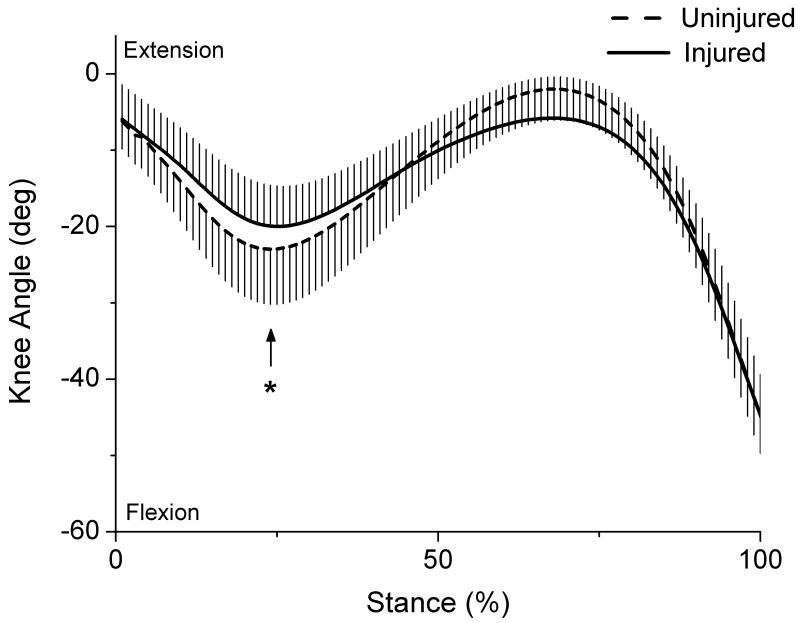
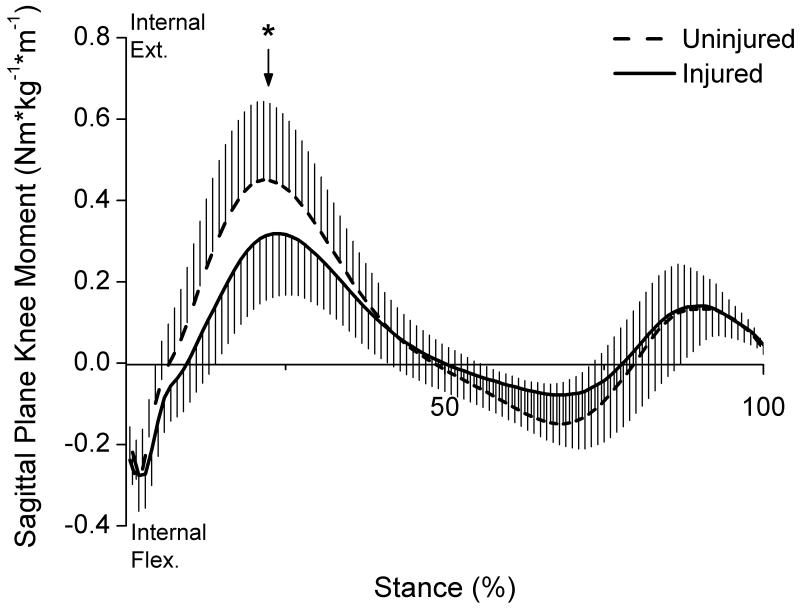
As expected, peak knee flexion angle during the first half of stance was significantly lower (p=0.028) for the injured limb (mean ± SD = 21.0 ± 5.0 °) compared to the uninjured limb (23.6 ± 7.4°) (Fig 2). Patients also displayed a significantly lower peak internal knee extensor moment (p=0.0004) for the injured limb (0.328 ± 0.154 Nm/kg*m) compared to the uninjured limb (0.459 ± 0.194 Nm/kg*m) (Fig 3).
Figure 2.
Knee flexion angle for each limb during stance. Arrow marks the occurrence of peak knee flexion angle.
Figure 3.
Knee extensor moments for each limb during stance (normalized to body mass × height; whiskers are SD). Arrow marks the occurrence of peak knee moment.
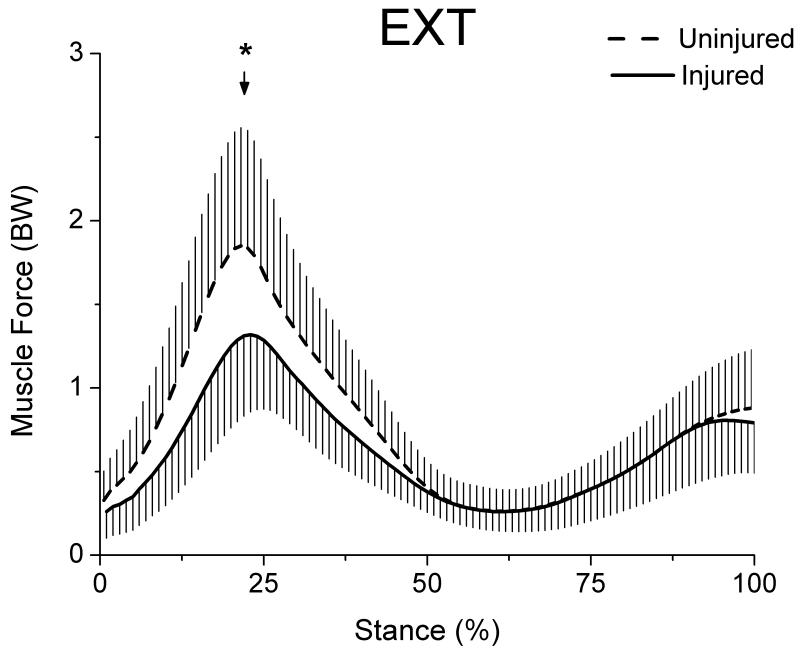
EXT muscle force was significantly lower (p=0.0001) for the injured limb (1.33 ± 0.42 BW) compared to the uninjured limb (1.87 ± 0.73 BW) at the point of peak knee moment (Fig 4). The mean difference in EXT force between limbs was 0.53 BW less for the injured limb.
Figure 4.
Extensor muscle force for both limbs during stance (whiskers are standard deviations). EXT group included VM, VL, VI and RF muscles. Arrow marks the occurrence of peak knee moment.
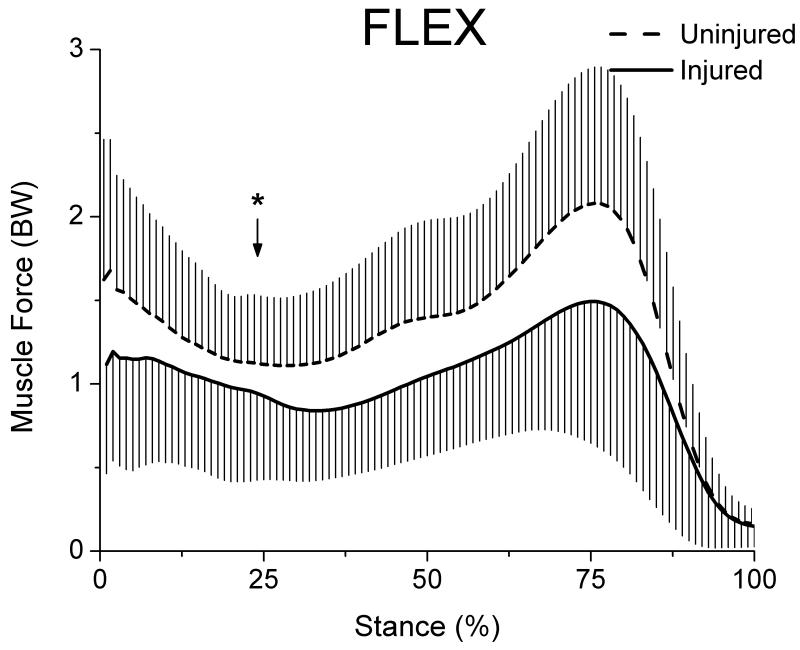
Likewise, FLEX muscle force was significantly lower (p=0.0103) for the injured limb (0.92 ± 0.45 BW) compared to the uninjured limb (1.15 ± 0.35 BW) at the point of peak knee moment (Fig 5). The mean difference in FLEX force between limbs was 0.23 BW less for the injured limb.
Figure 5.
Flexor muscle force for both limbs during stance (whiskers are standard deviations). The FLEX group included ST, SM, BFL, BFS, MG and LG muscles. Arrow marks the occurrence of peak knee moment.
DISCUSSION
Muscles are the effectors of gait adaptations in ACL-deficient individuals, and better understanding of the muscular stabilization strategy adopted after injury will aid in rehabilitation design for these patients. The aims of this study were to 1) confirm that our patients exhibited the typical decreased knee flexion and peak knee moment for the injured limb and 2) determine the muscular strategy causing the asymmetrical knee extensor moment using an EMG-driven modeling approach to estimate knee extensor and flexor muscle forces.
Consistent with previous reports (18, 22, 30, 31), patients in the present study demonstrated decreased peak knee flexion angles and internal extensor moments for their injured limbs during walking. These gait mechanics are typical for patients who demonstrate characteristic knee instability in their injured knee after ACL injury (noncopers (14)). The decreased knee moment is an important gait adaptation (18) and is the biomechanical hallmark of the noncoper (30).
Patients in this study accomplished the decreased peak knee moment for their injured limbs with decreased muscle force from both EXT and FLEX groups, rather than either of the expected strategies: isolated decrease in EXT force or isolated increase in FLEX force. The size of the difference in EXT force between limbs was greater than the difference in FLEX force at the point of comparison. This is not surprising because the force generated by the knee extensors is near its peak in early stance, whereas peak knee flexor force is near its minimum.
The strategy of decreased muscle force adopted by these patients may be explained, in part, by muscle weakness which frequently accompanies ACL injury. In acutely injured patients, quadriceps strength deficits for the injured limb range from 14-25%, when compared to the uninjured limb (9, 10, 36, 37). Hamstring strength deficits have also been reported for the injured limb, ranging from 14-19% (35, 36). Aberrant gait patterns are associated with quadriceps strength deficits of as low as 20% for the injured limb in patients with ACL deficiency (25), which demonstrates the potential for muscle weakness to impact the biomechanics of low demand activities. Although only about 30% of maximal thigh muscle strength is utilized during normal walking, strength deficits observed acutely after ACL injury may contribute to their low muscle force utilization during gait.
The observed decrease in injured limb muscle forces may also be explained by patients’ apprehension after injury. Patients with characteristic knee instability are those whose experience after injury is punctuated by low confidence in knee function and/or multiple episodes of giving-way (14). Despite having resolved pain, range of motion and obvious gait impairments, the patients in the present study may have been simply unwilling to place normal demands on their injured limb. Accordingly, these patients may have selected a gait strategy which ‘favors’ their injured limb, allowing less knee motion and requiring less muscle force. While restoring muscle strength is an important prerequisite for symmetrical movement (25) and is important for joint function in the long term (11), restoring confidence in knee function may also be an important component of rehabilitation after ACL injury. Fears associated with re-injury exist after reconstructive surgery (6, 24), and are coincident with low function (6). Although there is no evidence which directly relates psychosocial obstacles to altered movement patterns after injury, apprehension and lack of confidence may be factors which distinctly impact movement strategies following ACL injury.
The decreased EXT and FLEX forces at the point of peak knee moment were somewhat unexpected, given other studies which report that patients with ACL deficiency exhibit elevated muscle co-contraction in an attempt to stiffen and stabilize their injured knee (1, 3, 22, 30). Caution must be exercised when comparing muscle force results from this study to co-contraction findings previously reported for several reasons. Firstly, co-contraction calculations utilize EMG, which is a measure of neural command to muscle. Muscle force is the result of muscle contraction and is therefore not directly related to EMG. Secondly, EMG is typically interpreted after being normalized to reference value obtained during a volitional maximum test. As strength and activation deficits (esp. quadriceps) are common following ACL injury, one cannot assume that force production is equal between limbs during maximum volitional isometric strength testing. Therefore, the normalization of EMG to a maximal reference value does not accomplish normalization with respect to muscle force.
The present study is unique in its incorporation of in vivo muscle activity data in the estimation of muscle force. Predictions of muscle force for ACL-deficient gait are few. Noyes and colleagues (29) predicted muscle forces for varus-aligned, ACL-deficient knees during slow walking (1.1 m/s) and reported higher EXT and lower FLEX peak forces for the injured limb than the present study. EMG was not used. Muscle forces were obtained by reducing the number of muscles spanning each joint until the muscle force-joint moment problem could be solved uniquely. Such an approach assumes the absence muscular co-contraction, which is invalid for the case of ACL injury. The approach used here partitions force among muscles using recorded EMG and is therefore uniquely suited to investigate muscle forces following ACL injury.
Muscle forces have been estimated for gait in uninjured individuals using various modeling methods (12). Force trajectories and peak values for muscles crossing the knee were explicitly reported by Shelburne and colleagues during walking (33). Muscle forces were similar shape but lower in magnitude compared to the uninjured limbs in this study. The higher muscle forces for the uninjured limbs in this study were likely a result of the approach used to partition forces among muscles. These were obtained using a cost function during optimization which assumes that the goal adopted by the central nervous system during walking is to minimize muscle stress, or energy expenditure. This assumption inherently minimizes muscle co-contraction and consequently, higher muscle forces may be expected when using an EMG-driven approach. Muscle forces in uninjured individuals using an EMG-driven approach are similar in shape, timing and magnitude to the uninjured limbs in this study. Winby et al. (38) reported forces of about 18 N/kg for the quadriceps and about 10 N/kg for the hamstrings and gastrocnemeii at 25% of stance. Forces for the uninjured limb for this study were 18.3 and 11.2 N/kg, respectively.
The lower muscle force for the injured limbs of these patients affirms the existence of neuromuscular asymmetries following ACL injury. Considering that biomechanical mal-adaptations can persist even after reconstruction, an important goal of rehabilitation is to restore normal movement by re-training and strengthening the muscles affected by injury. Noncopers may benefit from a rehabilitation program which is successful in restoring muscle strength and confidence in the function of their injured limb. Future work should determine whether neuromuscular asymmetries resolve with the resolution of strength deficits and self-reported knee function.
One potential limitation of this study was the inclusion of only patients with acute ACL injury who demonstrated characteristic knee instability (noncopers (14)). This functionally unstable group represents the majority of all athletes with ACL-deficiency; however because of this inclusion criterion, the results of this study cannot be generalized to all patients with ACL-deficiency. Another potential limitation is the lack of an uninjured control group in this study. However, comparison with muscle forces estimated for uninjured controls using an EMG driven approach (38) are similar to the uninjured limbs in this study.
The resolution of pain, range of motion and obvious gait impairments prior to testing was an important strength of this study. Patients participated in an impairment treatment program until they met the following criteria per assessment by a physical therapist (21): minimal knee effusion, no knee extension deficit, no pain in the injured limb with hopping and no visually-identifiable gait impairments. The resolution of these impairments prior to testing ensured that they were not responsible for the gait adaptations captured in this study. Our goal was to identify the neuromuscular adaptations to injury associated with ACL-deficiency in our patients, rather than those associated with the acute effects of knee trauma.
An investigation of the clinically relevant factors which contribute to the gait and neuromuscular asymmetries in these patients is warranted. It is likely (as stated by Lewek et al. (25)) that quadriceps weakness contributes to but does not fully explain the gait and neuromuscular adaptations in individuals with ACL injury. Future work should examine the relationships between strength and self-reported fear/apprehension or lack of confidence and the neuromuscular adaptations described here. Also, muscles function primarily as joint accelerators and stabilizers and as such, they apply considerable loads to the articular surfaces. The adaptation of muscle forces during walking may impact joint loads, thereby playing a role in long-term joint health. Future work should also investigate knee loading after ACL injury in order to determine the impact of their muscular strategy on articular loads, and subsequently on long-term joint health.
ACL-deficient patients with characteristic knee instability employed a gait strategy which requires less global muscle force from their injured limb. They exhibited neither a ‘quadriceps avoidance’ nor a ‘hamstrings facilitation’ muscular stabilization strategy. Instead, they walked with decreased muscle force for both flexor and extensor muscle groups in their injured limbs. The strategy of employing lower muscle force may be explained in part by muscle weakness which frequently accompanies ACL injury, or by apprehension, low confidence and fear of further injury. The present study is unique in its incorporation of in vivo muscle activity data in the estimation of muscle force. These results affirm the existence of neuromuscular asymmetries which drive aberrant movement patterns after ACL injury.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01 AR46386 & AR48212, S10RR022396). The results of the present study do not constitute endorsement by ACSM.
Funding Disclosure: National Institutes of Health (R01 AR46386, R01 AR48212, S10RR022396).
Footnotes
CONFLICT OF INTEREST: The authors have no potential conflicts of interest to disclose.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Beard DJ, Soundarapandian RS, O’Connor JJ, Dodd CAF. Gait and electromyographic analysis of anterior cruciate ligament deficient subjects. Gait Posture. 1996;4:83–88. [Google Scholar]
- 2.Berchuck M, Andriacchi TP, Bach BR, Reider B. Gait adaptations by patients who have a deficient anterior cruciate ligament. J Bone Joint Surg Am. 1990;72A:871–877. [PubMed] [Google Scholar]
- 3.Boerboom AL, Hof AL, Halbertsma JPK, et al. Atypical hamstrings electromyographic activity as a compensatory mechanism in anterior cruciate ligament deficiency. Knee Surg Sports Traumatol Arthrosc. 2001;9:211–216. doi: 10.1007/s001670100196. [DOI] [PubMed] [Google Scholar]
- 4.Buchanan TS, Lloyd DG, Manal K, Besier TF. Neuromusculoskeletal modeling: Estimation of muscle forces and joint moments and movements from measurements of neural command. J Appl Biomech. 2004;20:367–395. doi: 10.1123/jab.20.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bulgheroni P, Bulgheroni MV, Andrini L, Guffanti P, Giughello A. Gait patterns after anterior cruciate ligament reconstruction. Knee Surg Sports Traumatol Arthrosc. 1997;5:14–21. doi: 10.1007/s001670050018. [DOI] [PubMed] [Google Scholar]
- 6.Chmielewski TL, Jones D, Day T, Tillman SM, Lentz TA, George SZ. The association of pain and fear of movement/reinjury with function during anterior cruciate ligament reconstruction rehabilitation. J Orthop Sports Phys Ther. 2008;38:746–753. doi: 10.2519/jospt.2008.2887. [DOI] [PubMed] [Google Scholar]
- 7.Delp SL, Loan JP, Hoy MG, Zajac FE, Topp EL, Rosen JM. An interactive graphics-based model of the lower extremity to study orthopaedic surgical procedures. IEEE Trans Biomed Eng. 1990;37:757–67. doi: 10.1109/10.102791. [DOI] [PubMed] [Google Scholar]
- 8.DeVita P, Hortobagyi T, Barrier J. Gait biomechanics are not normal after anterior cruciate ligament reconstruction and accelerated rehabilitation. Med Sci Sports Exerc. 1998;30:1481–8. doi: 10.1097/00005768-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Eastlack ME, Axe MJ, Snyder-Mackler L. Laxity, instability, and functional outcome after acl injury: Copers versus noncopers. Med Sci Sports Exerc. 1999;31:210–215. doi: 10.1097/00005768-199902000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Eitzen I, Eitzen TJ, Holm I, Snyder-Mackler L, Risberg MA. Anterior cruciate ligament-deficient potential copers and noncopers reveal different isokinetic quadriceps strength profiles in the early stage after injury. Am J Sports Med. 2010;38:586–593. doi: 10.1177/0363546509349492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eitzen I, Holm I, Risberg MA. Preoperative quadriceps strength is a significant predictor of knee function two years after anterior cruciate ligament reconstruction. Br J Sports Med. 2009;43:371–376. doi: 10.1136/bjsm.2008.057059. [DOI] [PubMed] [Google Scholar]
- 12.Erdemir A, McLean S, Herzog W, van den Bogert AJ. Model-based estimation of muscle forces exerted during movements. Clin Biomech. 2007;22:131–154. doi: 10.1016/j.clinbiomech.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 13.Ferber R, Osternig LR, Woollacott MH, Wasielewski NJ, Lee JH. Bilateral accommodations to anterior cruciate ligament deficiency and surgery. Clin Biomech. 2004;19:136–144. doi: 10.1016/j.clinbiomech.2003.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Fitzgerald GK, Axe MJ, Snyder-Mackler L. A decision-making scheme for returning patients to high-level activity with nonoperative treatment after anterior cruciate ligament rupture. Knee Surg Sports Traumatol Arthrosc. 2000;8:76–82. doi: 10.1007/s001670050190. [DOI] [PubMed] [Google Scholar]
- 15.Gao B, Zheng NQ. Alterations in three-dimensional joint kinematics of anterior cruciate ligament-deficient and -reconstructed knees during walking. Clin Biomech. 2010;25:222–229. doi: 10.1016/j.clinbiomech.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Goffe WL, Ferrier GD, Rogers J. Global optimization of statistical functions with simulated annealing. Journal of Econometrics. 1994;60:65–99. [Google Scholar]
- 17.Gokeler A, Schmalz T, Knopf E, Freiwald J, Blumentritt S. The relationship between isokinetic quadriceps strength and laxity on gait analysis parameters in anterior cruciate ligament reconstructed knees. Knee Surg Sports Traumatol Arthrosc. 2003;11:372–8. doi: 10.1007/s00167-003-0432-1. [DOI] [PubMed] [Google Scholar]
- 18.Hart JM, Ko JWK, Konold T, Pietrosimione B. Sagittal plane knee joint moments following anterior cruciate ligament injury and reconstruction: A systematic review. Clin Biomech. 2010;25:277–283. doi: 10.1016/j.clinbiomech.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Hartigan E, Axe MJ, Snyder-Mackler L. Perturbation training prior to acl reconstruction improves gait asymmetries in non-copers. J Orthop Res. 2009;27:724–729. doi: 10.1002/jor.20754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hefti F, Muller W, Jakob RP, Staubli HU. Evaluation of knee ligament injuries with the ikdc form. Knee Surg Sports Traumatol Arthrosc. 1993;1:226–34. doi: 10.1007/BF01560215. [DOI] [PubMed] [Google Scholar]
- 21.Hurd WJ, Axe MJ, Snyder-Mackler L. A 10-year prospective trial of a patient management algorithm and screening examination for highly active individuals with anterior cruciate ligament injury: Part 1, outcomes. Am J Sports Med. 2008;36:40–7. doi: 10.1177/0363546507308190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hurd WJ, Snyder-Mackler L. Knee instability after acute acl rupture affects movement patterns during the mid-stance phase of gait. J Orthop Res. 2007;25:1369–77. doi: 10.1002/jor.20440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar D, Rudolph K, Manal K. EMG-driven modeling approach to muscle force and joint load estimations: Case study in knee osteoarthritis. J Orthop Res. 2012;30(3):377–83. doi: 10.1002/jor.21544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kvist J, Ek A, Sporrstedt K, Good L. Fear of re-injury: A hindrance for returning to sports after anterior cruciate ligament reconstruction. Knee Surgery Sports Traumatology Arthroscopy. 2005;13:393–397. doi: 10.1007/s00167-004-0591-8. [DOI] [PubMed] [Google Scholar]
- 25.Lewek M, Rudolph K, Axe M, Snyder-Mackler L. The effect of insufficient quadriceps strength on gait after anterior cruciate ligament reconstruction. Clin Biomech. 2002;17:56–63. doi: 10.1016/s0268-0033(01)00097-3. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd DG, Besier TF. An emg-driven musculoskeletal model to estimate muscle forces and knee joint moments in vivo. J Biomech. 2003;36:765–776. doi: 10.1016/s0021-9290(03)00010-1. [DOI] [PubMed] [Google Scholar]
- 27.Majewski M, Susanne H, Klaus S. Epidemiology of athletic knee injuries: A 10-year study. Knee. 2006;13:184–8. doi: 10.1016/j.knee.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Manal K, Buchanan TS. A one-parameter neural activation to muscle activation model: Estimating isometric joint moments from electromyograms. J Biomech. 2003;36:1197–1202. doi: 10.1016/s0021-9290(03)00152-0. [DOI] [PubMed] [Google Scholar]
- 29.Noyes FR, Schipplein OD, Andriacchi TP, Saddemi SR, Weise M. The anterior cruciate ligament-deficient knee with varus alignment - an analysis of gait adaptations and dynamic joint loadings. Am J Sports Med. 1992;20:707–716. doi: 10.1177/036354659202000612. [DOI] [PubMed] [Google Scholar]
- 30.Rudolph KS, Axe MJ, Buchanan TS, Scholz JP, Snyder-Mackler L. Dynamic stability in the anterior cruciate ligament deficient knee. Knee Surg Sports Traumatol Arthrosc. 2001;9:62–71. doi: 10.1007/s001670000166. [DOI] [PubMed] [Google Scholar]
- 31.Rudolph KS, Eastlack ME, Axe MJ, Snyder-Mackler L. basmajian student award paper: Movement patterns after anterior cruciate ligament injury: A comparison of patients who compensate well for the injury and those who require operative stabilization. J Electromyogr Kinesiol. 1998;1998;8:349–62. doi: 10.1016/s1050-6411(97)00042-4. [DOI] [PubMed] [Google Scholar]
- 32.Shao Q, Bassett DN, Manal K, Buchanan TS. An emg-driven model to estimate muscle forces and joint moments in stroke patients. Comput Biol Med. 2009;39:1083–1088. doi: 10.1016/j.compbiomed.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shelburne KB, Pandy MG, Anderson FC, Torry MR. Pattern of anterior cruciate ligament force in normal walking. J Biomech. 2004;37:797–805. doi: 10.1016/j.jbiomech.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Thelen DG, Schultz AB, Ashtonmiller JA. Quantitative interpretation of lumbar muscle myoelectric signals during rapid cyclic attempted trunk flexions and extensions. J Biomech. 1994;27:157–167. doi: 10.1016/0021-9290(94)90204-6. [DOI] [PubMed] [Google Scholar]
- 35.Tsepis E, Vagenas G, Giakas G, Georgoulis A. Hamstring weakness as an indicator of poor knee function in acl-deficient patients. Knee Surg Sports Traumatol Arthrosc. 2004;12:22–29. doi: 10.1007/s00167-003-0377-4. [DOI] [PubMed] [Google Scholar]
- 36.Tsepis E, Vagenas G, Ristanis S, Georgoulis AD. Thigh muscle weakness in acl-deficient knees persists without structured rehabilitation. Clin Orthop Relat Res. 2006:211–218. doi: 10.1097/01.blo.0000223977.98712.30. [DOI] [PubMed] [Google Scholar]
- 37.Williams GN, Buchanan TS, Barrance PJ, Axe MJ, Snyder-Mackler L. Quadriceps weakness, atrophy, and activation failure in predicted noncopers after anterior cruciate ligament injury. Am J Sports Med. 2005;33:402–407. doi: 10.1177/0363546504268042. [DOI] [PubMed] [Google Scholar]
- 38.Winby CR, Lloyd DG, Besier TF, Kirk TB. Muscle and external load contribution to knee joint contact loads during normal gait. J Biomech. 2009;42:2294–300. doi: 10.1016/j.jbiomech.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 39.Zajac FE. Muscle and tendon: Properties, models, scaling, and application to biomechanics and motor control. Crit Rev Biomed Eng. 1989;17:359–411. [PubMed] [Google Scholar]