Abstract
In the past few years, there have been significant advances in the research on cancer stem cells (CSCs). The emerging evidences have demonstrated that CSCs and epithelial-mesenchymal transition (EMT)-type cells, which share molecular characteristics with CSCs, play critical roles in drug resistance, invasion, and metastasis. Pancreatic cancer (PC) has a high mortality due to both intrinsic (de novo) and extrinsic (acquired) drug resistance, leading to increased invasive and metastatic potential of PC cells. Therefore, targeting pancreatic CSCs and EMT-type cells could be a novel therapeutic strategy for the treatment of PC. In this article, we will review the current state of our knowledge on the role of pancreatic CSCs and EMT-type cells, and summarize the novel therapeutic strategies that could target pancreatic CSCs and EMT-type cells, leading to the reversal of EMT phenotype, the induction of drug sensitivity, and the inhibition of invasion and metastasis of PC, which is expected to yield better treatment outcome.
Keywords: pancreatic cancer, cancer stem cells, drug resistance, invasion, metastasis
1. Introduction
In the past few years, there have been significant advances in the research on cancer stem cells (CSCs). CSCs have been found in most types of hematopoietic and solid tumors including brain tumor, breast, head and neck, colon, lung, prostate, ovarian, and pancreatic cancers. It is now widely accepted that in tumors there is a subset of cancer cells, named as cancer stem cells, which has the capacity for extensive proliferation, self-renewal, multi-lineage differentiation, and high tumorigenic potential. Importantly, CSCs has been found to be resistant to conventional cancer therapies including radiotherapy and chemotherapy. This feature of CSCs could cause cancer recurrence after eliminating the most of cancer cells by conventional therapies. Moreover, CSCs show their high propensity of invasiveness and metastasis. It has been reported that CSCs contribute to the metastatic dissemination of solid tumors, induce epithelial-mesenchymal transition (EMT), and spread from primary site to distant metastatic place, homing in the new site with great capacity for self-renewal [1]. In addition, EMT-type cells, which share molecular characteristics with CSCs, also play critical roles in drug resistance and cancer metastasis [2,3]. Therefore, CSCs and EMT-type cells are believed to be the major culprits in cancer recurrence and metastasis.
Pancreatic cancer (PC) is an aggressive malignancy and the fourth leading cause of cancer death in the United States. Approximately, 44,030 people were expected to be diagnosed with PC and 37,660 people were expected to die from this disease in the US in 2011 [4]. The mortality of PC increased from 2003 to 2007 by 0.1% per year in women and by 0.7% per year in men. For all stages combined, the one year relative survival rate is 26% and the five year relative survival rate is only 6%. Even for the patients with local PC, the rate of five year survival is only 23% [4]. The lethal nature of PC is due to the propensity of PC cells to rapidly disseminate to the lymphatic system and distant organs. The intrinsic (de novo) and extrinsic (acquired) drug resistance characteristics of PC cells leads to resistance to all chemotherapeutic agents tested, which is another major cause of treatment failure in PC. The presence of pancreatic CSCs with the drug-resistant and high metastatic characteristics contributes to the treatment failure resulting in high mortality of patients diagnosed with PC. Therefore, it is important to elucidate the molecular mechanisms underlying drug resistance and metastasis with respect to CSCs and EMT in PC. Powered by the gain of such knowledge, novel therapeutic strategies could be devised for targeting pancreatic CSCs and EMT-type cells, which would result in increasing drug sensitivity and causing inhibition of invasion and metastasis. Such a novel strategy would be useful for the successful treatment of patients diagnosed with this devastating disease.
2. Discovery of Cancer Stem Cells especially Pancreatic Cancer Stem Cells
In 1997, the first evidence for the existence of CSCs was reported [5]. The study showed that subpopulation of leukemic cells (expected as leukemic stem cell) with cell surface marker CD34+ and CD38− were capable of self-renewing and initiating human acute myeloid leukemia in SCID mice. Since the biological characteristics of CSCs have some similarity with normal stem cells, CSC surface markers and stem cell surface markers overlap in the major epithelia, providing some clues to find CSCs. To identify and isolate CSCs from whole cancer cell population, several cell surface markers including CD24, CD34, CD44, CD133, CD139, CD166, and ESA have been used together with cell sorting by fluorescence-activated cell sorting (FACS) analysis [6,7]. Other molecules such as Aldh1, Lgr5, Bmi1, and Olfm4 have also been used as CSC markers. CD44, CD133, and ESA are more frequently used for the isolation of CSCs from different types of tumors. However, it is important to note that there is no unique marker which can be used for the isolation of CSCs from different types of cancers. It is known that combined use of several cell markers could increase the purity of isolated CSCs.
2.1. Discovery and molecular markers of pancreatic cancer stem cells (CSCs)
In 2007, two groups of researchers isolated and identified pancreatic CSCs from human PC using two different sets of cell surface markers [8,9]. By FACS analysis, Li et al firstly isolated the CD44+/CD24+/ESA+ pancreatic CSCs from PC tissue. These CD44+/CD24+/ESA+ cells counted for 0.2–0.8% of PC cells and showed the stem cell properties including self-renewal, the ability to produce differentiated progeny, and the increased expression of sonic hedgehog [9]. Moreover, the CD44+/CD24+/ESA+ cells had a 100-fold increased tumorigenic potential compared to marker-negative cancer cells. In an animal study, 50% of animals injected with as few as 100 CD44+/CD24+/ESA+ cells formed tumors that were histologically indistinguishable from the original PC [9]. Soon after that, Hermann et al reported that human PC tissue contained CSCs identified by CD133 expression [8]. They found that these CD133+ PC cells were highly tumorigenic. The evidences reported by these two groups clearly demonstrated that there is a small population of PC cells which possess stem cell surface markers and show the properties of self-renewal and increased tumorigenic potential, suggesting that these cells are pancreatic CSCs.
Moreover, other investigators also examined the distribution of CD133 in normal pancreas and pancreatic cancer tissues. They observed that CD133 was mainly located at the apical/endoluminal surface of glandular epithelia whereas pancreatic ductal adenocarcinomas showed a varying degree of apical cell surface CD133 expression [10]. Cytoplasmic staining was also seen in a few tumor cells [10]. In a study investigating the relationship between CD133 expression and clinicopathological factors in PC, it was found that 60% (48/80) of specimens were CD133-positive with less than 15% cells per specimen expressing CD133 [11]. CD133-positive cells were mainly located at the peripheral site of adenocarcinoma glandular structures. Importantly, the expression of CD133 was significantly associated with lymphatic metastasis, VEGF-C expression, and lower 5-year survival rate [11].
In another study, PC cell lines including Capan-1, Mia-PACA-2, Panc-1, and SNU-410 were analyzed for the expressions of CD133, CD44, and CD24 by flow cytometry [12]. The tumorigenicity was compared using tumor volumes and numbers of tumors formed in SCID mice. It was found that CD133 positive cells have higher tumorigenic and metastatic potential than CD44 and CD24 positive cells [12]. CD133 positive cells also showed high migration and invasion potential, suggesting that CD133 might be a meaningful cell surface marker of pancreatic CSCs [13]. The c-Met is another marker of pancreatic CSCs. It has been found that c-Met high expressing PC cells readily formed spheres and had increased tumorigenic potential whereas c-Met negative PC cells could not form spheres [14]. Moreover, c-Met inhibitor XL184 or c-Met knockdown significantly inhibited sphere formation and prevented metastasis, suggesting that the expression of c-Met may be a marker for pancreatic CSCs. In addition, the PC cells showing high expression of c-Met and CD44 had the highest capability of self-renewal capacity and tumorigenesis [14]. Further report also showed that another pancreatic CSC marker ALDH alone was sufficient for efficient tumor-initiation and had enhanced tumorigenic potential with CD133 positive expression [15]. It was also reported that ALDH+ and CD44+/CD24+ pancreatic CSCs are similarly tumorigenic, but ALDH+ cells are relatively more invasive [16]. It is worth mentioning that the expression of stem cell surface markers CD44 and CD24 in PC could be changed with their local microenvironment. The expression of CD44 and CD24 in PC sphere cells in serum free medium was up-regulated; however, the expression of CD44 and CD24 returned to initial levels once the medium was changed back to serum-containing medium [17]. These results collectively suggest that the combination of several stem cell markers should be used for the identification of pancreatic CSCs.
2.2. Pancreatic CSCs and EMT
Emerging evidences have shown that cancer stem cells share similar molecular characteristics with EMT-type cells. EMT is a process by which epithelial cells undergo morphological changes from epithelial cobblestone phenotype to elongated fibroblastic phenotype. EMT was originally known as a crucial differentiation and morphogenetic process during embryogenesis. In recent years, EMT is also recognized as pathological process during the progression of various diseases including inflammation and cancers. Alterations in the expression of EMT markers have been observed during the acquisition of EMT phenotype. During EMT, cells lose the expression of epithelial markers such as E-cadherin, γ-catenin, and zonula occludens-1 (ZO-1), and gain the expression of mesenchymal markers such as vimentin, fibronectin, α-smooth muscle actin (SMA), and N-cadherin. A number of transcription factors which repress E-cadherin and regulate other EMT markers also plays critical role in EMT during normal development and cancer progression. These transcription factors include the zinc finger binding transcription factor Snail homologues (Snail1, Snail2/Slug, and Snail3) and basic helix-loop-helix transcription factors such as Twist, ZEB1, ZEB2/SIP1, and TCF3/E47/E12 [18]. Similar to CSCs, the EMT-type cells are drug-resistant and have higher metastatic potential. Recently, in vitro studies have suggested that CSCs and EMT-type cell phenotypes overlap and that the properties and phenotypes of CSCs and EMT-type cells may be linked to some extent through shared molecular make-up [19].
The EMT phenomenon in PC cell lines and surgically resected pancreatic cancer tissues has been documented [3,20]. It was found that L3.6pl, Colo357, BxPC-3, HPAC, CFPAC-1, and SU86.86 PC cells expressed high levels of epithelial marker E-cadherin; however, MiaPaCa-2, Panc-1, Aspc-1, Hs766T, and MPanc96 cells had high level of expression of mesenchymal makers, vimentin and ZEB-1 [3,20]. In further studies using PC tumor tissues with immunohistochemical staining, a negative association between vimentin and E-cadherin expression was found [21]. Importantly, increased expression of fibronectin, vimentin, or N-cadherin and decreased expression of E-cadherin were correlated with invasion, metastasis, and poor survival [21]. These results suggest that a population of distinct PC cells, which show EMT phenotypes, does exist in PC and that these EMT cells in PC could promote the progression and aggressiveness of PC. We have also found that FOXM1 or Notch-1 could induce EMT in PC cells and that these EMT-type cells showed increased expression of ZEB1, ZEB2, Snail2, and vimentin resulting in higher pancreatosphere-forming capacity consistent with expression of CSC surface markers, CD44 and EpCAM [22,23]. These results suggest that EMT-type cells and CSCs shares similar molecular characteristics, and thus these cells are equipped with drug resistance phenotype with increased potential for invasion and metastasis.
2.3. Molecular pathways altered in CSCs and EMT-type cells
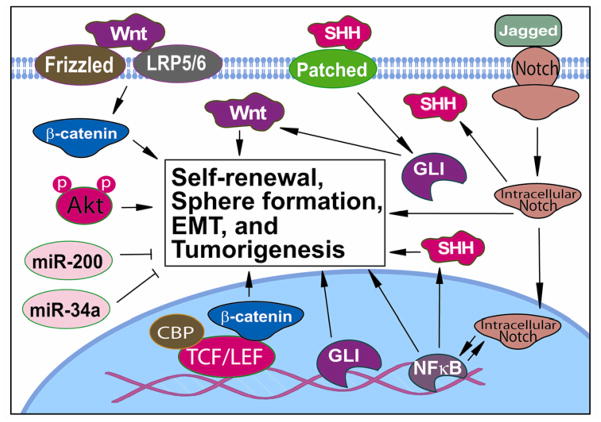
Similar to cancer cells, multiple signaling pathways are involved in CSCs and EMT-type cells; however, among multiple pathways Hedgehog, Notch, and Wnt are main signaling pathways that are altered in CSCs and EMT-type cells especially because they are important signaling pathways in pancreatic embryonic development and differentiation and they communicate with each other in the signaling network (Figure 1). Recent investigations also demonstrated the critical role of microRNAs (miRNAs) in the regulation of CSCs and EMT-type cells (Figure 1), and thus the deregulated expression of miRNAs and their target genes in the regulation and biology of CSCs and EMT is an emerging area of research.
Figure 1.
Signaling network altered in pancreatic CSCs and EMT-type cells. The cellular signal crosstalk and the deregulation of multiple cellular signaling pathways including Wnt, Notch, Hedgehog, Akt, NF-κB, and miRNA-regulated signaling pathways play critical roles in self-renewal of CSCs, sphere formation, EMT and tumorigenesis. Therefore, targeting these important signaling pathways could eliminate pancreatic CSCs and EMT-type cells, which could lead to a better treatment outcome of patients diagnosed with pancreatic cancer.
Hedgehog (Hh) signaling plays critical roles in embryonic pancreatic development. Hedgehog ligands including sonic hedgehog (Shh) are expressed in the endodermal epithelium at early embryonic stages but excluded from the region forming pancreas. Activation of Hh pathway has been found in various cancers including PC. Overexpression of Shh could promote pancreatic tumorigenesis. In the islets, acini, and ductal epithelium of normal pancreas, there was no detectable Shh expression [24]; however, Shh expression was detected in 70% of PC tissues [24], suggesting that up-regulation of Hh could contribute to the pancreatic cancer development and progression. Moreover, up-regulation of sonic hedgehog has been observed in pancreatic CSCs. Recent studies have shown down-regulation of Hh signaling by several inhibitors of Hh, which resulted in the inhibition of pancreatic CSCs and EMT-type cell growth and spheroid formation mediated by up-regulation of E-cadherin and down-regulation of Snail, Slug, ZEB1, and TCF/LEF transcriptional activity [25]. These observations clearly suggest the role of Hh signaling in CSCs and EMT phenotypic cells.
Notch signaling is another developmental signaling pathway which plays important roles in cell proliferation, survival, apoptosis, and differentiation. The activation of Notch signaling could promote EMT by the regulation of the several transcription factors and growth factors including Snail, Slug, and TGF-β. We found that Notch-2 and its ligand, Jagged-1, were highly up-regulated in gemcitabine resistant PC cells, which showed the acquisition of EMT phenotype. We also found that over-expression of Notch-1 increased the formation of pancreatospheres which showed higher expression of CSC surface markers CD44 and EpCAM [23]. These results clearly suggest that Notch signaling is critically involved in the formation of pancreatic CSCs and EMT-type cells.
Wnt signaling is critically involved in both self-renewal and carcinogenesis in various cancers. The activation of Wnt signaling also contributes to the formation of CSCs and EMT-type cells. It has been found that the inhibition of Wnt signaling could lead to the re-expression of epithelial markers and suppression of EMT related transcription factors, Slug and Twist [26]. In addition, overexpression of Snail increased the expression of Wnt target genes mediated through its interaction with β-catenin while down-regulation of Snail reduced target gene expression [27], suggesting the molecular links between Wnt signaling and snail which is an important regulator in EMT. Therefore, deregulation of Wnt signaling could influence the formation of pancreatic CSCs and EMT phenotype.
It has been well documented that miRNAs are critically involved in many normal biological processes including cell proliferation, differentiation, apoptosis, and stress resistance. Recently, miRNAs have received much attention in the field of cancer research. It has also been found that the aberrant expression of miRNAs could contribute to the development and progression of cancer [28]. More importantly, emerging evidences demonstrate the critical role of miRNAs in CSCs and EMT. The miRNAs could mediate EMT through the regulation of E-cadherin and other EMT related molecules. We have found that miR-200b, miR-200c, and miR-200a were down-regulated in gemcitabine-resistant PC cells, which showed the EMT phenotype [3]. We also found that many members of the tumor suppressor let-7 family were down-regulated in the gemcitabine-resistant EMT-type PC cells [3]. Moreover, introduction of miR-200 family into the EMT-type cells caused the up-regulation of epithelial marker E-cadherin and down-regulation of mesenchymal markers including ZEB1 and vimentin, suggesting reversal of EMT phenotype. Consistent with the alterations in EMT marker, the morphology of miR-200 transfected EMT-type cells was partially changed from elongated fibroblastoid to epithelial cobblestone-like appearance [3]. These results suggest the critical role of miRNA in the processes of EMT. Wellner et al reported that ZEB1 was necessary for the tumor-initiating capacity of pancreatic cancer [29]. They found that ZEB1 inhibited the expression of stemness-inhibiting miRNAs such as miR-200c and miR-203 which cooperate to suppress the expression of stem cell markers. Another recent study also showed differentially expressed miRNAs including miR-99a, miR-100, miR-125b, miR-192, and miR-429 in pancreatic CSCs [30]. These clusters of miRNAs were linked with the clusters of stem cell associated mRNAs in PC CSCs [30], and these observations provide clear rationale for designing CSC-targeted therapy.
3. Pancreatic CSCs and EMT-type cells are more drug resistant and metastatic
3.1. Pancreatic CSCs in drug resistance, invasion, and metastasis
Emerging evidences have demonstrated that CSCs are resistant to conventional chemotherapy. It was found that CSCs in mouse mammary tumors contributed to cisplatin resistance [31] and that CSCs in colorectal cancers were responsible for the resistance to chemotherapeutic drugs [32]. Using the stem cell marker CD133, Tadaro et al identified colon CSCs that account for around 2% of the cells in human colon cancer [32]. They also found that CD133+ cells produced and utilized IL-4 to protect themselves from apoptosis. Importantly, treatment with IL-4Rα antagonist or anti-IL-4 neutralizing antibody significantly enhanced the antitumor efficacy of chemotherapeutic drugs, suggesting that colon CSCs were drug-resistant due to the production of IL-4 [32]. A recent study using biopsy tissues from patients with breast cancer has shown that chemotherapy led to an increased percentage of CD44+/CD24− cells consistent with increased mammosphere-forming efficiency [33], suggesting the drug-resistant characteristics of CSCs. The CSCs also showed radio-resistance characteristics due to preferential activation of the DNA damage checkpoint response, and an increase in DNA repair capacity [34]. It was found that the fraction of glioma cells expressing CD133, a marker for brain cancer stem cells, was enriched after radiation in gliomas [34], suggesting that the CD133+ tumor cells represent glioma CSCs that are resistant to radiation.
CSCs also showed their propensity to invasion and metastasis, and it was reported that c-Jun, KLF-4, and c-kit signaling contributed to both self-renewal and invasion characteristics of CSCs [35,36]. Charafe-Jauffret et al isolated breast cancer cell lines which contained functional CSCs with high metastatic capacity consistent with alterations in the molecular signature of CSCs [37]. Similarly, Odoux also found that metastatic colon cancer contained CSCs with stem cell characteristics such as self-renewal and the ability to initiate tumors in animal [38]. These results suggest that CSCs are critically involved in cancer invasion and metastasis.
Similar to other CSCs, pancreatic CSCs also contribute to drug resistant, high invasive and metastatic features of PC. Gemicitabine is commonly used for the treatment of PC. In an in vitro study, high-dose of gemcitabine eliminated most of PC cells [39]; however, CD44+ CSCs proliferated and reconstituted the population of resistant cells. Clinical data showed that the patients with CD44-positive PC had poor prognosis [39]. In addition, pancreatic CD44+/CD24+ also demonstrated increased resistance to gemcitabine [40]. These findings suggest that pancreatic CD44+ or CD24+ CSCs were responsible for gemcitabine resistance and poor prognosis. Studies also showed that gemcitabine resistant PC cells were better capable in forming colonies and had a higher percentage of CD133+ cancer stem cells [41], and that human PC tissues contained pancreatic CD133+ CSCs that were highly tumorigenic and resistant to conventional chemotherapy [8]. Moreover, CD133+ cells showed enhanced migration and invasion, particularly when co-cultured with primary pancreatic stromal cells which express stromal derived factor-1, the ligand for CXCR4 [42]. Consistent with this observation, in vivo study showed that CD133+/CXCR4+ CSCs were located in the invasive front of pancreatic tumors, suggesting the higher invasive and metastatic phenotype of pancreatic CD133+/CXCR4+ CSCs [8]. Furthermore, removing these migrating CD133+/CXCR4+ CSCs significantly decreased the metastatic ability of pancreatic tumors [8]. In addition, pancreatic CD44+/CD24+ CSCs also showed increased migration ability [40]. These results collectively demonstrate that pancreatic CSCs are resistant to conventional therapeutics and contribute to cancer invasion and metastasis.
3.2. Role of EMT in PC drug resistance and metastasis
EMT has also been implicated in drug resistance, invasion, and metastasis. Studies have shown that TGF-β could induce EMT which leaded to erlotinib resistance. These erlotinib resistant cancer cells acquired mesenchymal phenotype and exhibited down-regulation of E-cadherin expression [43]. Another recent report has shown that over-expression of EMT-related transcription factor Twist and Snail could up-regulate the expression of ABC transporters, leading to drug resistance [44]. Therefore, the acquisition of EMT phenotype is important on conferring drug resistance characteristics to cancer cells against conventional chemotherapeutic agents. For most epithelial cancers, EMT-type cells could degrade the surrounding matrix to enable invasion and intravasation, leading to the formation of metastasis. It was found that cancer cells in the tumor center maintained high expression of E-cadherin and cytoplasmic β-catenin; however, the cancer cells in the periphery of tumor with propensity for invasion lost the expression of E-cadherin and gained the expression of vimentin, which are the typical characteristics of EMT phenotype [45]. Moreover, highly invasive breast cancer cells showed EMT characteristics associated with decreased levels of E-cadherin and increased levels of vimentin, fibronectin, Twist, and AKT2 expression [46], suggesting the critical role of EMT in cancer invasion. The role of EMT in metastasis has also been investigated in vitro and in vivo studies. Twist is an important regulator in EMT, and studies have shown that the suppression of Twist expression in highly metastatic mammary carcinoma cells could significantly inhibit cancer cell metastasis to the lung [47]. Moreover, ectopic expression of Twist caused loss of E-cadherinn, activation of mesenchymal markers, and induction of cell motility, suggesting that Twist could enhance metastasis through promoting EMT. Studies have also shown that human breast cancer cells, which metastasized to lung, had up-regulated Wnt signaling and EMT signatures, and that consequent inhibition of Wnt signaling re-expressed epithelial markers, inhibited EMT transcription factors Slug and Twist, and reduced the capacity of cancer cells to self-renew in vivo [48].
EMT occurred in PC also influences the sensitivity of PC cells to chemotherapeutic agents. We have previously reported that gemcitabine-sensitive PC cells including L3.6pl, Colo357, BxPC-3, and HPAC cells had strong expression of epithelial marker E-cadherin; however, gemcitabine-resistant PC cells such as MiaPaCa-2, PANC-1, and AsPC-1 showed strong expression of mesenchymal markers including vimentin and ZEB-1, suggesting that the gemcitabine-resistant cells elicits EMT phenotype which could in part be responsible for drug resistance [3]. Consistent with our findings, other investigators also found that L3.6pl, BxPC-3, CFPAC-1, and SU86.86 cells were drug-sensitive whereas PANC-1, Hs766T, AsPC-1, MIAPaCa-2, and MPanc96 cells, which showed EMT phenotype, were resistant to three commonly used chemotherapeutics such as gemcitabine, 5-fluorouracil (5-FU), and cisplatin [20]. EMT is also responsible for the higher invasive and metastatic ability of PC cells. It has been found that over-expression of Snail induced EMT in BxPC3 cells, which originally showed epithelial phenotype, and that Snail induced EMT promoted distant metastasis and invasiveness in vivo [49]. Another study also showed that up-regulation of Snail silenced E-cadherin expression, leading to the induction of EMT with increased metastatic potential in vivo [50]. These results demonstrate that the acquisition of EMT phenotype is in part responsible for higher propensity for invasion and metastasis in PC.
4. Targeting pancreatic CSCs and EMT-type cells to increase drug sensitivity and inhibit cancer cell invasion and metastasis
Recently, growing investigations have focused on the designing of novel therapeutic strategies to target CSCs and EMT-type cells in order to increase drug sensitivity and inhibition of cancer cell invasion and metastasis. These novel therapeutic strategies could have great potential for better treatment outcome in cancer therapy, especially for the treatment of PC because of intrinsic (de novo) and extrinsic (acquired) resistant characteristics of PC. Therefore, discovery of novel agents based on the molecular mechanisms underlying the regulation of CSCs and EMT would likely lead to the development of novel strategies for effective eradication of pancreatic CSCs/EMT-type cells and complete elimination of PC cells in patients diagnosed with this deadly disease.
In a drug screening study for agents that specifically kill CSCs, Gupta et al. have identified a selective agent salinomycin which showed high CSC-specific toxicity [51]. Salinomycin was able to reduce the proportion of CSCs by more than 100-fold relative to paclitaxel, a commonly used chemotherapeutic drug. Pre-clinical animal study showed that salinomycin could inhibit mammary tumor growth and induce epithelial differentiation of cancer cells [51]. Since salinomycin inhibited the growth of CSCs, a study was conducted in PC using salinomycin combined with gemcitabine which suppresses the viability of non-CSC PC cells. It was found that salinomycin combined with gemcitabine could eliminate the engraftment of human PC more effectively than either agents alone, suggesting that administration of salinomycin, which targets CSCs, may be a potential therapeutic strategy for improving the efficacy of gemcitabine for the treatment of patients diagnosed with PC [52].
EpCAM is another pancreatic CSC marker. Recently, a study has been conducted to investigate whether a targeted immunotherapy to EpCAM using EpCAM/CD3-bispecific T-cell-engaging antibody MT110 are able to eradicate pancreatic CSCs. It was found that the highly tumorigenic pancreatic CSCs were efficiently targeted by the antibody MT110 in vitro and in a mouse model of pancreatic cancer in vivo. Importantly, T-cells could be effectively redirected against highly tumorigenic pancreatic CSCs by T-cell-engaging EpCAM/CD3-bispecific antibody MT110, leading to the loss of tumorigenicity of pancreatic CSCs. These early findings clearly open newer avenues for designing novel immunotherapy for the treatment of PC mediated through eradication of CSCs [53]. In another study, it was found that RON was highly expressed and sustained in pancreatic CD24+/CD44+/ESA+ CSCs. To target pancreatic CD24+/CD44+/ESA+ CSCs, an anti-RON antibody with doxorubicin [anti-RON antibody Zt/c9-directing doxorubicin-immunoliposomes (Zt/c9-Dox-IL)] was engineered [54]. The authors have found that Zt/c9-Dox-IL specifically interacted with pancreatic CD24+/CD44+/ESA+ CSCs and rapidly caused RON internalization, leading to the uptake of liposome-coated doxorubicin. Importantly, Zt/c9-Dox-IL significantly reduced viability of pancreatic CD24+/CD44+/ESA+ CSCs [54], suggesting another novel agent targeting pancreatic CSCs in immunotherapy.
It has been reported that miR-34a is a tumor suppressor which inhibits tumorigenesis and cancer progression. The miR-34a could also participate in the regulation of CSCs especially because it was found that CSC maker CD44 was a direct target of miR-34a [55]. Studies showed that in CD44+ cells, the level of miR-34a was very low and that forced re-expression of miR-34a in CD44+ cells inhibited clonogenic expansion and metastasis. Moreover, animal studies have shown that systemically delivered miR-34a inhibited cancer metastasis and extended the survival of tumor-bearing mice [55]. Therefore, the strategies for re-expression of miR-34a by novel approaches could become newer therapeutic option for the treatment of cancers by targeted inhibition of CSCs to eliminate tumor metastasis. It has also been shown that demethylation agent 5-Aza-2′-deoxycytidine (5-Aza-dC) and HDAC inhibitor SAHA could up-regulate the expression of miR-34a in pancreatic CSCs [56]. The up-regulation of miR-34a caused inhibition of Bcl-2, CDK6 and SIRT1, which are the putative targets of miR-34a. In addition, SAHA also inhibited Notch signaling, leading to the inhibition of EMT consistent with up-regulation of E-cadherin and down-regulation of N-cadherin and ZEB1 expression [56]. 5-Aza-dC and SAHA also inhibited migration and invasion of pancreatic CSCs in vitro [56], suggesting that restoration of miR-34a expression by 5-Aza-dC and SAHA in pancreatic CSCs could be a novel strategy to eliminate pancreatic CSCs for the treatment of PC.
Nodal and Activin belong to the TGF-β superfamily and are overexpressed in pancreatic CSCs. A recent study has shown that the knock-down or inhibition of Nodal/Activin receptor Alk4/7 in CSCs could reduce self-renewal capacity, in vivo tumorigenicity, and overcome resistance to gemcitabine [57], suggesting that inhibition of Alk4/7 could be a therapeutic strategy for targeting pancreatic CSCs. Sorafenib (SO) is a multi-kinase inhibitor currently being used for the treatment of advanced kidney and liver cancers. It was found that the treatment of pancreatic CSCs with SO could inhibit CSC clonogenicity, spheroid formation, aldehyde dehydrogenase 1 (ALDH1) activity, and CSC growth in SCID mice; however, SO also caused activation of NF-κB, leading to survival and regrowth of spheroids. To enhance the effects of SO on CSCs, sulforaphane (SF) which could eliminate pancreatic CSCs by down-regulation of NF-κB was used in combination with SO. The authors have found that SF completely abrogated SO-induced NF-κB activation. Combination treatment inhibited clonogenicity, spheroid formation, ALDH1 activity, and migratory capacity. Moreover, the combination treatment also reduced tumor size in vivo in a synergistic manner, suggesting that the combination treatment with SO and SF is a promising therapeutic strategy for eliminating pancreatic CSCs [58].
Recently, natural agents have received much attention in the field of cancer therapy. Because of the non-toxic and anti-cancer characteristics of natural agents, these compounds could be used in combination with chemotherapeutic agents to enhance the efficacy of chemotherapeutics to achieve better treatment outcome. We have investigated whether natural agents including isoflavone, 3,3′-diindolylmethane (DIM), and CDF (curcumin analogue with greater bioavailability) could inhibit cancer stem-like cells and EMT-type cells through the regulation of important cell signaling molecules and miRNAs in PC. We found that DIM and isoflavone treatments increased the level of miR-200 family in EMT-type MiaPaCa-2 cells [3]. Moreover, DIM or isoflavone treatment also up-regulated epithelial marker E-cadherin and down-regulated mesenchymal markers, ZEB1, vimentin and slug, suggesting that these natural agents could revert the EMT phenotype, which would likely make these cells sensitive to conventional therapeutics. It has also been found that E-cadherin was re-distributed in the cytoplasm closer to the cell membrane after DIM and isoflavone treatments [3]. In addition, the morphology of MiaPaCa-2 cells changed from elongated fibroblastoid to epithelial cobblestone-like appearance, suggesting that DIM and isoflavone could reverse EMT phenotype as stated above. More importantly, we found that the sensitivity of EMT-type cells to gemcitabine was significantly increased after miR-200b transfection [3], and the cells transfected with miR-200b showed 20.8% to 38.2% more inhibition compared to control cells. Furthermore, pre-treatment of MiaPaCa-2 EMT-type cells with DIM or isoflavone also increased the sensitivity of MiaPaCa-2 EMT-type cells to gemcitabine [3]. These findings demonstrate that DIM or isoflavone treatment could partially increase the sensitivity of gemcitabine-resistant EMT-type cells to gemcitabine through miR-200 mediated reversal of EMT phenotype.
We also found that CDF could significantly inhibit sphere-forming ability of PC cells, increase disintegration of pancreatospheres, and attenuate the expression of CSC markers (CD44 and EpCAM) in gemcitabine-resistant (MIAPaCa-2) PC cells, which contain high proportion of CSCs and showed increased expression of miR-21 and decreased expression of miR-200 [59]. Since histone methyltransferase EZH2 is a regulator of CSC function, we tested the effects of CDF on EZH2 and we found that CDF could inhibit the expression of EZH2 which in part could be due to observed increased expression of tumor-suppressive miRNAs including let-7a, let-7b, let-7c, let-7d, miR-26a, miR-101, miR-146a, miR-200b, and miR-200c which are typically lost in PC [60]. Moreover, we found that re-expression of miR-101 inhibited the expression of EZH2 and EpCAM, and further studies in animal model showed that administration of CDF could inhibit tumor growth with reduced expression of EZH2, Notch-1, CD44, EpCAM, and Nanog, and increased expression of let-7, miR-26a, and miR-101 [60]. These findings suggest that CDF is a potent natural agent which could be used for the elimination of pancreatic CSCs, and thus CDF could become a newer agent for the treatment of PC.
Studies have shown that oral administration of metformin in diabetes patients are associated with reduced risk of PC. We found that metformin significantly decreased cell survival, clonogenicity, and sphere-forming capacity in PC cells. These effects of metformin could be mediated by decreased expression of CSC markers (CD44, EpCAM, EZH2, Notch-1, Nanog and Oct4) and increased expression of tumor suppressive miRNAs (let-7a, let-7b, miR-26a, miR-101, miR-200b, and miR-200c) [61]. DCAMKL-1 is another putative pancreatic stem cell marker, and it is found that DCAMKL-1 co-localized with vimentin and 14-3-3σ within premalignant PanIN lesions in an established k-Ras transgenic mouse model of PC. Knock-down of DCAMKL-1 in human PC cells induced EMT inhibitor miR-200a and down-regulated EMT-associated transcription factors ZEB1, ZEB2, Snail, Slug, and Twist, leading to the inhibition of EMT and tumorigenesis [62]. These results provide evidence establishing molecular link between DCAMKL-1, miR-200, and EMT in PC, and further suggesting that targeting DCAMKL-1 could be a therapeutic avenue for the treatment of PC. As mentioned in previous sections that Notch signaling is activated in CSCs and EMT-type cells. In our previous studies, we found that inactivation of Notch signaling in PC could partially reverse the EMT by down-regulation of vimentin, ZEB1, Slug, Snail, and NF-κB [63], suggesting that Notch inhibitors could suppress pancreatic CSCs and EMT-type cells. Collectively, all these findings demonstrate that targeting CSCs and EMT-type cells could be a novel strategy for the treatment of PC.
5. Conclusions
In the past few years, significant advances have been made in the field of pancreatic cancer CSCs. There is no doubt that pancreatic cancer contains CSCs and EMT-type cells, which are responsible for de novo and acquired drug-resistant and high invasive and metastatic propensity of PC. The molecular pathways altered in CSCs and EMT-type cells mainly include Hedgehog, Notch, Wnt, TGF-β, and miRNA regulated signaling. It is important to note that other signaling pathways such as Akt and NF-κB which are altered in cancers also participate in the regulation of CSCs and EMT-type cells in PC. With the discovery of CSC/EMT markers and molecular mechanisms underlying CSCs and EMT-type cell development and maintenance, novel therapeutic strategies could be envisaged to target CSCs and EMT-type cells in order to increase drug sensitivity and thereby eliminate the propensity of invasion and metastasis. Emerging investigations on targeting pancreatic CSCs and EMT-type cells have provided some novel agents that could become useful for the elimination of pancreatic CSCs and EMT-type cells; however, more in vivo animal studies and subsequent clinical trials are needed for testing the efficacy of these novel agents. It is worth mentioning that several natural agents have shown their promising inhibitory effects on pancreatic CSCs and EMT-type cells, and thus these non-toxic CSCs targeting agents could become novel approach for combination therapy with conventional therapeutics for the treatment of PC in order to achieve better treatment outcome. In conclusion, specific targeting of pancreatic CSCs and EMT-type cells would certainly open newer avenues toward better treatment of PC for which there is no curative therapy.
Acknowledgments
The authors’ work cited in this review article was funded by grants from the National Cancer Institute, NIH (5R01CA083695, 5R01CA108535, 5R01CA132794, 5R01CA131151, and 1R01CA154321 awarded to FHS). We also thank Puschelberg and Guido foundations for their generous contribution to support our research.
Footnotes
Conflict of Interest Statement
None Declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Borovski T, Melo De Sousa E, Vermeulen L, Medema JP. Cancer stem cell niche: the place to be. Cancer Res. 2011;71:634–639. doi: 10.1158/0008-5472.CAN-10-3220. [DOI] [PubMed] [Google Scholar]
- 2.Chen X, Lingala S, Khoobyari S, Nolta J, Zern MA, Wu J. Epithelial mesenchymal transition and hedgehog signaling activation are associated with chemoresistance and invasion of hepatoma subpopulations. J Hepatol. 2011;55:838–845. doi: 10.1016/j.jhep.2010.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li Y, VandenBoom TG, Kong D, Wang Z, Ali S, Philip PA, Sarkar FH. Up-regulation of miR-200 and let-7 by natural agents leads to the reversal of epithelial-to-mesenchymal transition in gemcitabine-resistant pancreatic cancer cells. Cancer Res. 2009;69:6704–6712. doi: 10.1158/0008-5472.CAN-09-1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 5.Bonnet D, Dick JE. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat Med. 1997;3:730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 6.Yi JM, Tsai HC, Glockner SC, Lin S, Ohm JE, Easwaran H, James CD, Costello JF, Riggins G, Eberhart CG, Laterra J, Vescovi AL, Ahuja N, Herman JG, Schuebel KE, Baylin SB. Abnormal DNA methylation of CD133 in colorectal and glioblastoma tumors. Cancer Res. 2008;68:8094–8103. doi: 10.1158/0008-5472.CAN-07-6208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patrawala L, Calhoun-Davis T, Schneider-Broussard R, Tang DG. Hierarchical organization of prostate cancer cells in xenograft tumors: the CD44+alpha2beta1+ cell population is enriched in tumor-initiating cells. Cancer Res. 2007;67:6796–6805. doi: 10.1158/0008-5472.CAN-07-0490. [DOI] [PubMed] [Google Scholar]
- 8.Hermann PC, Huber SL, Herrler T, Aicher A, Ellwart JW, Guba M, Bruns CJ, Heeschen C. Distinct populations of cancer stem cells determine tumor growth and metastatic activity in human pancreatic cancer. Cell Stem Cell. 2007;1:313–323. doi: 10.1016/j.stem.2007.06.002. [DOI] [PubMed] [Google Scholar]
- 9.Li C, Heidt DG, Dalerba P, Burant CF, Zhang L, Adsay V, Wicha M, Clarke MF, Simeone DM. Identification of pancreatic cancer stem cells. Cancer Res. 2007;67:1030–1037. doi: 10.1158/0008-5472.CAN-06-2030. [DOI] [PubMed] [Google Scholar]
- 10.Immervoll H, Hoem D, Sakariassen PO, Steffensen OJ, Molven A. Expression of the “stem cell marker” CD133 in pancreas and pancreatic ductal adenocarcinomas. BMC Cancer. 2008;8:48. doi: 10.1186/1471-2407-8-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maeda S, Shinchi H, Kurahara H, Mataki Y, Maemura K, Sato M, Natsugoe S, Aikou T, Takao S. CD133 expression is correlated with lymph node metastasis and vascular endothelial growth factor-C expression in pancreatic cancer. Br J Cancer. 2008;98:1389–1397. doi: 10.1038/sj.bjc.6604307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee HJ, You DD, Choi DW, Choi YS, Kim SJ, Won YS, Moon HJ. Significance of CD133 as a cancer stem cell markers focusing on the tumorigenicity of pancreatic cancer cell lines. J Korean Surg Soc. 2011;81:263–270. doi: 10.4174/jkss.2011.81.4.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding Q, Yoshimitsu M, Kuwahata T, Maeda K, Hayashi T, Obara T, Miyazaki Y, Matsubara S, Natsugoe S, Takao S. Establishment of a highly migratory subclone reveals that CD133 contributes to migration and invasion through epithelial-mesenchymal transition in pancreatic cancer. Hum Cell. 2011 doi: 10.1007/s13577-011-0037-9. [DOI] [PubMed] [Google Scholar]
- 14.Li C, Wu JJ, Hynes M, Dosch J, Sarkar B, Welling TH, Pasca di MM, Simeone DM. c-Met is a marker of pancreatic cancer stem cells and therapeutic target. Gastroenterology. 2011;141:2218–2227. doi: 10.1053/j.gastro.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 15.Kim MP, Fleming JB, Wang H, Abbruzzese JL, Choi W, Kopetz S, McConkey DJ, Evans DB, Gallick GE. ALDH activity selectively defines an enhanced tumor-initiating cell population relative to CD133 expression in human pancreatic adenocarcinoma. PLoS One. 2011;6:e20636. doi: 10.1371/journal.pone.0020636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rasheed Z, Wang Q, Matsui W. Isolation of stem cells from human pancreatic cancer xenografts. J Vis Exp. 2010 doi: 10.3791/2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wei HJ, Yin T, Zhu Z, Shi PF, Tian Y, Wang CY. Expression of CD44, CD24 and ESA in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary Pancreat Dis Int. 2011;10:428–434. doi: 10.1016/s1499-3872(11)60073-8. [DOI] [PubMed] [Google Scholar]
- 18.Moreno-Bueno G, Portillo F, Cano A. Transcriptional regulation of cell polarity in EMT and cancer. Oncogene. 2008;27:6958–6969. doi: 10.1038/onc.2008.346. [DOI] [PubMed] [Google Scholar]
- 19.Floor S, van Staveren WC, Larsimont D, Dumont JE, Maenhaut C. Cancer cells in epithelial-to-mesenchymal transition and tumor-propagating-cancer stem cells: distinct, overlapping or same populations. Oncogene. 2011;30:4609–4621. doi: 10.1038/onc.2011.184. [DOI] [PubMed] [Google Scholar]
- 20.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, Gallick GE, Logsdon CD, McConkey DJ, Choi W. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–5828. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Javle MM, Gibbs JF, Iwata KK, Pak Y, Rutledge P, Yu J, Black JD, Tan D, Khoury T. Epithelial-mesenchymal transition (EMT) and activated extracellular signal-regulated kinase (p-Erk) in surgically resected pancreatic cancer. Ann Surg Oncol. 2007;14:3527–3533. doi: 10.1245/s10434-007-9540-3. [DOI] [PubMed] [Google Scholar]
- 22.Bao B, Wang Z, Ali S, Kong D, Banerjee S, Ahmad A, Li Y, Azmi AS, Miele L, Sarkar FH. Over-expression of FoxM1 leads to epithelial-mesenchymal transition and cancer stem cell phenotype in pancreatic cancer cells. J Cell Biochem. 2011;112:2296–2306. doi: 10.1002/jcb.23150. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 23.Bao B, Wang Z, Ali S, Kong D, Li Y, Ahmad A, Banerjee S, Azmi AS, Miele L, Sarkar FH. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307:26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Thayer SP, di Magliano MP, Heiser PW, Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernandez-del CC, Yajnik V, Antoniu B, McMahon M, Warshaw AL, Hebrok M. Hedgehog is an early and late mediator of pancreatic cancer tumorigenesis. Nature. 2003;425:851–856. doi: 10.1038/nature02009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh BN, Fu J, Srivastava RK, Shankar S. Hedgehog signaling antagonist GDC-0449 (Vismodegib) inhibits pancreatic cancer stem cell characteristics: molecular mechanisms. PLoS One. 2011;6:e27306. doi: 10.1371/journal.pone.0027306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stemmer V, de CB, Berx G, Behrens J. Snail promotes Wnt target gene expression and interacts with beta-catenin. Oncogene. 2008;27:5075–5080. doi: 10.1038/onc.2008.140. [DOI] [PubMed] [Google Scholar]
- 27.Kong D, Wang Z, Sarkar SH, Li Y, Banerjee S, Saliganan A, Kim HR, Cher ML, Sarkar FH. Platelet-derived growth factor-D overexpression contributes to epithelial-mesenchymal transition of PC3 prostate cancer cells. Stem Cells. 2008;26:1425–1435. doi: 10.1634/stemcells.2007-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nicoloso MS, Spizzo R, Shimizu M, Rossi S, Calin GA. MicroRNAs--the micro steering wheel of tumour metastases. Nat Rev Cancer. 2009;9:293–302. doi: 10.1038/nrc2619. [DOI] [PubMed] [Google Scholar]
- 29.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur HA, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 30.Jung DE, Wen J, Oh T, Song SY. Differentially expressed microRNAs in pancreatic cancer stem cells. Pancreas. 2011;40:1180–1187. doi: 10.1097/MPA.0b013e318221b33e. [DOI] [PubMed] [Google Scholar]
- 31.Shafee N, Smith CR, Wei S, Kim Y, Mills GB, Hortobagyi GN, Stanbridge EJ, Lee EY. Cancer stem cells contribute to cisplatin resistance in Brca1/p53-mediated mouse mammary tumors. Cancer Res. 2008;68:3243–3250. doi: 10.1158/0008-5472.CAN-07-5480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Todaro M, Alea MP, Di Stefano AB, Cammareri P, Vermeulen L, Iovino F, Tripodo C, Russo A, Gulotta G, Medema JP, Stassi G. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 33.Li X, Lewis MT, Huang J, Gutierrez C, Osborne CK, Wu MF, Hilsenbeck SG, Pavlick A, Zhang X, Chamness GC, Wong H, Rosen J, Chang JC. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J Natl Cancer Inst. 2008;100:672–679. doi: 10.1093/jnci/djn123. [DOI] [PubMed] [Google Scholar]
- 34.Bao S, Wu Q, McLendon RE, Hao Y, Shi Q, Hjelmeland AB, Dewhirst MW, Bigner DD, Rich JN. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 35.Jiao X, Katiyar S, Willmarth NE, Liu M, Ma X, Flomenberg N, Lisanti MP, Pestell RG. c-Jun induces mammary epithelial cellular invasion and breast cancer stem cell expansion. J Biol Chem. 2010;285:8218–8226. doi: 10.1074/jbc.M110.100792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yu F, Li J, Chen H, Fu J, Ray S, Huang S, Zheng H, Ai W. Kruppel-like factor 4 (KLF4) is required for maintenance of breast cancer stem cells and for cell migration and invasion. Oncogene. 2011;30:2161–2172. doi: 10.1038/onc.2010.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Charafe-Jauffret E, Ginestier C, Iovino F, Wicinski J, Cervera N, Finetti P, Hur MH, Diebel ME, Monville F, Dutcher J, Brown M, Viens P, Xerri L, Bertucci F, Stassi G, Dontu G, Birnbaum D, Wicha MS. Breast cancer cell lines contain functional cancer stem cells with metastatic capacity and a distinct molecular signature. Cancer Res. 2009;69:1302–1313. doi: 10.1158/0008-5472.CAN-08-2741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Odoux C, Fohrer H, Hoppo T, Guzik L, Stolz DB, Lewis DW, Gollin SM, Gamblin TC, Geller DA, Lagasse E. A stochastic model for cancer stem cell origin in metastatic colon cancer. Cancer Res. 2008;68:6932–6941. doi: 10.1158/0008-5472.CAN-07-5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hong SP, Wen J, Bang S, Park S, Song SY. CD44-positive cells are responsible for gemcitabine resistance in pancreatic cancer cells. Int J Cancer. 2009 doi: 10.1002/ijc.24573. [DOI] [PubMed] [Google Scholar]
- 40.Yin T, Wei H, Gou S, Shi P, Yang Z, Zhao G, Wang C. Cancer stem-like cells enriched in panc-1 spheres possess increased migration ability and resistance to gemcitabine. Int J Mol Sci. 2011;12:1595–1604. doi: 10.3390/ijms12031595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu G, Li F, Ouyang K, Xie F, Tang X, Wang K, Han S, Jiang Z, Zhu M, Wen D, Qin X, Zhang L. Intrinsic gemcitabine resistance in a novel pancreatic cancer cell line is associated with cancer stem cell-like phenotype. Int J Oncol. 2011 doi: 10.3892/ijo.2011.1254. [DOI] [PubMed] [Google Scholar]
- 42.Moriyama T, Ohuchida K, Mizumoto K, Cui L, Ikenaga N, Sato N, Tanaka M. Enhanced cell migration and invasion of CD133+ pancreatic cancer cells cocultured with pancreatic stromal cells. Cancer. 2010;116:3357–3368. doi: 10.1002/cncr.25121. [DOI] [PubMed] [Google Scholar]
- 43.Suda K, Tomizawa K, Fujii M, Murakami H, Osada H, Maehara Y, Yatabe Y, Sekido Y, Mitsudomi T. Epithelial to mesenchymal transition in an epidermal growth factor receptor-mutant lung cancer cell line with acquired resistance to erlotinib. J Thorac Oncol. 2011;6:1152–1161. doi: 10.1097/JTO.0b013e318216ee52. [DOI] [PubMed] [Google Scholar]
- 44.Saxena M, Stephens MA, Pathak H, Rangarajan A. Transcription factors that mediate epithelial-mesenchymal transition lead to multidrug resistance by upregulating ABC transporters. Cell Death Dis. 2011;2:e179. doi: 10.1038/cddis.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brabletz T, Jung A, Reu S, Porzner M, Hlubek F, Kunz-Schughart LA, Knuechel R, Kirchner T. Variable beta-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A. 2001;98:10356–10361. doi: 10.1073/pnas.171610498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 2007;67:1979–1987. doi: 10.1158/0008-5472.CAN-06-1479. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 48.DiMeo TA, Anderson K, Phadke P, Feng C, Perou CM, Naber S, Kuperwasser C. A novel lung metastasis signature links Wnt signaling with cancer cell self-renewal and epithelial-mesenchymal transition in basal-like breast cancer. Cancer Res. 2009;69:5364–5373. doi: 10.1158/0008-5472.CAN-08-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishioka R, Itoh S, Gui T, Gai Z, Oikawa K, Kawai M, Tani M, Yamaue H, Muragaki Y. SNAIL induces epithelial-to-mesenchymal transition in a human pancreatic cancer cell line (BxPC3) and promotes distant metastasis and invasiveness in vivo. Exp Mol Pathol. 2010;89:149–157. doi: 10.1016/j.yexmp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 50.von BJ, Eser S, Paul MC, Seidler B, Brandl M, Messer M, von WA, Schmidt A, Mages J, Pagel P, Schnieke A, Schmid RM, Schneider G, Saur D. E-cadherin regulates metastasis of pancreatic cancer in vivo and is suppressed by a SNAIL/HDAC1/HDAC2 repressor complex. Gastroenterology. 2009;137:361–71. 371. doi: 10.1053/j.gastro.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 51.Gupta PB, Onder TT, Jiang G, Tao K, Kuperwasser C, Weinberg RA, Lander ES. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell. 2009;138:645–659. doi: 10.1016/j.cell.2009.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang GN, Liang Y, Zhou LJ, Chen SP, Chen G, Zhang TP, Kang T, Zhao YP. Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Lett. 2011;313:137–144. doi: 10.1016/j.canlet.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 53.Cioffi M, Dorado J, Baeuerle PA, Heeschen C. EpCAM/CD3-Bispecific T-cell Engaging Antibody MT110 Eliminates Primary Human Pancreatic Cancer Stem Cells. Clin Cancer Res. 2012 doi: 10.1158/1078-0432.CCR-11-1270. [DOI] [PubMed] [Google Scholar]
- 54.Padhye SS, Guin S, Yao HP, Zhou YQ, Zhang R, Wang MH. Sustained Expression of the RON Receptor Tyrosine Kinase by Pancreatic Cancer Stem Cells as a Potential Targeting Moiety for Antibody-Directed Chemotherapeutics. Mol Pharm. 2011;8:2310–2319. doi: 10.1021/mp200193u. [DOI] [PubMed] [Google Scholar]
- 55.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nalls D, Tang SN, Rodova M, Srivastava RK, Shankar S. Targeting epigenetic regulation of miR-34a for treatment of pancreatic cancer by inhibition of pancreatic cancer stem cells. PLoS One. 2011;6:e24099. doi: 10.1371/journal.pone.0024099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lonardo E, Hermann PC, Mueller MT, Huber S, Balic A, Miranda-Lorenzo I, Zagorac S, Alcala S, Rodriguez-Arabaolaza I, Ramirez JC, Torres-Ruiz R, Garcia E, Hidalgo M, Cebrian DA, Heuchel R, Lohr M, Berger F, Bartenstein P, Aicher A, Heeschen C. Nodal/Activin signaling drives self-renewal and tumorigenicity of pancreatic cancer stem cells and provides a target for combined drug therapy. Cell Stem Cell. 2011;9:433–446. doi: 10.1016/j.stem.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 58.Rausch V, Liu L, Kallifatidis G, Baumann B, Mattern J, Gladkich J, Wirth T, Schemmer P, Buchler MW, Zoller M, Salnikov AV, Herr I. Synergistic activity of sorafenib and sulforaphane abolishes pancreatic cancer stem cell characteristics. Cancer Res. 2010;70:5004–5013. doi: 10.1158/0008-5472.CAN-10-0066. [DOI] [PubMed] [Google Scholar]
- 59.Bao B, Ali S, Kong D, Sarkar SH, Wang Z, Banerjee S, Aboukameel A, Padhye S, Philip PA, Sarkar FH. Anti-tumor activity of a novel compound-CDF is mediated by regulating miR-21, miR-200, and PTEN in pancreatic cancer. PLoS One. 2011;6:e17850. doi: 10.1371/journal.pone.0017850. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 60.Bao B, Ali S, Banerjee S, Wang Z, Logna F, Azmi AS, Kong D, Ahmad A, Li Y, Padhye S, Sarkar FH. Curcumin Analogue CDF Inhibits Pancreatic Tumor Growth by Switching on Suppressor microRNAs and Attenuating EZH2 Expression. Cancer Res. 2012;72:335–345. doi: 10.1158/0008-5472.CAN-11-2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bao B, Wang Z, Ali S, Ahmad A, Azmi AS, Sarkar S, Banerjee S, Kong D, Li Y, Thakur S, Sarkar FH. Metformin inhibits cell proliferation, migration and invasion by attenuating CSC function mediated by deregulating miRNAs in pancreatic cancer cells. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-11-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sureban SM, May R, Lightfoot SA, Hoskins AB, Lerner M, Brackett DJ, Postier RG, Ramanujam R, Mohammed A, Rao CV, Wyche JH, Anant S, Houchen CW. DCAMKL-1 regulates epithelial-mesenchymal transition in human pancreatic cells through a miR-200a-dependent mechanism. Cancer Res. 2011;71:2328–2338. doi: 10.1158/0008-5472.CAN-10-2738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Z, Li Y, Kong D, Banerjee S, Ahmad A, Azmi AS, Ali S, Abbruzzese JL, Gallick GE, Sarkar FH. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]