Adipose tissue is not an inert organ simply for energy storage but is immunologically active, producing over 50 proteins called adipokines that regulate various body functions. These include energy-regulating adipokines, leptin and adiponectin, that play an important role in various inflammatory conditions such as diabetes mellitus and atherosclerosis. Recent research suggests that leptin and adiponectin may also play a role in inflammatory lung conditions such as asthma and chronic obstructive pulmonary disease (COPD). Although there is an increasing body of work supporting an additional role for these adipokines in obstructive sleep apnea, this review will primarily focus on pulmonary diseases. Further, for the purpose of this review, we conducted multiple PubMed searches using the following keywords: leptin and asthma; leptin and COPD; leptin and emphysema; leptin and pneumonia; leptin and lung cancer; leptin and inflammation; and repeated the same searches with adiponectin instead of leptin. We reviewed the manuscripts associated with these searches and summarized our findings in this review.
1. Leptin and Pulmonary Diseases
1.1 Introduction
Leptin, a protein product of the ob gene, is synthesized and secreted mainly by white adipose tissue [1–3]. Systemic leptin concentrations increase with meals; estrogen and progesterone treatment [4]; pregnancy [5]; and infectious and inflammatory states [6, 7]. Its systemic concentrations display a circadian rhythm, with a nadir at around 8 in the morning [8].
Leptin is a primarily pro-inflammatory adipokine that affects both innate and adaptive immune responses. Leptin differentially increases production of TH1 cytokines (Interleukin or IL-2, interferon- γ and Tumor Necrosis Factor or TNF-α) and suppresses production of TH2 cytokines (IL-4, IL-5, and IL-10). Leptin also increases the release of Vascular Endothelial Growth Factor (VEGF) by airway smooth muscle cells [9]. VEGF may stimulate subepithelial neovascularization and vascular permeability, key findings in pathogenesis of various lung inflammatory states such as asthma [9]. Leptin further increases natural killer cell function [10, 11]; CD4+ T-lymphocyte proliferation; macrophage phagocytosis [10–15]; and monocyte proliferation [10].
Leptin is expressed by human lung, including bronchial epithelial cells and alveolar type II pneumocytes and macrophages [16, 17]. Leptin concentration in bronchoalveolar lavage fluid is strongly correlated with systemic values [18], suggesting that leptin is also transported from blood into lung by mechanisms that are not clearly understood. The leptin receptor (Ob-R) is expressed by human bronchial and alveolar epithelial cells, bronchial smooth muscle cells, and bronchial submucosa [19, 20]. The lung is therefore a likely target organ for leptin signaling. The possible role for leptin in asthma, COPD, pneumonia and lung cancer is discussed below.
1.2 Leptin and Asthma
1.2.1 Mouse studies
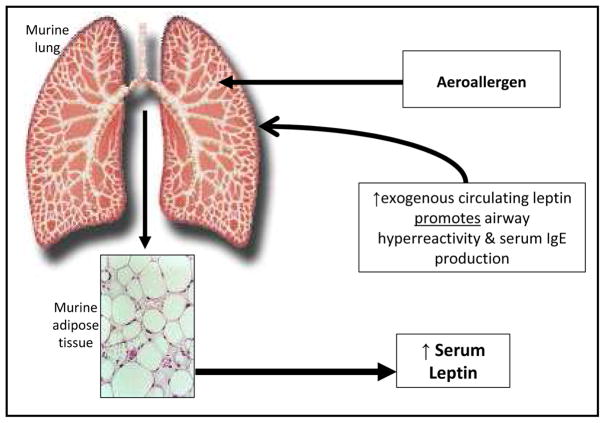
Obese mice have innate airway hyperresponsiveness that is at least partly explained by excess systemic leptin. Shore et al. demonstrated that administration of exogenous leptin in sensitized BALB/cJ mice augmented airway hyperreactivity following allergen challenge (Figure 1) [21].
Figure 1.
A schematic representation of the suggested role for leptin in murine asthma, based upon the work by Shore et al. [21]. Although these findings were not entirely reproduced in a human interventional study of mild atopic asthma [37], epidemiologic evidence suggests that greater serum leptin concentrations are associated with more severe asthma, particularly among prepubertal boys and peripubertal/postpubertal girls [31, 32].
1.2.2 Human Studies
Currently the human data regarding the independent association between serum leptin and asthma prevalence or severity remain inconclusive - although stronger among children than adults (Table 1).
Table 1.
A tabular summary of the evidence supporting a role for systemic leptin and adiponectin in asthma in humans
| Males | Females | ||||
|---|---|---|---|---|---|
| Population- based studies | Clinic/hospital based studies | Population- based studies | Clinic/hospital based studies | ||
| SYSTEMIC LEPTIN-ASTHMA ASSOCIATION | |||||
| Asthma prevalence | Adults | ↔ | ↔ | ↑/↔ | ↔ |
| Children | ↔ | ↑ (prepubertal) | ↔ | ↑ (peripubertal) | |
| Asthma severity | Adults | – | ↔ | – | ↔ |
| Children | – | ↑ (prepubertal) | – | ↑ (peri- & postpubertal) | |
| SYSTEMIC ADIPONECTIN-ASTHMA ASSOCIATION | |||||
| Asthma prevalence | Adults | ↑/↓/↔ | ↔ | ↓/↔ | ↔ |
| Children | ↔ | ↔ | ↔ | ↓ (peripubertal) | |
| Asthma severity | Adults | ↑ | ↔ | ↔ | ↔ |
| Children | – | ↓ (pre- & peripubertal) | – | ↔ | |
Key: ↑ means increased effect; ↓ means decreased effect; ↔ means no effect; – means no evidence is currently available.
1.2.3 Asthma Prevalence
Some but not all studies suggest that systemic leptin may be independently associated with greater odds for asthma prevalence, particularly among prepubertal boys, peripubertal/postpubertal girls, and premenopausal women.
1.2.3.1 Population-based studies
Sood et al. examined 5,876 participants of the U.S.-based Third National Health and Nutrition Examination Survey (NHANES III) and showed a positive association between the highest quartile of serum leptin concentration and odds for asthma in women [7], independent of triceps skin fold thickness [22]. These findings were however not confirmed by Sutherland et al. in a population-based birth cohort of approximately 1000 young adult New Zealanders [23] or by Jartti et al. [24] in a sequential cross-sectional study set within an established Finnish cohort.
1.2.3.2 Hospital-based and clinic–based studies
Adults
Several small case-control studies have failed to show a difference in serum or bronchoalveolar lavage fluid leptin concentrations between adult asthmatics and controls, after matching or adjusting for obesity [18, 25–27]. While Lessard et al. showed higher sputum leptin concentrations in adult asthmatics than controls, this association was confounded by the remarkably different body mass indices (BMI) between the groups [27].
Children
The leptin-asthma prevalence association is better demonstrated in case-control studies of children than adults. In two such studies of prepubertal children by Guler and Gurkan et al., asthma status was associated with higher serum leptin concentrations than controls, independent of BMI [28, 29]. Additionally, Guler found that this association was strongest among atopic boys [28] and Gurkan’s study included mostly boys as well [29]. In Mai’s study of peripubertal children, overweight asthmatics had twice as high levels of serum leptin as controls of similar BMI [30].
1.2.4 Asthma Severity
Some but not all studies suggest that systemic leptin may be associated with greater asthma severity, particularly among prepubertal boys and peripubertal/postpubertal girls.
Adults
In a small clinic-based case-control study, Holguin et al. showed no associations between concentrations of leptin in either serum or bronchoalveolar lavage fluid and lung inflammatory biomarkers [18].
Children
Unlike adults, the leptin-asthma severity association is better demonstrated in case-control studies of prepubertal boys and peripubertal/postpubertal girls. Serum leptin concentrations were positively associated with NHLBI category of asthma severity in a study of both sexes, regardless of BMI [31]. In a separate unadjusted analysis of prepubertal asthmatic children, mostly boys, serum leptin was associated with lower peak expiratory flow rates [29] and higher serum total IgE levels [28]. In another study of prepubertal asthmatic children, mostly boys, serum leptin was associated with greater severity of exercise induced bronchoconstriction, after adjustment for BMI [32]. Finally, Kattan et al. found a positive unadjusted correlation between serum leptin and maximum asthma symptom days in peripubertal/postpubertal asthmatic girls, although no sex interactions or BMI-adjustment were reported by the authors [33].
1.2.5 Subgroup effect
Among the studies showing an association between serum leptin and asthma prevalence or severity, the association seems to be stronger in specific population subgroups [28][22, 34–36] such as prepubertal boys, peripubertal or postpubertal girls, and premenopausal women. There are however no statistically significant interactions reported in the literature between serum leptin and either sex, menopause, age, atopy, or smoking on asthma outcomes.
Furthermore, even in studies demonstrating a leptin-asthma association, systemic leptin does not appear to be the only intermediary factor that explains the obesity-asthma association. This was reported by Sood et al. [22] where the association between BMI and asthma in women was only slightly attenuated after adjustment for serum leptin concentration. This suggests that other metabolic pathways and mechanical factors may be involved in the obesity-asthma association.
1.2.6 Interventional Studies
Exogenous leptin administration in sensitized mice exacerbates allergen-induced increase in airway hyperresponsiveness (Figure 1) [21]. Additionally, serum leptin concentrations increase following allergen challenge in sensitized mice [21]. The leptin-asthma relationship is therefore bidirectional in mice, whereby allergen inhalation affects serum leptin and exogenous leptin administration affects asthma (Figure 1). Among humans with mild atopic asthma, bronchoprovocation from inhalational allergen challenge does not acutely affect serum leptin concentrations [37].
There are many reasons for the observed discrepancy between murine and human results. First, there are key differences between murine asthma and human asthma [38]. Second, human studies are limited by the greater heterogeneity among subjects while murine studies are laboratory-based controlled experiments. Third, current epidemiologic studies are limited by lack of longitudinal and interventional data that are needed to establish a clear direction of association. Further, the only human interventional study [37] was small-sized; limited to subjects with mild atopic asthma; used relatively milder bronchoprovocation; and was not accompanied by a study of inflammatory cells and other cytokines.
To summarize, although leptin and its receptors are expressed in human airway cells, the leptin-asthma association is currently controversial, particularly among adults. The majority of the evidence among children however suggests that systemic leptin may be associated with greater asthma prevalence and severity, particularly among prepubertal boys and peripubertal/postpubertal girls. It is not currently known whether modulation of leptin, independent of BMI, may be helpful in asthma prevention or treatment.
1.3 Leptin and COPD
1.3.1 Laboratory studies
Leptin differentially affects airway innate and adaptive immune responses in mice after cigarette smoke exposure [39]. Mice deficient in leptin signalling pathway (i.e. ob/ob mice that lack leptin and db/db mice that lack the functional leptin receptor Ob-Rb), when exposed to cigarette smoke, demonstrate a greater recruitment of neutrophils (innate immune response) and lesser recruitment of CD4+ and CD8+ lymphocytes (adaptive immune response) to their lung tissue than similarly exposed wild type mice [39]. These findings suggest that leptin receptor activation is important in regulating the cigarette smoke-induced inflammatory response in the lung.
Cigarette smoke exposure also increases leptin expression in bronchial epithelial cells. This is demonstrated by in vivo experiments of wild type mice [39] and confirmed by in vitro experiments of cultured human primary bronchial epithelial cells [16]. Leptin-expressing bronchial epithelial cells are more frequently seen in the peripheral lung but less frequently seen in central airways of ever-smokers (with or without COPD) than never-smokers [16, 20].
Data on the effect of cigarette smoke exposure on leptin receptor expression in bronchial epithelial cells is more conflicting. Expression of leptin receptor is downregulated in exposed AKR/J mice, an in-bred strain of mice with an emphysema-like phenotype [40]. The murine findings are supported by a lower expression of leptin receptors by bronchial epithelial cells from central bronchi among smokers (with or without COPD) than never-smokers [20]. On the contrary, in vitro cigarette smoke exposure increases the expression of leptin receptor from cultured human primary bronchial epithelial cells [16].
The discrepant findings between studies using mice, cultured bronchial epithelial cells, and human lung tissue are not understood. Differences among species; among cigarette smoke exposure doses used; among in vitro and in vivo nature of experiments; and the wide heterogeneity of exposures among humans are some potential explanations. Further, there may even be differences in inflammatory characteristics between central and distal human airway epithelium [41] that may account for their different responses in leptin expression on exposure to cigarette smoke.
1.3.2 Human Studies
Currently the human data regarding the independent association between serum leptin concentrations and COPD prevalence or severity remain inconclusive and are possibly stronger among women than men (Table 2).
Table 2.
A tabular summary of the evidence supporting a role for systemic leptin and adiponectin in COPD in humans
| Male | Female | |||
|---|---|---|---|---|
| Population-based studies | Clinic/hospital based studies | Population- based studies | Clinic/hospital based studies | |
| SYSTEMIC LEPTIN-COPD ASSOCIATION | ||||
| COPD prevalence | – | ↔ | – | ↑ |
| COPD severity | – | ↑/↔ | ↑ | |
| SYSTEMIC ADIPONECTIN-COPD ASSOCIATION | ||||
| COPD prevalence | ↓* | ↑ | – | – |
| COPD severity | – | ↑ | – | – |
Key: ↑ means increased effect; ↓ means decreased effect; ↔ means no effect; – means no evidence is currently available.
Note 1:
This data was based on the association with lung function in healthy young adults [92].
1.3.3 COPD prevalence
BMI-adjusted serum leptin may predict COPD prevalence in women, as suggested by primarily small-sized cross-sectional or case-control studies.
1.3.3.1 Population-based longitudinal study
In a randomly selected subset of 429 European American current or former smokers from amongst 4,287 participants in the U.S.-based Lung Health Study, Hansel et al. demonstrated that 21 single nucleotide polymorphisms (SNPs) in leptin receptor gene were associated with a five-year decline in lung function, after adjustment for multiple comparisons and for covariates smoking and BMI [40].
1.3.3.2 Hospital-based and clinic–based studies
BMI-adjusted serum leptin concentrations may predict COPD prevalence in women. Breyer et al. found higher serum leptin concentrations among women with stable COPD than among BMI-matched healthy controls; this association was not seen in men (although a sex interaction term was not reported) [42]. Among men with and without COPD, no differences in systemic leptin, adjusted for fat mass, were noted in another small Korean case-control study [43]. In unadjusted results that likely reflect the confounding effect of low BMI of cachectic COPD subjects, Takabatake et al showed lower, rather than higher, serum leptin concentrations in men with COPD than without [44].
Airway leptin expression may predict COPD. COPD smokers show greater expression of both leptin and CD8 lymphocytes in their bronchial submucosa, as compared to healthy smokers or non-smokers, suggesting a role of leptin in regulating airway inflammation in COPD [20]. Similarly, peripheral lung specimens of current and former smokers with and without COPD demonstrate greater leptin expression in bronchial epithelial cells and alveolar macrophages than never smokers [16].
1.3.4 COPD severity
Systemic or airway leptin may be associated with greater COPD severity in primarily small-sized case-control studies.
Systemic leptin
Limited data suggest that systemic leptin is associated with greater disease severity in COPD. Breyer et al. demonstrated a positive unadjusted correlation between serum concentrations of leptin and C-reactive protein in women with COPD but not in men (although a sex interaction term was not provided) [42]. Schols et al. reported a positive correlation between plasma concentrations of leptin (adjusted for fat mass) and of soluble TNF receptor-55, a marker of systemic inflammation, among stable male patients with emphysema but not in chronic bronchitis [45]. On the other hand, other case-control studies did not confirm such an association between leptin and activity of the TNF-alpha system [42–44].
Airway leptin
Limited data suggest that airway leptin is associated with greater disease severity in COPD. Sputum leptin is positively correlated with other sputum inflammatory markers (i.e. C-reactive protein and TNF-alpha) in stable patients with COPD [46]. Further, the expression of leptin in bronchial submucosa of COPD patients is positively correlated with expression of CD8 T-lymphocytes (with decreased apoptotic death) and with GOLD stage of disease severity as well as inversely correlated with spirometric parameters [20].
1.3.5 Acute COPD exacerbations
Systemic leptin concentrations rise during acute COPD exacerbations and return to baseline several days to weeks later in the stable state following the resolution of the exacerbation [47–49]. These findings remain robust even after adjustment for percent fat mass [48, 49].
To summarize, systemic and airway leptin concentrations may be associated with greater odds of COPD prevalence, particularly among women, and reflect greater airway inflammation and disease severity. It is not currently known whether modulation of leptin, independent of BMI, may be helpful in COPD prevention and treatment.
1.4 Leptin and pneumonia
Ob/ob and db/db mice (with deficient leptin signaling) are more prone to bacterial infections and pneumonia than wild type mice [6, 7, 50–52]. The association between hyperleptinemia and pneumonia risk or mortality has not been directly studied in humans. Studies of obese subjects are conflicting regarding their increased risk for bacterial pneumonia compared to normal weight subjects [53–58]. On the other hand, hypoleptinemia, being a marker of poor nutritional status, is associated with poorer prognosis for pneumonia in one human study [59].
1.5 Leptin and non-small cell lung cancer
Shen et al. found that human lung cancer cell lines (A549 and H157) express leptin receptors and that leptin induces ‘immune escape’ of lung cancer cells by decreasing their apoptotic death [60]. Consistent with this finding, leptin expression in one study of non-small cell lung cancer specimens was associated with poor prognosis [61]. A human genetic study additionally demonstrated that a functional SNP of the leptin gene (LEP-2548 G/A) is associated with increased lung cancer prevalence [62]. Case-control studies however show variable associations between serum leptin concentrations and non-small cell lung cancer, partly due to the confounding effect of cancer cachexia [63–65]. Thus, the role of leptin in lung cancer is not entirely clear although the associated ‘immune escape’ of lung cancer cells is concerning.
To summarize, there is developing literature to suggest a potential role for leptin in inflammatory pulmonary conditions such as asthma, COPD, pneumonia and lung cancer. However, the current state of the literature suffers from many critical gaps that include a lack of adequately powered longitudinal and weight-intervention studies; inadequate adjustment for confounding effect of obesity; limited studies involving sputum or bronchoalveolar lavage fluid leptin or sophisticated physiological and inflammatory asthma/COPD outcomes; as well as unclear understanding of the tantalizing sex interactions.
2. Adiponectin and Pulmonary Diseases
2.1 Introduction
Adiponectin is a predominantly anti-inflammatory adipokine that inhibits proinflammatory cytokines (TNF-α, IL-6, and nuclear factor-κB) [66–68] and induces anti-inflammatory cytokines (IL-10 and IL-1 receptor antagonist) [68–70]. Under certain conditions, adiponectin however has pro-inflammatory effects as well [71, 72]. Although visceral adipocytes are its most important source [73], systemic adiponectin concentrations are reduced in obesity [74]. This may result from obesity-induced hypoxia-related necrosis of adipocytes. Necrotic adipocytes attract macrophages that collect and form syncytia around the adipocytes [75] and produce TNF-α and IL-6 that in turn, may inhibit the local production of adiponectin in a paracrine fashion [76].
In the circulation, adiponectin exists as low-, medium-, and high-molecular-weight complexes (LMW, MMW, and HMW, respectively) that may vary in efficacy regarding their effects on target tissues [77]. Recent studies suggest that the HMW isoform is the most biologically active isoform of systemic adiponectin in regulating insulin resistance [78, 79]. Whether the same is true for lung diseases is not known.
There is a gender difference in concentrations and isoform distribution of adiponectin with higher concentrations of total adiponectin, particularly the HMW isoform, among women than men [80]. These differences develop during puberty and are a result of inhibition of HMW adiponectin production by circulating testosterone [81].
Adiponectin and its receptors (AdipoR1, AdipoR2, T-cadherin, and calreticulin) are expressed on multiple cell types in the lung [82–85]. In addition, adiponectin is transported from blood into the alveolar lining fluid via the T-cadherin molecule on the endothelium [82]. Although human airway smooth muscle cells express adiponectin receptors, adiponectin does not regulate airway smooth muscle cell proliferation [9].
2.2 Adiponectin and Asthma
2.2.1 Mouse studies
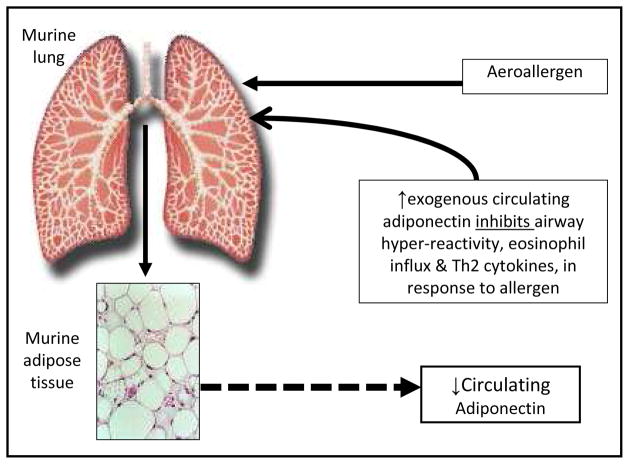
Shore et al. demonstrated that allergen bronchoprovocation among sensitized BALB/cJ mice reduced serum adiponectin concentrations (Figure 2) as well as the expression of adiponectin mRNA in adipose tissue and of adiponectin receptor mRNA in lung [86]. Shore further showed that exogenous adiponectin infusion attenuated allergic airway inflammation and airway hyperresponsiveness [86]. These findings were supported by another study of genetically adiponectin-deficient mice that develop greater allergic airway inflammation in response to allergen bronchoprovocation than wild type mice [87].
Figure 2.
A schematic representation of the suggested role for adiponectin in murine asthma, based upon the work by Shore et al. [86]. Although these findings were not entirely reproduced in a human interventional study of mild atopic asthma [37], epidemiologic evidence suggests that lower serum adiponectin concentrations are associated with increased odds for asthma, particularly among peripubertal girls and premenopausal women [34, 35]. Figure as originally published in ‘A. Sood, E. Dominic, C. Qualls, M.W. Steffes, B. Thyagarajan, L.J. Smith, C.E. Lewis, D.R. Jacobs, Jr., Serum Adiponectin is Associated with Adverse Outcomes of Asthma in Men but Not in Women, Front Pharmacol 2 (2011) 55’ is reproduced with permission [71].
2.2.2 Human Studies
Like leptin, the current human data regarding the independent association between serum adiponectin concentrations and asthma prevalence or severity remain inconclusive and possibly stronger among children than adults (Table 1).
2.2.3 Asthma prevalence
Some but not all studies demonstrate that serum adiponectin concentrations are inversely associated with asthma prevalence among premenopausal women and peripubertal girls.
2.2.3.1 Population based studies
One cross sectional study of American adults showed a protective association between serum adiponectin concentrations and odds for clinical diagnosis of asthma in premenopausal women, independent of BMI [35]. These findings were however not confirmed by Sutherland et al. in a population-based birth cohort of approximately 1,000 young adult New Zealanders [23] or by Jartti et al. [24] in a sequential cross-sectional study set within an established Finnish cohort. Surprisingly, serum adiponectin concentrations in the Sutherland study were positively associated with prevalent reversible airflow obstruction and inversely associated with exhaled nitric oxide among all men [23]. The former data suggest a pro-inflammatory effect of adiponectin and contradict the nitric oxide data which suggest an anti-inflammatory effect of adiponectin among men [23].
2.2.3.2 Clinic based studies
Adults
Several small case-control studies have shown no difference in serum or bronchoalveolar lavage fluid adiponectin concentrations between asthmatics and controls, after matching or adjusting for obesity [18, 25, 26].
Children
Nagel et al. demonstrated a protective association between serum adiponectin concentrations and risk for asthma in peripubertal girls, independent of BMI. This effect was stronger in nonatopic girls. These results were however not confirmed by a Korean study of similarly-aged children, mostly boys [88].
2.2.4 Asthma Severity
Although data are limited and confusing, serum adiponectin concentrations are inversely associated with asthma severity among boys and positively associated among men.
2.2.4.1 Population based studies
In a large community-based cross-sectional study, Sood et al. showed that serum adiponectin was positively associated with clinical measures of disease severity in men but not in women with asthma, with significant sex-specific interactions [71].
2.2.4.2 Clinic based studies
Adults
In a small case-control study, Holguin et al. showed no association between concentrations of serum or bronchoalveolar lavage fluid adiponectin and of lung inflammatory biomarkers [18].
Children
Serum adiponectin concentrations were associated with less severe exercise-induced bronchoconstriction in a study of pre-pubertal asthmatic children, mostly boys, after adjusting for BMI [32]. Serum adiponectin concentrations were also associated with fewer maximum asthma symptom days; fewer exacerbations; and higher FEV1/FVC ratio in a study including 14-year old boys with moderate to severe asthma [33]. Similarly, serum adiponectin was positively correlated with FEF25–75% in another study of prepubertal and peripubertal children, mostly boys [88].
2.2.5 Interventional Studies
Exogenous adiponectin administration in sensitized mice decreases allergen-induced increase in airway hyperresponsiveness (Figure 2). In addition, serum adiponectin concentrations decrease following allergen challenge in sensitized mice. The adiponectin-asthma relationship is therefore bidirectional in mice, whereby allergen inhalation affects serum adiponectin and exogenous adiponectin administration affects asthma. On the contrary, among humans with mild atopic asthma, bronchoprovocation from inhalational allergen challenge does not acutely affect serum adiponectin concentrations [37]. In support of this observation, asthma has no chronic effects on future serum adiponectin concentrations either [71]. This suggests that while the adiponectin-asthma association is bidirectional in mice, it may be unidirectional in humans.
The reasons for the observed discrepant results for adiponectin between murine and human interventional studies are the same as those discussed above for leptin [37, 86]. In addition, the human interventional study was powered to detect the effect sizes described in the murine study [37, 86]. Although this approach is statistically appropriate, it does not take into account the greater heterogeneity of human asthma as compared to murine asthma.
To summarize, although adiponectin and its receptors are expressed in human airway cells, the adiponectin-asthma association in humans is currently controversial. Some but not all studies, demonstrate that serum adiponectin concentrations are inversely associated with asthma prevalence among premenopausal women and peripubertal girls. On the other hand, serum adiponectin concentrations are favourably associated with asthma severity among boys and adversely associated among men. It is possible that pro-inflammatory effects of adiponectin dominate under certain physiologic conditions and anti-inflammatory effects under others. Furthermore, the obesity-asthma association does not appear to be explained by serum adiponectin alone [35], implying multiplicity of mechanistic pathways for the obesity asthma association. It is also not currently known whether modulation of adiponectin, independent of BMI, may be helpful in asthma prevention or treatment.
2.3 Adiponectin and COPD
2.3.1 Mouse studies
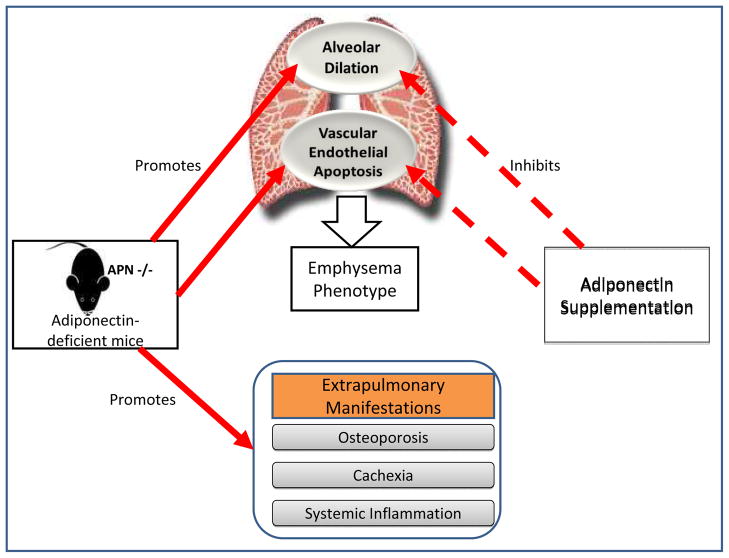
Genetically-induced adiponectin deficient mice (APN−/−) demonstrate greater expression of TNF-α and matrix metalloproteinases in their alveolar macrophages and abnormal alveolarization, resembling an emphysema-like phenotype (Figure 3) [89, 90]. These changes are reversed following adiponectin supplementation, supporting an anti-inflammatory role for adiponectin [90]. In addition, APN−/− mice demonstrate an increase in extrapulmonary inflammation; vascular endothelial dysfunction; and comorbidities (such as cachexia and osteoporosis). Yet, when the same APN−/− mice are additionally exposed to tobacco smoke, these mice do not demonstrate a further increase in lung inflammation and air space enlargement as would be expected and instead, show lesser degree of abnormality than similarly exposed wild-type mice [91]. Why adiponectin has pro-inflammatory effects under certain circumstances and anti-inflammatory under others is uncertain.
Figure 3.
A schematic representation of the suggested role for adiponectin in murine emphysema, based upon the work by Nakanishi et al. [90]
Intranasal elastase instillation among wild-type mice, while causing emphysema, reduces plasma adiponectin and lung vasculature expression of adiponectin as well as simultaneously increases bronchoalveolar lavage fluid adiponectin and adiponectin receptor expression on lung macrophages and epithelial cells [90]. Chronic tobacco smoke exposure in wild type mice has similar effects on bronchoalveolar lavage fluid adiponectin [83].
2.3.2 Human Studies
Currently the human data on the association between adiponectin and COPD prevalence or severity remain inconclusive and suggest both pro-inflammatory and anti-inflammatory effects of adiponectin in different population subgroups (Table 2).
2.3.3 COPD prevalence
Systemic adiponectin is positively associated with lung function in healthy adults [92] but inversely associated in subjects with COPD [93].
2.3.3.1 Population-based longitudinal study
Thyagarajan et al. showed a positive longitudinal association between serum adiponectin and spirometric lung function in young healthy adults, independent of sex, obesity and smoking [92]. Interestingly, the authors hypothesized that systemic adiponectin may affect lung growth during early adulthood rather than lung function decline. The attenuation of this association after adjustment for insulin resistance and systemic inflammation suggest that these covariates are on a causal pathway linking adiponectin and lung function.
2.3.3.2 Hospital/clinic-based studies
There is limited data in humans evaluating the predictive effect of serum adiponectin on risk for COPD, independent of BMI. Contrary to the direction of association in the Nakanishi’s mouse model [90], three small case-control human studies have demonstrated that serum adiponectin concentrations in male COPD patients were higher than those in controls [93–95]. Another study showed that levels of bronchoalveolar lavage adiponectin and adiponectin expression in airway epithelial cells in subjects with emphysema was greater than healthy (disproportionately female) non-smoking controls [83]. Interestingly, in contrast to subjects with emphysema who had increased levels of bronchoalveolar lavage adiponectin, current smokers without COPD had reduced levels of bronchoalveolar lavage adiponectin [83]. The molecular mechanism by which tobacco smoke exposure down-regulates adiponectin expression and the development of COPD up-regulates adiponectin expression is unknown.
2.3.4 COPD severity
Systemic adiponectin is associated with greater COPD severity in men and has not been studied among women.
One small case-control study showed plasma adiponectin to be associated with lower spirometric parameters in a BMI-adjusted analysis [93] while two others showed no correlation [94, 95]. Serum adiponectin concentrations were associated with greater serum TNF-α concentrations (only in the subgroup with elevated TNF-α concentrations) and greater static hyperinflation ( as measured by percent predicted residual volume) in unadjusted analyses of COPD patients [95].
2.3.5 Acute COPD exacerbation
Systemic adiponectin concentrations rise during acute COPD exacerbations and return to baseline several days to weeks later in the stable state following the resolution of the exacerbation [49].
To summarize, systemic and airway adiponectin concentrations are higher in case-control studies of primarily male COPD patients, although the direction of this association is not conclusively established in the absence of longitudinal studies. Systemic adiponectin is positively associated with lung function in healthy adults but inversely associated in subjects with COPD. Systemic adiponectin is associated with greater COPD severity in men and has not been studied among women. It is not currently known whether modulation of adiponectin, independent of BMI, may be helpful in COPD prevention and treatment.
2.4 Adiponectin and non-small cell lung cancer
The role of hypoadiponectinemia as a poor prognostic factor in non small cell lung cancer is not well established. While one study showed lower serum adiponectin concentrations in advanced cancer, as compared to limited stage disease [96], another study did not show any association in multivariable analyses [64].
The associations between adiponectin and inflammatory lung diseases suffer from many critical gaps in the literature. Other than Thyagarajan study that evaluated healthy adults [92], there are no large human studies that have evaluated the adiponectin-COPD association. Further, women with COPD have not been adequately studied. Adiponectin is both positively and inversely associated with lung function, depending upon the population subgroup studied. It is possible that adiponectin is anti-inflammatory in some subjects and pro-inflammatory in others. As with leptin, there is a lack of adequately powered longitudinal and weight-intervention studies; inadequate adjustment for confounding effect of obesity; lack of studies of adiponectin isoforms, and limited studies of sputum or bronchoalveolar lavage fluid adiponectin.
To summarize, there is developing literature to suggest a potential role for leptin and adiponectin in inflammatory pulmonary conditions such as asthma, COPD, pneumonia and lung cancer. Future research will determine whether modulation of leptin and adiponectin, independent of BMI, may allow novel ways to prevent or treat these pulmonary diseases.
Highlights.
Systemic leptin may be associated with greater asthma prevalence and severity in children.
Systemic leptin may be associated with greater COPD prevalence and severity.
Systemic adiponectin may be protective against odds for asthma among women and girls.
Systemic adiponectin concentrations are higher in COPD patients than controls.
Systemic adiponectin is associated with greater COPD severity in men.
Acknowledgments
NIH Source of Funding: This work was supported from funding by the National Institutes of Health (K23 HL 094531-01 and CTSA 1ULRR031977-01 for AS). The sponsor played no role in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
The authors would like to acknowledge the assistance provided by Mark Schuyler, M.D. at University of New Mexico in proof-reading and critiquing the article.
Abbreviations
- COPD
Chronic obstructive pulmonary disease
- IL
Interleukin
- TNF
Tumor Necrosis Factor
- VEGF
Vascular Endothelial Growth Factor
- NHANES III
Third National Health and Nutrition Examination Survey
- BMI
Body Mass Index
- NHLBI
National Heart Lung and Blood Institutes
- SNP
Single Nucleotide Polymorphism
- APN −/−
Genetically-induced Adiponectin Deficient Mice
- FEV1/FVC
Ratio of Forced Expiratory Volume in One Second to Forced Vital Capacity
- FEF25–75%
Maximum Mid-expiratory Flow
Footnotes
COI Disclosure: Nour Ali Assad, M.D. states that there is no personal or financial support or involvement with organization(s) with financial interest in the subject matter or any other actual or potential conflict of interest. Nour Ali Assad, M.D. also declares that she has materially participated in the article preparation including review of data, writing and editing of manuscript and creation of figures and has approved the final article.
Akshay Sood, M.D., M.P.H. states that there is no personal or financial support or involvement with organization(s) with financial interest in the subject matter or any other actual or potential conflict of interest. Akshay Sood, M.D., M.P.H. also declares that he has materially participated in the article preparation including review of data, writing and editing of manuscript and creation of figures and has approved the final article.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Nour Ali Assad, Email: nassad@salud.unm.edu.
Akshay Sood, Email: asood@salud.unm.edu.
References
- 1.Masuzaki H, Ogawa Y, Sagawa N, Hosoda K, Matsumoto T, Mise H, Nishimura H, Yoshimasa Y, Tanaka I, Mori T, Nakao K. Nonadipose tissue production of leptin: leptin as a novel placenta-derived hormone in humans. Nat Med. 1997;3:1029–1033. doi: 10.1038/nm0997-1029. [DOI] [PubMed] [Google Scholar]
- 2.Bado A, Levasseur S, Attoub S, Kermorgant S, Laigneau JP, Bortoluzzi MN, Moizo L, Lehy T, Guerre-Millo M, Le Marchand-Brustel Y, Lewin MJ. The stomach is a source of leptin. Nature. 1998;394:790–793. doi: 10.1038/29547. [DOI] [PubMed] [Google Scholar]
- 3.Larsson H, Ahren B. Short-term dexamethasone treatment increases plasma leptin independently of changes in insulin sensitivity in healthy women. J Clin Endocrinol Metab. 1996;81:4428–4432. doi: 10.1210/jcem.81.12.8954054. [DOI] [PubMed] [Google Scholar]
- 4.Messinis IE, Papageorgiou I, Milingos S, Asprodini E, Kollios G, Seferiadis K. Oestradiol plus progesterone treatment increases serum leptin concentrations in normal women. Hum Reprod. 2001;16:1827–1832. doi: 10.1093/humrep/16.9.1827. [DOI] [PubMed] [Google Scholar]
- 5.Holness MJ, Munns MJ, Sugden MC. Current concepts concerning the role of leptin in reproductive function. Mol Cell Endocrinol. 1999;157:11–20. doi: 10.1016/s0303-7207(99)00126-4. [DOI] [PubMed] [Google Scholar]
- 6.Hsu A, Aronoff DM, Phipps J, Goel D, Mancuso P. Leptin improves pulmonary bacterial clearance and survival in ob/ob mice during pneumococcal pneumonia. Clin Exp Immunol. 2007;150:332–339. doi: 10.1111/j.1365-2249.2007.03491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mancuso P. Leptin-Deficient Mice Exhibit Impaired Host Defense in Gram-Negative Pneumonia.pdf. 2002. [DOI] [PubMed] [Google Scholar]
- 8.WA, Mastronardi CA, Yu WH, Karanth S, Parlow AF, MSM The possible role of prolactin in the circadian rhythm of leptin secretion in male rats. Proc Soc Exp Biol Med. 2000;224:152–158. doi: 10.1046/j.1525-1373.2000.22414.x. [DOI] [PubMed] [Google Scholar]
- 9.Shin JH, Kim JH, Lee WY, Shim JY. The expression of adiponectin receptors and the effects of adiponectin and leptin on airway smooth muscle cells. Yonsei Med J. 2008;49:804–810. doi: 10.3349/ymj.2008.49.5.804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matarese G, Moschos S, Mantzoros CS. Leptin in immunology. J Immunol. 2005;174:3137– 3142. doi: 10.4049/jimmunol.174.6.3137. [DOI] [PubMed] [Google Scholar]
- 11.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 12.La Cava A, Alviggi C, Matarese G. Unraveling the multiple roles of leptin in inflammation and autoimmunity. J Mol Med (Berl) 2004;82:4–11. doi: 10.1007/s00109-003-0492-1. [DOI] [PubMed] [Google Scholar]
- 13.Otero M, Lago R, Gomez R, Dieguez C, Lago F, Gomez-Reino J, Gualillo O. Towards a proinflammatory and immunomodulatory emerging role of leptin. Rheumatology (Oxford) 2006;45:944– 950. doi: 10.1093/rheumatology/kel157. [DOI] [PubMed] [Google Scholar]
- 14.Baratta M. Leptin--from a signal of adiposity to a hormonal mediator in peripheral tissues. Med Sci Monit. 2002;8:RA282–292. [PubMed] [Google Scholar]
- 15.Lord G. Role of leptin in immunology. Nutr Rev. 2002;60:S35–38. doi: 10.1301/002966402320634913. discussion S68–84, 85–37. [DOI] [PubMed] [Google Scholar]
- 16.Vernooy JH, Drummen NE, van Suylen RJ, Cloots RH, Moller GM, Bracke KR, Zuyderduyn S, Dentener MA, Brusselle GG, Hiemstra PS, Wouters EF. Enhanced pulmonary leptin expression in patients with severe COPD and asymptomatic smokers. Thorax. 2009;64:26–32. doi: 10.1136/thx.2007.085423. [DOI] [PubMed] [Google Scholar]
- 17.Bruno A, Pace E, Chanez P, Gras D, Vachier I, Chiappara G, La Guardia M, Gerbino S, Profita M, Gjomarkaj M. Leptin and leptin receptor expression in asthma. J Allergy Clin Immunol. 2009;124:230–237. 237 e231–234. doi: 10.1016/j.jaci.2009.04.032. [DOI] [PubMed] [Google Scholar]
- 18.Holguin F, Rojas M, Brown LA, Fitzpatrick AM. Airway and plasma leptin and adiponectin in lean and obese asthmatics and controls. J Asthma. 2011;48:217–223. doi: 10.3109/02770903.2011.555033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nair P, Radford K, Fanat A, Janssen LJ, Peters-Golden M, Cox PG. The effects of leptin on airway smooth muscle responses. Am J Respir Cell Mol Biol. 2008;39:475–481. doi: 10.1165/rcmb.2007-0091OC. [DOI] [PubMed] [Google Scholar]
- 20.Bruno A, Chanez P, Chiappara G, Siena L, Giammanco S, Gjomarkaj M, Bonsignore G, Bousquet J, Vignola AM. Does leptin play a cytokine-like role within the airways of COPD patients? Eur Respir J. 2005;26:398–405. doi: 10.1183/09031936.05.00092404. [DOI] [PubMed] [Google Scholar]
- 21.Shore SA, Schwartzman IN, Mellema MS, Flynt L, Imrich A, Johnston RA. Effect of leptin on allergic airway responses in mice. J Allergy Clin Immunol. 2005;115:103–109. doi: 10.1016/j.jaci.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 22.Sood A, Ford ES, Camargo CA., Jr Association between leptin and asthma in adults. Thorax. 2006;61:300–305. doi: 10.1136/thx.2004.031468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland TJ, Sears MR, McLachlan CR, Poulton R, Hancox RJ. Leptin, adiponectin, and asthma: findings from a population-based cohort study. Ann Allergy Asthma Immunol. 2009;103:101– 107. doi: 10.1016/S1081-1206(10)60161-5. [DOI] [PubMed] [Google Scholar]
- 24.Jartti T, Saarikoski L, Jartti L, Lisinen I, Jula A, Huupponen R, Viikari J, Raitakari OT. Obesity, adipokines and asthma. Allergy. 2009;64:770–777. doi: 10.1111/j.1398-9995.2008.01872.x. [DOI] [PubMed] [Google Scholar]
- 25.Dixon AE, Johnson SE, Griffes LV, Raymond DM, Ramdeo R, Soloveichik A, Suratt BT, Cohen RI. Relationship of adipokines with immune response and lung function in obese asthmatic and nonasthmatic women. J Asthma. 2011;48:811–817. doi: 10.3109/02770903.2011.613507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jang AS, Kim TH, Park JS, Kim KU, Uh ST, Seo KH, Kim YH, Lim GI, Park CS. Association of serum leptin and adiponectin with obesity in asthmatics. J Asthma. 2009;46:59–63. doi: 10.1080/02770900802444203. [DOI] [PubMed] [Google Scholar]
- 27.Lessard A, St-Laurent J, Turcotte H, Boulet LP. Leptin and adiponectin in obese and non-obese subjects with asthma. Biomarkers. 2011;16:271–273. doi: 10.3109/1354750X.2010.550013. [DOI] [PubMed] [Google Scholar]
- 28.Guler N, Kirerleri E, Ones U, Tamay Z, Salmayenli N, Darendeliler F. Leptin: does it have any role in childhood asthma? J Allergy Clin Immunol. 2004;114:254–259. doi: 10.1016/j.jaci.2004.03.053. [DOI] [PubMed] [Google Scholar]
- 29.Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete N. Serum leptin levels in asthmatic children treated with an inhaled corticosteroid. Ann Allergy Asthma Immunol. 2004;93:277–280. doi: 10.1016/S1081-1206(10)61501-3. [DOI] [PubMed] [Google Scholar]
- 30.Mai XM, Bottcher MF, Leijon I. Leptin and asthma in overweight children at 12 years of age. Pediatr Allergy Immunol. 2004;15:523–530. doi: 10.1111/j.1399-3038.2004.00195.x. [DOI] [PubMed] [Google Scholar]
- 31.Tanju A, Cekmez F, Aydinoz S, Karademir F, Suleymanoglu S, Gocmen I. Association between clinical severity of childhood asthma and serum leptin levels. Indian J Pediatr. 2011;78:291–295. doi: 10.1007/s12098-010-0281-0. [DOI] [PubMed] [Google Scholar]
- 32.Baek HS, Kim YD, Shin JH, Kim JH, Oh JW, Lee HB. Serum leptin and adiponectin levels correlate with exercise-induced bronchoconstriction in children with asthma. Ann Allergy Asthma Immunol. 2011;107:14–21. doi: 10.1016/j.anai.2011.03.013. [DOI] [PubMed] [Google Scholar]
- 33.Kattan M, Kumar R, Bloomberg GR, Mitchell HE, Calatroni A, Gergen PJ, Kercsmar CM, Visness CM, Matsui EC, Steinbach SF, Szefler SJ, Sorkness CA, Morgan WJ, Teach SJ, Gan VN. Asthma control, adiposity, and adipokines among inner-city adolescents. J Allergy Clin Immunol. 2010;125:584–592. doi: 10.1016/j.jaci.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nagel G, Koenig W, Rapp K, Wabitsch M, Zoellner I, Weiland SK. Associations of adipokines with asthma, rhinoconjunctivitis, and eczema in German schoolchildren. Pediatr Allergy Immunol. 2009;20:81–88. doi: 10.1111/j.1399-3038.2008.00740.x. [DOI] [PubMed] [Google Scholar]
- 35.Sood A, Cui X, Qualls C, Beckett WS, Gross MD, Steffes MW, Smith LJ, Jacobs DR., Jr Association between asthma and serum adiponectin concentration in women. Thorax. 2008;63:877– 882. doi: 10.1136/thx.2007.090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sood A, Dawson BK, Eid W, Eagleton LE, Henkle JQ, Hopkins-Price P. Obesity is associated with bronchial hyper-responsiveness in women. J Asthma. 2005;42:847–852. doi: 10.1080/02770900500371047. [DOI] [PubMed] [Google Scholar]
- 37.Sood A, Qualls C, Seagrave J, Stidley C, Archibeque T, Berwick M, Schuyler M. Effect of specific allergen inhalation on serum adiponectin in human asthma. Chest. 2009;135:287–294. doi: 10.1378/chest.08-1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wenzel S, Holgate ST. The mouse trap: It still yields few answers in asthma. American journal of respiratory and critical care medicine. 2006;174:1173–1176. doi: 10.1164/rccm.2609002. discussion 1176–1178. [DOI] [PubMed] [Google Scholar]
- 39.Vernooy JH, Bracke KR, Drummen NE, Pauwels NS, Zabeau L, van Suylen RJ, Tavernier J, Joos GF, Wouters EF, Brusselle GG. Leptin modulates innate and adaptive immune cell recruitment after cigarette smoke exposure in mice. J Immunol. 2010;184:7169–7177. doi: 10.4049/jimmunol.0900963. [DOI] [PubMed] [Google Scholar]
- 40.Hansel NN, Gao L, Rafaels NM, Mathias RA, Neptune ER, Tankersley C, Grant AV, Connett J, Beaty TH, Wise RA, Barnes KC. Leptin receptor polymorphisms and lung function decline in COPD. Eur Respir J. 2009;34:103–110. doi: 10.1183/09031936.00120408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Battaglia S, Mauad T, van Schadewijk AM, Vignola AM, Rabe KF, Bellia V, Sterk PJ, Hiemstra PS. Differential distribution of inflammatory cells in large and small airways in smokers. Journal of clinical pathology. 2007;60:907–911. doi: 10.1136/jcp.2006.037002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Breyer MK, Rutten EP, Vernooy JH, Spruit MA, Dentener MA, van der Kallen C, vanGreevenbroek MM, Wouters EF. Gender differences in the adipose secretome system in chronic obstructive pulmonary disease (COPD): a pivotal role of leptin. Respir Med. 2011;105:1046–1053. doi: 10.1016/j.rmed.2011.02.001. [DOI] [PubMed] [Google Scholar]
- 43.Shin IH, Lee JH, Kim HC. Ubiquitous monitoring system for chronic obstructive pulmonary disease and heart disease patients. Conf Proc IEEE Eng Med Biol Soc. 2007;2007:3689–3692. doi: 10.1109/IEMBS.2007.4353132. [DOI] [PubMed] [Google Scholar]
- 44.Takabatake N, Nakamura H, Abe S, Hino T, Saito H, Yuki H, Kato S, Tomoike H. Circulating leptin in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1999;159:1215–1219. doi: 10.1164/ajrccm.159.4.9806134. [DOI] [PubMed] [Google Scholar]
- 45.Schols AM, Creutzberg EC, Buurman WA, Campfield LA, Saris WH, Wouters EF. Plasma leptin is related to proinflammatory status and dietary intake in patients with chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 1999;160:1220–1226. doi: 10.1164/ajrccm.160.4.9811033. [DOI] [PubMed] [Google Scholar]
- 46.Broekhuizen R, Vernooy JH, Schols AM, Dentener MA, Wouters EF. Leptin as local inflammatory marker in COPD. Respir Med. 2005;99:70–74. doi: 10.1016/j.rmed.2004.03.029. [DOI] [PubMed] [Google Scholar]
- 47.Creutzberg EC, Wouters EF, Vanderhoven-Augustin IM, Dentener MA, Schols AM. Disturbances in leptin metabolism are related to energy imbalance during acute exacerbations of chronic obstructive pulmonary disease. American journal of respiratory and critical care medicine. 2000;162:1239–1245. doi: 10.1164/ajrccm.162.4.9912016. [DOI] [PubMed] [Google Scholar]
- 48.Kythreotis P, Kokkini A, Avgeropoulou S, Hadjioannou A, Anastasakou E, Rasidakis A, Bakakos P. Plasma leptin and insulin-like growth factor I levels during acute exacerbations of chronic obstructive pulmonary disease. BMC Pulm Med. 2009;9:11. doi: 10.1186/1471-2466-9-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Krommidas G, Kostikas K, Papatheodorou G, Koutsokera A, Gourgoulianis KI, Roussos C, Koulouris NG, Loukides S. Plasma leptin and adiponectin in COPD exacerbations: associations with inflammatory biomarkers. Respir Med. 2010;104:40–46. doi: 10.1016/j.rmed.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 50.Ikejima S, Sasaki S, Sashinami H, Mori F, Ogawa Y, Nakamura T, Abe Y, Wakabayashi K, Suda T, Nakane A. Impairment of host resistance to Listeria monocytogenes infection in liver of db/db and ob/ob mice. Diabetes. 2005;54:182–189. doi: 10.2337/diabetes.54.1.182. [DOI] [PubMed] [Google Scholar]
- 51.Mandel MA, Mahmoud AA. Impairment of cell-mediated immunity in mutation diabetic mice (db/db) J Immunol. 1978;120:1375–1377. [PubMed] [Google Scholar]
- 52.Park S, Rich J, Hanses F, Lee JC. Defects in innate immunity predispose C57BL/6JLeprdb/ Leprdb mice to infection by Staphylococcus aureus. Infect Immun. 2009;77:1008–1014. doi: 10.1128/IAI.00976-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bochicchio GV, Joshi M, Bochicchio K, Nehman S, Tracy JK, Scalea TM. Impact of obesity in the critically ill trauma patient: a prospective study. J Am Coll Surg. 2006;203:533–538. doi: 10.1016/j.jamcollsurg.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 54.Newell MA, Bard MR, Goettler CE, Toschlog EA, Schenarts PJ, Sagraves SG, Holbert D, Pories WJ, Rotondo MF. Body mass index and outcomes in critically injured blunt trauma patients: weighing the impact. J Am Coll Surg. 2007;204:1056–1061. doi: 10.1016/j.jamcollsurg.2006.12.042. discussion 1062–1054. [DOI] [PubMed] [Google Scholar]
- 55.Yaegashi M, Jean R, Zuriqat M, Noack S, Homel P. Outcome of morbid obesity in the intensive care unit. J Intensive Care Med. 2005;20:147–154. doi: 10.1177/0885066605275314. [DOI] [PubMed] [Google Scholar]
- 56.Brandt M, Harder K, Walluscheck KP, Schottler J, Rahimi A, Moller F, Cremer J. Severe obesity does not adversely affect perioperative mortality and morbidity in coronary artery bypass surgery. Eur J Cardiothorac Surg. 2001;19:662–666. doi: 10.1016/s1010-7940(01)00647-9. [DOI] [PubMed] [Google Scholar]
- 57.Dossett LA, Heffernan D, Lightfoot M, Collier B, Diaz JJ, Sawyer RG, May AK. Obesity and pulmonary complications in critically injured adults. Chest. 2008;134:974–980. doi: 10.1378/chest.08-0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moulton MJ, Creswell LL, Mackey ME, Cox JL, Rosenbloom M. Obesity is not a risk factor for significant adverse outcomes after cardiac surgery. Circulation. 1996;94:II87–92. [PubMed] [Google Scholar]
- 59.Diez ML, Santolaria F, Tejera A, Aleman MR, Gonzalez-Reimers E, Milena A, de la Vega MJ, Martinez-Riera A. Serum leptin levels in community acquired pneumonia (CAP) are related to nutritional status and not to acute phase reaction. Cytokine. 2008;42:156–160. doi: 10.1016/j.cyto.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 60.Shen Y, Wang Q, Zhao Q, Zhou J. Leptin promotes the immune escape of lung cancer by inducing proinflammatory cytokines and resistance to apoptosis. Mol Med Report. 2009;2:295–299. doi: 10.3892/mmr_00000099. [DOI] [PubMed] [Google Scholar]
- 61.Xu YJ, Shao YF, Zhao X, Geng YT, Wang K, Yin YM. Expression and clinical significance of leptin, the functional receptor of leptin (OB-Rb) and HER-2 in non-small-cell lung cancer: a retrospective analysis. J Cancer Res Clin Oncol. 2011 doi: 10.1007/s00432-011-1054-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ribeiro R, Araujo AP, Coelho A, Catarino R, Pinto D, Araujo A, Calcada C, Lopes C, Medeiros R. A functional polymorphism in the promoter region of leptin gene increases susceptibility for non-small cell lung cancer. Eur J Cancer. 2006;42:1188–1193. doi: 10.1016/j.ejca.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 63.Aleman MR, Santolaria F, Batista N, de La Vega M, Gonzalez-Reimers E, Milena A, Llanos M, Gomez-Sirvent JL. Leptin role in advanced lung cancer. A mediator of the acute phase response or a marker of the status of nutrition? Cytokine. 2002;19:21–26. doi: 10.1006/cyto.2002.1051. [DOI] [PubMed] [Google Scholar]
- 64.Karapanagiotou EM, Tsochatzis EA, Dilana KD, Tourkantonis I, Gratsias I, Syrigos KN. The significance of leptin, adiponectin, and resistin serum levels in non-small cell lung cancer (NSCLC) Lung Cancer. 2008;61:391–397. doi: 10.1016/j.lungcan.2008.01.018. [DOI] [PubMed] [Google Scholar]
- 65.Carpagnano GE, Spanevello A, Curci C, Salerno F, Palladino GP, Resta O, Di Gioia G, Carpagnano F, Foschino Barbaro MP. IL-2, TNF-alpha, and leptin: local versus systemic concentrations in NSCLC patients. Oncol Res. 2007;16:375–381. doi: 10.3727/000000006783980900. [DOI] [PubMed] [Google Scholar]
- 66.Ajuwon KM, Spurlock ME. Adiponectin inhibits LPS-induced NF-kappaB activation and IL-6 production and increases PPARgamma2 expression in adipocytes. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1220–1225. doi: 10.1152/ajpregu.00397.2004. [DOI] [PubMed] [Google Scholar]
- 67.Masaki T, Chiba S, Tatsukawa H, Yasuda T, Noguchi H, Seike M, Yoshimatsu H. Adiponectin protects LPS-induced liver injury through modulation of TNF-alpha in KK-Ay obese mice. Hepatology. 2004;40:177–184. doi: 10.1002/hep.20282. [DOI] [PubMed] [Google Scholar]
- 68.Wulster-Radcliffe MC, Ajuwon KM, Wang J, Christian JA, Spurlock ME. Adiponectin differentially regulates cytokines in porcine macrophages. Biochem Biophys Res Commun. 2004;316:924–929. doi: 10.1016/j.bbrc.2004.02.130. [DOI] [PubMed] [Google Scholar]
- 69.Kumada M, Kihara S, Ouchi N, Kobayashi H, Okamoto Y, Ohashi K, Maeda K, Nagaretani H, Kishida K, Maeda N, Nagasawa A, Funahashi T, Matsuzawa Y. Adiponectin specifically increased tissue inhibitor of metalloproteinase-1 through interleukin-10 expression in human macrophages. Circulation. 2004;109:2046–2049. doi: 10.1161/01.CIR.0000127953.98131.ED. [DOI] [PubMed] [Google Scholar]
- 70.Wolf AM, Wolf D, Rumpold H, Enrich B, Tilg H. Adiponectin induces the anti-inflammatory cytokines IL-10 and IL-1RA in human leukocytes. Biochem Biophys Res Commun. 2004;323:630–635. doi: 10.1016/j.bbrc.2004.08.145. [DOI] [PubMed] [Google Scholar]
- 71.Sood A, Dominic E, Qualls C, Steffes MW, Thyagarajan B, Smith LJ, Lewis CE, Jacobs DR., Jr Serum Adiponectin is Associated with Adverse Outcomes of Asthma in Men but Not in Women. Front Pharmacol. 2011;2:55. doi: 10.3389/fphar.2011.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ehling A, Schaffler A, Herfarth H, Tarner IH, Anders S, Distler O, Paul G, Distler J, Gay S, Scholmerich J, Neumann E, Muller-Ladner U. The potential of adiponectin in driving arthritis. J Immunol. 2006;176:4468–4478. doi: 10.4049/jimmunol.176.7.4468. [DOI] [PubMed] [Google Scholar]
- 73.Steffes MW, Gross MD, Schreiner PJ, Yu X, Hilner JE, Gingerich R, Jacobs DR., Jr Serum adiponectin in young adults--interactions with central adiposity, circulating levels of glucose, and insulin resistance: the CARDIA study. Ann Epidemiol. 2004;14:492–498. doi: 10.1016/j.annepidem.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 74.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 75.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 76.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am J Physiol Endocrinol Metab. 2003;285:E527–533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 77.Waki H, Yamauchi T, Kamon J, Ito Y, Uchida S, Kita S, Hara K, Hada Y, Vasseur F, Froguel P, Kimura S, Nagai R, Kadowaki T. Impaired multimerization of human adiponectin mutants associated with diabetes. Molecular structure and multimer formation of adiponectin. J Biol Chem. 2003;278:40352–40363. doi: 10.1074/jbc.M300365200. [DOI] [PubMed] [Google Scholar]
- 78.Hara K, Horikoshi M, Yamauchi T, Yago H, Miyazaki O, Ebinuma H, Imai Y, Nagai R, Kadowaki T. Measurement of the high-molecular weight form of adiponectin in plasma is useful for the prediction of insulin resistance and metabolic syndrome. Diabetes Care. 2006;29:1357–1362. doi: 10.2337/dc05-1801. [DOI] [PubMed] [Google Scholar]
- 79.Pajvani UB, Hawkins M, Combs TP, Rajala MW, Doebber T, Berger JP, Wagner JA, Wu M, Knopps A, Xiang AH, Utzschneider KM, Kahn SE, Olefsky JM, Buchanan TA, Scherer PE. Complex distribution, not absolute amount of adiponectin, correlates with thiazolidinedione-mediated improvement in insulin sensitivity. J Biol Chem. 2004;279:12152–12162. doi: 10.1074/jbc.M311113200. [DOI] [PubMed] [Google Scholar]
- 80.Pajvani UB, Du X, Combs TP, Berg AH, Rajala MW, Schulthess T, Engel J, Brownlee M, Scherer PE. Structure-function studies of the adipocyte-secreted hormone Acrp30/adiponectin. Implications fpr metabolic regulation and bioactivity. J Biol Chem. 2003;278:9073–9085. doi: 10.1074/jbc.M207198200. [DOI] [PubMed] [Google Scholar]
- 81.Bottner A, Kratzsch J, Muller G, Kapellen TM, Bluher S, Keller E, Bluher M, Kiess W. Gender differences of adiponectin levels develop during the progression of puberty and are related to serum androgen levels. J Clin Endocrinol Metab. 2004;89:4053–4061. doi: 10.1210/jc.2004-0303. [DOI] [PubMed] [Google Scholar]
- 82.Hug C, Wang J, Ahmad NS, Bogan JS, Tsao TS, Lodish HF. T-cadherin is a receptor for hexameric and high-molecular-weight forms of Acrp30/adiponectin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:10308–10313. doi: 10.1073/pnas.0403382101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Miller B. Adiponectin and Functional Adiponectin Receptor 1 Are Expressed by Airway Epithelial Cells in Chronic Obstructive Pulmonary Disease.pdf. 2009. [DOI] [PubMed] [Google Scholar]
- 84.Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 86.Shore SA, Terry RD, Flynt L, Xu A, Hug C. Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J Allergy Clin Immunol. 2006;118:389–395. doi: 10.1016/j.jaci.2006.04.021. [DOI] [PubMed] [Google Scholar]
- 87.Medoff BD, Okamoto Y, Leyton P, Weng M, Sandall BP, Raher MJ, Kihara S, Bloch KD, Libby P, Luster AD. Adiponectin deficiency increases allergic airway inflammation and pulmonary vascular remodeling. Am J Respir Cell Mol Biol. 2009;41:397–406. doi: 10.1165/rcmb.2008-0415OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kim KW, Shin YH, Lee KE, Kim ES, Sohn MH, Kim KE. Relationship between adipokines and manifestations of childhood asthma. Pediatr Allergy Immunol. 2008;19:535–540. doi: 10.1111/j.1399-3038.2007.00690.x. [DOI] [PubMed] [Google Scholar]
- 89.Summer R, Little FF, Ouchi N, Takemura Y, Aprahamian T, Dwyer D, Fitzsimmons K, Suki B, Parameswaran H, Fine A, Walsh K. Alveolar macrophage activation and an emphysema-like phenotype in adiponectin-deficient mice. Am J Physiol Lung Cell Mol Physiol. 2008;294:L1035–1042. doi: 10.1152/ajplung.00397.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nakanishi K, Takeda Y, Tetsumoto S, Iwasaki T, Tsujino K, Kuhara H, Jin Y, Nagatomo I, Kida H, Goya S, Kijima T, Maeda N, Funahashi T, Shimomura I, Tachibana I, Kawase I. Involvement of endothelial apoptosis underlying chronic obstructive pulmonary disease-like phenotype in adiponectinnull mice: implications for therapy. American journal of respiratory and critical care medicine. 2011;183:1164–1175. doi: 10.1164/rccm.201007-1091OC. [DOI] [PubMed] [Google Scholar]
- 91.Miller M, Pham A, Cho JY, Rosenthal P, Broide DH. Adiponectin-deficient mice are protected against tobacco-induced inflammation and increased emphysema. Am J Physiol Lung Cell Mol Physiol. 2010;299:L834–842. doi: 10.1152/ajplung.00326.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Thyagarajan B, Jacobs DR, Jr, Smith LJ, Kalhan R, Gross MD, Sood A. Serum adiponectin is positively associated with lung function in young adults, independent of obesity: the CARDIA study. Respir Res. 2010;11:176. doi: 10.1186/1465-9921-11-176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chan KH. Elevated plasma adiponectin levels in patients with chronic obstructive pulmonary disease.pdf. Int J Tuberc Lung Dis. 2010 [PubMed] [Google Scholar]
- 94.Kirdar S, Serter M, Ceylan E, Sener AG, Kavak T, Karadag F. Adiponectin as a biomarker of systemic inflammatory response in smoker patients with stable and exacerbation phases of chronic obstructive pulmonary disease. Scand J Clin Lab Invest. 2009;69:219–224. doi: 10.1080/00365510802474400. [DOI] [PubMed] [Google Scholar]
- 95.Tomoda K, Yoshikawa M, Itoh T, Tamaki S, Fukuoka A, Komeda K, Kimura H. Elevated circulating plasma adiponectin in underweight patients with COPD. Chest. 2007;132:135–140. doi: 10.1378/chest.07-0227. [DOI] [PubMed] [Google Scholar]
- 96.Petridou ET, Mitsiades N, Gialamas S, Angelopoulos M, Skalkidou A, Dessypris N, Hsi A, Lazaris N, Polyzos A, Syrigos C, Brennan AM, Tseleni-Balafouta S, Mantzoros CS. Circulating adiponectin levels and expression of adiponectin receptors in relation to lung cancer: two case-control studies. Oncology. 2007;73:261–269. doi: 10.1159/000127424. [DOI] [PubMed] [Google Scholar]