Abstract
The construction of an inflammatory microenvironment provides the fuel for cancer development and progression. Hence, solid tumors promote the expansion and the recruitment of leukocyte populations, among which tumor-associated myeloid cells (TAMCs) represent a paradigm for cancer-promoting inflammation. TAMCs group heterogeneous phagocytic populations stemming from a common myeloid progenitor (CMP), that orchestrate various aspects of cancer, including: diversion and skewing of adaptive responses; immunosuppression; cell growth; angiogenesis; matrix deposition and remodelling; construction of a metastatic niche and actual metastasis. Several evidence indicate that TAMCs show plasticity and/or functional heterogeneity, suggesting that tumour-derived factors promote their functional “reprogramming” towards protumoral activities. While recent studies have attempted to address the role of microenvironment signals, the interplay between cancer cells, innate and adaptive immunity is now emerging as a crucial step of the TAMCs reprogramming. Here we discuss the evidence for the differentiation of TAMCs during the course of tumor progression and the molecular mechanisms that regulate such event.
Keywords: Tumor-associated myeloid cells (TAMCs), Polarized inflammation, Tumor-associated macrophages (TAMs), Myeloid-derived suppressor cells (MDSCs), Angiogenic monocytes Tie2+ (TEMs), Tumor-associated neutrophils (TANs)
Introduction
The cellular content of solid tumors comprises cancer cells and a heterogeneous group of cell populations, including fibroblasts, endothelial cells, pericytes and leukocytes. Among leukocytes, myeloid cell populations represent a prominent component, both in terms of number and functions, supporting tumor growth and progression [1]. Being part of the first line of immune defence mechanisms (innate immunity), the protumoral role of tumor-associated myeloid cells (TAMCs) appears a paradox of immunity, which finds its basis on the functional “plasticity” of myeloid cells, defined as the capability to express different functional programs in response to different signals (eg. cytokines, growth factors) [1] and/or microenvironment conditions (eg. acidosis, high interstitial pressure, low glucose levels) [2]. Consequently, new attention is directed towards mechanisms and molecules driving the protumoral skewing of TAMCs.
TAMCs include at least four different myeloid populations (Fig. 1): 1) tumor-associated macrophages (TAMs), considered crucial orchestrators of cancer-related inflammation [3], promoting angiogenesis, immunosuppression, tissue remodelling and metastasis [4]; 2) the angiogenic monocytes expressing the tunica internal endothelial kinase 2 (Tie2), the angiopoietin receptor, playing a key role in tumor angiogenesis [5]; 3) the Ly6G and Ly6C subsets of an heterogeneous population of immature myeloid cells, called myeloid-derived suppressor cells (MDSCs) for their ability to suppress T cells functions, which accumulate mainly in blood and lymphoid organs during tumor progression, but may also be recruited to the tumor site [6]; 4) tumor-associated neutrophils (TANs) that, despite their short half-life, have been recently proven to participate in tumor promotion by the expression of crucial pro-angiogenic factors [7].
Fig. 1.
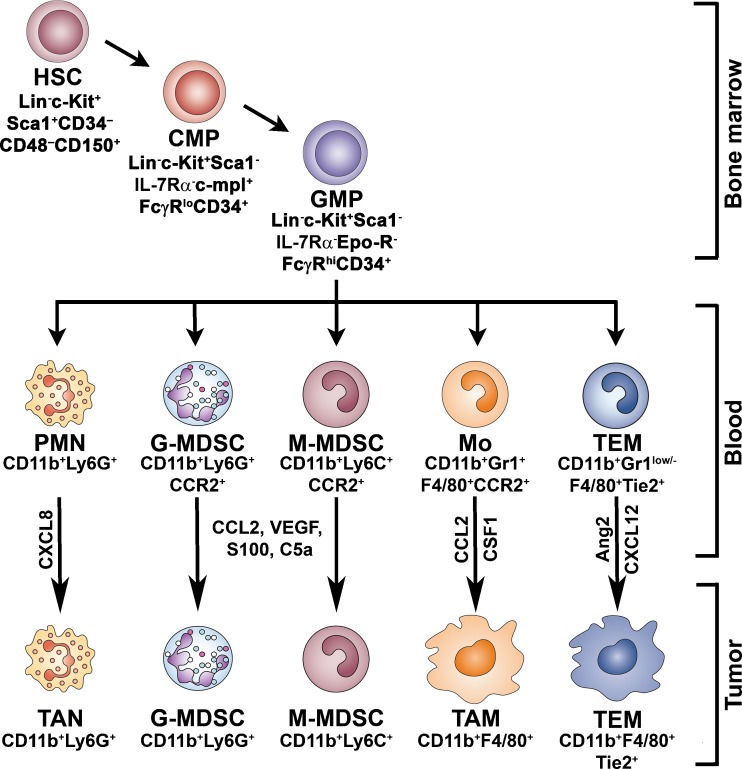
Mechanisms of differentiation and accumulation of TAMCs. In the bone marrow hematopoietic stem cell (HSC) differentiate into common myeloid progenitors (CMPs), which can subsequently differentiate into granulocyte/macrophage progenitors (GMPs). GMPs give rise to different subsets of circulating cells: monocytes (Mo), Tie2-expressing monocytes (TEM), neutrophils (PMN), and granulocytic and monocytic myeloid-suppressor cells (G-MDSC and M-MDSC). Tumors secrete factors which sustain myelopoiesis, promote the recruitment of circulating cells into both the tumor mass or secondary lymphoid organs (lymph nodes and spleen) and orientate their functional differentiation to their own advantage. TAMs are recruited into the tumor site by chemotactic factors (eg. CCL2, CSF-1) and represent the prominent phagocytes population orchestrating cancer-related inflammation. TEMs derive from circulating Tie2+ monocytes and are recruited in tumors by hypoxia-inducible chemoattractants, such as Ang2 and CXCL12. Tumor-associated neutrophils (TANs) stem from circulating neutrophils and are recruited in tumors by chemokines (e.g. CXCL8). TANs participate in tumor promotion by the expression of crucial pro-angiogenic factors. During tumour progression an heterogeneous population of myeloid cells (G-MDSC and M-MDSC) accumulate in blood and lymphoid organs. MDSCs may be recruited by selected chemoattractants (CCL2, S-100, VEGF, C5a) into the tumor microenvironment, where they contribute to suppression of the adaptive immunity
TAMCs originate in the bone marrow where hematopoietic stem cells (HSCs; Lin- c/Kit±/Sca1±/CD34-/CD48-/CD150-) differentiate into common myeloid progenitors (CMPs; Lin-/Sca1-/c-Kit±/IL-7Rα-/c-mpl±/FcγRlow/CD34±/SCL±/GATA-2±/NF-E2±/GATA-1±/GATA-3-), which can subsequently differentiate into granulocyte/macrophage progenitors (GMPs; Lin-/Sca1-/c-Kit±/IL-7Rα-/c-mpl±/FcγRhigh/CD34±/SCL±/GATA-2-/NF-E2-/GATA-1-/GATA-3-/EpoR-/C/EBPα±) [8]. GMPs give rise to different subsets of circulating cells: myeloid-derived suppressor cells (MDSCs) that can be further subdivided in a granulocytic (CD11b+/Ly6G+) and a monocytic (CD11b+/Ly6C+) subpopulation, monocytes (CD11b+/Gr1+/F4/80+/CCR2+), Tie2-expressing monocytes (CD11b+/Gr1low/-/Tie2+) and neutrophils (CD11b+/Ly6G+) [9]. Tumors secrete factors which sustain myelopoiesis, promote the recruitment of circulating cells into the tumor mass, and orientate their functional differentiation to their own advantage [6, 9]. Dendritic cells (DCs) belong to the family of myeloid cells stemming from CMPs. Cells with dendritic cell characteristics are present in neoplastic tissues [10]. Tumor-associated DCs (TADCs) generally show an immature phenotype and are poor inducers of effective responses to tumor antigens. The properties of these cells have been discussed elsewhere [11, 12] and are not included in the present review.
The functional skewing of myeloid populations is an emerging paradigm of tumor-mediated immunosuppression, where myeloid cell plasticity plays as a double-edged sword [1, 6]. The extreme versatility of mononuclear phagocytes is emphasized by different pathophysiological conditions (sepsis, cancer, obesity) in which the evolution of activating signals is paralleled by changes in the polarized activation of macrophages [13]. Recent studies have proved that the cross-talk between the different cellular components of the tumor mass (e.g. tumor cells, fibroblasts, innate and adaptive immune cells) shapes each other functions, resulting in “smouldering” inflammation mainly oriented to tune the adaptive immune response and to promote angiogenesis and tissue remodelling [1]. In this scenario, new efforts are needed to identify key regulators of myeloid cell plasticity during cancer development.
Mechanisms of TAMCs Recruitment
Chemokine (C-C motif) ligand 2 (CCL2) was the first tumor-derived chemotactic factor (TDCF) identified [14] and is currently recognized as the major chemoattractant for monocytes at the tumor site, in a variety of human tumors such as sarcomas, gliomas, melanomas, cancer of the breast, cervix and ovary [15, 16]. Many other different molecules, classically involved in the recruitment of monocytes to inflammatory sites, have also been involved in monocyte migration to neoplastic tissues; these include C-C and C-X-C chemokines (e.g. CCL3, CCL4, CCL5, CXCL12) [17–19], urokinase plasminogen activator (uPa) [20], growth factors (e.g. colony-stimulating factor-1, CSF-1; transforming growth factor-β, TGFβ; fibroblast growth factor, FGF; vascular endothelial growth factor, VEGF) [6, 21, 22] and antimicrobial peptides (β-defensin-3, BD-3) [23]. Many of these molecules correlate with TAM infiltration in different types of tumor, while others (eg. uPa, BD-3) are specifically associated with certain types of cancer, prostate and gastric cancer respectively [20, 23].
Tie2-expressing monocytes/macrophages (TEMs) are mainly clustered in hypoxic areas of solid tumors, in close proximity to nascent tumor vessels. They derive from circulating Tie2-expressing monocytes which are recruited in tumors by hypoxia-inducible chemotactic factors such as the CXCR4 ligand CXCL12 and Angiopoietin-2 (Ang-2) [5, 24–26].
MDSC recruitment and expansion are regulated by several cytokines, chemokines and transcription factors [6]. It has been demonstrated that among chemokine receptors, CCR2 plays a pivotal role in the recruitment and turnover of MDSC to the tumour site [27]. More recently, the C5a complement component, which interacts with a G protein-coupled receptor, has been shown to play a role in MDSC recruitment and activation in a cervix cancer model [28]. Moreover, some factors which are found in the tumour microenvironment, such as pro-inflammatory S-100 proteins, are crucial for MDSC recruitment. Sinha and co-workers demonstrated that MDSCs can produce S-100 proteins by themselves, providing evidence for an autocrine loop that promotes MDSC recruitment [29, 30].
Neutrophils may be recruited by chemotactic factors secreted by tumor cells. As an example, bronchoalveolar carcinoma cells produce CXCL8, a prototypic chemoattractant for neutrophils [31]. Further, TGFβ produced by different tumor cells promotes neutrophils migration both directly and indirectly, by regulating the expression of adhesion molecules in the endothelium [32].
TAMCs Functions
Tumour Associated Macrophages (TAMs)
Although macrophages were classically described as powerful inflammatory and cytotoxic cells, it has become evident that immunomodulatory signals such as IL-4 are more than simple inhibitors of macrophage activation, but actually induce an “alternative” or “M2” program of activation [33–35]. Whereas macrophage exposure to inflammatory cytokines (e.g. interferon-gamma, IFNγ) and bacterial moieties (e.g. Toll-like receptor ligands) triggers polarization of “M1” macrophages with anti-microbial and tissue destructive properties, M2-polarized macrophages tune inflammation, promote resistance against extracellular pathogens, angiogenesis, tissue remodelling and repair [35]. Within this scenario, M1- and M2-polarizations have been proposed as the extremes of a continuum of different states of polarized activation [35]. In addition to IL-4 several signals with M2-orienting properties have been identified. These include different classes of molecules, such as immunosuppressive (IL-10, TGFβ) and Th2-associated (IL-33, IL-21) cytokines , hormones (glucocorticoids, melanocortin, vaso-active intestinal peptide), growth factors (colony stimulating factors, CSFs), bacterial products (oedema toxin from Bacillus anthracis), immune complexes in combination with either lipopolysaccharide (LPS) or IL-1β [13, 33, 36].
Hence, it became clear that the M1 vs M2 dual subsets simplification offers a mechanistic model of the functional polarization of macrophages, whereas the tissue microenvironments are likely to elicit simultaneous activation of different signalling pathways with opposite influence on macrophage functions. Indeed, different macrophage phenotypes have been described with only partially overlapping functions with the original IFNγ (M1) and IL-4 (M2) induced phenotypes, indicating that differences in microenvironment milieu induce heterogeneous signalling events, contributing to the extensive heterogeneity in patterns of gene expression seen in macrophages [37–44]. The view that macrophage activation does not result in the expression of a single set of functions, but rather display a progression of functional changes in response to the changes occurring in its microenvironment, challenges the thesis that macrophages displaying unique tissue-specific or response-specific functional patterns, represent distinct lineages [42, 45]. For example, gene expression profiling studies highlighted that, upon human cytomegalovirus (HCMV) infection, the activation of both NF-κB and PI3K signalling pathways [46] drives monocytes toward an atypical M1/M2 reprogramming [47]. Similarly, CD11c± adipose tissue macrophages from obese mice have a mixed profile, with upregulation of several M1 and M2 gene transcripts [48]. Furthermore, a shift in monocyte-macrophage phenotypes during the course of several diseases such as sepsis, cancer and obesity has been reported [49–51].
New evidence indicate that polarized inflammation plays a central role during different stages of tumor development. In early phases, high production of M1 inflammatory mediators (e.g. tumor necrosis factor, TNF; reactive oxygen species, ROS) appears to support neoplastic transformation [6], whereas in established cancers the expression of M2-like phenotypes with immunosuppressive, pro-angiogenic and tissue remodelling activities promotes immune escape, tumor growth and malignancy [1, 6, 52–55]. Recent addition to the molecular repertoire of TAMs includes semaphorin 4D (Sema4D) [56] and growth arrest-specific 6 (Gas6) [57], which are respectively involved in promoting tumor angiogenesis and cancer cell proliferation. Of note, Src homology 2-containing inositol-5′-phosphatase-1 (SHIP1)-deficient mice, which exhibit a spontaneous macrophage drift towards M2 polarization, display increased growth of transplanted tumors [58]. Further, Notch signalling deficient macrophages, which show an M2-biased phenotype, inhibit T-cell activation and enhanced tumor growth when inoculated in solid tumors [59]. In contrast, p50 Nuclear factor-κB- (NF-κB) deficient mice, which showed a defective capacity to mount an M2 macrophage polarization [60], display increased tumor resistance [54]. Clinical studies suggest that the type of immunological profile expressed at the tumor site represents an independent prognostic factor. In particular, an established type-2 “suppressive” immunological profile correlates with poor prognosis, as shown in colorectal, hepatocellular and pancreatic carcinomas and in Hodgkin’s lymphoma [61–64]
Many studies have shed light on the intricate signalling network that drives myeloid cells towards M2-polarized activation. The acquisition of pro-tumoral M2 functions by TAM is driven by various cytokines and signals expressed within the tumor microenvironment [54]. Among these, IL-10, prostaglandin E2 (PGE2), TGF-β, IL-6, CCL2, migration-stimulating factor (MSF) and CSF-1 were reported to induce M2-like polarization [[54, 65–68]. Recent studies suggest that different circuits and cells, including innate immune cells and fibroblasts, participate in shaping TAM activities [13, 69–72] (Figure 2). As an example, in a model of mammary carcinoma, MDSCs were shown to contribute to tumor progression by suppressing T-cell activation and inducing an M2-like phenotype of TAMs [69]. Cancer associated fibroblasts (CAFs) have recently emerged as new players in cancer-related inflammation [71]. Using the K14-HPV16 mouse model of squamous carcinogenesis, Erez and colleagues demonstrated that CAFs express a distinct inflammatory gene signature associated with promotion of TAM recruitment, angiogenesis and tumor growth [71]. In a murine breast cancer model, in vivo ablation of CAFs by a DNA vaccination strategy, markedly inhibited recruitment of TAMs, MDSCs and regulatory T cells (Tregs), thus promoting a type 2 vs type 1 shift of tumor-associated inflammation [70].
Fig. 2.
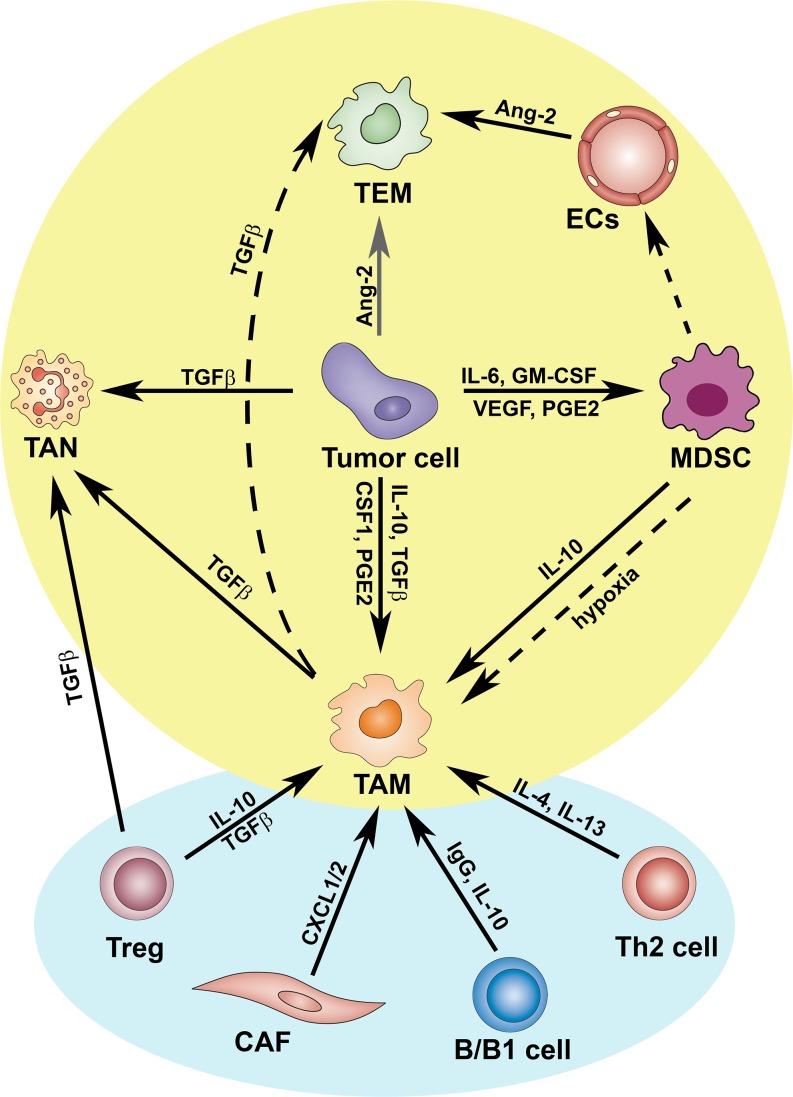
Pathways of polarized activation of tumor associated myeloid cells (TAMCs). As depicted, TAMCs reciprocally influence their protumoral differentiation, under the “remote control” of cancer-associated fibroblasts (CAFs), T and B lymphocytes. TAMs in concert with Tregs and tumor cells, produce TGFβ that induces the alternative (N2) activation of TANs. Differentiation of MDSCs is modulated by several tumor-derived factors, including GM-CSF, IL-6, VEGF and PGE2. Further, tumor microenvironmental signals can convert MDSCs in endothelial cells (ECs) or in TAMs, the latter event mainly regulated by hypoxia. Hypoxic ECs up-regulate Ang-2 that enhances activation of pro-angiogenic and M2-skewed TEMs
Further, the interplay between innate and adaptive immunity is emerging as a crucial step in this event [72]. Recently, utilizing the MMTV-PyMT model of mammary carcinogenesis, DeNardo et al. demonstrated that CD4+ T lymphocytes expressing the M2-polarizing cytokines IL-4 and IL-13 potentiate mammary adenocarcinoma metastasis by modulating the pro-tumor properties of TAMs [73]. In turn, TAMs enhance the invasive potential of malignant mammary epithelial cells.
The role of B cells in shaping the TAM phenotype was originally demonstrated in a K14-HPV16 mouse model of squamous carcinogenesis [74]. However, as B cells do not infiltrate the precancerous tissues, it was suggested that infiltration and functions of innate immune cells must be orchestrated remotely, suggesting that lymphocyte-derived cytokines and/or antibodies may drive the cancer-promoting inflammation [3]. This hypothesis has recently found a confirmation by the study of Andreu and co-workers. Using the same mouse model of squamous carcinogenesis, they showed that B cells and humoral immunity foster cancer development by activating Fcγ receptors (FcγR) on resident and recruited myeloid cells [75]. A recent report suggests that B1 cells, but not B2 cells, polarize peritoneal macrophages to an M2-like phenotype, characterized by impaired expression of LPS-induced pro-inflammatory genes (e.g. TNFα, CCL3, IL-1β) with up-regulation of the anti-inflammatory gene IL-10 [72, 76].
The observations identify the M2-like phenotype of TAM as a point of convergence of various pro-tumoral pathways (Figure 2).
Tie2-expressing Monocytes/Macrophages (TEMs)
TEMs are a small subset of myeloid cells characterized by the expression of the angiopoietin receptor Tie2 and powerful pro-angiogenic activity [24, 25, 77]. They derive from circulating Tie2-expressing monocytes which are recruited in tumors by hypoxia-induced endothelial-derived chemotactic factors, such as Ang-2 and CXCL12 [24–26, 78] The CXCL12-CXCR4 axis is a well known circuit driving accumulation of TAMs in hypoxic areas of solid tumors [18]. In addition, it has been demonstrated that pharmacological inhibition of CXCR4 is associated with a significant reduction of TEM recruitment into mammary tumors [26]. Despite representing only a small fraction of TAMs (Tie2- tumor-associated macrophages), both ablation and adoptive transfer studies have demonstrated that TEMs are crucial promoters of tumor angiogenesis [5, 25, 79]. In two models of mammary tumours and orthotopic human gliomas, Ganciclovir-driven ablation of Tie2+ monocytes induced a significant reduction of both tumour mass and vasculature, demonstrating their importance in tumour angiogenesis and growth [5, 25, 79]. In line, adoptive transfer studies demonstrated that subcutaneous co-injection of tumor cells with TEMs increases tumor vascularization [5]. Strikingly, gene expression analysis highlighted that TEMs are highly related to TAMs, but express a more pronounced M2-skewed gene signature, with higher expression of M2 genes, including arginase 1 (Arg1), scavenger receptors (CD163; Mannose receptor 1, Mrc1; Macrophage scavenger receptor 2, Msr2; stabilin-1) and lower levels of pro-inflammatory molecules (IL-1β; prostaglandin endoperoxide synthase 2/cyclooxygenase 2, PTGS2/COX2; IL-12; TNF; inducible nitric oxide synthase, iNOS; CCL5; CXCL10; CXCL11) [80]. These results suggested that Tie2+ monocytes could be a distinct lineage of myeloid cells, committed to execute physiologic pro-angiogenic and tissue-remodeling programs, which can be co-opted by tumors [75]. Noteworthy, human Tie2+ circulating monocytes express high levels of pro-angiogenic genes (e.g. VEGF-A; Matrix metallopeptidase 9, MMP9; COX2; wingless-related MMTV integration site 5A, WNT5A) and are powerful inducers of endothelial cells activation [81]. In agreement, sub-cutaneous tumors growing in Ang-2-overexpressing mice showed increased number of TEMs associated with enhanced microvessels density [81]. Tie2 engagement by Ang-2 in both mouse and human TEMs not only elicits a chemotactic response but also enhances their pro-tumoral activities [81]. It was also recently demonstrated that Ang-2 levels in 4T1 mammary tumors correlates with both TEM-derived IL-10 and Treg infiltration, resulting in suppression of T cells proliferation [78]. In contrast, Ang-2 inhibited the expression of M1 cytokines (IL-12 and TNFα) in TEMs exposed to hypoxia [24].
Myeloid-Derived Suppressor Cells (MDSCs)
MDSCs represent an heterogenous population of cells whose common characteristics are an immature state and the ability to suppress T-cell responses both in vitro and in vivo [82, 83]. MDSCs possess several mechanisms for immune suppression: 1) depletion of arginine, mediated by Arg1 and iNOS; 2) production of ROS; 3) post-translational modifications of T cell receptor (TCR) mediated by peroxynitrite generation; 4) depletion of cysteine; 5) production of TGFβ; 6) induction of Tregs [84–91]. In healthy individuals, myeloid progenitors differentiate in mature granulocytes, macrophages or dendritic cells, whereas in pathological conditions they expand into MDSCs. MDSCs have been observed in cancer, chronic infectious diseases, and autoimmunity. In tumor-bearing mice, MDSCs accumulate within primary and metastatic tumors, in the bone marrow, spleen and peripheral blood. In cancer patients, MDSCs have been identified in the blood. Recent studies have contributed to partially clarify the biology of MDSCs. In mice, two major subsets were identified on the basis of their morphology and the expression of Ly6 family glycoproteins: monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (G-MDSCs). M-MDSCs are CD11b+ Ly6G- Ly6Chigh cells with monocyte-like morphology, while G-MDSCs are CD11b+ Ly6G+ Ly6Clow with granulocyte-like morphology [83]. Cells with similar phenotype, precursors of myeloid cells, are present in physiological conditions, but they are devoid of an the immunosuppressive activity. Therefore, these cells should not be named MDSCs [92]. Other markers of MDSC subsets are: IL-4Rα (CD124), F4/80, CD80, and CSF-1R (CD115) [6]. The characterization of human MDSCs deeply suffers from the lack of specific markers. Human MDSCs are generally identified as cells expressing the common myeloid marker CD33, lacking the expression of markers of mature myeloid and lymphoid cells, and able to suppress T cell activation [93, 94]. The phenotype of human MDSCs seems to be dependent from the type of tumor born by the patient, suggesting that several cellular subsets may exist. MDSCs with different phenotype (reported here in brackets) were identified in patients affected by various tumor types, including: renal cancer (CD14- CD11b± CD15±) [95, 96], prostate cancer (CD14- HLA-DRlow/-) [97], advanced non-small cell lung cancer (CD14- CD33± CD11b± CD15±) [98], melanoma (CD14± CD11b± HLA-DRlow/-) [99, 100], and hepatocellular carcinoma (CD14± HLA-DRlow/-) [101]. Human MDSCs characterized by both monocytic and granulocytic morphology have been described [95, 99]. The ability to differentiate into mature DCs and macrophages in vitro has been shown to be restricted to M-MDSCs [84]. M-MDSC-mediated immune suppression does not require cell-cell contact, but utilizes up-regulation of iNOS and Arg1, as well as production of immunosuppressive cytokines [82]. On the contrary, G-MDSCs suppress antigen-specific responses using mechanisms, including the release of ROS, that require prolonged cell-cell contact between MDSC and T cell [82]. The C5a subunit of the complement system appears a key regulator of MDSC functions, by modulating their migration and ROS production [28].
Several factors produced by tumors have been implicated in the differentiation of MDSCs, including granulocyte monocytes-colony stimulating factor (GM-CSF), macrophage- monocytes-colony stimulating factor (M-CSF), IL-6, IL-1β, VEGF and PGE2 [82, 102]. The transcription factor CCAT/enhancer binding protein β (C/EBPβ) proved to be the key player in the process of MDSC development [102]. It has been proposed that two signals are needed for the expansion and function of MDSCs: one factor (e.g. GM-CSF) prevents the differentiation in mature myeloid cells, and a second signal, provided by pro-inflammatory molecules such as IFNγ, activate MDSCs [103]. A remarkable relation exists between MDSCs and TAMs. MDSCs are able to skew TAM differentiation toward a tumor-promoting type-2 phenotype [69]. The cross-talk between MDSCs and macrophages requires cell-cell contact, then MDSCs release IL-10 to reduce IL-12 production by macrophages. MDSCs from an IL-1β-enriched tumor microenvironment produce more IL-10 and are more potent down-regulators of macrophage-released IL-12 [104]. Circulating MDSCs can differentiate into Gr1- F4/80+ TAMs in the tumor site [105] and this conversion is driven by tumor hypoxia [106]. Because of their tumor-promoting activities, MDSCs are associated with type-2 immune responses, however accumulating evidence show that MDSCs have characteristics of both M1 and M2 macrophages [6]. As an example, MDSCs express both Arg1 and iNOS, where these enzyme are differentially expressed by M1 (iNOS) and M2 (Arg1) macrophages. A recent study, investigating the molecular mechanisms behind MDSC differentiation, demonstrated a essential role of paired-immunoglobulin receptors (PIRs) in the differentiation of M1 or M2 MDSCs [107]. The balance between PIR-A and PIR-B modulates MDSC polarization. In support of this, the growth of Lewis lung carcinoma was significantly retarded in PIR-B-deficient mice (Lilrb3−/−) and PIR-B-deficient M-MDSCs expressed high levels of iNOS and TNFα, therefore showing a M1 phenotype. Beside their immunosuppressive activities, MDSCs contribute to tumor growth also by nonimmune mechanisms, including the promotion of angiogenesis. MDSCs isolated from murine tumors express high levels of metalloproteases, including MMP9 [10]. MMP9 increases the bioavailability of VEGF sequestered in the extracellular matrix. Further in the tumor microenvironment and in proangiogenic culture conditions, MDSCs acquire endothelial markers such as CD31 and VEGF receptor 2 (VEGFR2) and the ability to directly incorporate into tumor endothelium [108]. In agreement, tumor refractoriness to anti-VEGF therapy was shown to be mediated by CD11b+GR1+ myeloid cells [109, 110].
Tumor-Associated Neutrophils (TANs)
Tumor-associated polymorphonuclear neutrophils (PMNs) have received little interest by immunologists, also based on their short life span. However, new evidence contradict this view, in that cytokines like IL-1 or microenvironment conditions such as hypoxia can prolong PMN survival [111]. Neutrophils are able to produce various cytokines and chemokines that can influence not only immune and antimicrobial responses, but other processes such as hematopoiesis, wound healing, and angiogenesis [112, 113]. Despite little attention has been paid to TANs, these cells are present in various tumors, including kidney, breast, colon, and lung [114]. Clinical evidence indicate that the presence of TANs is a negative prognostic indicator. A correlation between TANs infiltrate and poor outcome has been described in renal cell carcinoma, bronchoalveolar cell carcinoma, and breast cancer [115, 116]. In agreement, preclinical studies experimenting PMN depletion confirmed the detrimental nature of TANs [117, 118]. Neutrophils contribute to tumor growth by promoting angiogenesis, cell proliferation, and metastasis [114]. Similarly to macrophages, a recent report described the functional plasticity of neutrophils [7]. The authors investigated the effects of SM16, a TGFβ receptor kinase antagonist, in murine lung cancer and mesothelioma models using syngeneic tumor xenografts and the orthotopic LSL-K-ras tumor model. Depletion of neutrophils by a specific anti-Ly6G antibody resulted in a significantly reduced effect of SM16, suggesting that neutrophils participate to the antitumor activity of TGFβ blockade, most likely by the production of oxygen radicals. Also, depletion of neutrophils affected the activation of CD8+ CTLs. Fridlender and colleagues propose a new paradigm in which resident TANs acquire a protumor phenotype, largely driven by TGFβ, to become “N2 neutrophils”. If TGFβ is blocked, neutrophils acquire an antitumor phenotype to become “N1 neutrophils” [7].
It was suggested that N1- and N2-type neutrophils are neutrophils with a different degree of activation (i.e. fully activated or weakly activated neutrophils, respectively) rather than two alternatively activated cell subtypes [119]. It is also object of debate the existence of two distinct populations, namely N2-polarized TANs and granulocytic MDSCs, that seem to overlap for many characteristics. In the absence of specific markers, it cannot be determined if N2 neutrophils within the tumors are granulocytic MDSCs recruited from the spleen or whether they are blood-derived neutrophils converted to an N2 phenotype by the tumor microenvironment. In support to the existence of N2-polarized TANs, Fridlender et al. emphasize that TGFβ-blockade does not alter blood neutrophils, splenic myeloid cells (CD11b+), or splenic MDSCs, selectively acting on the intratumor activation of neutrophils. Also, TANs characterized in Fridlender’s study have clear features of mature neutrophils, while MDSCs mostly exhibit an immature morphology [120].
Molecular Determinants of TAMCs Functions
Nuclear Factor κB (NF-κB)
Several lines of evidence indicate the NF-κB system as a major regulator of the immune and inflammatory responses [121]. Two major signaling pathways control the activation of NF-κB [121, 122]. The classical pathway is stimulated by proinflammatory cytokines, such as TNF-α and IL-1, as well as by recognition of pathogen-associated molecular patterns (PAMPs), and is mostly involved in innate immunity [122]. In addition, an alternative pathway of NF-κB activation, mainly involved in adaptive immunity, is activated by certain members of the TNF cytokine family, but not by TNFα itself [122]. The NF-κB family consists of five members: NF-κB1 (p105/p50), NF-κB2 (p100/p52), RelA (p65), RelB and c-Rel, that may form different homo- and heterodimers associated with differential regulation of target genes [122]. Accumulation of inhibitory p50 homodimers has been observed in endotoxin tolerant macrophages [60], as well as in TAMs [54], suggesting an important role in the control and extinction of the inflammatory response [60]. Other negative regulators of NF-κB activation have been identified in disease and include the LPS-inducible splice variant of myeloid differentiation 88 (MyD88) termed MyD88s, the single immunoglobulin IL-1 receptor-related molecule/Toll-IL-1-R8 (SIGIRR/TIR8), suppressor of tumorigenicity 2 (ST2), interleukin 1 receptor accessory protein M (IRAK-M), suppressors of cytokine signalling 1 (SOCS1), and SHIP1 [123].
To the extent they have been investigated, TAMs display high accumulation of nuclear p50 homodimers and defective NF-κB activation in response to different pro-inflammatory signals [54], suggesting their tolerant phenotype.
Interestingly, TAM from p50−/− tumor-bearing mice express cytokines characteristic of M1 macrophages (eg. IL-12high/IL-10low) and their splenocytes produce increased levels of Th1 cytokines (eg. IFN-γ), which are associated with a delay in tumor growth [54]. By searching for the microenvironmental signals promoting accumulation of the p50 homodimer in macrophages, we demonstrated that IL-10, PGE2 and TGFβ, which are expressed by TAMs and promote M2-type polarized inflammation [124], induce p50 NF-κB homodimer activity [54]. A detailed analysis of the role of p50 NF-κB homodimer in macrophage functions revealed that its nuclear accumulation, both in TAMs and LPS-tolerant macrophages, not only mediates a status of unresponsiveness (tolerance) toward pro-inflammatory signals, but actually plays as key regulator of M2-driven inflammatory reactions, acting through inhibition of NF-κB-driven M1-polarizing IFNβ production and Signal Transducer and Activator of Transcription (STAT1) phosphorylation [60].
A microenvironment condition that appears to impact on NF-κB signaling in TAM is hypoxia (low oxygen tension). The presence of many areas of hypoxia is a hallmark feature of most forms of solid tumor [1] and TAMs have been shown to accumulate in these areas where hypoxia promotes their pro-tumor phenotype [10]. Hypoxia-inducible factor 1 (HIF-1) has been shown to control the cellular response to hypoxia. Hypoxia stabilizes HIF-1α, preventing posttranslational hydroxylation and subsequent degradation via the proteasome. More recently, short-term exposure of murine bone marrow–derived macrophages to hypoxia has been shown to up-regulate NF-κB activity, which in turn up-regulates HIF-1α levels [125].
NF-κB plays a key role also in the expansion and functional activation of MDSCs [103]. In a model of IL-1β–driven gastric inflammation and cancer it has been shown that activation of IL-1 receptor signalling is crucial for both MDSC recruitment and pre-neoplastic lesions development. Both in vitro and in vivo studies demonstrated that the IL-1β induced MDSC activation occurs through NF-κB signalling pathway [126]. In line, a recent study has demonstrated the importance MyD88-NF-κB pathway for MDSC accumulation in a mouse model of liver cancer [127]. In contrast, by deciphering the molecular mechanisms linking PIRs signalling with MDSC polarized activition, Ma and colleagues have demonstrated that PIR-A dependent activation of STAT-1 and NF-κB pathways drives MDSCs differentiation into an M1 anti-tumor phenotype [107].
Further studies addressing the relative contribution of individual NF-κB members (p65, c-Rel, p50, BCL3) and their combinatorial transcriptional partners, such as STATs and IRF3 will likely contribute to fully clarify its role in cancer-related inflammation.
Hypoxia
Hypoxia is a main trait of solid tumours [2] and hypoxic areas are sites of accumulation for infiltrating myeloid cells . As a consequence, the phenotype and functions of TAMCs are profoundly affected by activation of hypoxia inducible factors (HIFs) [128]. Hypoxia has divergent effects on cells of both innate and adaptive immunity [111]. Overall, HIF promotes pro-tumor behavior of leukocytes to the detriment of effective anti-tumor responses [10, 128]. Hypoxia has a significant impact on myeloid cells [129]. TAMs accumulate in tumor hypoxic areas and respond to the levels of hypoxia with a transcription program in which mitogenic, pro-invasive, pro-angiogenic, and pro-metastatic genes are up-regulated [130, 131]. We have shown that hypoxic induction of HIF-1α in TAMs influences the positioning and function of tumor cells, stromal cells and TAMs, by selectively up-regulating the expression of the chemokine receptor CXCR4 [18].
Furthermore, it has been shown that HIF-1 activation mediates expression of the CXCR4 ligand CXCL12, a chemokine involved in angiogenesis and cancer metastasis [132] and promotes recruitment of bone marrow-derived CD45+ myeloid cells Tie2+,VEGFR1+,CD11b+ and F4/80+ subpopulations [133]. Hypoxia induces upregulation of the Bombina variegata 8 kDa protein (Bv8), which attracts MDSCs in the tumor, where they can stimulate tumor angiogenesis [110] and mediate tumor refractoriness to anti-VEGF therapy [134].
The trophic action of pathological hypoxia on myeloid cells is also demonstrated by the increased presence and enhanced survival of neutrophils in hypoxic tissues [111, 135]. Both iNOS and Arg1 are controlled by hypoxia. As a consequence, hypoxia in TAMs and MDSCs influence the activation of adaptive immunity, as it promotes inhibition of lymphocyte functions by enhancing the expression of suppressive enzymes [6, 136, 137]. Noteworthy, Gabrilovich and colleagues demonstrated that hypoxia, via HIF-1α, reproduces the effect of tumor microenvironment on MDSCs and promotes their differentiation to TAMs [106].
Even if HIF-1 and HIF-2 are apparently similarly regulated and bind to the same hypoxic responsive element, they activate a distinct set of genes mediating unique biological functions [138]. HIF-alpha isoforms can differently regulate macrophage polarization. Johnson and colleagues proposed that HIF-2α stabilization induced by Th2 cytokines leads to type-2 polarization generating macrophages with pro-tumoral activities. Conversely, HIF-1α is induced by Th1 cytokines in M1 macrophage polarization [139]. This functional antagonism is due to a differential action on the iNOS and Arg1 genes. In contrast, Simon and colleagues reported that HIF-2α directly regulate the expression of proinflammatory cytokines [140]. Interestingly, the expression of HIF isoforms follows distinctive kinetic profiles that suggest a greater involvement of HIF-1α in acute responses and of HIF-2α in long-term responses [139]. HIF-2α was shown to regulate TAM infiltration in murine models of colon and hepatocellular carcinoma [140]. In a model of breast cancer, HIF-1α-deficient TAMs displayed defective capacity to suppress T cell functions [141]. These results endorse the great potential of HIF inhibition in cancer therapy.
Accumulating evidence suggests that intersections and compensatory pathways may exist between HIF and NF-κB systems in tumors [125, 142, 143]. Macrophages reveal a marked defect in HIF-1α expression following deletion of the NF-κB activity regulator IKKβ [125]. This cross-talk, triggered by hypoxic or pro-inflammatory signals, provides evidence of the close connection between immunity and the hypoxic response.
Signal Transducer and Activator of Transcription (STAT)
STATs are involved in several aspects of myeloid cell biology. MDSC differentiation and activation involves STAT1, STAT3, STAT5, and STAT6. STAT3 is the member of the family mainly responsible for MDSC expansion. STAT3 activation stimulates myelopoiesis and prevents the differentiation of myeloid precursor cells, most likely through the up-regulation of cyclin D, MYC, survivin, and B-cell lymphoma XL (BCL-CL) genes [82]. STAT3 also induce S100 calcium-binding protein 8 and 9 (S100A8/A9). S100A8 and S100A9 bind to receptors expressed on myeloid progenitors and impair their differentiation into mature cells, promoting differentiation expansion [29, 144]. In addition, these proteins chemoattract MDSCs to tumor sites through a NF-κB-dependent mechanism and potentiate ROS production [29, 30]. Importantly, MDSCs can produce S100A8/A9, generating a positive feedback loop that sustains MDSC expansion. STAT5 is involved in MDSC survival, while STAT1 is important for IFNγ-depending MDSC activation, especially in the case of M-MDSCs. IFNγ-induced STAT1 activation is fundamental in the up-regulation of Arg1 and iNOS [85, 103, 105, 145]. STAT6 is activated by the engagement of CD124, a marker that has been found on some subsets of MDSCs where this pathway controls Arg1 and TGFβ expression [91, 146]. STATs activation extremely diverges in macrophage differentiation: STAT1 activation results in M1 macrophage polarization, which promotes cytotoxic and inflammatory functions. In contrast, a predominance of STAT3 and STAT6 activation results in M2 macrophage polarization, which is associated with immune suppression and tumor progression [6]. Stat6−/− tumor–bearing mice display a M1 phenotype. Restored immunosurveillance in Stat6−/− mice facilitates survival against metastatic cancer via an IFN-γ-dependent mechanism [83]. Constitutive activation of STAT3 in tumor cells and in infiltrating myeloid cells creates the immunosuppressive environment that sustains tumor growth [147, 148]. For this reason, STAT3 is a prospective target for cancer therapy. Given that STAT3 is indispensable for hematopoiesis, an indiscriminate suppression of this pathway is not practicable. An attractive means to solve this problem has been recently proposed in a study utilizing a STAT3-targeting siRNA synthetically linked to a CpG oligonucleotide [149]. This macromolecule delivers the STAT3-inhibiting siRNA specifically to Toll-like receptor 9 (TLR9)-expressing cells (B cells and myeloid cells). Moreover, TLR9 activation synergizes with the block of STAT3 pathway, leading to an effective response. In vivo administration of the drug activate tumor-associated immune cells and provoking strong antitumor immune responses.
Therapeutic Approaches Targeting TAMCs
Activation of selected inflammatory programs provide protection in a preventive or therapeutic setting [3, 150, 151]. It was reported that dying tumor cells can be cross presented by dendritic cells and trigger a protective immune response via a TLR4-MyD88 pathway [152] and inflammasome activation and IL-1β production mediate activation of protective immunity [153].
A recent work showed that DNA vaccine against the M2-associated molecule legumain, a member of the asparaginyl endopeptidase family overexpressed by TAMs, induced a robust CD8+ T cell response against TAMs and led to a suppression of angiogenesis, tumor growth, and metastasis [154]. Rolny and co-workers have reported that by skewing TAM polarization away from the M2-like to a tumor-inhibiting M1-like phenotype, the host-produced histidine-rich glycoprotein (HRG) promotes antitumor immune responses and vessel normalization [155].
Alternatively, macrophages depletion has been obtained in vivo with the use of clodronate-encapsulated liposomes [156] or amino-bisphosphonate, resulting in reduced angiogenesis and tumor progression in several experimental tumor models [157]. However, as more evidence emerge for the signaling pathways involved in the ‘switch’ of macrophage polarization states in the early stages of tumor progression [1, 6], it may be possible to develop new therapies aimed at preventing this and/or re-orientating M2-like TAMs in favour of a more antitumoral phenotype.
Direct activation with IFNγ a prototypical M1-polarizing cytokine, has been shown to re-educate TAMs [158] and there is evidence for antitumor activity of this molecule in minimal residual disease [1]. In spontaneous breast cancer model, in vivo treatment with zoledronic acid, a well known anti-tumor drug, is able to revert TAM polarization from M2-like to M1 phenotype, as well as to inhibit mammary carcinogenesis [159].Taking this approach, combination of CpG plus an anti-IL-10 receptor antibody switched infiltrating macrophages from M2 to M1 and triggered innate response debulking large tumors within 16 h [160]. Moreover, TAMs lacking STAT6, the major mediator of IL-4 and IL-13 biological functions, display an M1 phenotype, with low level of Arg1 and high level of iNOS and rejected spontaneous mammary carcinoma by a process requiring adaptive immunity to cancer [161]. In analogy, inhibition of STAT3 activity, required for IL-10 biological functions and gene transcription, restored production of pro-inflammatory mediators (IL-12 and TNF-α) by infiltrating leukocytes and promoted tumour inhibition [147]. Recent results suggest that SHIP1 functions in vivo to repress M2 macrophage skewing. Consistent with this, Ship1−/− mice display enhanced tumor implant growth [58].
Targeting cytokines and cytotoxic proteins to tumors by means of gene modified cells represents a promising strategy to treat cancer. It was recently shown that TEMs could be used to deliver interferon-alpha (IFNα), a potent cytokine with angiostatic and antiproliferative activity [162], thanks to the preferential homing of TEMs to the tumors [77].
The translational potential of MDSC research is dual. The immunosuppressive activity of MDSCs could be exploited to inhibit immune responses in autoimmune diseases and organ transplantation. Conversely, elimination of MDSCs could be essential in cancer patients undergoing active (vaccination) or passive (adoptive transfer of ex-vivo expanded anti-tumor T cells) immunotherapy. A possible approach to contrast MDSC pro-tumoral activities consists in the promotion of MDSC differentiation into mature cells devoid of suppressive activity. Vitamin A represent an interesting candidate to restore immunosurveillance. In fact, Vitamin A metabolites stimulate the differentiation of myeloid progenitor cells into DCs and macrophages and reduce MDSC accumulation [163, 164]. A clinical trial testing the effects of all-trans-retinoic acid (ATRA) in patients with metastatic renal cell carcinoma showed the efficacy of this compound in reducing MDSCs in peripheral blood. The decrease in MDSC number correlated with improved-antigen-specific T cell responses [165]. It has been reported that some chemotherapeutic drugs, such as gemcitabine, are able to eliminate MDSCs, without affecting T cells, B cells, NK cells, and macrophages [166, 167]. Another strategy is aimed to inhibit MDSC suppressive function. Compounds under investigation for this ability belong to COX2 inhibitors, phosphodiesterase 5 (PDE5) inhibitors, and NO-releasing non-steroidal anti-inflammatory drugs (NSAIDs) [82]. Preclinical evidence support the use of IL-1 antagonists in treating human metastatic disease. Blocking IL-1 activity, mainly IL-1β, reduces both metastasis and tumor growth [168]. Recently, it was shown that the effect is also mediated by the decrease of MDSC accumulation and suppressive activity [83]. It has also been reported that CD11b+ Gr1+ cells enhance tumor refractoriness to anti-VEGF antibody (bevacizumab) treatment [109]. In this situation, MDSCs release the pro-angiogenic protein Bv8 that surrogates VEGF in the stimulation of tumor angiogenesis [110]. Because Bv8 is also important in MDSC mobilization and homing to the tumor site, this is an interesting candidate for cancer therapy.
TAN depletion represents a potential therapeutic approach for cancer cure [169]. However, since oncologic patients are already immunocompromized individuals, a complete ablation of neutrophils is not desirable. Alternatively, given that activated neutrophils can kill tumor cells through the release of toxic substances, it would be of interest to modulate TAN phenotype, with a switch from N2- towards N1-polarization. Nevertheless, this plan would lead to the generation of highly cytotoxic cells and could result in excessive tissue damage, potentially lethal. A more manageable therapeutic strategy can target neutrophils recruitment to tumors. Inhibition of CXCR2-mediated PMN chemotaxis with a specific antibody or a CXCR2 antagonist has been successfully tested in pre-clinical experimentation [119]. The description of the pivotal role of TGFβ in the promotion of a protumor phenotype of TAN suggests that therapies contrasting this cytokine could contribute to re-educate neutrophils in the tumor microenvironment [32]. Several strategies aimed to reduce or eliminate TAM recruitment have been developed. Two agents able to eliminate TAMs are clodronate (dichloromethylene–biphosphonate) and Yondelis® (trabectedin) [156, 170]. Interestingly, a recent study showed that the CCL2-driven accumulation of TAMs limits the influx of neutrophils in solid tumors by a yet unidentified mechanism. If TAM accumulation is suppressed, neutrophils are recruited to the tumor providing a secondary source of MMP-9. Therefore, in the absence of TAMs, TANs provide alternative paracrine support for tumor angiogenesis and progression [171]. Hence, the elimination of TAMs alone may be insufficient to eradicate myeloid cell support to tumor growth.
Concluding Remarks
Recent results indicate that tumour development promotes expansion and functional skewing of different myeloid cell populations, leading to accumulation of protumoral TAMC populations, which include TAMs, TEMs, MDSCs and TANs. New evidence also suggest that TAMCs reciprocally influence their protumoral differentiation, under the “remote control” of T and B lymphocytes [3, 13, 72, 172]. TAMs, TEMs, MDSCs and TANs display distinct specialized functions, as well as overlapping activities (eg. angiogenesis). New therapeutic strategies have been aimed at targeting single myeloid populations (Table 1). However, TAMCs appear to constitute a robust system and the functional elimination of a single myeloid population may be insufficient to eradicate myeloid cells support to tumor growth. It appears therefore necessary to identify strategies able to target different myeloid cell populations, simultaneously.
Table 1.
Anti-cancer strategies targeting tumor-associated myeloid cells
| Therapeutic strategy | Therapeutic agents | Refs |
|---|---|---|
| TAM depletion | Legumain based DNA vaccines | [154] |
| Clodronate | [156] | |
| Trabectedin | [170] | |
| Promotion of TAM switch from a M2-type to a M1-type phenotype | IFNγ | [158] |
| TLR9 agonists + IL-10 inhibition | [160] | |
| STAT3 inhibitors | [147, 149] | |
| STAT6 inhibitors | [161] | |
| p50 NF-κB homodimers antagonists | [60] | |
| SHIP1 activators | [58] | |
| Zoledronic acid | [159] | |
| Inhibition of TAM suppressive function | HIF-1 inhibitors | [111, 141] |
| Exploitation of cell tumor-homing aptitude for anti-tumor cytokine delivery | Engineereded TEMs | [162] |
| Inhibition of TEM recruitment and activation | Ang-2 antagonists | [81] |
| MDSC depletion | Gemcitabine | [166, 167] |
| Promotion of MDSC differentiation into mature cells devoid of suppressive functions | Vitamin A | [164, 165] |
| Inhibition of MDSC accumulation | S100A8/A9 inhibitors | [144] |
| STAT3 inhibitors | [147, 149] | |
| STAT6 inhibitors | [161] | |
| Inhibition of MDSC suppressive functions | COX2 inhibitors, | [82] |
| PDE5 inhibitors | [82] | |
| NO-releasing NSAIDs | [82] | |
| Inhibition of MDSC accumulation and angiogenic activity | Anti-Bv8 mAb | [110] |
| Prevention of neutrophils recruitment to tumors | CXCR2 antagonists | [119] |
| Blockade of M2-type and N2-type polarization, inhibition of MDSC suppressive functions | TGFβ inhibitors | [7, 32] |
TLR9 Toll-like receptor 9; SHIP1 Src homology 2-containing inositol 5′-phosphatase 1; HIF-1 Hypoxia inducible factor-1; ANG-2 Angiopoietin-2; COX2 Cyclooxygenase 2; PDE5 phosphodiesterase 5; NO-releasing NSAISs nitric oxide-releasing non-steroidal anti-inflammatory drugs; S100A8/A9 S100 calcium-binding protein 8 and 9; IL-1Ra Interleukin-1 receptor antagonist; Bv8 Bombina variegata 8 kDa protein; CXCR2 chemokine (C-X-C motif) receptor 2
Recent studies have highlighted that striking similarities exist among mechanisms governing both the transcriptional profile and the functional properties of TAMCs and it is becoming clear that pathways promoting polarized functions of either macrophages (eg. M1 vs M2) or neutrophils (N1 vs N2) may share common constituents [120]. This scenario suggests that key mechanisms may converge to promote the protumoral traits of different TAMC populations, thus potentially offering common target/s to therapeutically affect the protumoral networks established by cancer-associated myeloid cells.
Acknowledgments
This work was supported by Associazione Italiana Ricerca sul Cancro (AIRC), Italy; Fondazione Cariplo, Italy; by Ministero Università Ricerca (MUR), Italy; Ministero della Salute and by Regione Piemonte (project number 331, august 8th, 2009).
References
- 1.Mantovani A, Sica A. Macrophages, innate immunity and cancer: balance, tolerance, and diversity. Curr Opin Immunol. 2010;22:231–237. doi: 10.1016/j.coi.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Vaupel P, Mayer A. Hypoxia in cancer: significance and impact on clinical outcome. Cancer Metastasis Rev. 2007;26:225–239. doi: 10.1007/s10555-007-9055-1. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.Sica A. Role of tumour-associated macrophages in cancerrelated inflammation. Exp Oncol. 2010;32:153–158. [PubMed] [Google Scholar]
- 5.Palma M, Venneri MA, Galli R, Sergi Sergi L, Politi LS, Sampaolesi M, Naldini L. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 6.Sica A, Bronte V. Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest. 2007;117:1155–1166. doi: 10.1172/JCI31422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, Worthen GS, Albelda SM. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell. 2009;16:183–194. doi: 10.1016/j.ccr.2009.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- 9.Mantovani A, Sica A, Allavena P, Garlanda C, Locati M. Tumor-associated macrophages and the related myeloid-derived suppressor cells as a paradigm of the diversity of macrophage activation. Hum Immunol. 2009;70:325–330. doi: 10.1016/j.humimm.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 10.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 11.Chaput N, Conforti R, Viaud S, Spatz A, Zitvogel L. The Janus face of dendritic cells in cancer. Oncogene. 2008;27:5920–5931. doi: 10.1038/onc.2008.270. [DOI] [PubMed] [Google Scholar]
- 12.Ma Y, Aymeric L, Locher C, Kroemer G, Zitvogel L. The dendritic cell-tumor cross-talk in cancer. Curr Opin Immunol. 2011;23:146–152. doi: 10.1016/j.coi.2010.09.008. [DOI] [PubMed] [Google Scholar]
- 13.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 2010;11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 14.Bottazzi B, Polentarutti N, Acero R, Balsari A, Boraschi D, Ghezzi P, Salmona M, Mantovani A. Regulation of the macrophage content of neoplasms by chemoattractants. Science. 1983;220:210–212. doi: 10.1126/science.6828888. [DOI] [PubMed] [Google Scholar]
- 15.Pollard JW. Tumour-educated macrophages promote tumour progression and metastasis. Nat Rev Cancer. 2004;4:71–78. doi: 10.1038/nrc1256. [DOI] [PubMed] [Google Scholar]
- 16.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 17.Konishi T, Okabe H, Katoh H, Fujiyama Y, Mori A. Macrophage inflammatory protein-1 alpha expression in non-neoplastic and neoplastic lung tissue. Virchows Arch. 1996;428:107–111. doi: 10.1007/BF00193938. [DOI] [PubMed] [Google Scholar]
- 18.Schioppa T, Uranchimeg B, Saccani A, Biswas SK, Doni A, Rapisarda A, Bernasconi S, Saccani S, Nebuloni M, Vago L, Mantovani A, Melillo G, Sica A. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198:1391–1402. doi: 10.1084/jem.20030267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- 20.Zhang J, Sud S, Mizutani K, Gyetko MR, Pienta KJ. Activation of urokinase plasminogen activator and its receptor axis is essential for macrophage infiltration in a prostate cancer mouse model. Neoplasia. 2011;13:23–30. doi: 10.1593/neo.10728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin EY, Gouon-Evans V, Nguyen AV, Pollard JW. The macrophage growth factor CSF-1 in mammary gland development and tumor progression. J Mammary Gland Biol Neoplasia. 2002;7:147–162. doi: 10.1023/a:1020399802795. [DOI] [PubMed] [Google Scholar]
- 23.Jin G, Kawsar HI, Hirsch SA, Zeng C, Jia X, Feng Z, Ghosh SK, Zheng QY, Zhou A, McIntyre TM, Weinberg A. An antimicrobial peptide regulates tumor-associated macrophage trafficking via the chemokine receptor CCR2, a model for tumorigenesis. PLoS ONE. 2010;5:e10993. doi: 10.1371/journal.pone.0010993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Murdoch C, Tazzyman S, Webster S, Lewis CE. Expression of Tie-2 by human monocytes and their responses to angiopoietin-2. J Immunol. 2007;178:7405–7411. doi: 10.4049/jimmunol.178.11.7405. [DOI] [PubMed] [Google Scholar]
- 25.Venneri MA, Palma M, Ponzoni M, Pucci F, Scielzo C, Zonari E, Mazzieri R, Doglioni C, Naldini L. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood. 2007;109:5276–5285. doi: 10.1182/blood-2006-10-053504. [DOI] [PubMed] [Google Scholar]
- 26.Welford AF, Biziato D, Coffelt SB, Nucera S, Fisher M, Pucci F, Serio CD, Naldini L, Palma MD, Tozer GM, Lewis CE (2011) TIE2-expressing macrophages limit the therapeutic efficacy of the vascular-disrupting agent combretastatin A4 phosphate in mice. J Clin Investig [DOI] [PMC free article] [PubMed]
- 27.Sawanobori Y, Ueha S, Kurachi M, Shimaoka T, Talmadge JE, Abe J, Shono Y, Kitabatake M, Kakimi K, Mukaida N, Matsushima K. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood. 2008;111:5457–5466. doi: 10.1182/blood-2008-01-136895. [DOI] [PubMed] [Google Scholar]
- 28.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cheng P, Corzo CA, Luetteke N, Yu B, Nagaraj S, Bui MM, Ortiz M, Nacken W, Sorg C, Vogl T, Roth J, Gabrilovich DI. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med. 2008;205:2235–2249. doi: 10.1084/jem.20080132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sinha P, Okoro C, Foell D, Freeze HH, Ostrand-Rosenberg S, Srikrishna G. Proinflammatory S100 proteins regulate the accumulation of myeloid-derived suppressor cells. J Immunol. 2008;181:4666–4675. doi: 10.4049/jimmunol.181.7.4666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bellocq A, Antoine M, Flahault A, Philippe C, Crestani B, Bernaudin JF, Mayaud C, Milleron B, Baud L, Cadranel J. Neutrophil alveolitis in bronchioloalveolar carcinoma: induction by tumor-derived interleukin-8 and relation to clinical outcome. Am J Pathol. 1998;152:83–92. [PMC free article] [PubMed] [Google Scholar]
- 32.Flavell RA, Sanjabi S, Wrzesinski SH, Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10:554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 34.Stein M, Keshav S, Harris N, Gordon S. Interleukin 4 potently enhances murine macrophage mannose receptor activity: a marker of alternative immunologic macrophage activation. J Exp Med. 1992;176:287–292. doi: 10.1084/jem.176.1.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 36.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gratchev A, Kzhyshkowska J, Kannookadan S, Ochsenreiter M, Popova A, Yu X, Mamidi S, Stonehouse-Usselmann E, Muller-Molinet I, Gooi L, Goerdt S. Activation of a TGF-beta-specific multistep gene expression program in mature macrophages requires glucocorticoid-mediated surface expression of TGF-beta receptor II. J Immunol. 2008;180:6553–6565. doi: 10.4049/jimmunol.180.10.6553. [DOI] [PubMed] [Google Scholar]
- 38.Hu X, Chung AY, Wu I, Foldi J, Chen J, Ji JD, Tateya T, Kang YJ, Han J, Gessler M, Kageyama R, Ivashkiv LB. Integrated regulation of Toll-like receptor responses by Notch and interferon-gamma pathways. Immunity. 2008;29:691–703. doi: 10.1016/j.immuni.2008.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ravasi T, Wells C, Forest A, Underhill DM, Wainwright BJ, Aderem A, Grimmond S, Hume DA. Generation of diversity in the innate immune system: macrophage heterogeneity arises from gene-autonomous transcriptional probability of individual inducible genes. J Immunol. 2002;168:44–50. doi: 10.4049/jimmunol.168.1.44. [DOI] [PubMed] [Google Scholar]
- 40.Riches DW. Signalling heterogeneity as a contributing factor in macrophage functional diversity. Semin Cell Biol. 1995;6:377–384. doi: 10.1016/s1043-4682(05)80008-x. [DOI] [PubMed] [Google Scholar]
- 41.Riches DW, Henson PM, Remigio LK, Catterall JF, Strunk RC. Differential regulation of gene expression during macrophage activation with a polyribonucleotide. The role of endogenously derived IFN. J Immunol. 1988;141:180–188. [PubMed] [Google Scholar]
- 42.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–349. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 43.Tannenbaum CS, Koerner TJ, Jansen MM, Hamilton TA. Characterization of lipopolysaccharide-induced macrophage gene expression. J Immunol. 1988;140:3640–3645. [PubMed] [Google Scholar]
- 44.Wang L, Tassiulas I, Park-Min KH, Reid AC, Gil-Henn H, Schlessinger J, Baron R, Zhang JJ, Ivashkiv LB. ‘Tuning’ of type I interferon-induced Jak-STAT1 signaling by calcium-dependent kinases in macrophages. Nat Immunol. 2008;9:186–193. doi: 10.1038/ni1548. [DOI] [PubMed] [Google Scholar]
- 45.Geissmann F, Jung S, Littman DR. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity. 2003;19:71–82. doi: 10.1016/s1074-7613(03)00174-2. [DOI] [PubMed] [Google Scholar]
- 46.Chan G, Bivins-Smith ER, Smith MS, Yurochko AD. NF-kappaB and phosphatidylinositol 3-kinase activity mediates the HCMV-induced atypical M1/M2 polarization of monocytes. Virus Res. 2009;144:329–333. doi: 10.1016/j.virusres.2009.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chan G, Bivins-Smith ER, Smith MS, Smith PM, Yurochko AD. Transcriptome analysis reveals human cytomegalovirus reprograms monocyte differentiation toward an M1 macrophage. J Immunol. 2008;181:698–711. doi: 10.4049/jimmunol.181.1.698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shaul ME, Bennett G, Strissel KJ, Greenberg AS, Obin MS. Dynamic, M2-like remodeling phenotypes of CD11c + adipose tissue macrophages during high-fat diet--induced obesity in mice. Diabetes 59:1171–1181 [DOI] [PMC free article] [PubMed]
- 49.Biswas SK, Lopez-Collazo E. Endotoxin tolerance: new mechanisms, molecules and clinical significance. Trends Immunol. 2009;30:475–487. doi: 10.1016/j.it.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 50.Biswas SK, Sica A, Lewis CE. Plasticity of macrophage function during tumor progression: regulation by distinct molecular mechanisms. J Immunol. 2008;180:2011–2017. doi: 10.4049/jimmunol.180.4.2011. [DOI] [PubMed] [Google Scholar]
- 51.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dinapoli MR, Calderon CL, Lopez DM. The altered tumoricidal capacity of macrophages isolated from tumor-bearing mice is related to reduce expression of the inducible nitric oxide synthase gene. J Exp Med. 1996;183:1323–1329. doi: 10.1084/jem.183.4.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 54.Saccani A, Schioppa T, Porta C, Biswas SK, Nebuloni M, Vago L, Bottazzi B, Colombo MP, Mantovani A, Sica A. p50 nuclear factor-kappaB overexpression in tumor-associated macrophages inhibits M1 inflammatory responses and antitumor resistance. Cancer Res. 2006;66:11432–11440. doi: 10.1158/0008-5472.CAN-06-1867. [DOI] [PubMed] [Google Scholar]
- 55.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 56.Sierra JR, Corso S, Caione L, Cepero V, Conrotto P, Cignetti A, Piacibello W, Kumanogoh A, Kikutani H, Comoglio PM, Tamagnone L, Giordano S. Tumor angiogenesis and progression are enhanced by Sema4D produced by tumor-associated macrophages. J Exp Med. 2008;205:1673–1685. doi: 10.1084/jem.20072602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Loges S, Schmidt T, Tjwa M, Geyte K, Lievens D, Lutgens E, Vanhoutte D, Borgel D, Plaisance S, Hoylaerts M, Luttun A, Dewerchin M, Jonckx B, Carmeliet P. Malignant cells fuel tumor growth by educating infiltrating leukocytes to produce the mitogen Gas6. Blood. 2010;115:2264–2273. doi: 10.1182/blood-2009-06-228684. [DOI] [PubMed] [Google Scholar]
- 58.Rauh MJ, Ho V, Pereira C, Sham A, Sly LM, Lam V, Huxham L, Minchinton AI, Mui A, Krystal G. SHIP represses the generation of alternatively activated macrophages. Immunity. 2005;23:361–374. doi: 10.1016/j.immuni.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 59.Wang YC, He F, Feng F, Liu XW, Dong GY, Qin HY, Hu XB, Zheng MH, Liang L, Feng L, Liang YM, Han H. Notch signaling determines the M1 versus M2 polarization of macrophages in antitumor immune responses. Cancer Res. 2011;70:4840–4849. doi: 10.1158/0008-5472.CAN-10-0269. [DOI] [PubMed] [Google Scholar]
- 60.Porta C, Rimoldi M, Raes G, Brys L, Ghezzi P, Liberto D, Dieli F, Ghisletti S, Natoli G, Baetselier P, Mantovani A, Sica A. Tolerance and M2 (alternative) macrophage polarization are related processes orchestrated by p50 nuclear factor kappaB. Proc Natl Acad Sci U S A. 2009;106:14978–14983. doi: 10.1073/pnas.0809784106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Budhu A, Wang XW. The role of cytokines in hepatocellular carcinoma. J Leukoc Biol. 2006;80:1197–1213. doi: 10.1189/jlb.0506297. [DOI] [PubMed] [Google Scholar]
- 62.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 63.Kurahara H, Shinchi H, Mataki Y, Maemura K, Noma H, Kubo F, Sakoda M, Ueno S, Natsugoe S, Takao S (2009) Significance of M2-polarized tumor-associated macrophage in pancreatic cancer. J Surg Res [DOI] [PubMed]
- 64.Steidl C, Lee T, Shah SP, Farinha P, Han G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, Bast MA, Rosenwald A, Muller-Hermelink HK, Rimsza LM, Campo E, Delabie J, Braziel RM, Cook JR, Tubbs RR, Jaffe ES, Lenz G, Connors JM, Staudt LM, Chan WC, Gascoyne RD. Tumor-associated macrophages and survival in classic Hodgkin’s lymphoma. N Engl J Med. 2010;362:875–885. doi: 10.1056/NEJMoa0905680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;176:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 66.Solinas G, Schiarea S, Liguori M, Fabbri M, Pesce S, Zammataro L, Pasqualini F, Nebuloni M, Chiabrando C, Mantovani A, Allavena P. Tumor-conditioned macrophages secrete migration-stimulating factor: a new marker for M2-polarization, influencing tumor cell motility. J Immunol. 2010;185:642–652. doi: 10.4049/jimmunol.1000413. [DOI] [PubMed] [Google Scholar]
- 67.Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b + peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Martinez FO, Gordon S, Locati M, Mantovani A. Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol. 2006;177:7303–7311. doi: 10.4049/jimmunol.177.10.7303. [DOI] [PubMed] [Google Scholar]
- 69.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–983. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 70.Liao D, Luo Y, Markowitz D, Xiang R, Reisfeld RA. Cancer associated fibroblasts promote tumor growth and metastasis by modulating the tumor immune microenvironment in a 4T1 murine breast cancer model. PLoS ONE. 2009;4:e7965. doi: 10.1371/journal.pone.0007965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Erez N, Truitt M, Olson P, Arron ST, Hanahan D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-kappaB-dependent manner. Cancer Cell. 2010;17:135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 72.Sica A, Porta C, Riboldi E, Locati M. Convergent pathways of macrophage polarization: the role of B cells. Eur J Immunol. 2010;40:2131–2133. doi: 10.1002/eji.201040736. [DOI] [PubMed] [Google Scholar]
- 73.DeNardo DG, Barreto JB, Andreu P, Vasquez L, Tawfik D, Kolhatkar N, Coussens LM. CD4(+) T cells regulate pulmonary metastasis of mammary carcinomas by enhancing protumor properties of macrophages. Cancer Cell. 2009;16:91–102. doi: 10.1016/j.ccr.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Visser KE, Korets LV, Coussens LM. De novo carcinogenesis promoted by chronic inflammation is B lymphocyte dependent. Cancer Cell. 2005;7:411–423. doi: 10.1016/j.ccr.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 75.Andreu P, Johansson M, Affara NI, Pucci F, Tan T, Junankar S, Korets L, Lam J, Tawfik D, DeNardo DG, Naldini L, Visser KE, Palma M, Coussens LM. FcRgamma activation regulates inflammation-associated squamous carcinogenesis. Cancer Cell. 2010;17:121–134. doi: 10.1016/j.ccr.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wong SC, Puaux AL, Chittezhath M, Shalova I, Kajiji TS, Wang X, Abastado JP, Lam KP, Biswas SK. Macrophage polarization to a unique phenotype driven by B cells. Eur J Immunol. 2010;40:2296–2307. doi: 10.1002/eji.200940288. [DOI] [PubMed] [Google Scholar]
- 77.Palma M, Naldini L. Tie2-expressing monocytes (TEMs): novel targets and vehicles of anticancer therapy? Biochim Biophys Acta. 2009;1796:5–10. doi: 10.1016/j.bbcan.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 78.Coffelt SB, Chen YY, Muthana M, Welford AF, Tal AO, Scholz A, Plate KH, Reiss Y, Murdoch C, Palma M, Lewis CE. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J Immunol. 2011;186:4183–4190. doi: 10.4049/jimmunol.1002802. [DOI] [PubMed] [Google Scholar]
- 79.Palma M, Venneri MA, Roca C, Naldini L. Targeting exogenous genes to tumor angiogenesis by transplantation of genetically modified hematopoietic stem cells. Nat Med. 2003;9:789–795. doi: 10.1038/nm871. [DOI] [PubMed] [Google Scholar]
- 80.Pucci F, Venneri MA, Biziato D, Nonis A, Moi D, Sica A, Serio C, Naldini L, Palma M. A distinguishing gene signature shared by tumor-infiltrating Tie2-expressing monocytes, blood “resident” monocytes, and embryonic macrophages suggests common functions and developmental relationships. Blood. 2009;114:901–914. doi: 10.1182/blood-2009-01-200931. [DOI] [PubMed] [Google Scholar]
- 81.Coffelt SB, Tal AO, Scholz A, Palma M, Patel S, Urbich C, Biswas SK, Murdoch C, Plate KH, Reiss Y, Lewis CE. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 2010;70:5270–5280. doi: 10.1158/0008-5472.CAN-10-0012. [DOI] [PubMed] [Google Scholar]
- 82.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ostrand-Rosenberg S, Sinha P. Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol. 2009;182:4499–4506. doi: 10.4049/jimmunol.0802740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Youn JI, Nagaraj S, Collazo M, Gabrilovich DI. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J Immunol. 2008;181:5791–5802. doi: 10.4049/jimmunol.181.8.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Movahedi K, Guilliams M, Bossche J, Bergh R, Gysemans C, Beschin A, Baetselier P, Ginderachter JA. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood. 2008;111:4233–4244. doi: 10.1182/blood-2007-07-099226. [DOI] [PubMed] [Google Scholar]
- 86.Bronte V, Kasic T, Gri G, Gallana K, Borsellino G, Marigo I, Battistini L, Iafrate M, Prayer-Galetti T, Pagano F, Viola A. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J Exp Med. 2005;201:1257–1268. doi: 10.1084/jem.20042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nagaraj S, Gupta K, Pisarev V, Kinarsky L, Sherman S, Kang L, Herber DL, Schneck J, Gabrilovich DI. Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med. 2007;13:828–835. doi: 10.1038/nm1609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Srivastava MK, Sinha P, Clements VK, Rodriguez P, Ostrand-Rosenberg S. Myeloid-derived suppressor cells inhibit T-cell activation by depleting cystine and cysteine. Cancer Res. 2010;70:68–77. doi: 10.1158/0008-5472.CAN-09-2587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yang R, Cai Z, Zhang Y, Yutzy WHT, Roby KF, Roden RB. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1 + CD11b + myeloid cells. Cancer Res. 2006;66:6807–6815. doi: 10.1158/0008-5472.CAN-05-3755. [DOI] [PubMed] [Google Scholar]
- 90.Huang B, Pan PY, Li Q, Sato AI, Levy DE, Bromberg J, Divino CM, Chen SH. Gr-1 + CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 2006;66:1123–1131. doi: 10.1158/0008-5472.CAN-05-1299. [DOI] [PubMed] [Google Scholar]
- 91.Terabe M, Matsui S, Park JM, Mamura M, Noben-Trauth N, Donaldson DD, Chen W, Wahl SM, Ledbetter S, Pratt B, Letterio JJ, Paul WE, Berzofsky JA. Transforming growth factor-beta production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J Exp Med. 2003;198:1741–1752. doi: 10.1084/jem.20022227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Youn JI, Gabrilovich DI. The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol. 2010;40:2969–2975. doi: 10.1002/eji.201040895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- 94.Almand B, Clark JI, Nikitina E, Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: a mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. doi: 10.4049/jimmunol.166.1.678. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez PC, Ernstoff MS, Hernandez C, Atkins M, Zabaleta J, Sierra R, Ochoa AC. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 2009;69:1553–1560. doi: 10.1158/0008-5472.CAN-08-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zea AH, Rodriguez PC, Atkins MB, Hernandez C, Signoretti S, Zabaleta J, McDermott D, Quiceno D, Youmans A, O’Neill A, Mier J, Ochoa AC. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 2005;65:3044–3048. doi: 10.1158/0008-5472.CAN-04-4505. [DOI] [PubMed] [Google Scholar]
- 97.Vuk-Pavlovic S, Bulur PA, Lin Y, Qin R, Szumlanski CL, Zhao X, Dietz AB. Immunosuppressive CD14 + HLA-DRlow/- monocytes in prostate cancer. Prostate. 2010;70:443–455. doi: 10.1002/pros.21078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu CY, Wang YM, Wang CL, Feng PH, Ko HW, Liu YH, Wu YC, Chu Y, Chung FT, Kuo CH, Lee KY, Lin SM, Lin HC, Wang CH, Yu CT, Kuo HP. Population alterations of L-arginase- and inducible nitric oxide synthase-expressed CD11b+/CD14/CD15+/CD33+ myeloid-derived suppressor cells and CD8+ T lymphocytes in patients with advanced-stage non-small cell lung cancer. J Cancer Res Clin Oncol. 2009;136:35–45. doi: 10.1007/s00432-009-0634-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, Castelli C, Mariani L, Parmiani G, Rivoltini L. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. 2007;25:2546–2553. doi: 10.1200/JCO.2006.08.5829. [DOI] [PubMed] [Google Scholar]
- 100.Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14 + HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. 2010;70:4335–4345. doi: 10.1158/0008-5472.CAN-09-3767. [DOI] [PubMed] [Google Scholar]
- 101.Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, Greten TF, Korangy F. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4(+)CD25(+)Foxp3(+) T cells. Gastroenterology. 2008;135:234–243. doi: 10.1053/j.gastro.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 102.Marigo I, Bosio E, Solito S, Mesa C, Fernandez A, Dolcetti L, Ugel S, Sonda N, Bicciato S, Falisi E, Calabrese F, Basso G, Zanovello P, Cozzi E, Mandruzzato S, Bronte V. Tumor-induced tolerance and immune suppression depend on the C/EBPbeta transcription factor. Immunity. 2010;32:790–802. doi: 10.1016/j.immuni.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 103.Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011;32:19–25. doi: 10.1016/j.it.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bunt SK, Clements VK, Hanson EM, Sinha P, Ostrand-Rosenberg S. Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol. 2009;85:996–1004. doi: 10.1189/jlb.0708446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kusmartsev S, Gabrilovich DI. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J Immunol. 2005;174:4880–4891. doi: 10.4049/jimmunol.174.8.4880. [DOI] [PubMed] [Google Scholar]
- 106.Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P, Cho HI, Celis E, Quiceno DG, Padhya T, McCaffrey TV, McCaffrey JC, Gabrilovich DI. HIF-1alpha regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med. 2010;207:2439–2453. doi: 10.1084/jem.20100587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ma G, Pan PY, Eisenstein S, Divino CM, Lowell CA, Takai T, Chen SH. Paired immunoglobin-like receptor-B regulates the suppressive function and fate of myeloid-derived suppressor cells. Immunity. 2011;34:385–395. doi: 10.1016/j.immuni.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y, Matrisian LM, Carbone DP, Lin PC. Expansion of myeloid immune suppressor Gr + CD11b + cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell. 2004;6:409–421. doi: 10.1016/j.ccr.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 109.Shojaei F, Wu X, Malik AK, Zhong C, Baldwin ME, Schanz S, Fuh G, Gerber HP, Ferrara N. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b + Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 110.Shojaei F, Wu X, Zhong C, Yu L, Liang XH, Yao J, Blanchard D, Bais C, Peale FV, Bruggen N, Ho C, Ross J, Tan M, Carano RA, Meng YG, Ferrara N. Bv8 regulates myeloid-cell-dependent tumour angiogenesis. Nature. 2007;450:825–831. doi: 10.1038/nature06348. [DOI] [PubMed] [Google Scholar]
- 111.Sica A, Melillo G, Varesio L (2011) Hypoxia: a double-edged sword of immunity. J Mol Med [DOI] [PubMed]
- 112.Cassatella MA, Locati M, Mantovani A. Never underestimate the power of a neutrophil. Immunity. 2009;31:698–700. doi: 10.1016/j.immuni.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 113.Zhang X, Majlessi L, Deriaud E, Leclerc C, Lo-Man R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity. 2009;31:761–771. doi: 10.1016/j.immuni.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 114.Houghton AM. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle. 2010;9:1732–1737. doi: 10.4161/cc.9.9.11297. [DOI] [PubMed] [Google Scholar]
- 115.Jensen HK, Donskov F, Marcussen N, Nordsmark M, Lundbeck F, Maase H. Presence of intratumoral neutrophils is an independent prognostic factor in localized renal cell carcinoma. J Clin Oncol. 2009;27:4709–4717. doi: 10.1200/JCO.2008.18.9498. [DOI] [PubMed] [Google Scholar]
- 116.Yang P, Bamlet WR, Sun Z, Ebbert JO, Aubry MC, Krowka MJ, Taylor WR, Marks RS, Deschamps C, Swensen SJ, Wieben ED, Cunningham JM, Melton LJ, Andrade M. Alpha1-antitrypsin and neutrophil elastase imbalance and lung cancer risk. Chest. 2005;128:445–452. doi: 10.1378/chest.128.1.445. [DOI] [PubMed] [Google Scholar]
- 117.Tazawa H, Okada F, Kobayashi T, Tada M, Mori Y, Une Y, Sendo F, Kobayashi M, Hosokawa M. Infiltration of neutrophils is required for acquisition of metastatic phenotype of benign murine fibrosarcoma cells: implication of inflammation-associated carcinogenesis and tumor progression. Am J Pathol. 2003;163:2221–2232. doi: 10.1016/S0002-9440(10)63580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pekarek LA, Starr BA, Toledano AY, Schreiber H. Inhibition of tumor growth by elimination of granulocytes. J Exp Med. 1995;181:435–440. doi: 10.1084/jem.181.1.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Gregory AD, Houghton AM (2011) Tumor-associated neutrophils: new targets for cancer therapy. Cancer Res [DOI] [PubMed]
- 120.Mantovani A. The yin-yang of tumor-associated neutrophils. Cancer Cell. 2009;16:173–174. doi: 10.1016/j.ccr.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 121.Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- 122.Karin M. Nuclear factor-kappaB in cancer development and progression. Nature. 2006;441:431–436. doi: 10.1038/nature04870. [DOI] [PubMed] [Google Scholar]
- 123.O’Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 124.Mantovani A, Allavena P, Sica A. Tumour-associated macrophages as a prototypic type II polarised phagocyte population: role in tumour progression. Eur J Cancer. 2004;40:1660–1667. doi: 10.1016/j.ejca.2004.03.016. [DOI] [PubMed] [Google Scholar]
- 125.Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V, Johnson RS, Haddad GG, Karin M. NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature. 2008;453:807–811. doi: 10.1038/nature06905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Tu S, Bhagat G, Cui G, Takaishi S, Kurt-Jones EA, Rickman B, Betz KS, Penz-Oesterreicher M, Bjorkdahl O, Fox JG, Wang TC. Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell. 2008;14:408–419. doi: 10.1016/j.ccr.2008.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hu CE, Gan J, Zhang RD, Cheng YR, Huang GJ. Up-regulated myeloid-derived suppressor cell contributes to hepatocellular carcinoma development by impairing dendritic cell function. Scand J Gastroenterol. 2011;46:156–164. doi: 10.3109/00365521.2010.516450. [DOI] [PubMed] [Google Scholar]
- 128.Fang HY, Hughes R, Murdoch C, Coffelt SB, Biswas SK, Harris AL, Johnson RS, Imityaz HZ, Simon MC, Fredlund E, Greten FR, Rius J, Lewis CE. Hypoxia-inducible factors 1 and 2 are important transcriptional effectors in primary macrophages experiencing hypoxia. Blood. 2009;114:844–859. doi: 10.1182/blood-2008-12-195941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nizet V, Johnson RS. Interdependence of hypoxic and innate immune responses. Nat Rev Immunol. 2009;9:609–617. doi: 10.1038/nri2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lewis C, Murdoch C. Macrophage responses to hypoxia: implications for tumor progression and anti-cancer therapies. Am J Pathol. 2005;167:627–635. doi: 10.1016/S0002-9440(10)62038-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 132.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 133.Du R, Lu KV, Petritsch C, Liu P, Ganss R, Passegue E, Song H, Vandenberg S, Johnson RS, Werb Z, Bergers G. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Shojaei F, Wu X, Qu X, Kowanetz M, Yu L, Tan M, Meng YG, Ferrara N. G-CSF-initiated myeloid cell mobilization and angiogenesis mediate tumor refractoriness to anti-VEGF therapy in mouse models. Proc Natl Acad Sci U S A. 2009;106:6742–6747. doi: 10.1073/pnas.0902280106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Walmsley SR, Print C, Farahi N, Peyssonnaux C, Johnson RS, Cramer T, Sobolewski A, Condliffe AM, Cowburn AS, Johnson N, Chilvers ER. Hypoxia-induced neutrophil survival is mediated by HIF-1alpha-dependent NF-kappaB activity. J Exp Med. 2005;201:105–115. doi: 10.1084/jem.20040624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Albina JE, Mahoney EJ, Daley JM, Wesche DE, Morris SM, Jr, Reichner JS. Macrophage arginase regulation by CCAAT/enhancer-binding protein beta. Shock. 2005;23:168–172. doi: 10.1097/01.shk.0000148054.74268.e2. [DOI] [PubMed] [Google Scholar]
- 137.Melillo G, Musso T, Sica A, Taylor LS, Cox GW, Varesio L. A hypoxia-responsive element mediates a novel pathway of activation of the inducible nitric oxide synthase promoter. J Exp Med. 1995;182:1683–1693. doi: 10.1084/jem.182.6.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Chan DA, Krieg AJ, Turcotte S, Giaccia AJ. HIF gene expression in cancer therapy. Methods Enzymol. 2007;435:323–345. doi: 10.1016/S0076-6879(07)35016-7. [DOI] [PubMed] [Google Scholar]
- 139.Takeda N, O’Dea EL, Doedens A, Kim JW, Weidemann A, Stockmann C, Asagiri M, Simon MC, Hoffmann A, Johnson RS. Differential activation and antagonistic function of HIF-{alpha} isoforms in macrophages are essential for NO homeostasis. Genes Dev. 2010;24:491–501. doi: 10.1101/gad.1881410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Imtiyaz HZ, Williams EP, Hickey MM, Patel SA, Durham AC, Yuan LJ, Hammond R, Gimotty PA, Keith B, Simon MC. Hypoxia-inducible factor 2alpha regulates macrophage function in mouse models of acute and tumor inflammation. J Clin Invest. 2010;120:2699–2714. doi: 10.1172/JCI39506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Doedens AL, Stockmann C, Rubinstein MP, Liao D, Zhang N, DeNardo DG, Coussens LM, Karin M, Goldrath AW, Johnson RS. Macrophage expression of hypoxia-inducible factor-1 alpha suppresses T-cell function and promotes tumor progression. Cancer Res. 2010;70:7465–7475. doi: 10.1158/0008-5472.CAN-10-1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carbia-Nagashima A, Gerez J, Perez-Castro C, Paez-Pereda M, Silberstein S, Stalla GK, Holsboer F, Arzt E. RSUME, a small RWD-containing protein, enhances SUMO conjugation and stabilizes HIF-1alpha during hypoxia. Cell. 2007;131:309–323. doi: 10.1016/j.cell.2007.07.044. [DOI] [PubMed] [Google Scholar]
- 143.Mizukami Y, Jo WS, Duerr EM, Gala M, Li J, Zhang X, Zimmer MA, Iliopoulos O, Zukerberg LR, Kohgo Y, Lynch MP, Rueda BR, Chung DC. Induction of interleukin-8 preserves the angiogenic response in HIF-1alpha-deficient colon cancer cells. Nat Med. 2005;11:992–997. doi: 10.1038/nm1294. [DOI] [PubMed] [Google Scholar]
- 144.Foell D, Wittkowski H, Vogl T, Roth J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol. 2007;81:28–37. doi: 10.1189/jlb.0306170. [DOI] [PubMed] [Google Scholar]
- 145.Gallina G, Dolcetti L, Serafini P, Santo C, Marigo I, Colombo MP, Basso G, Brombacher F, Borrello I, Zanovello P, Bicciato S, Bronte V. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J Clin Invest. 2006;116:2777–2790. doi: 10.1172/JCI28828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Serafini P, Mgebroff S, Noonan K, Borrello I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 2008;68:5439–5449. doi: 10.1158/0008-5472.CAN-07-6621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kortylewski M, Kujawski M, Wang T, Wei S, Zhang S, Pilon-Thomas S, Niu G, Kay H, Mule J, Kerr WG, Jove R, Pardoll D, Yu H. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nat Med. 2005;11:1314–1321. doi: 10.1038/nm1325. [DOI] [PubMed] [Google Scholar]
- 148.Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, Bhattacharya R, Gabrilovich D, Heller R, Coppola D, Dalton W, Jove R, Pardoll D, Yu H. Regulation of the innate and adaptive immune responses by Stat-3 signaling in tumor cells. Nat Med. 2004;10:48–54. doi: 10.1038/nm976. [DOI] [PubMed] [Google Scholar]
- 149.Kortylewski M, Swiderski P, Herrmann A, Wang L, Kowolik C, Kujawski M, Lee H, Scuto A, Liu Y, Yang C, Deng J, Soifer HS, Raubitschek A, Forman S, Rossi JJ, Pardoll DM, Jove R, Yu H. In vivo delivery of siRNA to immune cells by conjugation to a TLR9 agonist enhances antitumor immune responses. Nat Biotechnol. 2009;27:925–932. doi: 10.1038/nbt.1564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–1149. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Nickoloff BJ, Ben-Neriah Y, Pikarsky E. Inflammation and cancer: is the link as simple as we think? J Invest Dermatol. 2005;124:x–xiv. doi: 10.1111/j.0022-202X.2005.23724.x. [DOI] [PubMed] [Google Scholar]
- 152.Apetoh L, Ghiringhelli F, Tesniere A, Obeid M, Ortiz C, Criollo A, Mignot G, Maiuri MC, Ullrich E, Saulnier P, Yang H, Amigorena S, Ryffel B, Barrat FJ, Saftig P, Levi F, Lidereau R, Nogues C, Mira JP, Chompret A, Joulin V, Clavel-Chapelon F, Bourhis J, Andre F, Delaloge S, Tursz T, Kroemer G, Zitvogel L. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–1059. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 153.Ghiringhelli F, Apetoh L, Tesniere A, Aymeric L, Ma Y, Ortiz C, Vermaelen K, Panaretakis T, Mignot G, Ullrich E, Perfettini JL, Schlemmer F, Tasdemir E, Uhl M, Genin P, Civas A, Ryffel B, Kanellopoulos J, Tschopp J, Andre F, Lidereau R, McLaughlin NM, Haynes NM, Smyth MJ, Kroemer G, Zitvogel L. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med. 2009;15:1170–1178. doi: 10.1038/nm.2028. [DOI] [PubMed] [Google Scholar]
- 154.Lewen S, Zhou H, Hu HD, Cheng T, Markowitz D, Reisfeld RA, Xiang R, Luo Y. A Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother. 2008;57:507–515. doi: 10.1007/s00262-007-0389-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C, Squadrito ML, Segura I, Li X, Knevels E, Costa S, Vinckier S, Dresselaer T, Akerud P, Mol M, Salomaki H, Phillipson M, Wyns S, Larsson E, Buysschaert I, Botling J, Himmelreich U, Ginderachter JA, Palma M, Dewerchin M, Claesson-Welsh L, Carmeliet P. HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PlGF. Cancer Cell. 2011;19:31–44. doi: 10.1016/j.ccr.2010.11.009. [DOI] [PubMed] [Google Scholar]
- 156.Gazzaniga S, Bravo AI, Guglielmotti A, Rooijen N, Maschi F, Vecchi A, Mantovani A, Mordoh J, Wainstok R. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- 157.Morgan G, Lipton A. Antitumor effects and anticancer applications of bisphosphonates. Semin Oncol. 2010;37(Suppl 2):S30–S40. doi: 10.1053/j.seminoncol.2010.10.005. [DOI] [PubMed] [Google Scholar]
- 158.Duluc D, Corvaisier M, Blanchard S, Catala L, Descamps P, Gamelin E, Ponsoda S, Delneste Y, Hebbar M, Jeannin P. Interferon-gamma reverses the immunosuppressive and protumoral properties and prevents the generation of human tumor-associated macrophages. Int J Cancer. 2009;125:367–373. doi: 10.1002/ijc.24401. [DOI] [PubMed] [Google Scholar]
- 159.Coscia M, Quaglino E, Iezzi M, Curcio C, Pantaleoni F, Riganti C, Holen I, Monkkonen H, Boccadoro M, Forni G, Musiani P, Bosia A, Cavallo F, Massaia M. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14:2803–2815. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP. Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res. 2005;65:3437–3446. doi: 10.1158/0008-5472.CAN-04-4262. [DOI] [PubMed] [Google Scholar]
- 161.Ostrand-Rosenberg S, Grusby MJ, Clements VK. Cutting edge: STAT6-deficient mice have enhanced tumor immunity to primary and metastatic mammary carcinoma. J Immunol. 2000;165:6015–6019. doi: 10.4049/jimmunol.165.11.6015. [DOI] [PubMed] [Google Scholar]
- 162.Palma M, Mazzieri R, Politi LS, Pucci F, Zonari E, Sitia G, Mazzoleni S, Moi D, Venneri MA, Indraccolo S, Falini A, Guidotti LG, Galli R, Naldini L. Tumor-targeted interferon-alpha delivery by Tie2-expressing monocytes inhibits tumor growth and metastasis. Cancer Cell. 2008;14:299–311. doi: 10.1016/j.ccr.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 163.Gabrilovich DI, Velders MP, Sotomayor EM, Kast WM. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J Immunol. 2001;166:5398–5406. doi: 10.4049/jimmunol.166.9.5398. [DOI] [PubMed] [Google Scholar]
- 164.Kusmartsev S, Cheng F, Yu B, Nefedova Y, Sotomayor E, Lush R, Gabrilovich D. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 2003;63:4441–4449. [PubMed] [Google Scholar]
- 165.Mirza N, Fishman M, Fricke I, Dunn M, Neuger AM, Frost TJ, Lush RM, Antonia S, Gabrilovich DI. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 2006;66:9299–9307. doi: 10.1158/0008-5472.CAN-06-1690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ko HJ, Kim YJ, Kim YS, Chang WS, Ko SY, Chang SY, Sakaguchi S, Kang CY. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 2007;67:7477–7486. doi: 10.1158/0008-5472.CAN-06-4639. [DOI] [PubMed] [Google Scholar]
- 167.Suzuki E, Kapoor V, Jassar AS, Kaiser LR, Albelda SM. Gemcitabine selectively eliminates splenic Gr-1+/CD11b + myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res. 2005;11:6713–6721. doi: 10.1158/1078-0432.CCR-05-0883. [DOI] [PubMed] [Google Scholar]
- 168.Dinarello CA. Why not treat human cancer with interleukin-1 blockade? Cancer Metastasis Rev. 2010;29:317–329. doi: 10.1007/s10555-010-9229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tazzyman S, Lewis CE, Murdoch C. Neutrophils: key mediators of tumour angiogenesis. Int J Exp Pathol. 2009;90:222–231. doi: 10.1111/j.1365-2613.2009.00641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Allavena P, Signorelli M, Chieppa M, Erba E, Bianchi G, Marchesi F, Olimpio CO, Bonardi C, Garbi A, Lissoni A, Braud F, Jimeno J, D’Incalci M. Anti-inflammatory properties of the novel antitumor agent yondelis (trabectedin): inhibition of macrophage differentiation and cytokine production. Cancer Res. 2005;65:2964–2971. doi: 10.1158/0008-5472.CAN-04-4037. [DOI] [PubMed] [Google Scholar]
- 171.Pahler JC, Tazzyman S, Erez N, Chen YY, Murdoch C, Nozawa H, Lewis CE, Hanahan D. Plasticity in tumor-promoting inflammation: impairment of macrophage recruitment evokes a compensatory neutrophil response. Neoplasia. 2008;10:329–340. doi: 10.1593/neo.07871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Johansson M, Denardo DG, Coussens LM. Polarized immune responses differentially regulate cancer development. Immunol Rev. 2008;222:145–154. doi: 10.1111/j.1600-065X.2008.00600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]