Abstract
Purpose
There are risks of common bile duct (CBD) stones in acute cholecystitis, and there is a move among surgeons to identify choledocholithiasis before surgery. Magnetic resonance cholangiopancreaticography (MRCP) has the potential to accurately detect choledocholithiasis in patients with acute cholecystitis. The aim of this study was to evaluate the predictive values of MRCP and elevated biochemical predictors for choledocholithiasis in patients with acute cholecystitis.
Methods
Between September 2006 and August 2008, of 84 patients with acute cholecystitis based on the diagnosis criteria of the Tokyo guidelines, 57 had MRCP preoperatively. The predictive values of six biochemical predictors for choledocholithiasis were also evaluated.
Results
Of the 57 patients, seven (12.28%) had choledocholithiasis, of whom three had CBD stones in nondilated ducts. The smallest stone detected in a dilated CBD and nondilated duct was 3.19 and 4.55 mm in diameter, respectively. None of our patients whose MRCP showed a clear CBD returned with symptomatic choledocholithiasis during the follow-up period. All biochemical predictors and CBD diameter had limited positive predictive values.
Conclusions
Magnetic resonance cholangiopancreaticography is a reliable evaluation technique for the detection of choledocholithiasis. It reduces the misdiagnosis of retained choledocholithiasis with normal biochemical predictors and prevents the risk of overlooking choledocholithiasis. No single predictor or combined markers have been found to be reliable for including/excluding the presence of choledocholithiasis.
Keywords: Magnetic resonance cholangiopancreatography, Acute cholecystitis, Choledocholithiasis, Liver enzymes, Ultrasonography
Introduction
Of patients undergoing cholecystectomy for cholelithiasis, 3–33% will also harbor common bile duct (CBD) stones [1, 2], and the incidence of those with symptoms suggestive of choledocholithiasis will be even higher. Risk factors for choledocholithiasis are well recognized [3], and there is a move among clinicians to identify CBD stones before surgery. The serum hepatobiliary biochemical index and findings on abdominal ultrasonography images have commonly been used to initially predict CBD stones [1–6]. However, the biochemical predictive models may be affected by inflammatory gallstone disease due to abnormally elevated predictor levels secondary to acute transient hepatocellular injury [7], thus disguising biliary obstruction owing to CBD stones. In addition, the low accuracy of ultrasonography in early extrahepatic obstruction [6, 8] limits its reliability in predicting the necessity for CBD exploration. Magnetic resonance cholangiopancreaticography (MRCP) is a non-invasive technique that has the potential to accurately evaluate choledocholithiasis in the preoperative acute calculous cholecystitis setting. Although MRCP is reportedly beginning to replace diagnostic endoscopic retrograde cholangiopancreatography (ERCP) for the early assessment of suspected biliary obstruction due to its comparable accuracy [9], its cost-effectiveness is still under debate. In cases of acute cholecystitis, we routinely investigate biliary demographics preoperatively using medical imaging, mostly with MRCP. The aims of the study reported here were to evaluate the predictive value of elevated hepatobiliary biochemical predictors for CBD stones and the influence of the MRCP results on perioperative management.
Materials and methods
This study was conducted in an urban university teaching hospital that provides surgical services and primary, secondary, and tertiary care. Eighty-four patients diagnosed with acute cholecystitis based on the diagnostic criteria of the Tokyo guidelines for acute cholecystitis [10] between September 2006 and August 2008 were identified. In our department, all patients routinely undergo MRCP, abdominal computed tomography (CT), ERCP, or percutaneous transhepatic gallbladder drainage (PTGBD) tube-cholangiography preoperatively to demonstrate the complete biliary anatomy. The surveys of those who had only preoperative abdominal CT, ERCP and/or post-PTGBD tube-cholangiography were excluded from our study.
MRCP scans were performed on a Siemens 1.5T Magnetom Sonata scanner (Siemens, Munich, Germany) using a T2-weighted Turbo Spin Echo sequence acquired with a non-breath-hold in the coronal plane. On hospital admission, blood samples were collected for the laboratory analysis of serum hepatobiliary biochemical predictor levels prior to the MRCP. The abnormal cut-off levels were as follows: aspartate aminotransferase (AST >38 U/L), alanine aminotransferase (ALT >37 U/L), total bilirubin (TB >1.2 mg/dL), direct bilirubin (DB >0.2 mg/dL), alkaline phosphatase (ALP >122 U/L), and gamma glutamyl transferase (GGT >49 U/L). The clinical definition of the presence/absence of CBD stones is based on the identification of CBD stones on the MRCP. Patients with CBD stones detected on the MR image were managed preoperatively with ERCP. The treatment choices were given by one surgeon who used consistent criteria for the assignment of patients to specific courses of treatment according to the Tokyo guidelines for biliary infection. Intraoperative ultrasonographic cholangiography was used to confirm the patient free of CBD stones. All patients were advised to consult the authors if they developed symptoms such as abdominal pain, fever, nausea, vomiting, anorexia, or jaundice. The ambulatory follow-up was conducted for at least 12 months
All statistical analyses were performed with SPSS for Windows ver. 12.0 (SPSS, Chicago, IL). Test characteristics were determined for all clinical parameters (CBD size on the MR image, CBD size on the ultrasonographic image, serum hepatobiliary biochemistry examination), including sensitivity, specificity, likelihood ratio, prevalence, accuracy, positive predictive values, and negative predictive value for each potential parameter for CBD stones. The CBD diameter based on the MRCP was analyzed categorically using subjective assessments of enlargement and a cut-off value of >10 mm. The Fisher’s exact test was used to determine the clinical variables associated with the presence of CBD stones. A p value of <0.05 was considered to be statistically significant. All significant factors from the univariate analysis were subsequently included in the multivariate analysis, which was carried out by logistic regression to determine the independent parameter.
Results
In this study, 57 MRCP examinations were performed prior to a cholecystectomy. All patients who underwent MRCP examinations were included in the analysis. The mean age of the patient cohort was 55.79 ± 14.66 (range 26.33–81.19) years, and 49.12% (n = 28) of the patients were women (Table 1).
Table 1.
Demographic findings
| Characteristics | Total | CBD stones (+) | CBD stones (−) |
|---|---|---|---|
| Sex (male:female) | 29:28 | 5:2 | 24:26 |
| Age (years) | |||
| ≥55 | 32 | 6 | 26 |
| <55 | 25 | 1 | 24 |
| American Society of Anesthesiologists physical status | |||
| ≤II | 45 | 5 | 40 |
| >II | 12 | 2 | 10 |
| Tokyo guideline severity assessment | |||
| Mild | 30 | 3 | 27 |
| Moderate | 23 | 4 | 19 |
| Severe | 4 | 0 | 4 |
CBD Common bile duct
Of the 84 patients diagnosed with acute cholecystitis during the study period, 12 (14.28%) had CBD stones, and only eight of the latter had jaundice. Of the 57 enrolled participants (with MRCP examinations), jaundice was identified in three patients with CBD stones and in 21 patients without. Of the seven patients with stones detected by MR, one patients had all normal biochemical predictors; in comparison, 14 patients without choledocholithiasis had normal serum biochemical levels. According to the diagnostic criteria of the Tokyo guidelines for acute cholangitis, an elevated serum hepatobiliary index is also suitable for making the diagnosis of acute cholangitis. It would have been dangerous to have defined the patient with a positive MR for CBD stones but normal biochemical predictors as having a silent stone, as the patient was symptomatic with proven systemic inflammation at admission.
All patients had a cholecystectomy at a mean of 3.35 days post-MRCP (range 0–17 days), mainly laparoscopically. Fifty-two patients underwent a laparoscopic cholecystectomy with a conversion rate of 6.12%. Five patients had an open cholecystectomy because of previous upper abdominal surgery. Our patients had a mean postoperative hospitalization stay of 2.96 (range 1–8) days.
MRCP findings
The MRCP results were positive for CBD stones in seven of the 57 patients (12.28%) (four with a single stone; three with multiple stones); six of these seven patients were >55 years of age. The stones ranged in maximum diameter from 3.19 to 9 mm (mean 6.47 mm), whereas the diameter of the bile ducts ranged from 5.07 to 21.05 mm (mean 9.07 mm). In three patients, the stones were located in a nondilated duct. The smallest stone detected with MRCP had a diameter of 3.19 mm and was located in a dilated extrahepatic bile duct (Fig. 1a, diameter 11.53 mm). The smallest stone located in a nondilated duct (Fig. 1b, diameter 7.74 mm) was 4.55 mm in diameter. All seven patients with stones detected by MR were further proven and managed preoperatively with ERCP, which the absence of false positive; the overall specificity of MRCP was 100%. The possibility of CBD sludge or undetectable stones smaller than 3 mm is always taken into account. A false negative of such small debris may exist, but spontaneous evacuation of such debris can be expected and further postoperative follow-up is necessary. The fact that none of our patients whose MRCP showed a clear CBD returned with symptomatic CBD stones at the clinical follow-up 1 week and a year after discharge also demonstrated the overall high sensitivity of MRCP.
Fig. 1.
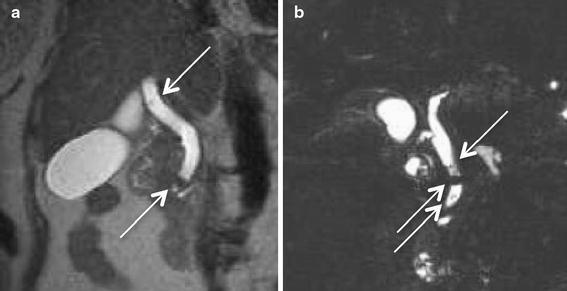
Magnetic resonance cholangiopancreaticography (MRCP) image demonstrates common bile duct (CBD) calculi (arrows). a T2-weighted short echo time sequence (TR/TE 2000/79 ms, slice thickness 5 mm, scan time 3 min 44 s). The smallest stone was 3.19 mm in a dilated CBD. b T2-weighted turbo spin-echo sequence (TR/TE 1800/635 ms, slice thickness 1.15 mm, scan time 3 min 44 s). The smallest stone in a nondilated duct was 4.55 mm in diameter
Description of clinical predictors
The frequency and mean serum levels of the different parameters are shown in Table 2. Mean CBD diameters based on the MRCP findings were 12.55 (range 7.74–21.05) mm for patients with acute cholecystitis with choledocholithiasis and 8.59 (range 5.07–13.72) mm for patients without CBD stones. In 23% (n = 13) of our patients, the CBD could not be fully observed; in none of the patients were CBD stones directly identified based on transabdominal ultrasonography findings. The diameter of the ultrasonographically detected bile ducts ranged from 5 to 14 (mean 8) mm, with a mean bile duct diameter of 10.2 mm (n = 6) in choledocholithiasis patients and 7.6 mm (n = 39) in patients without CBD stones. Measurements of the diameter of the bile duct based on MRCP and transabdominal ultrasonography were found to be similar in terms of diagnostic accuracy in predicting CBD stones (Table 2), with low sensitivities of 57% [95% confidence interval (CI) 20–94] and 50% (95% CI 10–90), respectively. Among the six biochemical predictors, ALT, GGT, and DB had the highest sensitivity at 86, 83, and 80%, respectively. The specificity was highest for AST (86%) and ALP (76%). All biochemical predictors had a limited positive predictive value, while ALT, ALP, and AST had the highest negative predictive value at 97% (95% CI 91–100), 95% (95% CI 88–100), and 93% (95% CI 86–100), respectively.
Table 2.
Patients with dilated CBD and abnormal serum biochemical parameters
| Component | Mean (range) | Abnormal results/no. of patients |
|---|---|---|
| MR CBD size (mm) | 9.08 (5.07–21.05) | 14/57 |
| U/S CBD size (mm) | 7.99 (5.00–14.00) | 10/44 |
| AST (U/L) | 28.98 (10–105) | 11/56 |
| ALT (U/L) | 45.75 (7–172) | 24/57 |
| TB (mg/dL) | 1.34 (0.4–8) | 24/57 |
| DB (mg/dL) | 5.95 (0–3.9) | 15/26 |
| ALP (U/L) | 109.29 (35–407) | 16/56 |
| GGT (U/L) | 133.04 (1.4–1141) | 17/34 |
AST Aspartate aminotransferase, ALT alanine aminotransferase, TB total bilirubin, DB direct bilirubin, ALP alkaline phosphotase, GGT gamma glutamyl transferase, MR magnetic resonance cholangiopancreaticography, U/S transabdominal ultrasonography
Accuracy of the clinical parameters
Table 3 shows the analysis of the predictors of CBD stones. Both CBD diameter and elevated serum biochemical parameters were found to have only a limited positive predictive value for the presence of CBD stones. Among the six non-invasive biochemical markers evaluated in our study, the analysis of each predictor revealed that elevated AST, ALT, and ALP were statistically significant predictors. Multiple logistic regression analysis of these three predictors revealed that they were not jointly significant: AST, 1.78 (95% CI 0.21–14.85); ALT, 5.25 (95% CI 0.43–63.45); ALP, 3.46 (95% CI 0.47–25.24). A backward stepwise logistic regression model was created with the presence or absence of CBD stones as the dependent variable and the previously determined statistically significant variables (AST, ALT, and ALP) as independent variables. The backward stepwise model begins with all factors entered in the model and then removes nonsignificant factors in a stepwise fashion, such that only statistically significant factors are retained in the final model. In the final model, ALT [odds ratio (OR) 9.412, 95% CI 1.02–87.19, p = 0.048] was a significant predictor. As mentioned above, the false negative of MRCP undetectable sludge or small debris might be possible. The potential for a degree of error in the accuracy of CBD size and biochemical predictors does exist because the data for the calculations on biochemical predictor sensitivities and specificities originate from a pool of patients who had MRCP-based findings by virtue of the enrolment criteria.
Table 3.
Predictors of common bile duct stones in patients with acute cholecystitis undergoing magnetic resonance cholangiopancreaticography
| Clinical predictors | Sensitivity (%) | Specificity (%) | Likelihood ratio | PPV | NPV | p value |
|---|---|---|---|---|---|---|
| MR CBD size (n = 57) | 57 (20–94) | 80 (69–91) | 2.86 (1.22–6.67) | 29 | 93 | 0.0541 |
| U/S CBD size (n = 44) | 50 (10–90) | 82 (69–94) | 2.71 (0.96–7.70) | 30 | 91 | 0.1197 |
| AST (n = 56) | 57 (20–94) | 86 (76–96) | 4.00 (1.56–10.23) | 36 | 93 | 0.0223 |
| ALT (n = 57) | 86 (60–100) | 64 (51–77) | 2.38 (1.48–3.84) | 25 | 97 | 0.0343 |
| Total bilirubin (n = 57) | 43 (6–80) | 58 (44–72) | 1.02 (0.41–2.55) | 13 | 88 | 1 |
| Direct bilirubin (n = 26) | 80 (45–100) | 48 (26–69) | 1.53 (0.84–2.78) | 27 | 91 | 0.3562 |
| ALP (n = 56) | 67 (29–100) | 76 (64–88) | 2.78 (1.31–5.88) | 25 | 95 | 0.0494 |
| GGT (n = 34) | 83 (54–100) | 57 (39–75) | 1.94 (1.11–3.4) | 29 | 94 | 0.1748 |
Sensitivity, specificity, and likelihood ratio are given as a percentage, with the 95% confidence intervals (95% CIs) given in parentheses
PPV Positive predictive value, NPV negative predictive value
Follow-up of patients and prognosis
Patients were advised to consult us if they developed symptoms such as abdominal pain, fever, nausea, vomiting, anorexia, or jaundice, and the ambulatory follow-up was conducted for at least 12 months. However, only 96.49% (n = 55) of the 57 patients returned to the clinic for the 1-week postoperative follow-up, and only 43.86% (n = 25) returned for the 12-month follow-up.
The mean period of postoperative follow-up was 9.09 months (range 5 days to 45.30 months). During the follow-up period, the following clinical symptoms were observed: surgical site infection (6 patients, 10.5%), umbilical hernia (1 patient, 1.7%), diarrhea (1 patient, 1.7%), and postoperative cholangitis (1 patient, 1.7%) that occurred 6 months postoperatively. In the latter case, the patient was admitted to hospital for 1 week where there was a good response to medical treatment. In the follow-up period, a retained CBD stone was found in one of the patients with CBD dilation 12 months postoperatively. The patient had had preoperative endoscopic stone removal following the identification of CBD stones by MR (MR CBD size 16.44 mm; multiple stones size of 3.09 and 8.50 mm, respectively), and all preoperative biochemical predictors were normal. As a result, postoperative ERCP was performed in only one case.
Discussion
The probability of a patient having CBD stones should be stratified on the basis of clinical, biochemical, and radiological parameters into low and high risk groups for CBD stones, which can then dictate further work-up and management. However, the “a priori” probability of CBD stones is difficult to assign and, therefore, it is not easy to estimate the specific risk to an individual patient.
Eighty-four patients diagnosed with acute cholecystitis were identified in this study according to the diagnostic criteria of the Tokyo guidelines for cholecystitis. Based on the strength and quality of the diagnostic evidence, all our reported patients with acute cholecystitis were admitted to the surgical service by the same surgeon in our hospital. A number of published studies have combined data from patients with acute cholecystitis with those having simple acute biliary colic. These are two different entities, with each having a different incidence of CBD stones; consequently, they should be discussed separately. In 2005, Peng et al. reported that of 243 acute biliary colic cases and 142 acute cholecystitis patients, all of whom had had a prior laparoscopic cholecystectomy, 7.7 and 16.5% had choledocholithiasis, respectively [11]. Nebiker et al. [12] also reported that patients with cholecystitis had a higher frequency of CBD stones (9.1%) than those without signs of acute inflammation (6.6%), which motivated us to analyze patients with acute calculous cholecystitis. The inflammatory process may also result in elevated serum biochemical predictor levels secondary to acute transient hepatocellular injury, thereby interfering with the accuracy by which serum biochemical predictors can be used to detect CBD stones [7]. Based on our limited number of patients, we found that patients testing positive for CBD stones in the biochemical tests did not necessarily present more severe clinical symptoms according to Tokyo guideline classification of severity.
Although serum biochemical predictors have been reported to be reliable for use as an initial screening modality, the specific biochemical predictors reported to be accurate and their cut-off levels vary from institution to institution [1–3, 5]. Our recommended time interval for a cholecystectomy is within 96 h after presentation of initial symptoms; consequently, blood samples are collected upon admission to enable the laboratory analysis of serum hepatobiliary biochemical predictor levels. The univariate analysis carried out in our study demonstrated that AST, ALT, and ALP are independent noninvasive predictors for the detection of CBD stones. In the final backward stepwise logistic regression model, ALT was found to be the only significant predictor. When all three predictors were entered into the model, the analysis revealed that the three predictors were not jointly significant statistically. In terms of cost-effectiveness, ALT is noted to be the most cost-effective of probability markers of CBD stones, and the utility of all three predictors in the detection of CBD stones may carry a higher risk of overlooking CBD stones. As a result, no single predictor or combined markers have been found to be reliable enough to be used to include or exclude the presence of CBD stones.
All patients had preoperative transabdominal ultrasonography, which has been reported to be a reliable screening modality in terms of CBD stone detection. However, this technique often provides only limited or indirect CBD data and only rarely enables the direct inspection of CBD stones [8]. The CBD was undetectable in about 25% of our patients, and transabdominal ultrasonography was unable to directly identify CBD stones in any of our patients. Some reports emphasize that about one-third of CBD stones detected ultrasonographically occur in nondilated biliary systems [13, 14], which can be easily missed [15]. Ultrasonography is also reported to be less sensitive for choledocholithiasis, with a sensitivity ranging from 12 to 55% [16]. However, CT is unlikely to provide more information than ultrasonography in choledocholithiasis [8]. Of the 57 patients testing negative for CBD stones, nine also had a preoperative CT that showed a mean CBD diameter of 10.21 mm (range 7.33–12.41 mm), which was relatively larger than the measurements calculated on the basis of MRCP measurements. In addition to the 57 patients who underwent a MRCP, other subjects were examined with different isolated investigative modalities, such as CT (n = 26), percutaneous cholangiography (PTC) (n = 19), and ERCP (n = 12); the data on these patients were excluded from this study—mainly because ERC and PTC are considered to be invasive techniques and limited to therapeutic purposes. It should be possible to predict CBD stones using noninvasive tests that avoid unnecessary and risky procedures. The accuracy of CT in diagnosing CBD stones remains a challenge, with a reported sensitivity varying from 50 to 90% [17, 18], as only calcified stones can be visualized and cholesterol stones can have the same density as bile [19].
MR imaging (MRI) was first applied in 1986 for the diagnosis of biliary disease, demonstrating only the anatomy of the dilated bile ducts and the location of an obstruction [20]. With advances in technology and the refinement of MR cholangiographic sequences, MRI has become a more reliable diagnostic tool for detecting CBD stones [21], although the presence of CBD sludge or undetectable stones <3 mm in diameter remains unknown and the spontaneous passage of such small debris is possible [22, 23]. Not one patient whose MRCP showed a clear CBD returned with symptomatic CBD stones at the clinical follow-up 1 week and a year after discharge, which suggests a high negative predictive value for the MRCP and intraoperative ultrasonography. All cholecystectomized patients in this study were confirmed to be free of CBD stones by intraoperative ultrasonographic cholangiography. CBD stones were found in seven of the 57 patients (12.28%) (four with single stones; three with multiple stones). The stones ranged in diameter from 3.19 to 9.0 (mean 6.47 mm) mm, whereas the diameter of the bile ducts ranged from 5.07 to 21.05 mm (mean 9.07 mm). The patients with CBD stones had a wider MR bile duct mean diameter (12.55 mm) than those without CBD stones (8.59 mm). However, the location of the CBD stones in three patients was in a nondilated duct that could be missed by an over-dependence on the predictor of common duct diameters. The smallest stone detected with MRCP was 3.19 mm in diameter and was located in a dilated extrahepatic bile duct; the smallest stone detected in a nondilated duct was 4.55 mm in diameter. A prospective study of 265 patients by Fulcher et al. [24] showed a sensitivity of 100% and a specificity of 98–100%, with excellent resolution of MRCP for the detection of CBD stones: the calculi detected range from 2 to 20 mm in diameter (average diameter 9 mm).
MRCP is a reliable and noninvasive procedure for detecting or excluding the presence of CBD stones [25, 26]. It also has the potential to reduce the number of invasive preoperative diagnostic procedures [27, 28] and their associated risks and overall healthcare costs [29]. MRCP can also allow the surgeon to verify the state of the patient’s biliary ductal demographic condition, and even in severe inflammation cases the cystic duct can be cannulated more confidently based on a more thorough understanding of a specific patient’s characteristics preoperatively [12]. Severe inflammation is reported as one of the most important reasons for bile duct injury [30], as the presence of inflammation in the acute setting may obscure the view of Calot’s triangle.
Surgeons are usually more skilled in using an imaging modality to exclude the presence of CBD stones; however, an MRI is usually not recommended for this purpose due to economic considerations [12]. There is some evidence that MRCP is more accurate than a diagnostic ERCP, although the quality of these studies has been criticized [31]. As MRCP yields only static reconstructed images, our measurements of the size of the CBD based on MRI findings may be less accurate than those based on diagnostic ERCP findings. ERCP is still considered to be the ‘gold standard’ for the diagnosis of pancreatic and biliary ductal pathology [32]. In addition to medical considerations, we must also consider the costs, and although the costs of diagnostic modalities differ markedly by country and by healthcare system, transabdominal ultrasound and serum biochemical predictors are universally less expensive than MRCP.
Conclusion
Magnetic resonance cholangiopancreaticography is a reliable and noninvasive evaluation for the detection or exclusion of CBD stones. Rather than indicating a need for CBD exploration, MRCP is more useful in determining when not to explore and for avoiding retained CBD stones in small CBD. No single predictor or combined markers have been found to be the best evidence to include or exclude the presence of CBD stones if the stone is directly inspected by cholangiography.
Acknowledgments
We would like to express our deep gratitude to Associate Professor Lee Hong-Shen who provided us with great support and guidance in the statistical analysis. We also truly appreciate our colleague Ms Liou Chih-Chen for the data preparation.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Contributor Information
Hon-Phin Wong, Email: wdolphin@ms23.hinet.net.
Yu-Lun Chiu, Email: holaclaire@yahoo.com.tw.
Bei-Hao Shiu, Email: bearpro2007@gmail.com.
Lu-Chang Ho, Phone: +886-4-24739595 ext 34601, FAX: +886-4-24756347, Email: cshe032@csh.org.tw.
References
- 1.Barkun AN, Barkun JS, Fried GM, Ghitulescu G, Steinmetz O, Pham C, et al. Useful predictors of bile duct stones in patients undergoing laparoscopic cholecystectomy. McGill Gallstone Treatment Group. Ann Surg. 1994;220:32–39. doi: 10.1097/00000658-199407000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang MH, Chen TH, Wang SE, Tsai YF, Su CH, Wu CW, et al. Biochemical predictors for absence of common bile duct stones in patients undergoing laparoscopic cholecystectomy. Surg Endosc. 2008;22:1620–1624. doi: 10.1007/s00464-007-9665-2. [DOI] [PubMed] [Google Scholar]
- 3.Hauer-Jensen M, Karesen R, Nygaard K, Solheim K, Amlie E, Havig O, et al. Predictive ability of choledocholithiasis indicators. A prospective evaluation. Ann Surg. 1985;202:64–68. doi: 10.1097/00000658-198507000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Peng WK, Sheikh Z, Paterson-Brown S, Nixon SJ. Role of liver function tests in predicting common bile duct stones in acute calculous cholecystitis. Br J Surg. 2005;92:1241–1247. doi: 10.1002/bjs.4955. [DOI] [PubMed] [Google Scholar]
- 5.Alponat A, Kum CK, Rajnakova A, Koh BC, Goh PM. Predictive factors for synchronous common bile duct stones in patients with cholelithiasis. Surg Endosc. 1997;11:928–932. doi: 10.1007/s004649900489. [DOI] [PubMed] [Google Scholar]
- 6.Lapis JL, Orlando RC, Mittelstaedt CA, Staab EV. Ultrasonography in the diagnosis of obstructive jaundice. Ann Intern Med. 1978;89:61–63. doi: 10.7326/0003-4819-89-1-61. [DOI] [PubMed] [Google Scholar]
- 7.Chang CW, Chang WH, Lin CC, Chu CH, Wang TE, Shih SC. Acute transient hepatocellular injury in cholelithiasis and cholecystitis without evidence of choledocholithiasis. World J Gastroenterol. 2009;15:3788–3792. doi: 10.3748/wjg.15.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pasanen PA, Partanen KP, Pikkarainen PH, Alhava EM, Janatuinen EK, Pirinen AE. A comparison of ultrasound, computed tomography and endoscopic retrograde cholangiopancreatography in the differential diagnosis of benign and malignant jaundice and cholestasis. Eur J Surg. 1993;159:23–29. [PubMed] [Google Scholar]
- 9.Brisbois D, Plomteux O, Nchimi A, Hock D, Dupont P, Delforge M, et al. Value of MRCP for detection of choledocholithiasis in symptomatic patients: one-year experience with a standardized high resolution breath-hold technique. JBR-BTR. 2001;84:258–261. [PubMed] [Google Scholar]
- 10.Hirota M, Takada T, Kawarada Y, Nimura Y, Miura F, Hirata K, et al. Diagnostic criteria and severity assessment of acute cholecystitis: Tokyo guidelines. J Hepatobiliary Pancreat Surg. 2007;14:78–82. doi: 10.1007/s00534-006-1159-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peng WK, Sheikh Z, Nixon SJ, Paterson-Brown S. Role of laparoscopic cholecystectomy in the early management of acute gallbladder disease. Br J Surg. 2005;92:586–591. doi: 10.1002/bjs.4831. [DOI] [PubMed] [Google Scholar]
- 12.Nebiker CA, Baierlein SA, Beck S, von Flue M, Ackermann C, Peterli R. Is routine MR cholangiopancreatography (MRCP) justified prior to cholecystectomy? Langenbecks Arch Surg. 2008;394:1005–1010. doi: 10.1007/s00423-008-0447-7. [DOI] [PubMed] [Google Scholar]
- 13.Laing FC, Jeffrey RB., Jr Choledocholithiasis and cystic duct obstruction: difficult ultrasonographic diagnosis. Radiology. 1983;146:475–479. doi: 10.1148/radiology.146.2.6849098. [DOI] [PubMed] [Google Scholar]
- 14.Cronan JJ, Mueller PR, Simeone JF, O’Connell RS, van Sonnenberg E, Wittenberg J, et al. Prospective diagnosis of choledocholithiasis. Radiology. 1983;146:467–469. doi: 10.1148/radiology.146.2.6849096. [DOI] [PubMed] [Google Scholar]
- 15.Parulekar SG, McNamara MP., Jr Ultrasonography of choledocholithiasis. J Ultrasound Med. 1983;2:395–400. doi: 10.7863/jum.1983.2.9.395. [DOI] [PubMed] [Google Scholar]
- 16.Laing FC, Jeffrey RB, Wing VW. Improved visualization of choledocholithiasis by sonography. AJR Am J Roentgenol. 1984;143:949–952. doi: 10.2214/ajr.143.5.949. [DOI] [PubMed] [Google Scholar]
- 17.Neitlich JD, Topazian M, Smith RC, Gupta A, Burrell MI, Rosenfield AT. Detection of choledocholithiasis: comparison of unenhanced helical CT and endoscopic retrograde cholangiopancreatography. Radiology. 1997;203:753–757. doi: 10.1148/radiology.203.3.9169700. [DOI] [PubMed] [Google Scholar]
- 18.Baron RL. Diagnosing choledocholithiasis: how far can we push helical CT? Radiology. 1997;203:601–603. doi: 10.1148/radiology.203.3.9169674. [DOI] [PubMed] [Google Scholar]
- 19.Brink JA, Kammer B, Mueller PR, Balfe DM, Prien EL, Ferrucci JT. Prediction of gallstone composition: synthesis of CT and radiographic features in vitro. Radiology. 1994;190:69–75. doi: 10.1148/radiology.190.1.8259431. [DOI] [PubMed] [Google Scholar]
- 20.Dooms GC, Fisher MR, Higgins CB, Hricak H, Goldberg HI, Margulis AR. MR imaging of the dilated biliary tract. Radiology. 1986;158:337–341. doi: 10.1148/radiology.158.2.3941860. [DOI] [PubMed] [Google Scholar]
- 21.Wallner BK, Schumacher KA, Weidenmaier W, Friedrich JM. Dilated biliary tract: evaluation with MR cholangiography with a T2-weighted contrast-enhanced fast sequence. Radiology. 1991;181:805–808. doi: 10.1148/radiology.181.3.1947101. [DOI] [PubMed] [Google Scholar]
- 22.Tranter SE, Thompson MH. Spontaneous passage of bile duct stones: frequency of occurrence and relation to clinical presentation. Ann R Coll Surg Engl. 2003;85:174–177. doi: 10.1308/003588403321661325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins C, Maguire D, Ireland A, Fitzgerald E, O’Sullivan GC. A prospective study of common bile duct calculi in patients undergoing laparoscopic cholecystectomy: natural history of choledocholithiasis revisited. Ann Surg. 2004;239:28–33. doi: 10.1097/01.sla.0000103069.00170.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998;207:21–32. doi: 10.1148/radiology.207.1.9530295. [DOI] [PubMed] [Google Scholar]
- 25.Dalton SJ, Balupuri S, Guest J. Routine magnetic resonance cholangiopancreatography and intra-operative cholangiogram in the evaluation of common bile duct stones. Ann R Coll Surg Engl. 2005;87:469–470. doi: 10.1308/003588405X51137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demartines N, Eisner L, Schnabel K, Fried R, Zuber M, Harder F. Evaluation of magnetic resonance cholangiography in the management of bile duct stones. Arch Surg. 2000;135:148–152. doi: 10.1001/archsurg.135.2.148. [DOI] [PubMed] [Google Scholar]
- 27.Topal B, Van de Moortel M, Fieuws S, Vanbeckevoort D, Van Steenbergen W, Aerts R, et al. The value of magnetic resonance cholangiopancreatography in predicting common bile duct stones in patients with gallstone disease. Br J Surg. 2003;90:42–47. doi: 10.1002/bjs.4025. [DOI] [PubMed] [Google Scholar]
- 28.Dwerryhouse SJ, Brown E, Vipond MN. Prospective evaluation of magnetic resonance cholangiography to detect common bile duct stones before laparoscopic cholecystectomy. Br J Surg. 1998;85:1364–1366. doi: 10.1046/j.1365-2168.1998.00957.x. [DOI] [PubMed] [Google Scholar]
- 29.Carlos RC, Scheiman JM, Hussain HK, Song JH, Francis IR, Fendrick AM. Making cost-effectiveness analyses clinically relevant: the effect of provider expertise and biliary disease prevalence on the economic comparison of alternative diagnostic strategies. Acad Radiol. 2003;10:620–630. doi: 10.1016/S1076-6332(03)80080-6. [DOI] [PubMed] [Google Scholar]
- 30.Richardson MC, Bell G, Fullarton GM. Incidence and nature of bile duct injuries following laparoscopic cholecystectomy: an audit of 5913 cases. West of Scotland Laparoscopic Cholecystectomy Audit Group. Br J Surg. 1996;83:1356–1360. doi: 10.1002/bjs.1800831009. [DOI] [PubMed] [Google Scholar]
- 31.Kaltenthaler E, Vergel YB, Chilcott J, Thomas S, Blakeborough T, Walters SJ, et al. A systematic review and economic evaluation of magnetic resonance cholangiopancreatography compared with diagnostic endoscopic retrograde cholangiopancreatography. Health Technol Assess. 2004; 8:iii1–89. [DOI] [PubMed]
- 32.Sahai AV, Devonshire D, Yeoh KG, Kay C, Feldman D, Willner I, et al. The decision-making value of magnetic resonance cholangiopancreatography in patients seen in a referral center for suspected biliary and pancreatic disease. Am J Gastroenterol. 2001;96:2074–2080. doi: 10.1111/j.1572-0241.2001.03965.x. [DOI] [PubMed] [Google Scholar]


