Conspectus
The ability of gene or RNA interference (RNAi) delivery to increase or decrease virtually any protein in a cell opens the path for cures to most diseases that afflict humans. However, their high molecular weight, anionic nature, and instability in the presence of enzymes, pose major obstacles to nucleic acid delivery and frustrates their use as human therapies.
This Account describes current ideas on the mechanisms in non-viral nucleic acid delivery and how lipidic and polymeric carriers overcome some of the critical barriers to delivery. A multitude of polymeric and lipidic vectors have been developed over the last 20 years, only a small fraction of them have progressed into clinical trials. Given that none of these vectors has received FDA approval, indicates that the current vectors do not yet have suitable properties for effective in vivo nucleic acid delivery.
Nucleic acid delivery is a multistep process and inefficiencies at any stage result in a dramatic decrease in gene delivery or gene silencing. Despite this, the majority of studies investigating synthetic vectors focus solely on optimization of endosomal escape. A small number of studies address how to improve uptake via targeted delivery. A smaller fraction examine the intracellular fate of the delivery systems and nucleic acid cargo. The internalization of genes into the cell nucleus remains an inefficient and mysterious process. In the case of DNA delivery, strategies to increase and accelerate the migration of DNA through the cytoplasm and transport it through the nuclear membrane are required.
The barriers to siRNA delivery are fewer: siRNA is more readily released from the carrier, siRNA is more resistant to enzymatic degradation and the target is in the cytoplasm; hence, siRNA delivery systems are becoming a clinical reality. With regard to siRNA therapy, the exact cytoplasmic location of RISC formation and activity is unknown. This makes specific targeting of the RISC for more efficient siRNA delivery difficult. Furthermore, identifying the factors favoring the binding of siRNA to Ago-2 and understanding how the half-life of siRNA and Ago-2/siRNA complex in the cytoplasm can be modulated without interfering with RISC functions that are essential for normal cell activity could increase siRNA delivery efficiency.
In this manuscript we concisely review the current synthetic vectors and for a few of these, propose alternative strategies. We suggest how certain cellular mechanisms might be exploited to improve gene transfection and silencing. Finally, we raise the question if some carriers are delivering the siRNA to cells capable of repackaging the siRNA into exosomes. The exosomes would then transport the siRNA into a subsequent population of cells where the siRNA effect is manifest. This piggy-back mechanism may be responsible for reported deep tissue siRNA effects using certain carriers.
Introduction
The application of gene and RNAi therapy in the clinic requires safe and efficient vectors. To date the two main approaches for nucleic acid therapy are based on viral and non-viral vectors. There are a number of safety concerns associated with viral vectors: risks of induced immune responses, unwanted mutagenesis, and cancer. Thus, tremendous effort has gone into developing non-viral vectors based on lipidic and/or polymeric carriers. In 1965, Vaheri and Pagano introduced diethylaminoethyl modified dextran, the first polymer for gene delivery1. In 1987, Felgner introduced DOTMA, the first cationic lipid for DNA transfection2. Since then, a multitude of different lipids and polymers have been developed. These, along with technologies for encapsulating nucleic acids in nanosized vesicles3, have been extensively reviewed and thus will not be discussed in this Account. By encapsulating, complexing, or binding the nucleic acid into particles, these vectors are able to protect the nucleic acid from degradation and deliver it into certain cellular compartments.
While the optimization of biomaterials for gene delivery was ongoing, the discovery of small interfering RNA (siRNA) by Fire and Mello in 1998 introduced new prospects for the treatment of incurable diseases4. Sequence specific siRNA molecules were shown to target complementary mRNA and induce silencing of the encoded protein. A decade later, tremendous progress has been made in the field of gene silencing, with several RNAi-therapeutics in clinical trials. Among the RNAi-therapeutics currently being assessed in the clinic are lipid-based or polymeric-based formulations for the systemic treatment of TTR-mediated amyloidosis5 and cancer6-8. Despite these successes, improvements still need to be made to non-viral gene and siRNA vectors.
This Account will focus on the mechanistic aspects involved in lipid-based and polymeric-based gene and siRNA delivery. We discuss the shortcomings of current practices and propose alternative mechanistic approaches which may offer potential means of improving non-viral delivery with synthetic vectors.
Changes in the composition of lipoplexes or polyplexes upon in vivo administration
For reproducible in vitro and in vivo gene and siRNA delivery, the formulation of liposomes, lipoplexes, and polyplexes containing nucleic acids requires precise composition of the transfection reagents. Although several studies have investigated how blood components can destabilize lipidic and polymeric nanoparticles9, 10, little is known about the final composition of the delivery systems that mediates gene delivery or silencing in the cells. The composition of polyplexes and lipoplexes undergo constant changes after systemic administration into the bloodstream. Excessive polymer chains or liposome components not strongly attached to the complexes11, 12 will be shed from the particles and new components, such as lipoproteins, can adhere to the surface of the complexes. This can not only lead to destabilization of the particles, but can also alter the biodistribution or promote clearance in vivo13. Understanding how polyplex and lipoplex composition changes at each stage of delivery in vivo (at the administration site, during circulation in the blood stream, in the extracellular matrix of organs and tissues, and finally upon entry into target cells) could potentially allow for the design of synthetic vector systems with higher stability. Although single particle tracking in whole animals is not yet possible with the current resolution of luminescence and fluorescence imaging, advances in multi-photon excitation microscopy or confocal microscopy may allow lipoplex and polyplex composition to be fully characterized from administration to final destination in the target cells.
Controlling intracellular uptake by less explored mechanisms
Non-viral, synthetic vectors are shown to enter cells by endocytosis. This can be divided into clathrin-mediated endocytosis, caveolae-mediated endocytosis, macropinocytosis, and clathrinand caveolae-independent endocytosis. In contrast to viruses, synthetic vectors still suffer from low intracellular uptake and low transfection efficiency. One strategy to increase intracellular uptake is to attach targeting ligands to non-viral vectors to increase receptor-mediated endocytosis. These ligands mostly target nutrient uptake receptors such as transferrin, folate, and low-density lipoprotein receptors (LDL)12,13. Although this leads to improvement in cellular uptake and gene delivery, other more powerful strategies should be taken into consideration. Invading viruses often activate signaling cascades of cells to induce their intracellular uptake14,15. Enhanced uptake is triggered by binding to signaling receptors that regulate the endocytic machinery. Influenza viruses, for example, initiate the formation of new clathrin coated pits (CCP) at their site of binding. There is 20 times as much CCP formation at the site of virus binding than at other sites of the cells16,17. Vaccinia viruses trigger their uptake via macropinocytosis by activating kinases and GTPases18. The challenge remains to engineer synthetic vectors with ligands that bind to signaling receptors to enhance endocytosis without inducing an immune response, a severe effect often caused by viruses. The type of ligands can also be used to influence intracellular fate and to sort vectors into two distinct populations of early endosomes: a fast maturing population that is transported rapidly on microtubules towards the perinuclear region and a static population that hardly moves19. The population that is targeted will determine how quickly the vectors will be transported to the perinuclear region (important aspect for DNA delivery) or how long they will stay at the cell periphery. This might have consequences on the efficiency of gene silencing or expression. Influenza viruses preferentially choose the fast maturing populations, most likely as a means of transportation to the perinuclear region as fast as possible19.
Endosomal escape
Polymeric vectors: The proton sponge effect and the umbrella hypothesis
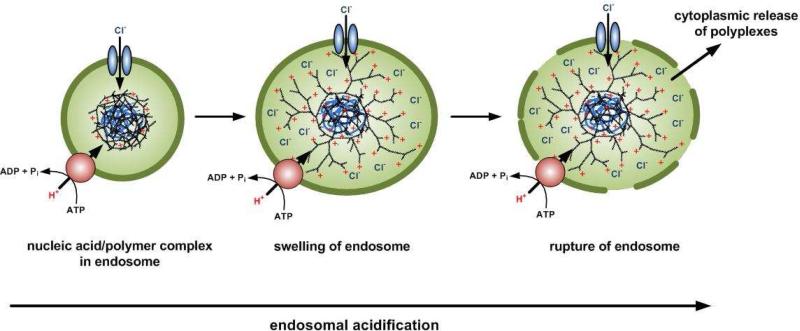
Once taken up into the cells, polyplexes have to escape from the endosomal pathway to release the siRNA or DNA into the cytoplasm. The endosomal pathway starts with the early endosomes, which become progressively acidic as they mature into late endosomes. The proton pump vacuolar ATPase generates acidification by accumulating protons in the vesicle until the pH drops to pH 5-6. Usually this would end with the fusion of the late endosomes with the lysosomes, where the pH reaches 4-5 and the content would be degraded by enzymes. The ability of many cationic polymers to mediate efficient nucleic acid delivery is mainly attributed to their strong buffering capacity in the pH range from 5 to 7. It is hypothesized that these strongly buffering polyamines prevent acidification of the endosomes by acting as ‘proton sponges’ (Figure 1). This leads to an increase in proton influx followed by an enhanced accumulation of Cl- and osmotic swelling20-22.
Figure 1.
Endosomal rupture is mediated by polymers with a buffer capacity in the endosomal pH range which can trigger (a) the proton-sponge effect and (b) polymer swelling according to the umbrella hypothesis.
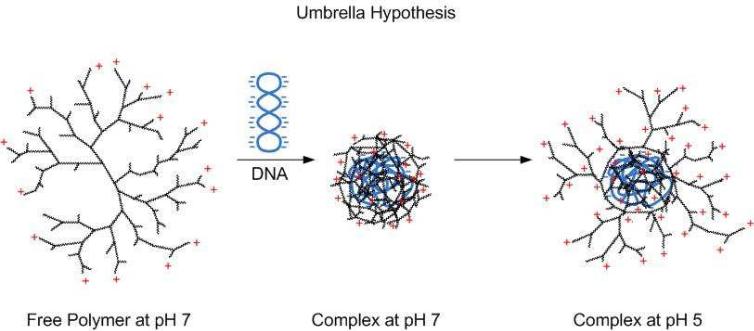
An extension of the proton-sponge effect was introduced with the umbrella hypothesis, which describes the ability of polymers to expand volumetrically when protonated at lower pH (pH 5-6). According to the ‘umbrella effect’ the polymer unfolds from a collapsed state into an extended conformation after protonation of the amine groups (Figure 2). When the polymer forms complexes with DNA, compaction of both into small particles based on electrostatic interactions occur. After being taken up into cells and transported along the endosomal pathway, excess protons in the endosome lead to protonation of the tertiary amines in the interior of the polyplexes. Due to the electrostatic repulsion of the neighboring charged amine groups, the terminal branches of the polymer spread out and adopt a fully extended conformation if not restrained by steric hindrances. Tang et al. have shown that this increase in volume and space caused by polymer swelling contributes to endosomal escape of the polyplexes. This was shown using a set of intact and fractured polyamidoamine (PAMAM) dendrimers that differ in their degree of flexibility and their ability to volumetrically expand with decreasing pH. It was found that fractured dendrimers with optimal flexibility of their branches mediate superior transfection efficiency compared to intact dendrimers that are sterically constrained. Taken together, the proton-sponge hypothesis and the umbrella effect suggest that the requirements for endosomal escape of cationic polymers are titratable amine groups at pH 5-7 and a highly flexible structure that can increase in volume after protonation in the endosome23.
Figure 2.
Schematic representation of the umbrella hypothesis induced by polymer swelling. Cationic polymers form a complex with negatively charged nucleic acid. At lower pH in the endosomes, the complex partially unfolds. Due to the protonation of the terminal amine groups and electrostatic repulsion, the terminal branches of the polymer spread out and adopt a fully extended conformation.
Lipidic vectors: membrane destabilization, ion pair formation and nucleic acid release
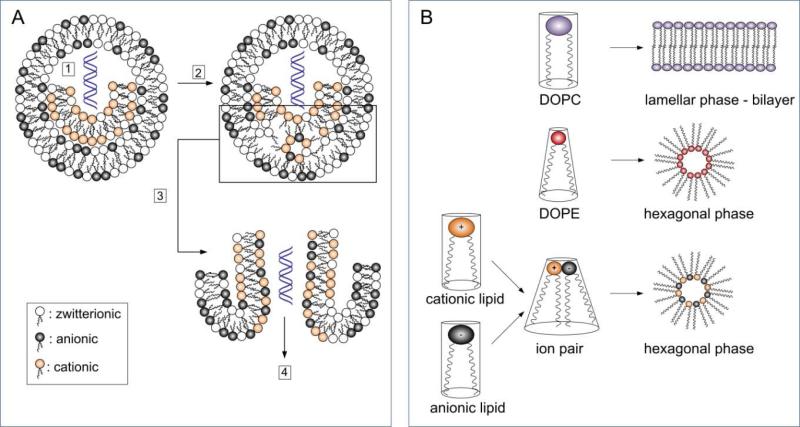
The escape of cationic lipidic vectors from endosomes is mainly mediated by their interactions with anionic phospholipids from the endosomes and their ability to transit from the lamellar to the hexagonal phase. This model introduced by Xu and Szoka identified factors important for cytoplasmic delivery (Figure 3)24. First, the nucleic acid/lipid complexes are transferred into endosomal vesicles where the close proximity of the lipoplex and endosomal membrane promotes an electrostatic interaction between the cationic lipids in the lipoplex and anionic lipids in the endosomal membrane. The lipid bilayers are destabilized due to the formation of cationic-anionic ion pairs and the nucleic acid is released from the lipoplex. The ion pairs adopt a molecular cone shape which promotes the transition from a lamellar phase to an inverted hexagonal phase with delivery of the nucleic acid into the cytoplasm25,26. The ability of cationic liposomal systems to mediate transition into the hexagonal phase is generally triggered by lipids whose molecular shapes exhibit high curvature. This can be controlled and enhanced by the following factors: (i) the geometry of the headgroup and the lipid tail of the cationic lipid; (ii) the addition of helper lipids, such as DOPE and cholesterol, to further enhance the adoption of a non-bilayer structure; and (iii) the bilayer-to-HII transition temperature of the liposomal formulation8, 25, 27.
Figure 3.
A) Proposed mechanisms of cationic lipid/nucleic acid complexes after endocytosis along the endosomal pathway: (1) Cationic lipids interact with anionic lipids in endosomes by forming ion-pairs; (2) The lipid bilayer is destabilized; (3) The hexagonal phase is formed; and (4) The nucleic acid is released into the cytoplasm. B) The formation of the hexagonal phase is triggered by lipid molecules with a cone shape shown here for DOPE and a charged lipid ion-pair that transits from a cylindrical shape into a molecular cone shape. Helper lipids (i.e. DOPC) with a cylindrical shape mediate bilayer stabilization8, 24, 26.
The same endosomal escape mechanisms proposed for cationic liposomes can be applied to ionizable lipids with DLin-DMA headgroups. The headgroup of DLin-DMA lipids contains tertiary amines (pka 6-7). These are protonable at lower pH and become cationic in the endosomes. Due to their neutral charge at physiological pH, they appear to be less immunoreactive than cationic lipids with a fixed charge such as DOTAP8.
DNA delivery: cytoplasmic transport to the nucleus and the impact of spatio-temporal factors
Endosomal escape is often believed to be the most critical step in gene delivery and vectors are designed to mediate endosomal release at the earliest time possible to avoid enzymatic degradation in the late endosomes or lysosomes. A closer look at the endosomal pathway and the vectors’ journey from the cell periphery towards the nucleus, however, indicates that particles or DNA released at the perinuclear region have the best chance of entering the nucleus28, 29. The transport of endocytic vesicles is organized by a network of microtubules (MT). MTs radiate from a MT organizing center (MTOC) near the nucleus towards the periphery of the cells. Transport along the MT is regulated by motor proteins such as dynein and kinesin30. Particles that escape from the endosomes prematurely close to the cell membrane have to travel the longest distance before reaching the nucleus. Passive diffusion of particles or DNA through the highly viscous cytoplasm is slow and constrained by the high concentrations of proteins in the cell. Prolonged exposure of particles or DNA to the cytoplasmic environment can lead to destabilization of the complexes and degradation of the DNA by DNase31. However, as long as particles stay inside endosomes, they can exploit the endosomal pathway to receive active directional transport along the MT towards the nucleus.
The optimal time for endosomal escape would be when the complexes reach the perinuclear region, right before the lysosomes start to accumulate into clusters32. When the lysosomes concentrate and aggregate, the speed of diffusion decreases and the escape of complexes from clustered lysosomes becomes more difficult28. In contrast to viruses, current synthetic vectors are not capable of fully utilizing the cell machinery to their advantage: (1) the timing and location of endosomal escape of synthetic vectors is not optimized; (2) once released from the endosomes, synthetic vectors are not capable of utilizing motor proteins for active transport on MT; (3) because the complexes formed by synthetic vectors are large (80-500 nm), passive diffusion through the cytoplasm is slow.
One way to define the optimal time-point for endosomal escape is to incorporate a pH-sensor into the synthetic vectors, a mechanism used by the adenovirus serotype 7 (Ad7). It was suggested that the fiber protein of the Ad7 serves as a pH-sensor and triggers lysosomal escape at ~ pH 5. This ensures that the Ad7 accumulates in the perinuclear region, enabling efficient nuclear entry33. Vectors designed to target the lysosomal pathway for gene delivery need to protect the DNA against degrading enzymes in the harsh environment of the lysosomes.
If the synthetic vectors are designed to escape the endosomes at an early time-point at the cell periphery - a pathway exploited by the adenovirus serotype 5 - incorporating a mechanism facilitating MT transport would improve migration through the cytoplasm. Ligands with high affinity to dynein as a motor protein (adenovirus hexon monomers are one example34) may be capable of mediating active transport along the MT through the cytoplasm. Directional transport could be further accelerated by stimulating the signaling cascades of cells such as protein kinase A (PKA) and P38/MAPK pathways35. Adenoviruses utilize this mechanism to boost minus-end motility of the MT network for transport towards the nuclear region.
According to Stokes-Einstein, diffusion is a function of diameter, hence smaller particles move faster than larger ones. Thus, another way to optimize gene delivery to the nucleus would be to decrease the size of the particles to increase the velocity of passive diffusion through the cytoplasm. It has been shown that viruses and synthetic particles, which are small in diameter (< 30 nm), are able to move through the cytoplasm independently from the MT network36, 37. Since DNA plasmids are large in size, packaging and complexing them into small particles can be difficult and would require more sophisticated encapsulation methods. The passage through the nuclear pore complex (NPC) is another critical barrier in gene delivery. Strategies to overcome the NPC has been thoroughly described by Lam et al.38.
Once inside the nucleus the copy number of DNA and its accessibility for the transcription machinery determines the level of transgene expression. Studies have shown that the reported minimum number of plasmids delivered to the nucleus required for measurable transgene expression depends on the type of vectors and varies between 75 and 4000 plasmid copies39, 40. Comparisons between different delivery vehicles showed that higher copy numbers of DNA molecules in the nucleus do not necessarily correlate with higher transfection efficiency. At similar plasmid/nucleus copies, lipofectamine mediated 10-fold higher transfection efficiency than PEI. This suggests that the DNA delivered by PEI is biologically less active than the DNA delivered by lipofectamine. It also emphasizes that a deeper understanding of the nuclear events in gene delivery is required for future progress.
With advances in proteomics, single particle tracking, and electron microscopy, it should be possible to fully identify every single component and protein participating in molecular motor binding and the MT transport in the near future. This will aid in a mechanism-based design of synthetic gene delivery systems.
Suitability of 2D in vitro cell cultures for predictability of in vivo results
Vectors mediating high transfection efficiency in vitro often fail to achieve similar results in vivo. One possible reason for this is that lipidic and polymeric vectors are optimized in vitro using 2-dimensional (2D) cultures that lack extracellular in vivo barriers and do not realistically reflect in vivo conditions. While cells in vitro grow in monolayers, cells in vivo grow in 3D tissue layers held together by the extracellular matrix. Vectors delivered in vivo by systemic administration do not only have to withstand the blood stream but also overcome the cellular matrix to reach all cell layers of the tissue. While large particles seem to have an advantage in in vitro transfection due to a sedimentation effect on cells, efficient delivery of particles deep into organs requires particles < 100 nm. Small particles (40 nm) diffuse faster and more effectively in the extracellular matrix and inner layers of tissues whereas larger particles (>100 nm) are restricted by steric hindrance41.
Another aspect that influences nucleic acid delivery is the difference in cell geometry and morphology between in vitro and in vivo environments. Cells grown on 2D cultures are monolayers and usually adapt a flattened morphology. This results in cells with reduced thicknesses but larger widths and lengths. Particles which are taken up directly above the nucleus (supranuclear region) have the shortest transport distance to the nucleus (4-8 μm) and hence a greater chance of delivery success. Dinh et al. have shown that size and morphology of cells have a large influence on the spatiotemporal distribution of carriers and on transfection efficiency (transfection of flattened cells is up to an order of magnitude greater than spherical cells for the same input of DNA)28. The spatiotemporal distribution of carriers, however determines the optimal time for endosomal escape and the optimal intracellular pathway28. As most cells in vivo are more spherical and not flattened, no particular region of the cellular membrane is especially close to the nucleus. Consequently, optimization of carriers under in vitro conditions may not be applicable to in vivo conditions. 3D in vitro models that culture cells in an extracellular matrix and that take the spatial organization of cells into account may present a more viable cell culture method for the optimization of synthetic vectors42.
siRNA delivery: targeting to specific locations of RISC activity and assembly
The extensive investigations and optimization of DNA delivery systems has translated into rapid progress being made for siRNA delivery. However, similar to DNA delivery, a major limitation of siRNA-based therapeutics is the inability to deliver a significant fraction of the dose to the target site after intravenous administration. The most easily accessed organ is the liver and even in this tissue recent studies have shown that less than 0.1% of the total siRNA dose reaches the target site, the remaining 99.9% of the siRNA is being degraded or lost on its way to the cytosol of the target cells43, 44. Hence, strategies that do more than optimizing endosomal escape are needed to further increase gene silencing efficiency.
One strategy is to better identify the cytoplasmic location of the RNA-induced silencing complex (RISC) which contains different Argonaute (Ago) family proteins responsible for mRNA knockdown. After escaping from the endosomes, the siRNA needs to be released from the polymeric or lipidic particles into the cytosol to be able to bind to the RISC. Upon loading into RISC, the passenger strand of the siRNA is degraded. The guide strand base-pairs with the target, complementary mRNA sequence and mediates its cleavage and degradation4. Of the different Ago family proteins, only Ago2 is capable of catalyzing mRNA degradation45. Although great progress has been made in characterizing and identifying the main components of RISC, little is known about where in the cytoplasm RISC assembly and activity takes place. With current synthetic vectors, siRNA is released into some random locations of the cytoplasm. The cytoplasm constitutes a large part of the entire cell with different organelles and compartments including the golgi apparatus, endoplasmic reticulum, mitochondria, endocytic vesicles, and perinuclear region. Thus, knowledge about the exact intracellular location of RISC and Ago2 would provide powerful insights into how to effectively target the siRNA to the site of action. Several groups have shown that RISC and Ago2 are not randomly distributed in the cytoplasm, but are concentrated in specific centers of the cytoplasm46-48. Sen et al. demonstrated that Ago2, a main component of RISC, is localized to cytoplasmic P-bodies - regions where mRNA degradation occurs. They suggest two models for the RISC location. In the first, Ago2/RISC stays permanently in P-bodies (Figure 4). In the second model, the Ago2/RISC binds to the siRNA and mRNA in the cytoplasm and serves as a shuttle between the cytoplasm and P-bodies. Where the Ago2/RISC binding of siRNA occurs and whether the process is signaling-based or stochastic-based are unknown factors. More recent studies suggest that the endosomal trafficking pathway is involved in silencing by small RNAs47. They found Ago2 and RISC co-localized with GW-bodies associated with late endosomes called multivesicular bodies (MVB)46,47,49. Lee et al. suggest that MVBs control gene silencing by promoting the turnover of RISC and its loading competence for miRNA or siRNA47. Thus, in analogy to DNA targeting into the nucleus, siRNA targeting into the RISC rather than depending upon diffusion in the cytoplasm might enhance activity.
Figure 4.
Models of RISC location and activity in the cytoplasm: (1) RISC is concentrated in cytoplasmic bodies, such as P-bodies; (2) RISC is associated with late endosomes (MVBs); and (3) RISC freely diffuse in the cytoplasm.
Another issue that needs to be addressed is that non-proportional amounts of siRNA are required for increased levels of knockdown: in a liver targeting mouse model, 370 copies of siRNA mediated 50% of knockdown, whereas 2200 copies of siRNA were required for 80% gene silencing43. Several factors will need to be investigated to understand this: siRNA delivery needs to be optimized with regard to the kinetics of siRNA loading into Ago2; the half-life of Ago2/siRNA complexes must be identified; and the factors that determine the binding affinity of siRNA to Ago2 should be explored.
Exosomal pathway as potential means for deep tissue delivery of nucleic acids
One of the limitations of current nucleic acid delivery systems is their inability to deeply penetrate tissues and organs such as solid tumors and brain. Typically, only the outer layer of cells can be reached and transfected thus resulting in poor therapeutic efficacy. The Epstein-Barr-Virus (EBV), a human tumor pathogen, seems to overcome this by hijacking the exosomes for intercellular communication in the tumor microenvironment. Exosomes are small membrane vesicles (40-200 nm) of endocytic origin. After being released into the extracellular environment, they can fuse with neighboring cells because of the presence of cell recognition molecules on their surface50. Hence, it is hypothesized that by going through several cycles of cell internalization and release, exosomes are able to cross several layers of tissues51. Exosomes serve as shuttles of mRNA, small RNAs (miRNA), and signaling factors between cells. They can be secreted by a number of cells, including tumor cells, dendritic cells, B cells, T cells, epithelial cells and neurons50, 52. Furthermore, it has been suggested that exosomes may be used by viruses (such as EBV and HIV) and other pathogens (such as prions) to promote infectivity53. Recently it was observed that EBV repackages its viral miRNA and viral-encoded proteins into exosomes to infect neighboring cells in the tumor microenvironment and to manipulate tumor growth54.
This process might already be responsible for the observed silencing effect of certain siRNA carriers. If not, synthetic vectors might be engineered to exploit the pathway to create carriers capable of delivering nucleic acids from the periphery to the center of tumor tissues or other organs. One potential way to utilize the exosomal pathway would be to repackage liposomal nucleic acid into exosomes. This could be achieved by targeting membrane proteins specific to exosomes such as tetraspanins and annexins and initiating a fusion event between the liposomes and exosomes55. A deeper understanding of the biogenesis of exosomes and the factors that control their formation may open possibilities for engineering vectors capable of piggybacking on the exosomal pathway.
Summary and Perspectives
While tremendous progress on non-viral vectors for DNA and siRNA delivery has been made over the past three decades, clinical advances have been slow to arrive. A major limitation of current systems in vivo is their inability to effectively deliver a high dose to the target site. Thus, we believe the following developments - which can be divided into cellular transport and in vivo cellular accessibility - will be necessary to advance gene and RNAi therapy in humans:
- New strategies to enhance uptake of particles into cells should be developed. The signaling receptors and cascades that regulate the endocytotic machinery of cells might be exploitable for this end.
- A more detailed understanding of RISC functioning must be developed to improve siRNA delivery. This will entail identifying the exact cytoplasmic location of RISC formation and RISC activity as well as the factors regulating RISC loading, RISC turnover, and the affinity of siRNA to Ago2.
- The passive diffusion of particles or nucleic acids through the cytoplasm needs to be minimized. Novel mechanisms which utilize the active transport of particles or nucleic acids through the cytoplasm along the microtubules are required.
- More representative in vitro cell culture models that precisely predict in vivo nucleic acid transfer should be developed.
- For the precise engineering of nucleic acid delivery systems, new in vivo imaging technologies are required so that changes in the composition of particles can be characterized in circulation after intravenous administration.
- Novel strategies for the deep tissue delivery of nucleic acids must be identified. Repackaging nucleic acids into exosomes of circulating cells in the body may provide a way of accomplishing this.
- A challenge remains for researchers to develop systems with few components capable of performing multiple functions (such as nucleic acid encapsulation, targeting, and transfer) in parallel. This is especially important for product manufacturing given the analytical and stability challenges that exist for even the current non-targeted nanomedicines, such as the FDA-approved Doxil56.
The optimization of synthetic delivery systems has been largely based on empirical approaches. A better understanding of the intracellular mechanisms and molecular bases of nucleic acid transfer, however, will enable a more rational and mechanism-based design of vectors. This knowledge will provide the missing pieces of the puzzle for effective nucleic acid delivery in animals and humans.
Figure 5.
Cytoplasmic transport of synthetic vectors. (1) After endocytosis, synthetic vectors are released from the early endosomes at the cell periphery into the cytoplasm. For efficient transport of synthetic vectors through the cytoplasm, a mechanism for facilitating MT transport would be required. (2) Lysosomes are transported along the MT pathway to the perinuclear region. Synthetic vectors designed to exploit the lysosomal pathway have to be able to protect the DNA against the degrading enzymes in the lysosomes. (3) To migrate efficiently through the cytoplasm by passive diffusion, synthetic vectors need to be smaller than 30 nm.
Figure 6.
Comparison of cells grown under 2D conditions in vitro and cells under in vivo conditions: Cells cultured under 2D conditions grow in a monolayer and exhibit a flattened morphology. Particles taken up in the supranuclear region have the shortest distance to the nucleus, whereas particles taken up in the cytoplasmic area have to travel a longer distance. In contrast, cells grown under 3D cell culture conditions exhibit a more circular geometry, where no points at the cell membrane are particularly close to the cell nucleus28.
Acknowledgments
We thank the National Institutes of Health for financial support (2R01EB003008-08). Juliane Nguyen is a recipient of the research fellowship by the Deutsche Forschungsgemeinschaft (DFG). We gratefully acknowledge support from the Pfizer-UCSF QB3 consortium.
Biographies
Juliane Nguyen received her pharmacy degree and her PhD in Pharmaceutical Sciences from the Philipps-Universität Marburg (Germany). She is currently a post-doctoral fellow in the research group of Professor Dr. Szoka, University of California San Francisco. Her research interests include nanoparticle-based nucleic acid delivery systems using biodegradable polymeric and lipidic carriers.
Francis C. Szoka received his M.S. (Microbiology) from the University of Maryland and his Ph.D. (Biochemistry) from the State University of New York (SUNY). His major research interests include applying chemical, biochemical, and biophysical approaches to the study of membrane fusion/destabilization and developing drug/gene delivery systems based upon defined physicochemical mechanisms of membrane destabilization and intracellular trafficking.
References
- 1.Pagano JS, Vaheri A. Enhancement of infectivity of poliovirus RNA with diethylaminoethyl-dextran (DEAE-D). Arch Gesamte Virusforsch. 1965;17:456–464. doi: 10.1007/BF01241201. [DOI] [PubMed] [Google Scholar]
- 2.Felgner PL, Gadek TR, Holm M, Roman R, Chan HW, Wenz M, et al. Lipofection: a highly efficient, lipid-mediated DNA-transfection procedure. Proc Natl Acad Sci U S A. 1987;84:7413–7417. doi: 10.1073/pnas.84.21.7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li W, Szoka FC., Jr. Lipid-based nanoparticles for nucleic acid delivery. Pharm Res. 2007;24:438–449. doi: 10.1007/s11095-006-9180-5. [DOI] [PubMed] [Google Scholar]
- 4.Fire A, Xu S, Montgomery MK, Kostas SA, Driver SE, Mello CC. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature. 1998;391:806–811. doi: 10.1038/35888. [DOI] [PubMed] [Google Scholar]
- 5.Akinc A, Goldberg M, Qin J, Dorkin JR, Gamba-Vitalo C, Maier M, et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol Ther. 2009;17:872–879. doi: 10.1038/mt.2009.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aleku M, Schulz P, Keil O, Santel A, Schaeper U, Dieckhoff B, et al. Atu027, a liposomal small interfering RNA formulation targeting protein kinase N3, inhibits cancer progression. Cancer Res. 2008;68:9788–9798. doi: 10.1158/0008-5472.CAN-08-2428. [DOI] [PubMed] [Google Scholar]
- 7.Davis ME. The first targeted delivery of siRNA in humans via a self-assembling, cyclodextrin polymer-based nanoparticle: from concept to clinic. Mol Pharm. 2009;6:659–668. doi: 10.1021/mp900015y. [DOI] [PubMed] [Google Scholar]
- 8.Semple SC, Akinc A, Chen J, Sandhu AP, Mui BL, Cho CK, et al. Rational design of cationic lipids for siRNA delivery. Nat Biotechnol. 2010;28:172–176. doi: 10.1038/nbt.1602. [DOI] [PubMed] [Google Scholar]
- 9.Cullis PR, Chonn A, Semple SC. Interactions of liposomes and lipid-based carrier systems with blood proteins: Relation to clearance behaviour in vivo. Adv Drug Deliv Rev. 1998;32:3–17. doi: 10.1016/s0169-409x(97)00128-2. [DOI] [PubMed] [Google Scholar]
- 10.Li S, Tseng WC, Stolz DB, Wu SP, Watkins SC, Huang L. Dynamic changes in the characteristics of cationic lipidic vectors after exposure to mouse serum: implications for intravenous lipofection. Gene Ther. 1999;6:585–594. doi: 10.1038/sj.gt.3300865. [DOI] [PubMed] [Google Scholar]
- 11.Fahrmeir J, Gunther M, Tietze N, Wagner E, Ogris M. Electrophoretic purification of tumor-targeted polyethylenimine-based polyplexes reduces toxic side effects in vivo. J Control Release. 2007;122:236–245. doi: 10.1016/j.jconrel.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 12.Boeckle S, von Gersdorff K, van der Piepen S, Culmsee C, Wagner E, Ogris M. Purification of polyethylenimine polyplexes highlights the role of free polycations in gene transfer. J Gene Med. 2004;6:1102–1111. doi: 10.1002/jgm.598. [DOI] [PubMed] [Google Scholar]
- 13.Crielaard BJ, Yousefi A, Schillemans JP, Vermehren C, Buyens K, Braeckmans K, et al. An in vitro assay based on surface plasmon resonance to predict the in vivo circulation kinetics of liposomes. J Control Release. 2011 doi: 10.1016/j.jconrel.2011.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Feng SZ, Cao WS, Liao M. The PI3K/Akt pathway is involved in early infection of some exogenous avian leukosis viruses. J Gen Virol. 2011;92:1688–1697. doi: 10.1099/vir.0.030866-0. [DOI] [PubMed] [Google Scholar]
- 15.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 16.Rust MJ, Lakadamyali M, Zhang F, Zhuang X. Assembly of endocytic machinery around individual influenza viruses during viral entry. Nat Struct Mol Biol. 2004;11:567–573. doi: 10.1038/nsmb769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johannessen LE, Pedersen NM, Pedersen KW, Madshus IH, Stang E. Activation of the epidermal growth factor (EGF) receptor induces formation of EGF receptor- and Grb2-containing clathrin-coated pits. Mol Cell Biol. 2006;26:389–401. doi: 10.1128/MCB.26.2.389-401.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mercer J, Helenius A. Vaccinia virus uses macropinocytosis and apoptotic mimicry to enter host cells. Science. 2008;320:531–535. doi: 10.1126/science.1155164. [DOI] [PubMed] [Google Scholar]
- 19.Lakadamyali M, Rust MJ, Zhuang X. Ligands for Clathrin-Mediated Endocytosis Are Differentially Sorted into Distinct Populations of Early Endosomes. Cell. 2006;124:997–1009. doi: 10.1016/j.cell.2005.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Behr J-P. The Proton Sponge: a Trick to Enter Cells the Viruses Did Not Exploit. CHIMIA International Journal for Chemistry. 1997;51:34–36. [Google Scholar]
- 21.Sonawane ND, Szoka FC, Jr., Verkman AS. Chloride accumulation and swelling in endosomes enhances DNA transfer by polyamine-DNA polyplexes. J Biol Chem. 2003;278:44826–44831. doi: 10.1074/jbc.M308643200. [DOI] [PubMed] [Google Scholar]
- 22.Haensler J, Szoka FC., Jr. Polyamidoamine cascade polymers mediate efficient transfection of cells in culture. Bioconjug Chem. 1993;4:372–379. doi: 10.1021/bc00023a012. [DOI] [PubMed] [Google Scholar]
- 23.Tang MX, Redemann CT, Szoka FC., Jr. In vitro gene delivery by degraded polyamidoamine dendrimers. Bioconjug Chem. 1996;7:703–714. doi: 10.1021/bc9600630. [DOI] [PubMed] [Google Scholar]
- 24.Xu Y, Szoka FC., Jr. Mechanism of DNA release from cationic liposome/DNA complexes used in cell transfection. Biochemistry. 1996;35:5616–5623. doi: 10.1021/bi9602019. [DOI] [PubMed] [Google Scholar]
- 25.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8:1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 26.Zelphati O, Szoka FC., Jr. Mechanism of oligonucleotide release from cationic liposomes. Proc Natl Acad Sci U S A. 1996;93:11493–11498. doi: 10.1073/pnas.93.21.11493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cullis PR, Hope MJ, Tilcock CP. Lipid polymorphism and the roles of lipids in membranes. Chem Phys Lipids. 1986;40:127–144. doi: 10.1016/0009-3084(86)90067-8. [DOI] [PubMed] [Google Scholar]
- 28.Dinh AT, Pangarkar C, Theofanous T, Mitragotri S. Understanding intracellular transport processes pertinent to synthetic gene delivery via stochastic simulations and sensitivity analyses. Biophys J. 2007;92:831–846. doi: 10.1529/biophysj.106.095521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lukacs GL, Haggie P, Seksek O, Lechardeur D, Freedman N, Verkman AS. Size-dependent DNA mobility in cytoplasm and nucleus. J Biol Chem. 2000;275:1625–1629. doi: 10.1074/jbc.275.3.1625. [DOI] [PubMed] [Google Scholar]
- 30.Welte MA. Bidirectional transport along microtubules. Curr Biol. 2004;14:R525–537. doi: 10.1016/j.cub.2004.06.045. [DOI] [PubMed] [Google Scholar]
- 31.Lechardeur D, Sohn KJ, Haardt M, Joshi PB, Monck M, Graham RW, et al. Metabolic instability of plasmid DNA in the cytosol: a potential barrier to gene transfer. Gene Ther. 1999;6:482–497. doi: 10.1038/sj.gt.3300867. [DOI] [PubMed] [Google Scholar]
- 32.Matteoni R, Kreis TE. Translocation and clustering of endosomes and lysosomes depends on microtubules. J Cell Biol. 1987;105:1253–1265. doi: 10.1083/jcb.105.3.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miyazawa N, Crystal RG, Leopold PL. Adenovirus serotype 7 retention in a late endosomal compartment prior to cytosol escape is modulated by fiber protein. J Virol. 2001;75:1387–1400. doi: 10.1128/JVI.75.3.1387-1400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carlisle RC, Bettinger T, Ogris M, Hale S, Mautner V, Seymour LW. Adenovirus hexon protein enhances nuclear delivery and increases transgene expression of polyethylenimine/plasmid DNA vectors. Mol Ther. 2001;4:473–483. doi: 10.1006/mthe.2001.0472. [DOI] [PubMed] [Google Scholar]
- 35.Suomalainen M, Nakano MY, Boucke K, Keller S, Greber UF. Adenovirus-activated PKA and p38/MAPK pathways boost microtubule-mediated nuclear targeting of virus. EMBO J. 2001;20:1310–1319. doi: 10.1093/emboj/20.6.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babcock HP, Chen C, Zhuang X. Using single-particle tracking to study nuclear trafficking of viral genes. Biophys J. 2004;87:2749–2758. doi: 10.1529/biophysj.104.042234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lai SK, Hida K, Man ST, Chen C, Machamer C, Schroer TA, et al. Privileged delivery of polymer nanoparticles to the perinuclear region of live cells via a non clathrin, non-degradative pathway. Biomaterials. 2007;28:2876–2884. doi: 10.1016/j.biomaterials.2007.02.021. [DOI] [PubMed] [Google Scholar]
- 38.Lam AP, Dean DA. Progress and prospects: nuclear import of nonviral vectors. Gene Ther. 2010;17:439–447. doi: 10.1038/gt.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cohen RN, van der Aa MA, Macaraeg N, Lee AP, Szoka FC., Jr. Quantification of plasmid DNA copies in the nucleus after lipoplex and polyplex transfection. J Control Release. 2009;135:166–174. doi: 10.1016/j.jconrel.2008.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hama S, Akita H, Iida S, Mizuguchi H, Harashima H. Quantitative and mechanism-based investigation of post-nuclear delivery events between adenovirus and lipoplex. Nucleic Acids Res. 2007;35:1533–1543. doi: 10.1093/nar/gkl1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ng CP, Pun SH. A perfusable 3D cell-matrix tissue culture chamber for in situ evaluation of nanoparticle vehicle penetration and transport. Biotechnol Bioeng. 2008;99:1490–1501. doi: 10.1002/bit.21698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang H, Lee MY, Hogg MG, Dordick JS, Sharfstein ST. Gene delivery in three-dimensional cell cultures by superparamagnetic nanoparticles. ACS Nano. 2010;4:4733–4743. doi: 10.1021/nn9018812. [DOI] [PubMed] [Google Scholar]
- 43.Pei Y, Hancock PJ, Zhang H, Bartz R, Cherrin C, Innocent N, et al. Quantitative evaluation of siRNA delivery in vivo. RNA. 2010;16:2553–2563. doi: 10.1261/rna.2255810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei J, Jones J, Kang J, Card A, Krimm M, Hancock P, et al. RNA-Induced Silencing Complex-Bound Small Interfering RNA Is a Determinant of RNA Interference-Mediated Gene Silencing in Mice. Molecular Pharmacology. 2011;79:953–963. doi: 10.1124/mol.110.070409. [DOI] [PubMed] [Google Scholar]
- 45.Liu J, Carmell MA, Rivas FV, Marsden CG, Thomson JM, Song JJ, et al. Argonaute2 is the catalytic engine of mammalian RNAi. Science. 2004;305:1437–1441. doi: 10.1126/science.1102513. [DOI] [PubMed] [Google Scholar]
- 46.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat Cell Biol. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 47.Lee YS, Pressman S, Andress AP, Kim K, White JL, Cassidy JJ, et al. Silencing by small RNAs is linked to endosomal trafficking. Nat Cell Biol. 2009;11:1150–1156. doi: 10.1038/ncb1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sen GL, Blau HM. Argonaute 2/RISC resides in sites of mammalian mRNA decay known as cytoplasmic bodies. Nat Cell Biol. 2005;7:633–636. doi: 10.1038/ncb1265. [DOI] [PubMed] [Google Scholar]
- 49.Gibbings D. Continuous density gradients to study Argonaute and GW182 complexes associated with the endocytic pathway. Methods Mol Biol. 2011;725:63–76. doi: 10.1007/978-1-61779-046-1_5. [DOI] [PubMed] [Google Scholar]
- 50.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 51.Lakhal S, Wood MJA. Exosome nanotechnology: An emerging paradigm shift in drug delivery. BioEssays. 2011 doi: 10.1002/bies.201100076. n/a-n/a. [DOI] [PubMed] [Google Scholar]
- 52.Lachenal G, Pernet-Gallay K, Chivet M, Hemming FJ, Belly A, Bodon G, et al. Release of exosomes from differentiated neurons and its regulation by synaptic glutamatergic activity. Mol Cell Neurosci. 2011;46:409–418. doi: 10.1016/j.mcn.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Couzin J. Cell biology: The ins and outs of exosomes. Science. 2005;308:1862–1863. doi: 10.1126/science.308.5730.1862. [DOI] [PubMed] [Google Scholar]
- 54.Meckes DG, Jr., Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proc Natl Acad Sci U S A. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schorey JS, Bhatnagar S. Exosome function: from tumor immunology to pathogen biology. Traffic. 2008;9:871–881. doi: 10.1111/j.1600-0854.2008.00734.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. http://www.ashp.org/DrugShortages/Current/Bulletin.aspx?id=806.