In vivo brain imaging with positron emission tomography (PET) has helped to quantify and localize functional brain deficits in Alzheimer's disease (AD), and to understand disease mechanisms, in relation to dementia severity and cognitive profile. In their PNAS article, Small et al. (1) now conclude that such imaging done twice within a 2-year period can identify pathologically affected but not demented subjects “at risk” for AD, who have memory complaints and an apolipoprotein E4 (APOE-4) allele. Although Small et al. (1) speculate that serial imaging “will assist in response monitoring during experimental treatments,” its potential for success remains to be demonstrated.
Their paper is a sequel to several cross-sectional PET studies showing significant brain metabolic abnormalities in genetically at-risk subjects. In their earlier report, APOE-4 carriers with memory complaints and a positive family history for dementia, compared with controls, had a lower regional cerebral metabolic rate for glucose, rCMRglc, in the parietal lobes (2). In another study, asymptomatic subjects with a chromosome-14 linkage, an APP mutation, or other evidence of “familial” AD demonstrated reduced mean global and parietotemporal rCMRglc, but with considerable overlap with individual control data (3). In a third, cognitively normal homozygous APOE-4 carriers evidenced an AD-like pattern on cortical projection maps of rCMRglc (4).
Small et al. (1) do not indicate whether their APOE-4 subjects were hypertensive, despite evidence that well treated hypertension can engender brain metabolic abnormalities, that hypertension and APOE-4 are risk factors for vascular dementia, and that vascular dementia itself can produce an AD-like brain metabolic pattern (5–7). They normalized regional brain radioactivity to global brain radioactivity, when using a region-of-interest analysis or a voxel-based smoothing procedure called statistical parametric mapping, but in neither case did they correct for the brain atrophy that can occur in at-risk subjects, at least in limbic regions (8–10).
Small et al. (1) demonstrated significant initial metabolic group differences between their APOE-4 carriers and noncarriers, as expected (see above). From 2-year follow-up scans of 10 APOE-4 carriers and 10 noncarriers, they found a significant metabolic decline by region-of-interest analysis in the left posterior cingulate cortex of the carriers. Statistical parametric mapping gave a 5% rate decline in inferior parietal and lateral temporal cortices in both groups, but statistically significant only in the carriers after a multiple comparison correction. Noncarriers had a significant rate of decline in frontal cortex, which they attributed somewhat arbitrarily to “normal aging,” although frontal lobe metabolic deficits and neuropathology are not uncommon in AD (11, 12). In no case was between-group significance estimated.
Taken together, however, prior PET cross-sectional studies and the longitudinal study by Small et al. (1) indicate that statistically significant mean rCMRglc deficits are found in at-risk subjects, and thus that rCMRglc is more sensitive to the AD process than are cognitive tests. Additional evidence for greater sensitivity comes from reports that right-left hemispheric metabolic asymmetries, if present in mildly demented AD subjects with only a memory deficit, predict appropriate discrepancies in visuospatial compared with language deficits that appear 1–3 years later. Consistent with principles of functional neuroanatomy, right lower than left metabolism predicts worse visuospatial than language scores, and vice versa (13). Similarly, early frontal-parietal metabolic gradients predict later failure of abstract reasoning compared with complex attention (14).
Clearly, longitudinal PET assessment of brain metabolism is a better “biomarker” of AD diagnosis and progression than cross-sectional assessment. The same is true for longitudinal compared with cross-sectional assessment of brain atrophy by computer assisted tomography or magnetic resonance imaging (MRI) (9, 10, 15). These longitudinal biomarkers, when subjected to power analyses to estimate sample size, can assess drug effects more reliably than cognitive or behavioral measures of “symptomatic” relief (10, 16).
Because the population frequency of APOE-4 is about 0.16 (17) and mutations account for only 5% of AD cases (18), longitudinal PET scans in all subjects with only a memory complaint, including noncarriers and carriers, would require very large numbers to demonstrate drug efficacy, in view of the small and overlapping rate changes noted by Small et al. (1). Fewer subjects would be required if efficacy were evaluated in only genetically vulnerable at-risk subjects with a memory complaint, or once an AD diagnosis were firmly established (13, 14). Additionally, a more informed estimate of disease in any one individual with a memory complaint might be provided by applying a discriminant analysis with multiple regression to longitudinal PET data. This statistical procedure constructs a linear combination of observed variables to best describe group differences and to classify group membership of any individual (19). It affords a probabilistic statement regarding the likelihood of a single scan or other data set being similar to data sets from controls compared with diagnosed AD patients.
For example, a discriminant function, derived from values of rCMRglc in diagnosed AD patients and controls, classified subjects with 87% accuracy. This function then identified as “pathological” an apparently normal PET scan from an at-risk subject; 1 year later, a second scan and the appearance of dementia confirmed the AD diagnosis (20). The same function correctly classified 10 older demented Down syndrome subjects as having AD, as well as 2 of 4 older nondemented subjects known to be at risk for AD (21, 22). The potential of discriminant functions can be enhanced by considering genetic, anatomic, or cerebrospinal fluid measurements with PET measurements (23, 24).
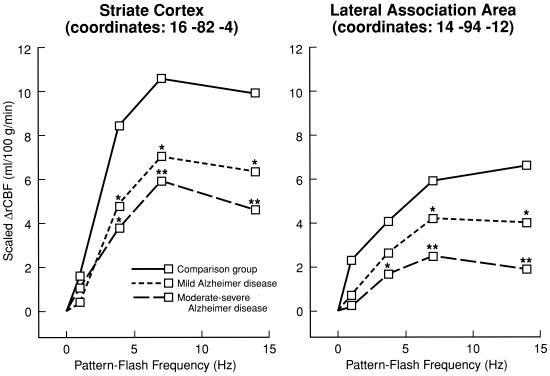
Cross-sectional activation studies to “stress” the brain have been performed with early diagnosis and disease progression in mind, when the stimulus “parameter” was varied. This approach, if used in a longitudinal design, may quantify the progression of synaptic changes in AD and enhance our ability to estimate drug efficacy (12, 25). In one “parametric” study, goggles were used to administer alternating patterned light flashes at frequencies of 0–14 Hz, and PET and H215O were used to quantify rCBF responses at each frequency (26, 27). Widespread cortical areas were activated in control subjects, including the middle temporal gyrus (V5/MT) at 1 Hz when pattern motion was apparent, and primary (striate) and association visual cortices at all stimulation frequencies (Fig. 1). Activation was reduced in mildly demented AD patients and to a greater extent in moderately-severely demented patients, particularly at the higher frequencies, and MT was not activated at 1 Hz in either dementia group. These data suggested that more vulnerable, higher frequency-responding synapses within the magnocellular visual system are affected earlier in AD than are lower frequency-responding synapses in the parvocellular visual system (28).
Figure 1.
rCBF responses in primary (striate) and association visual cortical areas, measured with PET and H215O during patterned flash stimulation at different frequencies, in control subjects and in Alzheimer's disease patients with mild dementia or moderate-severe dementia. Scaled increment in blood flow, ΔrCBF, corrects for global flow difference between groups. *, P < 0.05; **, P < 0.01, differs significantly from control mean. Coordinates (in millimeters) are in stereotactic (Talairach) space. Passive “parametric” stimulus paradigms such as this might be used to examine synaptic integrity in at-risk subjects, as well as drug effects on synaptic transmission in relation to dementia severity (see text). Modified from ref. 27. (The American Journal of Psychiatry 155, 785–794, 1998. Copyright 1998, the American Psychiatric Association. Reprinted by permission.)
Cognitive tasks performed by at-risk or mildly demented AD patients can activate more widespread brain areas than in controls, suggesting compensatory neural recruitment with increased effort (29–31), although less extensive activation also may occur (32). Exemplifying the former case, during the recall of eight-word lists, prefrontal cortical activation was more widespread in mildly demented patients than in controls (30). Greater prefrontal activation, in elderly compared with young healthy subjects performing a memory task, is correlated with a prolonged reaction time. Both frontal activation and reaction time are reduced proportionately in response to physostigmine, suggesting their dependence on cholinergic integrity (33).
In the future, methods need to be developed to image brain signal transduction “beyond the receptor,” which postmortem evidence suggests is abnormal in AD (34). For example, arachidonic acid, an important second messenger, is released from brain phospholipids via G-protein activation of phospholipase A2, after transmitter occupancy of muscarinic M1 and M3, dopaminergic D2, or serotoninergic 5HT2 receptors (35). Brain uptake of intravenously injected labeled arachidonate from plasma reflects local phospholipase A2 activation and might be measured in at-risk subjects by using [11C]arachidonic acid and PET (36). Uptake is stimulated in response to an M1 muscarinic agonist into ipsilateral cortex of rats with a chronic unilateral lesion of the nucleus basalis, despite fewer cortical M1 receptors in this animal model of cholinergic loss in AD (37–40). As another example, receptor-initiated phospholipase C-mediated signaling might be studied, using magnetic resonance spectroscopy to measure the brain concentration of myo-inositol, a component of the phosphoinositide cycle (35). Brain myo-inositol is elevated in diagnosed AD and in older nondemented Down syndrome subjects at risk for AD (21, 41, 42); it is reduced by lithium, an inhibitor of inositol monophosphatase (43).
In summary, additional clinical data, together with discriminant, power, and other statistical procedures, will be necessary to estimate the extent to which longitudinal PET or functional MRI studies, at rest or during activation, can identify early-affected AD patients and be used to estimate drug efficacy. Novel in vivo methods addressing signal transduction also should be developed for these purposes.
Footnotes
See companion article at www.pnas.org/cgi/doi/10.1073/pnas.090106797
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.120178897.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.120178897
References
- 1.Small, G. W., Ercoli, L. M., Silverman, D. H. S., Huang, S.-C., Komo, S., Bookheimer, S. Y., Lavretsky, H., Miller, K., Siddarth, P., Rasgon, N. L., et al. (May 16, 2000) Proc. Natl. Acad. Sci. USA, 10.1073/pnas.090106797. http://www.pnas.org/cgi/doi/10.1073/pnas.090106797 [DOI] [PMC free article] [PubMed]
- 2.Small G W, Mazziotta J C, Collins M T, Baxter L R, Phelps M E, Mandelkern M A, Kaplan A, La Rue A, Adamson C F, Chang L, et al. J Am Med Assoc. 1995;273:942–947. [PubMed] [Google Scholar]
- 3.Kennedy A M, Frackowiak R S J, Newman S K, Bloomfield P M, Seaward J, Roques P, Lewington G, Cunningham V J, Rossor M N. Neurosci Lett. 1995;186:17–20. doi: 10.1016/0304-3940(95)11270-7. [DOI] [PubMed] [Google Scholar]
- 4.Reiman E M, Caselli R J, Yun L S, Chen K, Bandy D, Minoshima S, Thibodeau S N, Osborne D. N Engl J Med. 1996;334:752–758. doi: 10.1056/NEJM199603213341202. [DOI] [PubMed] [Google Scholar]
- 5.Duara R, Barker W, Loewenstein D, Pascal S, Bowen B. Eur Neurol. 1989;29, Suppl. 3:9–15. doi: 10.1159/000116474. [DOI] [PubMed] [Google Scholar]
- 6.Frisoni G B, Calabresi L, Geroldi C, Bianchetti A, D'Acquarica A L, Govoni S, Sirtori C R, Trabucchi M, Franceschini G. Dementia. 1994;5:240–242. doi: 10.1159/000106730. [DOI] [PubMed] [Google Scholar]
- 7.Salerno J A, Mentis M J, Gonzalez-Aviles A, Grady C, Wagner E, Schapiro M B, Rapoport S I. J Gerontol. 1995;50:M147–M154. doi: 10.1093/gerona/50a.3.m147. [DOI] [PubMed] [Google Scholar]
- 8.Ibáñez V, Pietrini P, Alexander G E, Furey M L, Teichberg D, Rajapakse J C, Rapoport S I, Schapiro M B, Horwitz B. Neurology. 1998;50:1585–1593. doi: 10.1212/wnl.50.6.1585. [DOI] [PubMed] [Google Scholar]
- 9.Jack Jr C R, Petersen R C, Xu Y C, O'Brien P C, Smith G E, Ivnik R J, Boeve B F, Waring S C, Tangalos E G, Kokmen E. Neurology. 1999;52:1397–1403. doi: 10.1212/wnl.52.7.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox N C, Cousens S, Scahill R, Harvey R J, Rossor M N. Arch Neurol. 2000;57:339–344. doi: 10.1001/archneur.57.3.339. [DOI] [PubMed] [Google Scholar]
- 11.Grady C L, Haxby J V, Schapiro M B, Gonzalez-Aviles A, Kumar A, Ball M J, Heston L, Rapoport S I. J Neuropsychiatry Clin Neurosci. 1990;2:373–384. doi: 10.1176/jnp.2.4.373. [DOI] [PubMed] [Google Scholar]
- 12.DeKosky S T, Scheff S W. Ann Neurol. 1990;27:457–464. doi: 10.1002/ana.410270502. [DOI] [PubMed] [Google Scholar]
- 13.Haxby J V, Grady C L, Koss E, Horwitz B, Heston L, Schapiro M, Friedland R P, Rapoport S I. Arch Neurol. 1990;47:753–760. doi: 10.1001/archneur.1990.00530070043010. [DOI] [PubMed] [Google Scholar]
- 14.Haxby J V, Grady C L, Koss E, Horwitz B, Schapiro M, Friedland R P, Rapoport S I. Neurology. 1988;38:1853–1863. doi: 10.1212/wnl.38.12.1853. [DOI] [PubMed] [Google Scholar]
- 15.Luxenberg J S, Haxby J V, Creasey H, Sundaram M, Rapoport S I. Neurology. 1987;37:1135–1140. doi: 10.1212/wnl.37.7.1135. [DOI] [PubMed] [Google Scholar]
- 16.Leber P. Alzheimer Dis Assoc Disord. 1997;11, Suppl. 5:S10–S21. [PubMed] [Google Scholar]
- 17.Strittmatter W J, Saunders A M, Schmechel D, Pericak-Vance M, E nghild J, Salvesen G S, Roses A D. Proc Natl Acad Sci USA. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cruts M, Van Broeckhoven C. Ann Med. 1998;30:560–565. doi: 10.3109/07853899809002605. [DOI] [PubMed] [Google Scholar]
- 19.Clark C M, Ammann W, Martin W R, Ty P, Hayden M R. J Cereb Blood Flow Metab. 1991;11:A96–A102. doi: 10.1038/jcbfm.1991.44. [DOI] [PubMed] [Google Scholar]
- 20.Pietrini P, Azari N P, Grady C L, Salerno J A, Gonzales-Aviles A, Heston L L, Pettigrew K D, Horwitz B, Haxby J V, Schapiro M B. Dementia. 1993;4:94–101. doi: 10.1159/000107349. [DOI] [PubMed] [Google Scholar]
- 21.Mann D M, Yates P O, Marcyniuk B. Neuropathol Appl Neurobiol. 1984;10:185–207. doi: 10.1111/j.1365-2990.1984.tb00351.x. [DOI] [PubMed] [Google Scholar]
- 22.Azari N P, Pettigrew K D, Pietrini P, Horwitz B, Schapiro M B. Dementia. 1994;5:69–78. doi: 10.1159/000106700. [DOI] [PubMed] [Google Scholar]
- 23.DeCarli C, Murphy D G M, McIntosh A R, Teichberg D, Schapiro M B, Horwitz B. Psychiatry Res. 1995;57:119–130. doi: 10.1016/0165-1781(95)02651-c. [DOI] [PubMed] [Google Scholar]
- 24.Hampel H, Teipel S J, Padberg F, Haslinger A, Riemenschneider M, Schwarz M J, Kotter H U, Scheloske M, Buch K, Stubner S, et al. Brain Res. 1999;823:104–112. doi: 10.1016/s0006-8993(99)01146-4. [DOI] [PubMed] [Google Scholar]
- 25.Rapoport S I, Grady C L. Int J Neurosci. 1993;70:39–56. doi: 10.3109/00207459309000559. [DOI] [PubMed] [Google Scholar]
- 26.Mentis M J, Horwitz B, Grady C L, Alexander G E, VanMeter J W, Maisog J M, Pietrini P, Schapiro M B, Rapoport S I. Am J Psychiatry. 1996;153:32–40. doi: 10.1176/ajp.153.1.32. [DOI] [PubMed] [Google Scholar]
- 27.Mentis M J, Alexander G E, Krasuski J, Pietrini P, Furey M L, Schapiro M B, Rapoport S I. Am J Psychiatry. 1998;155:785–794. doi: 10.1176/ajp.155.6.785. [DOI] [PubMed] [Google Scholar]
- 28.Livingstone M, Hubel D. Science. 1988;240:740–749. doi: 10.1126/science.3283936. [DOI] [PubMed] [Google Scholar]
- 29.Grady C L, Haxby J V, Horwitz B, Gillette J, Salerno J A, Gonzalez-Aviles A, Carson R E, Herscovitch P, Schapiro M B, Rapoport S I. Neurobiol Aging. 1993;14:35–44. doi: 10.1016/0197-4580(93)90018-7. [DOI] [PubMed] [Google Scholar]
- 30.Becker J T, Mintun M A, Aleva K, Wiseman M B, Nichols T, DeKosky S T. Neurology. 1996;46:692–700. doi: 10.1212/wnl.46.3.692. [DOI] [PubMed] [Google Scholar]
- 31.Woodard J L, Grafton S T, Votaw J R, Green R C, Dobraski M E, Hoffman J M. Neuropsychology. 1998;12:491–504. doi: 10.1037//0894-4105.12.4.491. [DOI] [PubMed] [Google Scholar]
- 32.Smith C D, Andersen A H, Kryscio R J, Schmitt F A, Kindy M S, Blonder L X, Avison M J. Neurology. 1999;53:1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- 33.Furey M L, Pietrini P, Haxby J V, Alexander E, Lee H C, Van Meter J, Grady C L, Shetty U, Rapoport S I, Schapiro M B, Freo U. Proc Natl Acad Sci USA. 1997;94:6512–6516. doi: 10.1073/pnas.94.12.6512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferrari-DiLeo G, Mash D C, Flynn D D. Mol Chem Neuropathol. 1995;24:69–91. doi: 10.1007/BF03160113. [DOI] [PubMed] [Google Scholar]
- 35.Cooper J R, Bloom F E, Roth R H. The Biochemical Basis of Neuropharmacology. Oxford: Oxford Univ. Press; 1996. [Google Scholar]
- 36.Rapoport S I, Chang M C, Connolly K, Kessler D, Bokde A, Carson R E, Herscovitch P, Channing M, Eckelman W C. J Neurochem. 2000;74:S21. [Google Scholar]
- 37.Whitehouse P J, Price D L, Struble R G, Clark A W, Coyle J T, DeLong M R. Science. 1982;215:1237–1239. [Google Scholar]
- 38.Nariai T, DeGeorge J J, Lamour Y, Rapoport S I. Brain Res. 1991;559:1–9. doi: 10.1016/0006-8993(91)90279-5. [DOI] [PubMed] [Google Scholar]
- 39.Bogdanovic N, Islam A, Nilsson L, Bergstrom L, Winblad B, Adem Å. Exp Brain Res. 1993;97:225–232. doi: 10.1007/BF00228691. [DOI] [PubMed] [Google Scholar]
- 40.Rapoport S I, Purdon D, Shetty H U, Grange E, Smith Q, Jones C, Chang M C J. Ann NY Acad Sci. 1997;820:56–74. doi: 10.1111/j.1749-6632.1997.tb46189.x. [DOI] [PubMed] [Google Scholar]
- 41.Miller B L, Moats R A, Shonk T, Ernst T, Woolley S, Ross B D. Radiology. 1993;187:433–437. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- 42.Huang W, Alexander G E, Daly E M, Shetty H U, Krasuski J S, Rapoport S I, Schapiro M B. Am J Psychiatry. 1999;156:1879–1886. doi: 10.1176/ajp.156.12.1879. [DOI] [PubMed] [Google Scholar]
- 43.Moore G J, Bebchuk J M, Parrish J K, Faulk M W, Arfken C L, Strahl-Bevacqua J, Manji H K. Am J Psychiatry. 1999;156:1902–1908. doi: 10.1176/ajp.156.12.1902. [DOI] [PubMed] [Google Scholar]