Abstract
β-tubulin, calmodulin, internal transcribed spacer and partial lsu-rDNA, RNA polymerase 2, DNA replication licensing factor Mcm7, and pre-rRNA processing protein Tsr1 were amplified and sequenced from numerous isolates belonging to Aspergillus sect. versicolor. The isolates were analyzed phylogenetically using the concordance model to establish species boundaries. Aspergillus austroafricanus, A. creber, A. cvjetkovicii, A. fructus, A. jensenii, A. puulaauensis, A. subversicolor, A. tennesseensis and A. venenatus are described as new species and A. amoenus, A. protuberus, A. sydowii, A. tabacinus and A. versicolor are accepted as distinct species on the basis of molecular and phenotypic differences. PCR primer pairs used to detect A. versicolor in sick building syndrome studies have a positive reaction for all of the newly described species except A. subversicolor.
Keywords: Aspergillus amoenus, Aspergillus austroafricanus, Aspergillus creber, Aspergillus cvjetkovicii, Aspergillus fructus, Aspergillus jensenii, Aspergillus protuberus, Aspergillus puulaauensis, Aspergillus subversicolor, Aspergillus sydowii, Aspergillus tabacinus, Aspergillus tennesseensis, Aspergillus venenatus, Aspergillus versicolor, concordance analysis, phylogeny, systematics
INTRODUCTION
Aspergillus section Versicolores was originally erected as the Aspergillus versicolor group by Thom & Church (1926) and was subsequently revised by Thom & Raper (1945) to contain four species. Raper & Fennell (1965) revised the genus Aspergillus and accepted 18 species in the A. versicolor group. Gams et al. (1985) formalized the sectional taxonomy of Raper & Fennell’s (1965) groups. Using scanning electron microscopy (SEM), Kozakiewicz (1989) examined conidial surface ornamentation of most species of the section and removed seven species from section Versicolores. Klich (1993) revised the section based on morphological and other characteristics and accepted the seven species previously removed by Kozakiewicz (1989) from section Versicolores. Peterson (2008) accepted four phylogenetically distinct species in the section based on multilocus DNA sequence analysis, placing the other 14 species in different clades of Aspergillus.
Aspergillus versicolor is the most widely reported and studied species in section Versicolores. It has been isolated from soil (Domsch et al. 1980), indoor environments (Samson et al. 2001, Shelton et al. 2002, Engelhart et al. 2002, Amend et al. 2010, Anderson et al. 2011), various foods and feeds (Pitt & Hocking 2009) and hypersaline water (Kis-Papo et al. 2003, Mbata 2008), and is associated with many health issues of humans and animals (Jussila 2003, Perri et al. 2005, Baddley et al. 2009, Edmondson et al. 2009, Pitt & Hocking 2009, Moreno & Arenas 2010). It is a producer of the mycotoxin sterigmatocystin that is a precursor of aflatoxin B1 (Mills & Abramson 1986, Tuomi et al. 2000, Nielsen 2003, Veršilovskis & Saeger 2010).
Environmental isolates of section Versicolores species exhibit great variation in macro-phenotypic ones but few differences in micro-phenotypic characters (Domsch et al. 1980, Klich 2002, Raper & Fennell 1965, Thom & Church 1926, Vesonder & Horn 1985), leading us to conduct a DNA-based phylogenetic study. to determine the limits of variation within species, we amplified and sequenced DNA from 6 loci and used concordance analysis to identify species boundaries (Dettman et al. 2003) within section Versicolores. The species described and accepted are monophyletic.
MATERIALS AND METHODS
Fungal isolates
The provenance of fungal isolates examined in this study is detailed in Table 1 and these cultures are available from the Agricultural Research Service Culture Collection (NRRL), Peoria, Illinois (http://nrrl.ncaur.usda.gov).
Table 1. Provenance of fungal isolates used.
| NRRL number | Provenance | |
|---|---|---|
| Aspergillus amoenus MycoBank MB250654 | ||
| 226 | USA: isol. ex mammary gland, 1913. | |
| 236 | Germany: Munster, isol. ex a Berberis sp. fruit, 1930, M. Roberg. | |
| 658 | UK: isol. ex brined meat, 1929, G. A. Ledingham. | |
| 4838 | Equivalent to NRRL 236, received from Centraalbureau voor Schimmelcultures, 1962, ex-type. | |
| 35600 | USA: Hawaii, Kapuka Pauula, isol. ex the basidiomata of Gandoderma australe, 2005, D.T. Wicklow. | |
| A-23228 | India: Karnataka, isol. ex coffee berry, 1978, B. Muthappa. | |
| Aspergillus asperescens Stolk MycoBank MB292835 | ||
| 4770 | Ex-type, out-group species. | |
| Aspergillus austroafricanus sp. nov., MycoBank MB800597 | ||
| 233 | South Africa: Capetown, unknown, 1922, sent by V. A. Putterill, ex-type. | |
| Aspergillus creber sp. nov., MycoBank MB800598 | ||
| 231 | South Africa: Capetown, unknown, 1922, sent by V. A. Putterill. | |
| 6544 | Atlantic Ocean: isol. ex a floating tar ball, 1979, A. Wellman. | |
| 25627 | Japan: Ibaraki, isol. ex tea field soil, 1996, T. Goto. | |
| 58583 | USA: Pennsylvania, isol. ex indoor air sampler, 2008, Z. Jurjevic. | |
| 58584 | USA: California, isol. ex indoor air sample, 2008, Z. Jurjevic. | |
| 58587 | USA: California, isol. ex indoor air sample, 2008, Z. Jurjevic. | |
| 58592 | USA: California, isol. ex indoor air sample, 2008, Z. Jurjevic, ex-type. | |
| 58597 | USA: New Jersey, isol. ex indoor air sample, 2008, Z. Jurjevic. | |
| 58601 | USA: New Jersey: isolated from indoor air sample, 2009, Z. Jurjevic. | |
| 58606 | USA: Pennsylvania, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58607 | USA: Pennsylvania, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58612 | USA: New Jersey, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58670 | USA: New Jersey, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58672 | USA: Georgia, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58673 | USA: Georgia, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58675 | USA: Ohio, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| Aspergillus cvjetkovicii sp. nov., MycoBank MB800599 | ||
| 227 | USA: New Jersey, isol. ex soil, 1915, G.W. Wilson, ex-type. | |
| 230 | China: isol. ex soy sauce, 1917, Round. | |
| 4642 | Unknown: sent to NRRL, 1969, D. I. Fennell as WB4642. | |
| 58593 | USA: California, isol. ex indoor air sample, 2008, Z. Jurjevic. | |
| Aspergillus fructus sp. nov., MycoBank MB800600 | ||
| 239 | USA: California, isol. ex date fruit, 1939, Bliss, ex-type. | |
| 241 | Unknown: isol. ex pomegranate fruit, 1916, L. McCulloch. | |
| Aspergillus jensenii sp. nov., MycoBank MB800601 | ||
| 225 | UK: unknown, 1913, sent to C. Thom by Dade. | |
| 235 | UK: London, isol. ex paraffin, 1930, H. Raistrick. | |
| 240 | USA: New York, Ithaca, isol. ex the rhizosphere of pepper plants, 1911, C. N. Jensen, sent to C. Thom by Whetzel as type strain of A. globosus. | |
| 58582 | USA: Montana, isol. ex indoor air sample, 2008, Z. Jurjevic. | |
| 58600 | USA: Montana, isol. ex indoor air sample, 2008, Z. Jurjevic, ex-type. | |
| 58671 | USA: Pennsylvania, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58674 | USA: Ohio, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| Aspergillus multicolor Sappa MycoBank MB292849 | ||
| 4775 | Ex-type, out-group species. | |
| Aspergillus protuberus MycoBank MB326650 | ||
| 661 | UK: isol. ex brined meat, 1929, G. A. Ledingham. | |
| 3505 | Yugoslavia, isol. ex rubber coated electrical cables, ca 1968, ex-type. | |
| 58613 | USA: New Jersey, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58747 | USA: New Jersey, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58748 | USA: New Jersey, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58990 | USA: Connecticut, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 58991 | USA: Connecticut, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| Aspergillus puulaauensis sp. nov., MycoBank MB800602 | ||
| 35641 | USA: Hawaii, Pu’u la’au Highway 200, isol. ex dead hardwood branch, 2003, D. T. Wicklow, ex-type. | |
| 58602 | USA: West Virginia, isol. ex indoor air sample, 2009, Z. Jurjevic. | |
| 62124 | USA: Hawaii, mesic mountain forest, isol. ex basidiomata of Inonotus sp., 2003, D. T. Wicklow. | |
| 62516 | Canada: Alberta, isol. ex air sample in bee house, ca 1990, S. P. Abbot, equivalent to UAMH 7651. | |
| Aspergillus subversicolor sp. nov., MycoBank MB800603 | ||
| 58999 | India: Karnataka, isol. ex coffee berry, 1970, B. Muthappa, ex-type. | |
| Aspergillus sydowii MycoBank MB279636 | ||
| 250 | Unknown: prior to 1930, sent to C. Thom by M. Swift. | |
| 254 | USA: Georgia, Waycross, clinical isolate, 1940, M. M. Harris. | |
| 4768 | USA: California, isol. ex soil, 1969. | |
| 62450 | Thailand: isol. ex dead plant stem, 1977, E. G. Simmons. | |
| Aspergillus tabacinus MycoBank MB539544 | ||
| 659 | UK: isol. ex brined meat, 1929, G. A. Ledingham. | |
| 4791 | Unknown: isol. ex tobacco, 1934, Y. Nakazawa, ex-type. | |
| 5031 | Unknown: type isolate of A. versicolor var. magnus Sasaki, received from IFO, 1962. | |
| 62481 | Nepal: Kathmandu, isol. ex maize, 1977. | |
| Aspergillus tennesseensis sp. nov., MycoBank MB800604 | ||
| 229 | Unknown: sent to C. Thom, 1917, by R. Thaxter. | |
| 234 | USA: Maryland, Beltsville, isol. ex chestnut seed, 1927, C. Thom. | |
| 13150 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn, ex-type. | |
| 13152 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn. | |
| Aspergillus venenatus sp. nov., MycoBank MB800605 | ||
| 13147 | USA: Tennessee: isolated from toxic dairy feed, 1984, B. W. Horn, ex-type. | |
| 13148 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn. | |
| 13149 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn. | |
| 62457 | USA: Missouri, isol. ex corn, 1989, D. T. Wicklow. | |
| Aspergillus versicolor MycoBank MB172159 | ||
| 238 | USA: isol. ex unrecorded substrate, 1935, V. K. Charles, ex-type. | |
| 5219 | South Africa: Pretoria, received 1970, from J. P. van der Walt. | |
| 13144 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn. | |
| 13145 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn. | |
| 13146 | USA: Tennessee, isol. ex toxic dairy feed, 1984, B. W. Horn. | |
| Aspergillus species, undescribed | ||
| 530 | East Indies: isol. ex natural rubber, 1938, Shumard. | |
| 13151 | USA: Tennessee: isol. ex toxic dairy feed, 1984, B. W. Horn. | |
Culture methods
Cultures were grown on Czapek yeast extract agar (CYA) at 5 °C, 25 °C, and 37 °C and on malt extract agar (MEA), CY20S, M40Y and M60Y, all at 25 °C for 10 d in darkness (Pitt 1980, Klich 2002). M40Y contained 2 % malt extract, 0.5 % yeast extract and 40 % sucrose; M60Y contained 2 % malt extract, 0.5 % yeast extract and 60 % sucrose. Colony diameters and appearance were recorded and photographs were made from 10-d culture plates incubated at 25 °C. Color names are from Ridgway (1912) and are referred to with plate number, e.g. R45.
Microscopy
Microscopic examination was performed by teasing apart a small amount of mycelium in a drop of 0.1 % Triton X-100 and examining the preparation under bright field or DIF illumination. Additional microscopic samples were made by gently pressing a ca 20 × 5 mm piece of transparent tape onto a colony, rinsing the tape with one or two drops of 70 % ethanol and mounting the tape in lactic acid with fuchsin dye. A Leica DM 2500 microscope with bright field, phase contrast and DIF contrast optics was used to view the slides. The Spot camera with spot imaging software was mounted on the microscope and used for photomicrography. A Nikon digital SLR camera with D70 lens was used for colony photography. Photographs were resized and fitted into plates with Microsoft PowerPoint 2003 or Adobe Photoshop.
DNA methods
Conidia from agar slant cultures were used to inoculate 125-mL Erlenmeyer flasks containing 25 mL of malt extract broth. Cultures were grown on a rotary platform (200 rpm) for 2–3 d at 25 °C. Biomass was collected by vacuum filtration, and then frozen and freeze-dried in microfuge tubes. Dry mycelium was ground to a powder, rehydrated with CTAB buffer and extracted with chloroform; the phases were separated by centrifugation and DNA was precipitated from the aqueous phase with an equal volume of isopropanol. Total nucleic acids were collected by centrifugation, the pellet was rinsed with 70 % ethanol, and the nucleic acids were dissolved in 100 μL sterile deionized water.
DNA was diluted ca 1:100 with sterile deionized water for use in amplifications. β-tubulin (BT2), calmodulin (CF), ITS and partial lsu-rDNA (ID), RNA polymerase 2 (RPB2), DNA replication licensing factor (Mcm7), and pre-rRNA processing protein (Tsr1) were amplified with primers used by Peterson et al. (2010). Standard buffer and conditions were used with a thermal profile of 95 °C for 2 min followed by 35 cycles of 96 °C for 30 sec; 51 °C for 60 sec; 72 °C for 60 sec; and a final extension phase of 72 °C for 5 min. Occasionally, multiple amplification bands were obtained and a higher annealing temperature was used to obtain single amplification bands. DNA sequencing was performed on both template strands using dye terminator technology (v3.1) and an ABI 3730 sequencer, both from Applied Biosystems (http://www.appliedbiosystems.com/). Raw sequences (bi-directional) were corrected using Sequencher (http://www.genecodes.com/). Corrected sequences were aligned for phylogenetic analysis using CLUSTALW (Thompson et al. 1994). Sequences were deposited in GenBank as accessions JN853798–JN854131, EF652176, EF652178, EF652185–EF652187, EF652196, EF652203, EF652209–EF652211, EF652214–EF652216, EF652226, EF652264, EF652266, EF652273–EF652275, EF652284, EF652291, EF652297–EF652299, EF652302–EF652304, EF652314, EF652352, EF652354, EF652361–EF652363, EF652372, EF652379, EF652385–EF652387, EF652390–EF652392, EF652402, EF652440, EF652442, EF652449–EF652451, EF652460, EF652467, EF652473–EF652475, EF652478–EF652480, EF652490 and JQ301889–JQ301896.
Parsimony analysis was conducted using PAUP* 4.0b10 (Swofford 2003). For single-locus data sets, the criterion was parsimony, addition order was random (5000 replications), branch swapping was NNI (nearest neighbor interchange) and max trees was set at 5000. The set of trees generated was used as the starting point for parsimony analysis with addition order “as is” and TBR branch swapping. Bootstrap analysis was conducted with “as is” addition order and TBR branch swapping for 1000 replications.
Bayesian posterior probabilities were calculated using MrBayes 3.12 (Huelsenbeck & Ronquist 2001, Ronquist & Huelsenbeck 2003). The Mcm7, Tsr1 and RPB2 data sets included only protein-coding sequences and each data set was partitioned into codon positions 1, 2, and 3. The BT2 and CF loci included protein-coding and intron regions and the data were partitioned into intron and exon data. A GTR (general time-reversible) model was used with a proportion of invariant sites and a gamma-shaped distribution of rates across the sites. Markov chain Monte Carlo (MCMC) analysis was conducted for up to 5 × 106 generations until the chains converged.
Concordance analysis was based on the exclusionary principle of Baum & Shaw (1995) and the genealogical concordance phylogenetic species recognition concepts of Taylor et al. (2000). Clades were recognized as independent evolutionary lineages if 1) the clade was present in the majority of single-locus genealogies (majority rule consensus) or 2) if a clade was strongly supported by both parsimony and Bayesian analysis in at least one locus, and was not contradicted by another strongly supported locus (Dettman et al. 2003). Strong support was assessed as >70 % bootstrap and >0.95 posterior probability (Dettman et al. 2003).
The primers used for identification of A. versicolor in a PCR amplification (Dean et al. 2005) were tested using the primer sequences and amplification thermal profile recommended, but in a uniplex rather than multiplex amplification system (Dean et al. 2005).
RESULTS
Phylogenic analysis of sequence data
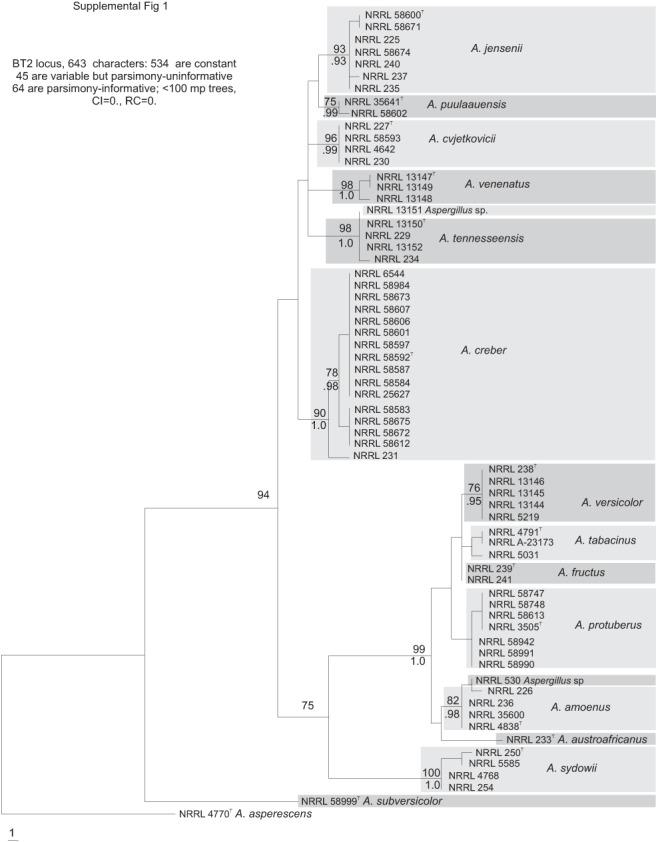
Sixteen independent evolutionary lineages were detected using both criteria for concordance (Dettman et al. 2003). The accepted species (Peterson 2008) A. versicolor, A. tabacinus, A. amoenus, A. protuberus and A. sydowii each were identified as independent lineages (Fig. 1). Four lineages contained a single isolate. Two of these single-isolate lineages, A. subversicolor and A. austroafricanus, were sufficiently distinct phenotypically from other species in the section and are described as new. The other two single-isolate lineages (NRRL 13151 and NRRL 530) were phenotypically difficult to distinguish from their siblings, and species descriptions were not accorded them.
Fig. 1.

Phylogenetic tree calculated from DNA sequence data from four concatenated loci. The section Versicolores contains three subclades, the A. versicolor subclade, the A. sydowii subclade and the A. subversicolor subclade. Thick branches indicate >90 % bootstrap and >0.90 Bayesian posterior probability for the node. Isolate NRRL 13151 is similar in colony appearance to A. tennesseensis but may represent a distinct species. Isolate NRRL 530 is similar in colony appearance to A. amoenus but also may represent a distinct species.
The section Versicolores clade contained three subclades (Fig. 1): the A. sydowii subclade containing A. sydowii, A. creber, A. venenatus, A. tennesseensis, A. cvjetkovicii, A. jensenii and A. puulaauensis; the A. versicolor subclade containing A. versicolor, A. tabacinus, A. fructus, A. protuberus, A. amoenus and A. austroafricanus; and the A. subversicolor subclade containing the single species A. subversicolor. Single-locus trees placed A. sydowii in the A. sydowii subclade, in the A. versicolor subclade or in a distinct clade containing only A. sydowii (Figs S1–S5, Supplementary Information, online only) with low confidence levels. The Mcm7 locus from A. sydowii was not amplified despite numerous attempts and thus A. sydowii does not appear in Fig. S3 (Supplementary Information, online only). The combined data tree (Fig. 1) depicts A. sydowii as a member of the A. sydowii subclade with strong statistical support. In the combined data tree, each species’ group of isolates resides on a branch with >90 % bootstrap proportion and >0.90 Bayesian posterior probability.
TAXONOMY
Previously described species
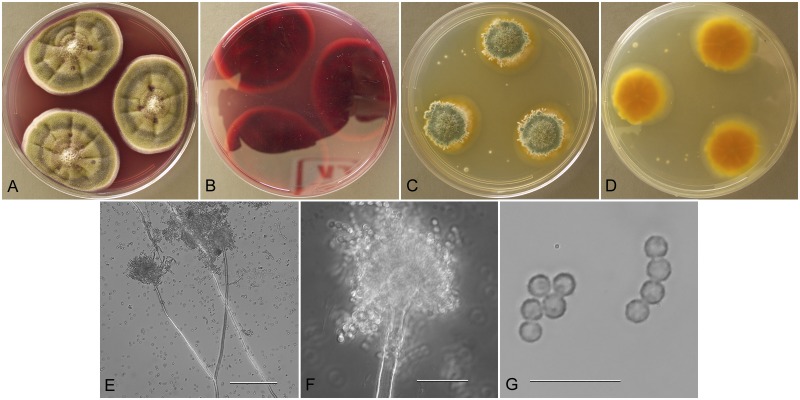
Aspergillus amoenus M. Roberg, Hedwigia 70:138 (1931).
MycoBank MB250654
Fig. 2.
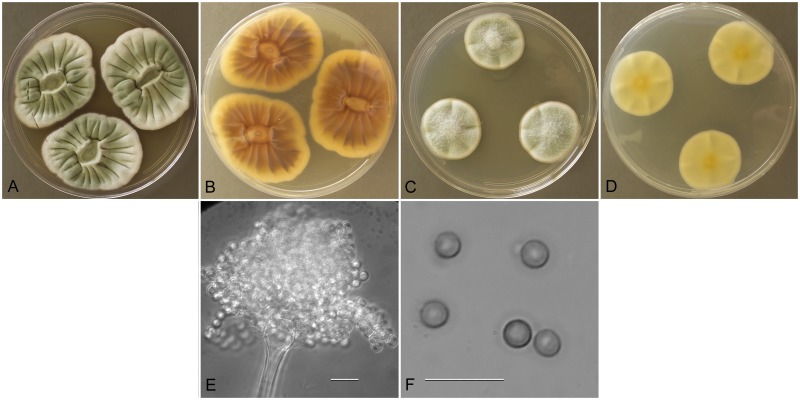
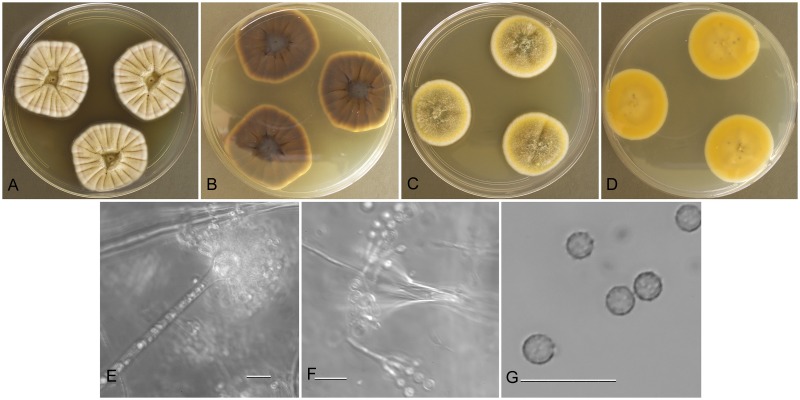
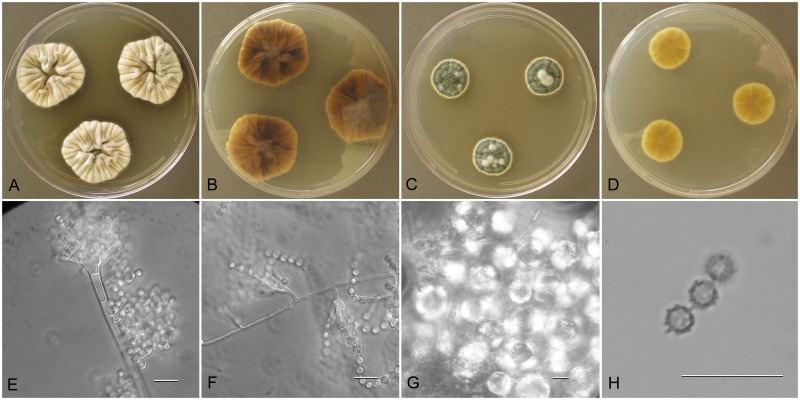
Aspergillus amoenus (NRRL 4838), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, subglobose vesicle, and conidia, bar=10 μm. f. Globose, smooth-walled conidia, bar=10 μm.
Type: Germany: Munster, isol. ex Berberis sp. fruit, 1930. M. Roberg (NRRL 4838—ex holotype culture).
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 2a–b) attained 25–40 mm diam, radially sulcate, centrally raised or sunken 3–4 mm, one older isolate (NRRL 226) plane, sporulating moderately to well, conidial heads in grayish green shades near tea green (R47), clear to pale orange exudate present in some isolates, faint reddish soluble pigment present in some isolates, reverse mostly reddish brown hues, with some isolates uncolored. Colonies grown 10 d on MEA at 25 °C (Fig. 2c–d) attained 23–33 mm diam, low, velutinous, some isolates with shallow sulcations, colony center often with funicular hyphal aggregates, sporulation in blue-green to gray-green shades, no soluble pigment except NRRL 226 with pale brown pigment, no exudate, reverse colored light orange yellow to pale yellow red. Incubation for 7 d on CYA at 5 °C produced no growth or germination of conidia. Incubation for 7 d on CYA at 37 °C commonly produced growth up to 6 mm diam.
Stipes (Fig. 2e) smooth walled, hyaline to yellow with brownish shades, (35−)100–600(−1100) × (2.5−)4–7(−8) μm, vesicles pyriform to spatulate, (4−)7–17(−21) μm diam, conidial heads biseriate, metulae covering 1/3 to entire vesicle, 3–6(−8) × 2.5–4.0(−5.5) μm, phialides (5−)6–8(−11) × 2–3 μm, fragmentary heads resembling penicillate fructifications abundant, conidia (Fig. 2f) spherical to subspherical, occasionally ellipsoidal, 2.5–3.5(−5) μm, smooth walled, NRRL 35600 produced globose hülle cells 12–22 μm diam when grown on M40Y medium, other isolates did not.
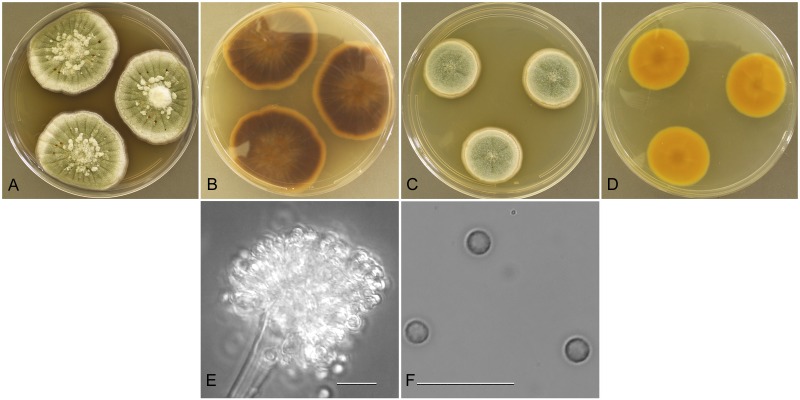
Aspergillus protuberus Muntañola-Cvetkoviæ, Mikrobiologija 5: 119 (1968).
MycoBank MB326650
Fig. 3.
Aspergillus protuberus (NRRL 3505), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Stipe, subglobose to clavate vesicle, and conidia, bar=10 μm. f. Roughened surface of stipe, bar=10 μm. g. Globose hülle cell, bar=10 μm. h. Globose, finely roughened conidia, bar=10 μm.
Synonym: Aspergillus versicolor var. protuberus (Muntañola-Cvetkoviæ) Kozak., Mycol. Pap. 161: 139 (1989).
Type: Yugoslavia: isol. ex rubber coated electrical cables, ca 1968 (NRRL 3505—ex holotype culture).
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 3a–b) attained 28–34 mm diam, radially and concentrically sulcate, wrinkled, centrally raised 2–4 mm, clumped aerial hyphae give a mealy appearance in some areas of some isolates, sporulation moderate with conidial heads often creamy white but sometimes patches of yellow-green conidia (celandine green R47) are present, scarlet red (R1) exudate moderately abundant, vinaceous-fawn (R40) to pale yellow soluble pigment present, reverse brownish red or orange cinnamon (R20), one isolate brazil red (R1). Colonies grown 10 d on MEA at 25 °C (Fig. 3c–d) attained 27–32 mm diam, floccose, mounded 4–5 mm centrally, radially sulcate, no exudate, no soluble pigment, reverse light pinkish yellow to pinkish yellow. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 3e–f) smooth to tuberose, hyaline to yellow or occasionally with brownish shades, (120−)300–800(−1250) × 4–10 μm, occasionally terminating with two vesicles, vesicles pyriform to spatulate, rarely subspherical, (6−)10–24(−27) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, (3−)4–7(−8) × 2.5–4.5(−5.5) μm, phialides (4−)5–8(−11) × 2–3(−3.5) μm, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 3h) spherical to subspherical or occasionally ellipsoidal to pyriform, (2.0−)2.5–3.5(−5) μm, finely roughened wall, hülle cells (Fig. 3g) globose sometimes present.
Aspergillus sydowii (Bain. & Sart.) Thom & Church Aspergilli:147 (1926).
MycoBank MB279636
Fig. 4.
Aspergillus sydowi (NRRL 250), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, subglobose vesicle, and conidia, bar=10 μm. f. Penicillate conidiophore from aerial hyphae, bar=10 μm. g. Subglobose, spinulose conidia, bar=10 μm.
Basionym: Sterigmatocystis sydowi Bainer & Sartory, Ann. Mycol. 11: 25 (1913).
Type: Sine loc.: sent to C. Thom, prior to 1930, M. Swift (NRRL 250—culture ex neotype).
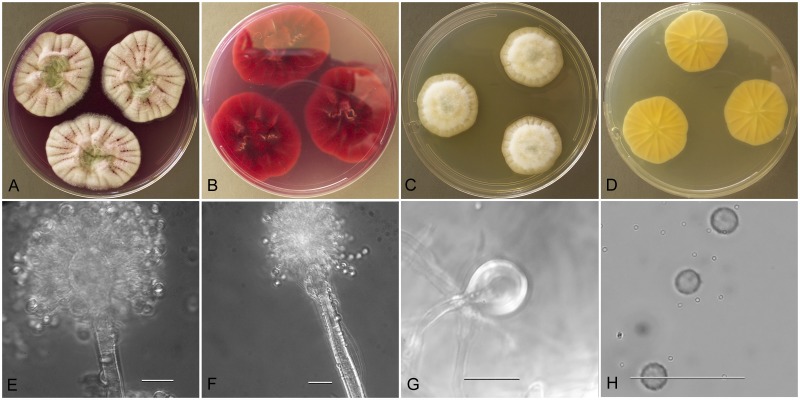
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 4a–b) attained 27–37 mm diam, velutinous, radially sulcate, sporulating well, conidial heads deep bluish gray-green (R42), exudate moderate to abundant, clear to yellowish to reddish brown, reddish-brown soluble pigment, reverse tawny olive (R39) to orange cinnamon (R29) on the periphery. Colonies grown 10 d on MEA at 25 °C (Fig. 4c–d) attained 37–48 mm diam, velutinous, some isolates with shallow sulcations, sporulating in dark grayish blue-green color, funicular hyphal aggregates often seen centrally, no exudate, no soluble pigment, reverse unpigmented to brownish pink in NRRL 4768. Incubation for 7 d on CYA at 5 °C produced no growth or germination of conidia. Incubation at 37 °C produced colonies 10–17 mm diam in 10 d.
Stipes (Fig. 4e) smooth, colorless, 100–500 μm × 4–7 μm, vesicles subglobose, 5–10 (−15) μm diam, conidial heads biseriate, metulae covering most of the vesicle, 6–7 × 2–3 μm, phialides 7–10 × 2.0–2.5 μm, fragmentary heads (Fig. 4f) resembling penicillate fructifications abundant, conidia (Fig. 4g) globose to subglobose, 2.5–3.0 (−5) μm, spinulose.
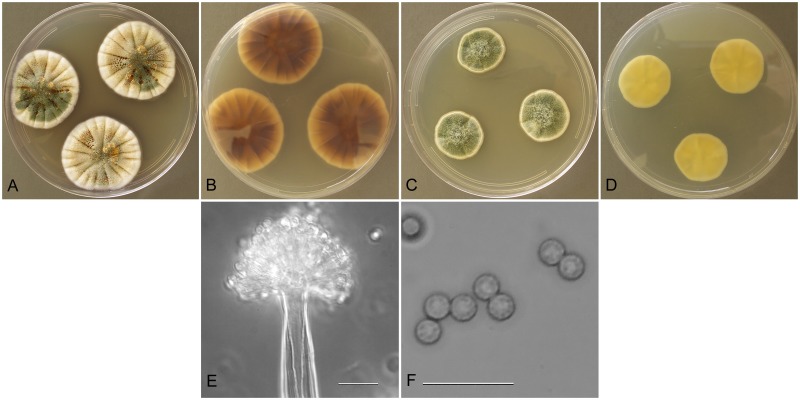
Aspergillus tabacinus Nakaz et al., J. Agr. Chem. Soc. Japan 10: 177 (1934).
MycoBank MB539544
Fig. 5.
Aspergillus tabacinus (NRRL 4791), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, with clavate vesicle, and conidia, bar=10 μm. f. Globose, smooth-walled conidia, bar=10 μm.
Synonym: Aspergillus versicolor var. magnus Sasaki, J. Fac. Agric. Hokkaido Univ. 49: 144 (1950).
Type: Sine loc.: isol. ex tobacco, 1934, Y. Nakazawa (NRRL 4791—culture ex neotype).
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 5a–b) attained 30–32 mm diam, sulcate, centrally raised 2–3 mm, often sporulating heavily throughout but sometimes sporulation is delayed, conidial heads artemisia green (R47), sporulation from aerial branches pronounced, exudate clear when present, no soluble pigment, reverse uncolored in NRRL 5031, or brown in other isolates. Colonies grown 10 d on MEA at 25 °C (Fig. 5c–d) attained 17–30 mm diam, NRRL 4791 is velutinous and covered with funicular hyphal aggregates, NRRL 5031 and NRRL 62481 are floccose, sporulation in bluish-green shades, no exudate, no soluble pigment, reverse uncolored to cream or very pale yellow. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes smooth walled (Fig. 5e), septate, hyaline to yellow with brownish tint, (70−)300–700(−900) × 4–8(−9) μm, vesicles pyriform to spatulate, (5−)8–15(−22) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, 3–8(−9)um × 2.5–4.5(−5.5) μm, phialides 5–8(−11) × 2–3(−3.5) μm, fragmentary heads resembling penicillate fructifications abundant, conidia (Fig. 5f) spherical to subspherical, occasionally ellipsoidal, (2.5−)3–4(−7) μm, smooth walled.
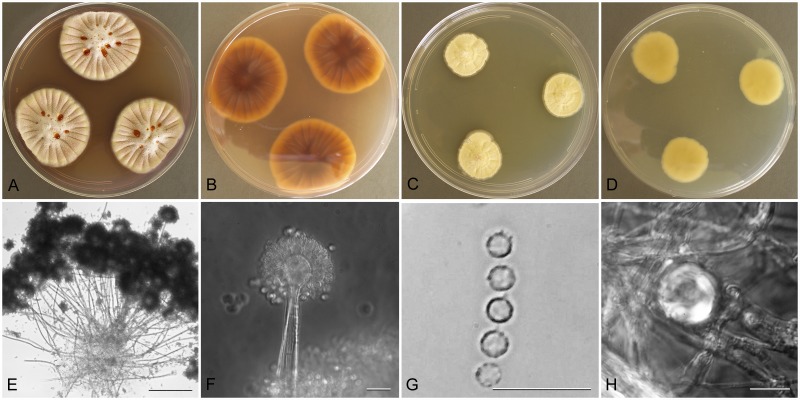
Aspergillus versicolor (Vuill.) Tirab., Annali Bot. 7: 9 (1908).
MycoBank MB172159
Fig. 6.
Aspergillus versicolor (NRRL 238), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Bifurcating stipe producing two conidiophores, bar=50 μm. f. Smooth stipe, subglobose vesicle, and conidia, bar=10 μm. g. Globose conidia with roughened walls, bar=10 μm.
Basionym: Sterigmatocystis versicolor Vuill., in Mirsky, Thèse de médicine (Nancy) 27:15 (1903).
Type: Sine loc.: 1935, V. K. Charles (NRRL 238—culture ex neotype).
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 6a–b) attained 28–36 mm diam, sulcate, centrally raised 4–5 mm, sporulating well, conidial heads pale grayish green near tea green (R47), central area mealy from aggregated aerial hyphae, exudate present in mostly clear to pale pink shades (brownish red in one isolate), faint to very obvious pinkish soluble pigment, reverse vinaceous or brown or scarlet (NRRL 238). Colonies grown 10 d on MEA at 25 °C (Fig. 6c–d) attained 21–31 mm diam, low, with funicular hyphal aggregates, sometimes dominating colony appearance, sporulating in pale to dark bluish green to gray green color, no exudate seen, soluble pigment yellow in some isolates, not present in others, reverse pale yellow, yellow orange or orange. Incubation for 7 d on CYA at 5 °C produced no growth or germination of conidia. Incubation for 7 d on CYA at 37 °C produced growth up to 8 mm diam.
Stipes (Fig. 6e–f) smooth, occasionally lightly tuberose, hyaline to yellow with brownish shades, (45−)200–750(−1050) × (4−)5–8(−12) μm, vesicles pyriform to spatulate, (6−)9–17(−20) μm in diam, conidial heads biseriate, metulae covering half to entire vesicle, 3–6(−9) × 2.5–4.5 μm, phialides (4−)5–7(−11) × 2–3 μm, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 6g) spherical to subspherical, occasionally ellipsoidal, (2−) 2.5–3.5(−6.5) μm, finely roughened wall, hülle cells globose, produced by NRRL 5219 when grown on M40Y medium, but not other isolates.
Observations: The ex-neotype culture NRRL 238 (isolated in 1935) is quite different in appearance, particularly in production of dark red soluble pigment and scarlet colony reverse on CYA, from the more recent isolates that were placed in the ARS Culture Collection between 1970 and 1984. The more recent isolates (NRRL 5219, NRRL 13144, NRRL 13145 and NRRL 13146) are quite similar in appearance and are the primary basis of the phenotypic description. Although there is phenotypic distinction, all five isolates are A. versicolor based on DNA sequence analysis.
New species
Aspergillus austroafricanus Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800597
Fig. 7.
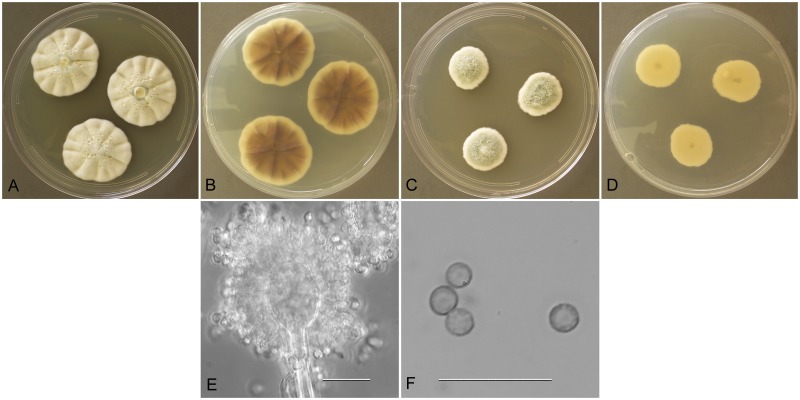
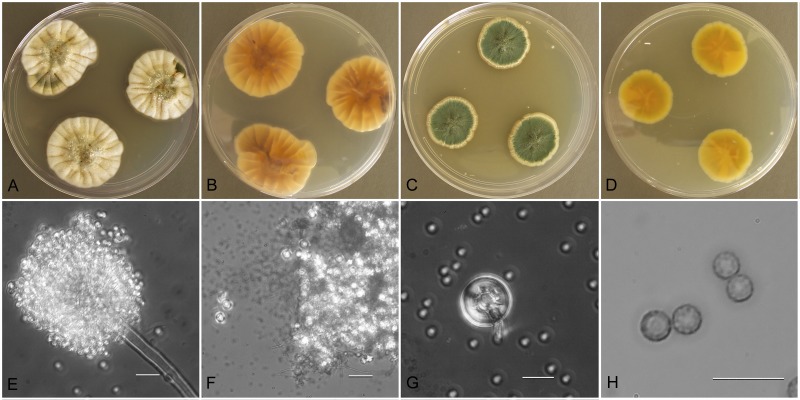
Aspergillus austroafricanus (NRRL 233), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, subglobose vesicle and conidia, bar=10 μm. f. Globose, smooth-walled conidia, bar=10 μm.
Etymology: Isolated from soil in South Africa.
Type: South Africa: Capetown, sent to C. Thom, 1922, V. A. Putterill ( BPI 880914 – holotype [from dried colonies of NRRL 233 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Conidia smooth-walled, no growth at 37 °C, produces reddish brown soluble pigment when grown on CYA.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 7a–b) attained 23–24 mm diam, mounded, shallowly sulcate, overgrowth by clumped hyphae making surface appear mealy, sporulating well, conidial heads near sage green (R47), sparse clear exudate, soluble pigment reddish brown, reverse dull brown. Colonies grown 10 d on MEA at 25 °C (Fig. 7c–d) attained 27 mm diam, velutinous, sporulation pale blue green, central hyphal tufts, no exudate, no soluble pigment, reverse yellowish orange. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 7e) smooth walled, hyaline to yellowish, (40−)100–350(−500) μm × 3–5(−6) μm, vesicles pyriform to spatulate, (4−)6–12(−15) μm diam, conidial heads biseriate, metulae covering 1/3 to entire vesicle, 3–7(−9)um × 2.5–4.5 μm, phialides (4−)5–7(−9) × (2−)2.5–3(−4) μm, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 7f) spherical to subspherical, 2.5–3.5 (−4.5) μm, smooth walled.
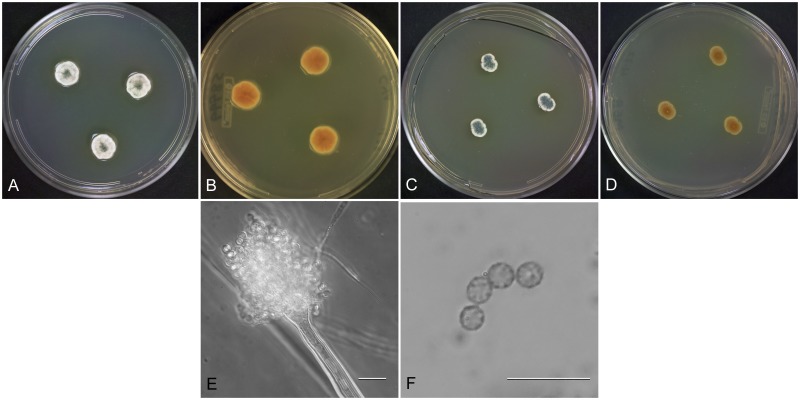
Aspergillus creber Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800598
Fig. 8.
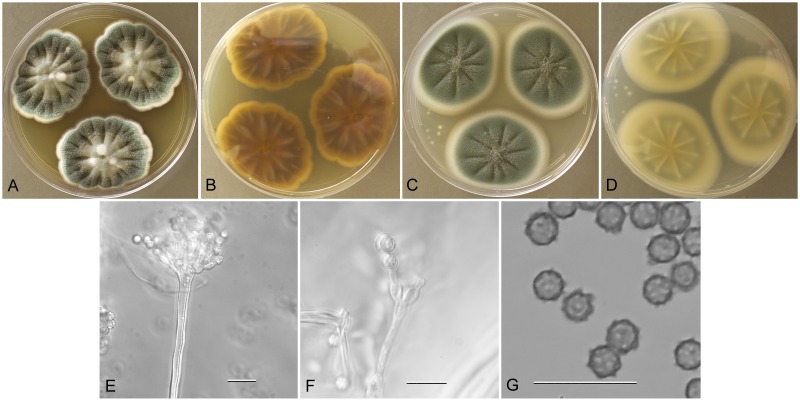
Aspergillus creber (NRRL 58583), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, subglobose vesicle, and conidia, bar=10 μm. f. Globose, finely roughened conidia, bar=10 μm.
Etymology: From the Latin word creber meaning numerous or frequent.
Type: USA: California: isol. ex air sample, Nov. 2008, Z. Jurjevic (BPI 800912 – holotype; [from dried colonies of NRRL 58592 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Produces rough-walled conidia, no growth at 37 °C, no soluble pigments formed on CYA or MEA, conidial color pea green or sage green on CYA and MEA.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 8a–b) attained 18–26 mm diam, radially sulcate, raised 3–5 mm centrally, peripheral areas white or yellow, central area sporulating well, conidial heads pea green to artemisia green (R47), exudate when present yellowish to reddish, no soluble pigment, reverse clay colored to cinnamon or reddish brown (R29). Colonies grown 10 d on MEA at 25 °C (Fig. 8c–d) attained 18–22 mm diam, low to 1–2 mm mounded, often overgrown centrally with hyphae aggregated into funicles, sporulation in yellow-green shades (pea green to sage green R47), with ca 1 mm white border, one isolate (NRRL 231) with vivid brown soluble pigment, other isolates no soluble pigment, no exudate, reverse pale yellow orange or olive drab or orange brown. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 8e) smooth walled, (10−)70–450(−650) x (3−) 4–7(−8) μm, vesicles pyriform to spatulate and occasionally subglobose, (4−)7–17(−25) μm diam, conidial heads biseriate, metulae (3−)4–6(−8) x 2.5–4.5(−5) μm, phialides (4−)5–8(−10) x 2–3(−4) μm, conidia (Fig. 8f) spherical to subspherical, occasionally ellipsoidal to pyriform, (2.5−)3–4(−9) μm, finely roughened wall.
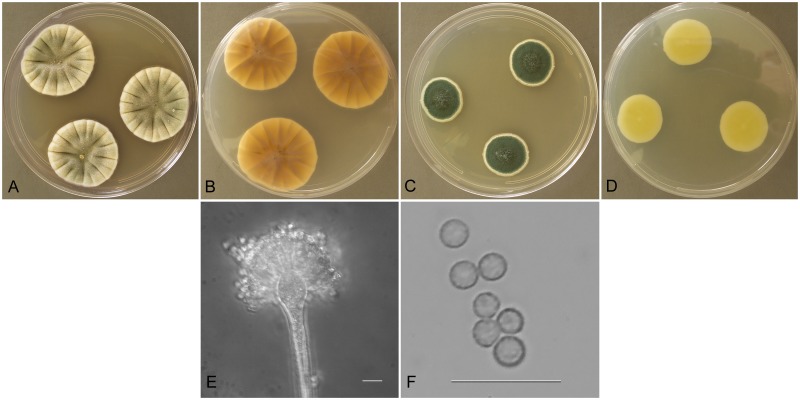
Aspergillus cvjetkovicii Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800599
Fig. 9.
Aspergillus cvjetkovicii (NRRL 4642), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Numerous conidiophores arising from the basal colony, bar=50 μm. f. Stipe, subglobose vesicle, and conidia, bar=10 μm. g. Globose, spinulose conidia, bar=10 μm. h. Globose hülle cell, bar=10 μm.
Etymology: Named in honor of Bogdan Cvjetkoviæ (University of Zagreb); pronunciation \`chet-kO-``vi-chi\.
Type: USA: New Jersey: isol. ex soil, 1915, W. Wilson (BPI 880909 – holotype [from dried colonies of NRRL 227 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Produces spinulose conidia, no growth at 37 °C, colonies producing red exudate and red soluble pigment on CYA.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 9a–b) attained 24–29 mm diam, radially sulcate, either centrally sunken or raised (2–3 mm), sporulating well, conidial heads white to cream in most isolates, pea green (R47) in NRRL 58593, exudate generally abundant, reddish brown to orange cinnamon, reddish brown soluble pigment, reverse yellowish red shades near orange cinnamon (R29) or tawny olive (R39). Colonies grown 10 d on MEA at 25 °C (Fig. 9c–d) attained 17–36 mm diam, low, slightly sulcate, sporulating throughout in creamy yellow shades, NRRL 58593 conidia are yellowish green, NRRL 227 and NRRL 230 produce brown soluble pigment while NRRL 4642 and 58593 do not produce soluble pigment, reverse brownish orange or pale creamy yellow. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 9e–f) smooth walled, hyaline to yellow, (40−)200–700(−850) × (3−)4–7(−8) μm, vesicles pyriform to spatulate, rarely subspherical, (5−)9–18(−23) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, 3–6(−8) × 2.5–4.5 μm, phialides 5–8(−10) × 2–3(−4) μm, occasionally solitary phialides present up to 32 μm long, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 9g) spherical to subspherical, occasionally ellipsoidal, (2−)2.5–3.5(−5) μm, spinulose, hülle cells (Fig. 9h) globose, sometimes present.
Aspergillus fructus Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800600
Fig. 10.
Aspergillus fructus (NRRL 239), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, spathulate vesicle, and conidia, bar=10 μm. f. Globose, finely roughened conidia, bar=10 μm. g. Globose hülle cell, bar=10 μm.
Etymology: From fruit.
Type: USA: California: isol. ex date fruit, 1939, Bliss (BPI 880915 – holotype [from dried colonies of NRRL 239 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Resembling A. versicolor growth at 37 °C, but forming shorter conidiophores 150–400 μm versus 200–750 μm conidiophores in A. versicolor.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 10a–b) attained 29–39 mm diam, sulcate, centrally raised 4–5 mm, funicular clumps of aerial hyphae abundant, sporulating well, conidial heads celandine green (R47), exudate clear to yellow, moderately abundant, soluble pigment clear, orange red in NRRL 239, reverse uncolored or mahogany red to orange-rufous (R2). Colonies grown 10 d on MEA at 25 °C (Fig. 10c–d) attained 22–32 mm diam, slightly sulcate, centrally covered by hyphal tufts, sporulation in yellow-green hues near artemisia green (R47), no exudate, no soluble pigment, reverse uncolored or drab orange. NRRL 241 was floccose on MEA. Incubation for 7 d on CYA at 5 °C produced no growth or germination of conidia. Incubation for 7 d on CYA at 37 °C produced growth up to 4 mm diam.
Stipes (Fig. 10e) smooth walled, hyaline to yellow, (50−) 150–400(−500) × 4–7 μm, vesicles pyriform to spatulate, (6−)9–17(−21) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, (2−)3–7(−9) × 2.5–4.5(−7) μm, phialides (5−)6–8(−11) × 2–3(−4) μm, fragmentary heads resembling penicillate fructifications abundant, conidia (Fig. 10f) spherical to subspherical, occasionally ellipsoidal, (2−) 2.5–3.5(−4.5) μm, finely roughened wall, hülle cells (Fig. 10g) globose, sometimes present.
Aspergillus jensenii Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800601
Fig. 11.
Aspergillus jensenii (NRRL 58671), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, subglobose vesicle, metulae and phialides, bar=10 μm. f. Penicillate conidiogenous cells from aerial hyphae, bar=10 μm. g. Globose, finely roughened conidia, bar=10 μm.
Etymology: Named in honor of C. N. Jensen who first reported this species as Aspergillus globosus Jensen, a later homonym of A. globosus Link.
Type: USA: Montana: isol. ex air sample, Oct. 2008, Z. Jurjevic (BPI 880910 – holotype [from dried colonies of NRRL 58600 grown 7 d at 25 °C on CYA and MEA]).
Synonym: Aspergillus globosus Jensen, Cornell University Agricultural Experiment Station Bulletin 315: 482 (1912); non Link 1809.
Diagnosis: Conidial walls roughened, no growth at 37 °C, conidial color near celandine, tawny olive to dark umber colony reverse on CYA.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 11a–b) attained 20–27 mm diam, radially sulcate, centrally raised or sunken, with clumped hyphal aggregates common in some isolates, sporulating moderately well, conidial heads celandine (R47) centrally and often white peripherally, exudate when present reddish brown or yellow brown, soluble pigment faint or intense yellow brown, in one case reddish brown, reverse tawny olive (R39) to dark brown near dark umber (R3). Colonies grown 10 d on MEA at 25 °C (Fig. 11c–d) attained 17–30 mm diam, low, plane, most isolates have funicular tufts of aerial hyphae centrally, sporulating well in yellowish blue-green shades, no exudate seen, soluble pigment either light brown or reddish brown, brownish orange in one isolate, reverse pale yellow or orange or brownish red. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 11e) smooth walled, hyaline to yellow, occasionally with brownish shades, (45−)200–700(−1000) × (−3)4–7(−8) μm, vesicles pyriform to spatulate, rarely subspherical, (5−)7–16(−22) μm diam, conidial heads biseriate, metulae covering 1/3 to entire vesicle, 3–8 × 2.5–4(−5) μm, phialides (4−)5–8(−11) × 2–3 μm, rarely solitary phialides present up to 32 μm long and up to 4.5 μm diam, fragmentary heads resembling penicillate fructifications (Fig. 11f) commonly present, conidia (Fig. 11g) spherical to subspherical, occasionally ellipsoidal to pyriform, (2.5−)3–4.5(−7) μm, finely roughened wall, globose hülle cells 15–20 μm diam produced by NRRL 58582 but not other isolates.
Aspergillus puulaauensis Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800602
Fig. 12.
Aspergillus puulaauensis (NRRL 35641), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, vesicle, and conidia, bar=10 μm. f. Mass of hülle cells, bar=50 μm. g. Hülle cell, bar=10 μm. h. Globose conidia with finely roughened walls, bar=10 μm.
Etymology. Isolated near the Pu’u la’au Highway on Hawaii; pronunciation \pU-U-la-U-en-sis\
Type: USA: Hawaii: isol. ex dead hardwood branch, 2003, D.T. Wicklow (BPI 880911 – holotype [from dried colonies of NRRL 35641 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Isolates produce abundant hülle cells when grown on M40Y agar, no growth at 37 °C,.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 12a–b) attained 22–25 mm diam, sulcate, centrally raised 5–6 mm with funicular hyphal clumps, sporulation light, conidial heads artemisia green (R47), exudate when present clear or reddish, soluble pigment when present brown, reverse yellowish to clay color (R39) or cinnamon (R29). Colonies grown 10 d on MEA at 25 °C (Fig. 12c–d) attained 21–25 mm diam, sulcate or plane, low, velutinous, deep green (artemisia to lily green R47), no exudate seen, no soluble pigment, reverse pale yellow near chamois or pale orange. Incubation for 7 d on CYA at 5 °C and 37 °C produced no growth or germination of conidia.
Stipes (Fig. 12e) smooth walled, hyaline to yellow, (35−) 100–500(−700) × (3−)4–7 μm, vesicles pyriform to spatulate, occasionally subspherical, (5−)8–18(−21) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, (3−)4–7(−9) × 2.5–4 μm, phialides 5–7(−10) × 2–3 μm, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 12h) spherical to ellipsoidal, (2.5−)3–4(−5.5) μm, finely roughened wall, hülle cells (Fig. 12f–g) spherical 11–19 μm diam seen in all isolates when grown on M40Y medium.
Aspergillus subversicolor Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800603
Fig. 13.
Aspergillus subversicolor (NRRL 58999), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, subglobose vesicle, and conidia, bar=10 μm. f. Subglobose, finely roughened conidia, bar=10 μm.
Etymology: Beneath or at the foot of Aspergillus versicolor.
Type: India: Karnataka: isol. ex green coffee berries, 1970, B. Muthappa (BPI 880918 – holotype [from dried colonies of NRRL 58999 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Conidia rough-walled, no growth at 37 °C, growing slowly on all media, producing yellow soluble pigment on CYA but no exudate.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 13a–b) attained 18–20 mm diam, sulcate, raised 5–6 mm centrally, wrinkled, sporulating sparsely, conidial heads artemisia green (R47), no exudate, soluble pigment faint yellow, reverse tawny (R15) to ochraceous orange. Colonies grown 10 d on MEA at 25 °C (Fig. 13c–d) attained 12–14 mm diam, low, plane, velutinous, sporulating in bluish green color (artemisia R47), no exudate, no soluble pigment, reverse brownish orange. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 13e) smooth walled, hyaline to slightly brownish, (60−) 250–450 (−550) × 4–7(−10) μm, vesicles pyriform to subglobose (6−)10–17(−22) μm diam, conidial heads biseriate, metulae covering half to entire or rarely 1/3 of vesicle, (3−)4–7(−9) × (2−)2.5–4 μm, bearing 2–3 ampuliform phialides, 5–8(−10) × 2–3 μm, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 13f) spherical to subspherical, occasionally ellipsoidal to pyriform, (2.5−)3–4(−7) μm, finely roughened wall.
Aspergillus tennesseensis Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800604
Fig. 14.
Aspergillus tennesseensis (NRRL 13150), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, pyriform vesicle, and conidia, bar=10 μm. f. Globose, finely roughened conidia, bar=10 μm.
Etymology: Isolated in Tennessee.
Type: USA: Tennessee: isol. ex toxic dairy feed, 1984, B.W. Horn (BPI 880917 – holotype [from dried colonies of NRRL 13150 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Producing rough-walled conidia, no growth at 37 °C, conidial color slate green when grown on MEA.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 14a–b) attained 22–30 mm diam, composed of a loose hyphal mat, radially sulcate, centrally raised or sunken, overgrown by clumps of aerial hyphae in some isolates, sporulating well centrally, pea green to artemisia green (R47), scant clear exudate usually present, soluble pigment absent, reverse in brownish orange shades near honey yellow or chamois (R30). Colonies grown 10 d on MEA at 25 °C (Fig. 14c–d) attained 20–46 mm diam, low, plane, velutinous, sporulating in dark green color near slate green (R47), no exudate, no soluble pigment, reverse uncolored, pale lemon yellow, or pale brown. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 14e) smooth walled, hyaline to yellowish with brownish shades, (35−)100–300(−400) × 4–7 μm, vesicles pyriform, (7−)10–16(−18) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, 4–6(−8) × 2.5–4 μm, phialides 5–8(−11) × 2–3 μm, fragmentary heads resembling penicillate fructifications occasionally present, conidia (Fig. 14f) spherical to subspherical, occasionally ellipsoidal to pyriform, (2.5−)3–4(−8) μm, finely roughened wall.
Aspergillus venenatus Jurjevic, S. W. Peterson & B. W. Horn, sp. nov.
MycoBank MB800605
Fig. 15.
Aspergillus venenatus (NRRL 13147), culture plates are 9 cm diam, colonies grown at 25 °C for 10 d. a. CYA colonies. b. CYA colony reverse. c. MEA colonies. d. MEA colony reverse. e. Smooth stipe, spathulate vesicle, and conidia, bar=10 μm. f. Penicillate conidiogenous cells on aerial hyphae, bar=10 μm. g. Globose hülle cells, bar=10 μm. h. Globose, spinulose conidia, bar=10 μm.
Etymology: Producing toxins.
Type: USA: Tennessee: isol. ex toxic dairy feed, 1984, B.W. Horn (BPI 880916 – holotype [from dried colonies of NRRL 13147 grown 7 d at 25 °C on CYA and MEA]).
Diagnosis: Producing spinulose conidia, no growth at 37 °C, producing no exudate or soluble pigments on CYA or MEA.
Description: Colonies grown 10 d on CYA at 25 °C (Fig. 15a–b) attained 22–31 mm diam, radially sulcate, sporulating centrally in artemisia green (R47) to deep bluish gray-green (R42) in one isolate, no exudate, no soluble pigment, reverse deep olive buff to tawny or brown (R15). Colonies grown 10 d on MEA at 25 °C (Fig. 15c–d) attained 17–24 mm diam, lightly sulcate, low, central tufted funicular aggregates of aerial hyphae, sporulating well in deep green color near slate green (R47), no exudate, no soluble pigment, reverse pale lemon yellow, chamois, or light olive drab. Incubation for 7 d on CYA at 5 °C or 37 °C produced no growth or germination of conidia.
Stipes (Fig. 15e) smooth walled, hyaline to yellow with brownish shades, (20−)100–400(−500) × 4–7 μm, vesicles pyriform to spatulate, (6−)9–17(−21) μm diam, conidial heads biseriate, metulae covering half to entire vesicle, (3−) 4–7(−9) × 2.5–4(−5) μm, phialides (5−)6–8(−11) × 2–3(3.5) μm, fragmentary heads resembling penicillate fructifications (Fig. 15f) commonly present, hülle cells (Fig. 15g) spherical, present in some isolates, conidia (Fig. 15h) spherical to subspherical, occasionally ellipsoidal to pyriform, 3–4(−6) μm diam, spinulose.
Phenotypic species recognition.
Growth rates of species on different media are presented in Table 2.
Table 2. Colony diameters (mm) of section Versicolores species on various media after 7d. Incubation at 25 °C except where noted.
| Species | CYA | MEA | CY20 | M40Y | M60Y | CYA at 37 °C |
|---|---|---|---|---|---|---|
| A. amoenus | 20–29 | 11–15 | 11–20 | 14–22 | 15–24 | 6 |
| A. austroafricanus | 18–19 | 16–17 | 24–25 | 18–19 | 17–18 | - |
| A. creber | 17–23 | 11–15 | 15–22 | 18–23 | 19–24 | - |
| A. cvjetkovicii | 15–21 | 14–17 | 17–20 | 16–24 | 15–25 | - |
| A. fructus | 13–20 | 10–16 | 9–17 | 10–23 | 10–20 | 4 |
| A. jensenii | 16–20 | 9–13 | 15–20 | 21–26 | 22–28 | - |
| A. protuberus | 17–25 | 11–18 | 14–22 | 21–24 | 20–24 | - |
| A. puulaauensis | 18–21 | 11–12 | 17–19 | 19–22 | 18–21 | - |
| A. subversicolor | 13–14 | 6–7 | 10–11 | 15–16 | 16–18 | - |
| A. sydowii | 20–25 | 20–25 | 21–26 | 23–26 | 23–27 | 8 |
| A. tabacinus | 21–26 | 12–16 | 21–26 | 8–23 | 8–23 | - |
| A. tennesseensis | 20–22 | 12–14 | 17–19 | 19–22 | 19–22 | - |
| A. venenatus | 14–17 | 8–10 | 15–16 | 19–23 | 17–21 | - |
| A. versicolor | 20–26 | 10–18 | 19–22 | 21–26 | 22–25 | 8 |
Phenotypic recognition of species in section Versicolores is based on smooth, roughened or spinulose conidia, conidial color, exudate and soluble pigment colors on CYA and MEA, growth rates and ability to grow at 37 °C, and on the uniform presence of hülle cells in one species.
Aspergillus cvjetkovicii, A. sydowii and A. venenatus isolates produce spinulose conidia. A. sydowii isolates grow at 37 °C, while A. cvjetkovicii and A. venenatus isolates do not. A. cvjetkovicii isolates produce reddish exudate and soluble pigment on CYA, while A. venenatus isolates produce no exudate or soluble pigment.
Aspergillus amoenus, A. austroafricanus and A. tabacinus produce smooth-walled conidia. Of these only A. amoenus isolates grow at 37 °C. A. tabacinus isolates produce no soluble pigment and A. austroafricanus produces reddish brown soluble pigment when grown on CYA.
The remaining eight species produce conidia with noticeably roughened walls, but the ornamentation is not pronounced enough to be considered spinulose. Two of the eight species, A. versicolor and A. fructus, have roughened conidial walls and grow at 37 °C. These two species are very similar but have somewhat distinct stipe lengths of 150–400 μm in A. fructus versus 200–750 μm in A. versicolor. We examined only two A. fructus isolates and five A. versicolor isolates and while separation of these species using phenotype on standard media appears possible, until more isolates are seen, it is recommended that strains be identified from gene sequences such as beta tubulin or calmodulin. Genealogical concordance species recognition clearly distinguishes these sibling species (Fig. 1).
Species with roughened conidia that do not grow at 37 °C are A. protuberus, A. creber, A. jensenii, A. puulaauensis, A. subversicolor and A. tennesseensis. Aspergillus protuberus isolates on CYA produce a red exudate (near scarlet R1) and a vinaceous or yellow soluble pigment, and MEA cultures are floccose. A. jensenii isolates produce brown CYA colony reverse colors from tawny olive to dark umber, and conidial color is near celandine green (R47). All A. puulaauensis isolates produce spherical hülle cells when grown on M40Y medium and the species is distinguished by this consistent character. One isolate each of A. versicolor and A. amoenus (both grow at 37 °C) and one isolate of A. jensenii also produced hülle cells on M40Y. A. subversicolor isolates are relatively slow growing on MEA and M40Y (Table 2) and produce faint yellow soluble pigment on CYA. A. tennesseensis, when grown on MEA produce very dark green conidial areas (near slate green R47) not produced by other rough-spored species in the section. Aspergillus creber isolates produce no soluble pigment on either CYA or MEA, and conidial color on either medium is pea green to sage green (R47).
There is considerable variation in colony appearance within species and considerable overlap in colony appearance between species, making species separation within section Versicolores challenging. In addition, some of the isolates included in this study were propogated in vitro for several decades prior to preservation by lyophilization. Among those isolates, several appear to have mutated and consequently produce colonies that have a wet appearance when grown on CYA or produce only moist aerial aggregates of hyphae with little sporulation. Identification of these degenerate strains relies on DNA sequence analysis. DNA sequence analysis is the most reliable means for identifying species within this section.
ITS region genotypes from species in section Versicolores are presented in Table 3. Some genotypes are shared by two or more species. Genotype A is present in isolates of five different species and genotype D is present in four different species of the section. Isolates of some species, such as A. creber (genotypes I, K, L N), display two to four ITS genotypes within species.
Table 3. Predicted species identity based on ITS genotype and correlation of ITS genotypes and species in section Versicolores. ITS geneotypes were assigned arbitrary letter designations and species are determined by genealogical concordance.
| ITS genotype | Predicted species |
|---|---|
| A | A. amoenus, A. fructus, A. protuberus, A. tabacinus, A. versicolor |
| B | A. subversicolor |
| C | A. austroafricanus |
| D | A. cvjetkovicii, A. jensenii, A. tennesseensis, A. venenatus |
| E | A. sydowii |
| F | A. sydowii |
| G | A. amoenus |
| H | A. tabacinus |
| I | A. creber, A. versicolor |
| J | A. puulaauensis |
| K | A. creber |
| L | A. creber |
| M | A. jensenii |
| N | A. creber |
DISCUSSION
Initial phenotypic examination of Aspergillus section Versicolores isolates was made using CYA cultures grown for 7 d at 25 °C (Klich & Pitt 1988). Those cultures did not provide sufficient data to reliably identify the species. Subsequently we tried culturing the isolates for 10 d at 25 °C on CYA to allow for further development of exudate, soluble pigment and conidial color. Raper & Fennell (1965) used incubation times of generally 10–14 d. We found that incubation for 10 d is necessary for characterizing isolates of section Versicolores.
Only four of the available genetic loci were used in preparing the combined data tree (Fig. 1). The ITS region was not included because it contained few informative nucleotides and because its veracity as a phylogenetic indicator is questionable (Galagan et al. 2005). The ITS data themselves however may be of interest for bar-coding studies (discussed later). The beta tubulin sequences from section Versicolores are of the “two intron” type and probably have a different evolutionary origin than the “three intron” type of beta tubulin found in the out-group species (Peterson 2008). Because of the suspected paralogy of this molecule, it was not included in the combined data tree. It was included as a possible target for DNA sequence-based identification of isolates.
Henig (1966) in his work on systematics required that all taxa be monophyletic. When working with phenotypic characters in section Versicolores, it was difficult to identify the informative characters that could satisfy Henig’s requirement. Analysis of DNA sequences from unlinked loci using concordance (Taylor et al. 2000, Dettman et al. 2003) makes it possible to define monophyletic groups. Phylogenetic recognition of species occasionally makes it necessary to accept cryptic species (Perrone et al. 2011) because the phenotypic characters of the species overlap with their siblings to such an extent that the species cannot be reliably identified without molecular tools. For NRRL 530 and NRRL 13151 that form single isolate lineages, reliable characters to define the species have not been found, but with the identification of additional isolates it may be possible to phenotypically characterize and subsequently name these species. For A. versicolor and A. fructus the limited number of isolates and the observed intraspecific variation reduce confidence in the current phenotypic recognition of the species, but the phylogenetic data are unequivocal and so A. fructus was described as new.
Prior to this publication A. versicolor was a species with documented genetic and phenotypic variation that did not resolve into clearly recognizable species. Fourteen species are now known in section Versicolores and the ITS region variation is ca. 3 % as calculated from the data herein. By comparison ca. 4 % variation is found in the Petromyces clade (Aspergillus sect. Flavi) between P. flavus and P. nomius and 14 species have been named (Varga et al. 2009). In the Petromyces clade, one species may possess a phenotype very similar to another species (Kurtzman et al. 1987, Peterson et al. 2001, Soares et al., 2012). While the validity of some species in the Petromyces clade have been questioned (Varga et al. 2009), phylogenetic distinction has served to validate species (Peterson 2008, Varga et al. 2009) regardless of the phenotypic similarities or overlapping character states of the species. Peterson (2008) suggested that sect. Versicolores could easily be dropped from Aspergillus taxonomy. This much broader study of A. versicolor sensu lato isolates suggests that section Versicolores should be retained as a monophyletic and useful subgeneric designation.
Aspergillus versicolor is the most reported fungal species in section Versicolores from damp indoor environments (Jussila 2003, Rydjord et al. 2005) and its presence is used as an indicator of Sick Building Syndrome (SBS) (Schwab & Straus 2004). We amplified each newly described species using A. versicolor-specific primers (Dean et al. 2005; data not shown) and obtained a positive signal in all cases except for A. subversicolor and A. sydowii; therefore the primer set retains its usefulness. In A. creber and A. jensenii some isolates did not amplify even though the genotypes were identical with isolates that did amplify, suggesting degradation or incorrect quantitation of the genomic DNA.
Twenty-four sect. Versicolores isolates in this study were obtained by one of us (ZJ) from air samples in buildings, but none comprised A. versicolor sensu stricto (Table 1). Of the five A. versicolor isolates examined, three were isolated from a single lot of toxic cattle feed in the USA and the substrate for the other two isolates, one from the USA and the other from South Africa, was not recorded. The species is widespread geographically, but was not commonly encountered among the isolates used in this study. Aspergillus creber was the most frequently isolated species from indoor air samples in the USA (13 strains from six states), followed by A. protuberus (five strains from two states) and A. jensenii (four strains from three states). Aspergillus versicolor sensu stricto may not be common in buildings. Two other species, A. cvjetkovicii and A. puulaauensis, were each isolated once from indoor air. The other newly described species, A. fructus, A. austroafricanus, A. subversicolor, A. tennesseensis and A. venenatus, were isolated from plant material or had unknown sources (Table 1). Amend et al. (2010) reported that fungi isolated from indoor air sources (e.g., dust, carpet) are highly diverse in the temperate regions of the world and are much less diverse in tropical regions. Therefore, our strains from indoor air samples from the USA in addition to culture collection strains from many regions of the world may represent much of the diversity present in section Versicolores.
There is considerable interest in using ITS sequences for bar-coding identification of fungi, particularly for large-scale ecological studies (Begerow et al. 2010, Schoch et al. 2012). In section Versicolores species, one particular ITS genotype is present in isolates of five different species and another genotype is found in four different species (Table 3). Because ITS genotypes do not uniquely identify species in this section, use of multiple loci is the most reliable means of DNA sequence-based identification in section Versicolores (Peterson 2012).
Viable propagules of A. versicolor have been recovered from the highly saline Dead Sea (Kis-Papo et al. 2003), showing an ability to survive conditions of salinity or drying. The ARS Culture Collection contains a few putative A. versicolor isolates obtained from brined meats in the UK. Upon sequence and phenotypic analysis, these isolates were identified as three species, A. amoenus, A. tabacinus and A. protuberus, all of which occur in the A. versicolor subclade (Fig. 1). Additionally, A. creber NRRL 6544, from the A. sydowii subclade, was isolated from a tar ball floating in the Atlantic Ocean. High tolerance to salinity may extend to other species in section Versicolores. Aspergillus versicolor has also been identified from dust collected in the International Space Station (Vesper et al. 2008). In addition to CYA and MEA we used high sugar content media (CY20S, M40Y and M60Y) containing 20, 40 or 60 % sucrose, respectively. All isolates grew well on all of the media (Table 2), with no noticable reduction in growth rates even on M60Y medium. Species from section Versicolores have a remarkably broad tolerance for a wide range of water activity of their substrates.
Aspergillus versicolor isolates produce the aflatoxin precursor sterigmatocystin, a compound that is mutagenic and tumorigenic (Veršilovskis & Saeger 2010). Animal feed infested with three morphotypes of A. versicolor, all of which produce sterigmatocystin, have been implicated in dairy animal toxicosis, but it is unknown whether sterigmatocystin caused the toxicosis (Vesonder & Horn 1985). Those three morphotypes are now identified as A. versicolor, A. tennesseensis and A. venenatus, and as these species occur in the two main subclades of section Versicolores (Fig. 1), sterigmatocystin production may be present in additional species. The distribution of section Versicolores species in agricultural commodities and their role in stigmatocystin toxicoses require additional study. In addition to sterigmatocystin, recent studies have revealed numerous metabolites with biological activities (Finefield et al. 2011, Lee et al. 2011) from A. versicolor sensu lato Jaio et al. (2007) discovered novel nucleotide analogs from A. puulaauensis which was reported under the name A. versicolor.
Aspergillus versicolor has been implicated as the causitive agent of disseminated aspergillosis in dogs (Zhang et al. 2012), has probably caused aspergillosis in transplant recipients (Baddley et al. 2009), and has been isolated from the infected eye of a patient suffering from HIV (Perri et al. 2005). We included two section Versicolores clinical isolates in our study. NRRL 254 was identified as A. sydowii and NRRL 226, originally identified as A. versicolor, is here identified as A. amoenus. Because of different sensitivities of fungal species to fungal antibiotics, a more detailed study of A. versicolor clinical isolates might be of value to guide appropriate therapeutic regimens (Pfaller et al. 2011).
Supplementary Information, online only
Fig. S1.
Phylogenetic tree based on beta tubulin sequences; Bayesian posterior probabilities and bootstrap values based on 1000 replicates are placed on the internodes where values are significant.
Fig. S2.
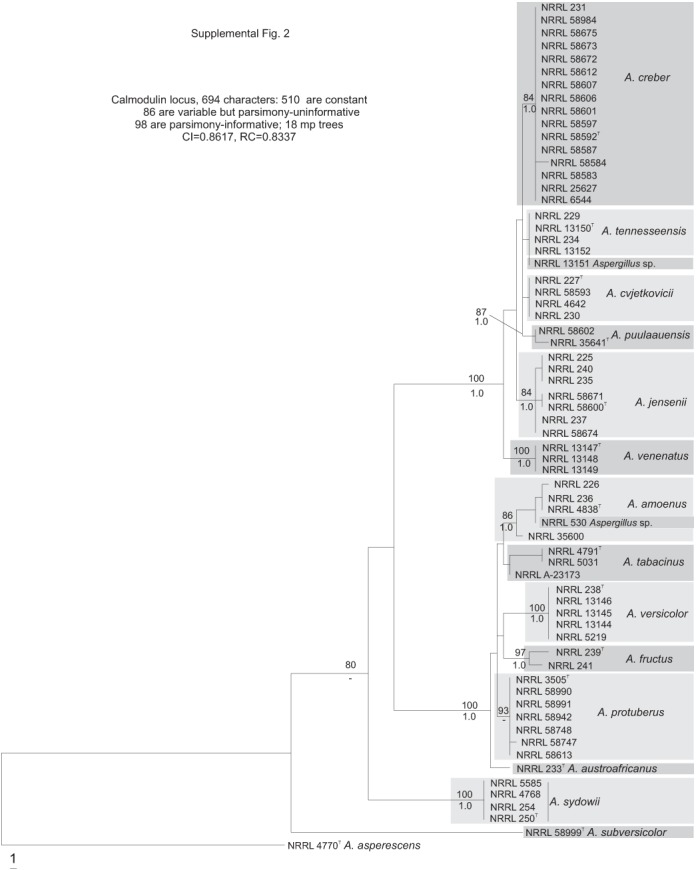
Phylogenetic tree based on calmodulin sequences; Bayesian posterior probabilities and bootstrap values based on 1000 replicates are placed on the internodes where values are significant.
Fig. S3.
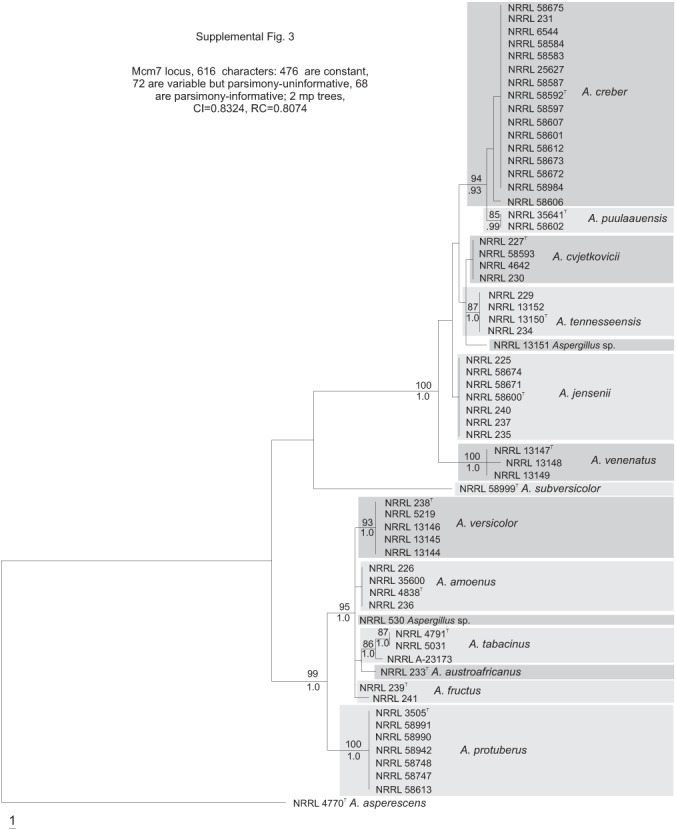
Phylogenetic tree based on Mcm7 locus sequences; Bayesian posterior probabilities and bootstrap values based on 1000 replicates are placed on the internodes where values are significant.
Fig. S4.
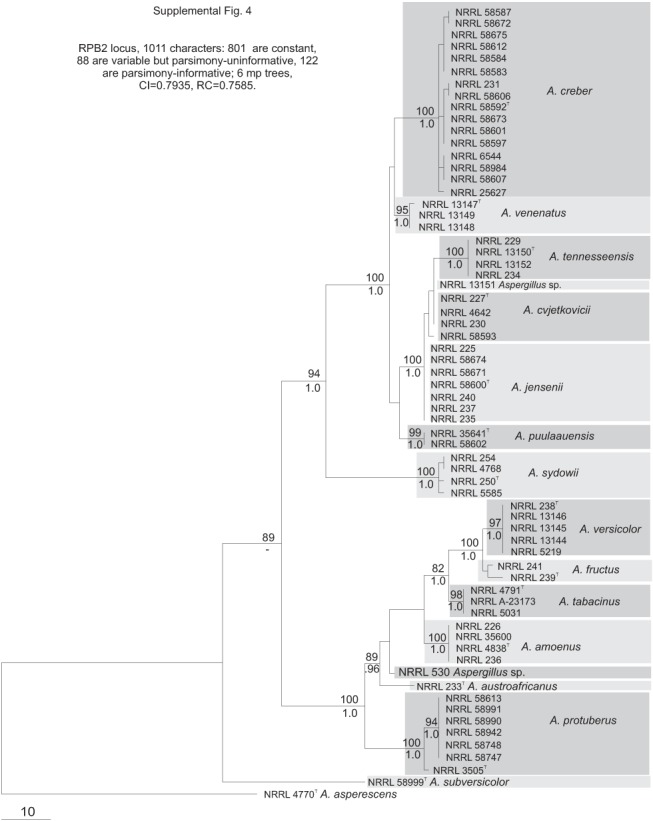
Phylogenetic tree based on RPB2 locus sequences; Bayesian posterior probabilities and bootstrap values based on 1000 replicates are placed on the internodes where values are significant.
Fig. S5.

Phylogenetic tree based on Tsr1 locus sequences; Bayesian posterior probabilities and bootstrap values based on 1000 replicates are placed on the internodes where values are significant.
Acknowledgments
We thank Amy E. McGovern for valuable technical support. Patricia Eckel kindly advised us on Latin usage. David L. Hawksworth advised us on some nomenclatural issues. The authors thank Donald T. Wicklow (USDA, Peoria, IL) and Lynne Sigler (University of Alberta, Edmonton, Alberta) for the gift of Hawaiian and Canadian isolates. Mention of a trade name, proprietary product, or specific equipment does not constitute a guarantee or warranty by the United States Department of Agriculture and does not imply its approval to the exclusion of other products that may be suitable. USDA is an equal opportunity provider and employer.
REFERENCES
- Amend AS, Seifert KA, Samson R, Bruns TD. (2010) Indoor fungal composition is geographically patterned and more diverse in temperate zones than in the tropics. Proceedings of the National Academy of Sciences, USA 107: 13748–13753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson B, Frisvad JC, Sondergaard I, Rasmussen IB, Larsen LS. (2011) Associations between fungal species and water-damaged building materials. Applied and Environmental Microbiology 77: 4180–4188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baddley JW, Marr KA, Andes DR, Walsh TJ, Kauffman CA, Kontoyiannis DP, Ito JI, Balajee SA, Pappas PG, Moser SA. (2009) Patterns of susceptibility of Aspergillus isolates recovered from patients enrolled in the transplant-associated infection surveillance network. Journal of Clinical Microbiology 47: 3271–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum DA, Shaw KL. (1995) Genealogical perspectives on the species problem. In: Experimental and Molecular Approaches to Plant Biosystematics (Hoch PC, Stephenson AG, eds): 289–303 St Louis. Mo: Missouri Botanical Garden; [Google Scholar]
- Begerow D, Nilsson H, Unterseher M, Maier W. (2010) Current state and perspectives of fungal DNA barcoding and rapid identification procedures. Applied Microbiology and Biotechnology 87: 99–108 [DOI] [PubMed] [Google Scholar]
- Dean TR, Roop B, Betancourt D, Menetrez MY. (2005) A simple multiplex polymerase chain reaction assay for the identification of four environmentally relevant fungal contaminants. Journal of Microbiological Methods 61: 9–16 [DOI] [PubMed] [Google Scholar]
- Dettman JR, Jacobson DJ, Turner E, Pringle A, Taylor JW. (2003) Reproductive isolation and phylogenetic divergence in Neurospora: comparing methods of species recognition in a model eukaryote. Evolution 57: 2721–2741 [DOI] [PubMed] [Google Scholar]
- Domsch KH, Gams W, Anderson TH. (1980) Compendium of Soil Fungi Vol. 1. Academic Press, UK: [Google Scholar]
- Edmondson DA, Barrios CS, Brasel TL, Straus DC, Kurup VP, Fink JN. (2009) Immune response among patients exposed to molds. International Journal of Molecular Sciences 10: 5471-5484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelhart S, Loock A, Skutlarek D, Sagunski H, Lommel A, Farber H, Exner M. (2002) Occurrence of toxigenic Aspergillus versicolor isolates and sterigmatocystin in carpet dust from damp indoor environments. Applied and Environmental Microbiology 68: 3886–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finefield JM, Sherman DH, Tsukamoto S, Williams RM. (2011) Studies on the biosynthesis of the notoamides: synthesis of an isotopomer of 6-hydroxydeoxybrevianamide E and biosynthetic incorporation into notoamide J. Journal of Organic Chemistry 76: 5954–5958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galagan JE, Calvo SE, Cuomo C, Ma L-J, Wortman JR, Batzoglou S, Lee S-I, Batürkmen M, Spevak CC, Clutterbuck J, Kapitonov V, Jurka J, Scazzocchio C, Farman M, Butler J, Purcell S, Harris S, Braus GH, Draht O, Busch S, D’Enfert C, Bouchier C, Goldman GH, Bell-Pedersen D, Griffiths-Jones S, Doonan JH, Yu J, Vienken K, Pain A, Freitag M, Selker EU, Archer DB, Peñalva MA, Oakley BR, Momany M, Tanaka T, Kumagai T, Asai K, Machida M, Nierman WC, Denning DW, Caddick M, Hynes M, Paoletti M, Fischer R, Miller B, Dyer P, Sachs MS, Osmani SA, Birren BW. (2005) Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature 438: 1105–1115 [DOI] [PubMed] [Google Scholar]
- Gams W, Christensen M, Onions AHS, Pitt JI, Samson RA. (1985) Infrageneric taxa of Aspergillus. In: Advances in Penicillium and Aspergillus Systematics (Samson RA, Pitt JI, eds): 55–64 New York: Plenum Press; [Google Scholar]
- Hennig W. (1966) Phylogenetic Systematics. [English translation.] Urbana, IL: University of Illinois Press; [Google Scholar]
- Huelsenbeck JP, Ronquist F. (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754–755 [DOI] [PubMed] [Google Scholar]
- Jiao P, Murdur SV, Gloer JB, Wicklow DT. (2007) Kipukasins, nucleoside derivatives from Aspergillus versicolor. Journal of Natural Products 70: 1308–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jussila J. (2003) Inflammatory Responses in Mice after Intratracheal Instillation of Microbes Isolated from Moldy Buildings. Helsinki, Finland: National Public Health Institute; [Google Scholar]
- Kis-Papo T, Kirzhner V, Wasser SP, Nevo E. (2003) Evolution of genomic diversity and sex at extreme environments: fungal life under hypersaline Dead Sea stress. Proceedings of the National Academy of Sciences, USA 100: 14970–14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klich MA. (1993) Morphological studies of Aspergillus section Versicolor and related species. Mycologia 85: 100–107 [Google Scholar]
- Klich MA. (2002) Identification of Common Aspergillus Species. Utrecht: Centraalbureau voor Schimmelcultures; [Google Scholar]
- Klich MA, Pitt JI. (1988) A Laboratory Guide to Common Aspergillus Species and Their Teleomorphs. North Ryde, NSW: CSIRO; [Google Scholar]
- Kozakiewicz Z. (1989) Aspergillus species on stored products. Mycological Papers 161: 1–188 [Google Scholar]
- Kurtzman CP, Horn BW, Hesseltine CW. (1987) Aspergillus nomius, a new aflatoxin-producing species related to Aspergillus flavus and Aspergillus tamarii. Antonie van Leeuwenhoek 53: 147–158 [DOI] [PubMed] [Google Scholar]
- Lee YM, Dang HT, Li J, Zhang P, Hong J, Lee CO, Jung JH. (2011) A cytotoxic fellutamide analogue from the sponge-derived fungus Aspergillus versicolor. Bulletin of the Korean Chemical Society 32: 3817–3820 [Google Scholar]
- Mbata TI. (2008) Isolation of fungi in hyper saline Dead Sea water. Sudanese Journal of Public Health 3: 170–172 [Google Scholar]
- Mills JT, Abramson D. (1986) Production of sterigmatocystin by isolates of Aspergillus versicolor from western Canadian stored barley and rapeseed/canola. Canadian Journal of Plant Pathology 82: 151–153 [Google Scholar]
- Moreno G, Arenas R. (2010) Other fungi causing onychomycosis. Clinics in Dermatology 28: 160–163 [DOI] [PubMed] [Google Scholar]
- Nielsen KF. (2003) Mycotoxin production by indoor molds. Fungal Genetics and Biology 39: 103–117 [DOI] [PubMed] [Google Scholar]
- Perri P, Campa C, Incorvaia C, Parmeggiani F, Lamberti G, Costagliola C, Sebastian A. (2005) Endogenous Aspergillus versicolor endophthalmitis in an immuno-competent HIV-positive patient. Mycopathologia 160: 259–261 [DOI] [PubMed] [Google Scholar]
- Perrone G, Stea G, Epifani F, Varga J, Frisvad JC, Samson RA. (2011) Aspergillus niger contains the cryptic phylogenetic species A. awamori. Fungal Biology 115: 1138–1150 [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2008) Phylogenetic analysis of Aspergillus species using DNA sequences from four loci. Mycologia 100: 205–226 [DOI] [PubMed] [Google Scholar]
- Peterson SW. (2012) Aspergillus and Penicillium identification using DNA sequences: barcode or MLST? Applied Microbiology and Biotechnology DOI 10.1007/s00253-012-4165-2. [DOI] [PubMed] [Google Scholar]
- Peterson SW, Ito Y, Horn BW, Goto T. (2001) Aspergillus bombycis, a new aflatoxigenic species and genetic variation in its sibling species, A. nomius. Mycologia 93: 689–703 [Google Scholar]
- Peterson SW, Jurjevic Z, Bills GF, Stchigel AM, Guarro J, Vega FE. (2010) The genus Hamigera, six new species and multilocus DNA sequence based phylogeny. Mycologia 102: 847–864 [DOI] [PubMed] [Google Scholar]
- Pfaller MA, Castanheira M, Diekema DJ, Messer SA, Jones RN. (2011) Triazole and echinocandin MIC distributions with epidemiological cutoff values for differentiation of wild-type strains from non-wild-type strains of six uncommon species of Candida. Journal of Clinical Microbiology 49: 3800–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitt JI. (1980) [“1979”] The Genus Penicillium and Its Teleomorph States Eupenicillium and Talaromyces. London: Academic Press; [Google Scholar]
- Pitt JI, Hocking AD. (2009) Fungi and Food Spoilage. 3rd edn London: Springer Verlag; [Google Scholar]
- Raper KB, Fennell DI. (1965) The Genus Aspergillus. Baltimore, MD: Williams & Wilkins; [Google Scholar]
- Ridgway R. (1912) Color Standards and Color Nomenclature. Washington, DC: Ridgway; [Google Scholar]
- Ronquist F, Huelsenbeck JP. (2003) MrBayes3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574 [DOI] [PubMed] [Google Scholar]
- Rydjord B, Hetlandy G, Wikerz HG. (2005) Immunoglobulin G antibodies against environmental moulds in a Norwegian healthy population shows a bimodal distribution for Aspergillus versicolor. Scandinavian Journal of Immunology 62: 281–288 [DOI] [PubMed] [Google Scholar]
- Samson RA, Houbraken J, Summerbell RC, Flannigan B, Miller JD. (2001) Common and important species of fungi and actinomycetes in indoor environments. In: Microorganisms in Home and Indoor Work Environments (Flannigan B., Samson RA, Miller JD, eds): 287–292 London: Taylor and Francis; [Google Scholar]
- Schoch CL, Seifert KA, Huhndorf S, Robert V, Souge JL, Levesque CA, Chen W, Fungal Barcoding Consortium (2012) Nuclear ribosomal internal transcribed spacer (ITS) region as a universal DNA barcode marker for Fungi. Proceedings of the National Academy of Sciences, USA 109: 6241–6246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab CJ, Straus DC. (2004) The roles of Penicillium and Aspergillus in sick building syndrome. Advances in Applied Microbiology 55: 215–238 [DOI] [PubMed] [Google Scholar]
- Shelton BG, Kirkland KH, Dana W. (2002) Profiles of airborne fungi in buildings and outdoor environments in the United States. Applied and Environmental Microbiology 68: 1743–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares C, Rodrigues P, Peterson SW, Lima N, Venâncio A. (2012) Three new species of Aspergillus section Flavi isolated from almonds and maize in Portugal. Mycologia: doi:10.3852/11-088. [DOI] [PubMed] [Google Scholar]
- Swofford DL. (2003) PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4. Sunderland, MA: Sinauer Associates; [Google Scholar]
- Taylor JW, Jacobson DJ, Kroken S, Kasuga T, Geiser DM, Hibbett DS, Fisher MC. (2000) Phylogenetic species recognition and species concepts in fungi. Fungal Genetics and Biology 31: 21–32 [DOI] [PubMed] [Google Scholar]
- Thom C, Church MB. (1926) The Aspergilli. Baltimore, MD: Williams & Wilkins; [Google Scholar]
- Thom C, Raper KB. (1945) Manual of the Aspergilli. Baltimore, MD: Williams & Wilkins; [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ. (1994) CLUSTALW: improving the sensitivity of progressive multiple sequence alignments through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Research 22: 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuomi T, Reijula K, Johnsson T, Hemminki K, Hintikka EL, Llindroos O, Kalso S, Koukila-Kähkölä P, Mussalo-Rauhamaa H, Haahtela T. (2000) Mycotoxins in crude building materials from water-damaged buildings. Applied and Environmental Microbiology 66: 1899–1904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varga J, Frisvad JC, Samson RA. (2009) A reappraisal of fungi producing aflatoxins. World Mycotoxin Journal 2: 263−277 [Google Scholar]
- Veršilovskis A, Saeger SD. (2010) Sterigmatocystin: occurrence in foodstuffs and analytical methods – an overview. Molecular Nutrition and Food Research 54: 136–147 [DOI] [PubMed] [Google Scholar]
- Vesonder RF, Horn BW. (1985) Sterigmatocystin in dairy cattle feed contaminated with Aspergillus versicolor. Applied and Environmental Microbiology 49: 234–235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vesper SJ, Wong W, Kuo CM, Pierson DL. (2008) Mold species in dust from the International Space Station identified and quantified by mold-specific quantitative PCR. Research in Microbiology 159: 32–435 [DOI] [PubMed] [Google Scholar]
- Zhang S, Corapi W, Quist E, Griffin S, Zhang M. (2012) Aspergillus versicolor, a new causative agent of canine disseminated aspergillosis. Journal of Clinical Microbiology 50: 187–191 [DOI] [PMC free article] [PubMed] [Google Scholar]