Abstract
Objective: To assess the feasibility, acceptability, and preliminary impact of a telepharmacy intervention in an underserved, rural asthma patient population. Subjects and Methods: Patients with asthma were randomized to receive either standard care or telephone consultations from pharmacists regarding asthma self-management over a 3-month period. Qualitative interviews were conducted to identify participants' attitudes/opinions regarding the intervention. Baseline and follow-up surveys assessed asthma control, patient activation, and medication utilization. Results: Ninety-eight adults were recruited (78% accrual); 83 completed the study (15% dropout). Participants reported positive opinions and believed the intervention improved their asthma self-management. The intervention group had significantly higher patient activation compared with the control (p<0.05). There were no significant between-group differences regarding asthma control. However, within-group analyses of the intervention group showed an improvement in asthma control (p<0.01) and medication adherence (p<0.01). No within-group differences were found for the control group. Conclusions: This telepharmacy intervention is feasible and showed indicators of effectiveness, suggesting the design is well suited for a robust study to evaluate its impact in uncontrolled asthma patients. Pharmacists helping patients manage asthma through telecommunications may resolve access barriers and improve care.
Key words: patient–pharmacist communication, asthma, telepharmacy, intervention, rural, underserved
Introduction
Over 24 million adults, or 8.2% of the U.S. population, had a diagnosis of asthma in 2009.1 The prevalence of lifetime asthma is increasing at a similar rate among urban and rural populations in the United States, but it is a particular problem for rural residents of some states.2 The cost associated with caring for people with asthma is approximately $20.7 billion, $5.6 billion of which is accounted for by prescription drugs.3 Optimal medication use—especially with long-term controller (LTC) medicines—is vital to improving patients' asthma control; however, up to 80% of patients misuse asthma medications.4,5
Patients' problems with asthma medication use may include underuse of controller medications, overuse of rescue medications, and improper inhaler technique. These are associated with inadequate asthma control, poor quality of life, and increased emergency and non-emergency health services utilization.5,6 Factors such as incomplete understanding of a treatment plan, low motivation and self-efficacy, and forgetfulness contribute to asthma medication use problems.5,7 However, patient activation (i.e., asthma-related knowledge, self-efficacy, and self-management) and good communication with healthcare providers may improve appropriate medication use.5,6,8,9
Pharmacists' patient care services can prevent and solve drug therapy problems and improve asthma outcomes.4,10–12 However, fewer data are available regarding pharmacist services' impact on rural populations in the United States. Rural patients often face limited access to services that could improve their medication use and asthma control, because of economic and supply disparities.13,14 Data, although limited, also suggest patients living in rural areas receive substandard care for their asthma.13,15 Communication technologies such as telepharmacy and telemedicine show promise for improving rural healthcare by extending patient–provider communication and improving healthcare efficiency.16
The use of telecommunications to monitor medication use may improve patients' access to healthcare and asthma control.17 A literature review of telemedicine asthma interventions found that services commonly included an initial face-to-face introductory session and used doctors and nurses to deliver the main intervention.18 International research has demonstrated feasibility and cost-effectiveness for pharmacists to provide patient care services using telemedicine.19–22 In the United States, Bynum et al.23 used a Web-based interactive compressed video to teach metered-dose inhaler technique to a rural, adolescent U.S. population and found that knowledge increased. However, limited research has evaluated pharmacists' care for underserved rural, adult patients with asthma implemented solely through telecommunications (i.e., without face-to-face contact).
The objective of this study was to conduct a pilot test of the Patient And phaRmacist Telephonic Encounters (PARTE) intervention to improve underserved rural asthma patients' asthma control. The primary aim was to assess the feasibility and acceptability of implementing this intervention. Secondary aims included the exploration of the intervention's impact on asthma control, patient activation, and the use of LTC medications.
Materials and Methods
Study Design
A randomized controlled trial was conducted to assess the feasibility, acceptability, and initial impact of the PARTE intervention.24 Participants randomized to the intervention group received telephone consultation from pharmacists regarding their asthma self-management and medication use. Five pharmacists incorporated the intervention into their usual practice. Participants randomized to the control group received usual care, which included mail receipt of a prescription refill with written medication use instructions. Baseline and 3-month post-intervention follow-up (6 months from baseline) telephone surveys were conducted to assess asthma control, patient activation, and medication utilization. Interviews were conducted with a subset of intervention group participants to explore their attitudes about the intervention. The study was approved by the Marshfield Clinic Research Foundation Institutional Review Board and the Health Sciences Institutional Review Board at the University of Wisconsin–Madison.
Participants
Patients with asthma who received their medications from the Family Health Center of Marshfield, Inc. (FHC), a federally qualified health center, 340B mail-order pharmacy were invited to participate. Inclusion criteria included the following: participation in the Community Health Access (a charity program sponsored by the Marshfield Clinic and supported by the FHC) or FHC programs (a federally funded program to assist underserved, uninsured, and underinsured individuals in the northern Wisconsin area), age ≥19 years, English-speaking, receipt of one or more asthma medication(s) dispensed in the 6-month period ending January 31, 2009, and a diagnosis of asthma. The Community Health Access and FHC programs have financial screening criteria of income less than or equal to 200% of the federal poverty level. The FHC service area is located within an 11-county region in north central Wisconsin (8,228 square miles). This predominantly rural area comprises 254 municipalities, 78% of which are populated by less than 1,000 people. Eighty-six percent of the service area population resides in communities that have been designated by the federal government as a medically underserved and/or a health professional shortage area. Exclusion criterion was the enrollment in the FHC Pharmacy medication auto-refill program. Electronic health records were reviewed to identify potential participants. Participants were reimbursed $75 for study participation: $50 at the beginning and $25 at study completion.
Study Procedures
A pool of 576 individuals who met the enrollment criteria was identified; 25% were randomly selected to be included in the recruitment effort. Letters, including information sheets describing the study and consent forms, were mailed to prospective participants to introduce the study. About 4–5 days later, research assistants contacted prospective participants to determine their willingness to enroll in the study and answer any questions. The research assistants screened for the study exclusion criterion. If an individual was interested in participating, the research assistant obtained oral consent and conducted a baseline survey.
Research assistants then forwarded participant contact information to a data manager for random assignment to the intervention or the control group. The data manager forwarded intervention group participants' contact information to the FHC pharmacy manager for allocation to pharmacists. Following the intervention period, the FHC pharmacy manager sent participants' information to the data manager. The data manager then compiled a list of intervention and control group participants and sent this list to the research assistants for the 3-month post-intervention follow-up (6 months from baseline) telephone surveys. Thus, the research assistants and researchers were blinded to the allocation of participants to the intervention and control groups.
Intervention
Participants randomized to the intervention received three telephone consultations from trained pharmacists regarding asthma self-management and medication use over a 3-month period (approximately one call per month). Each pharmacist was assigned 9 or 10 participants. Each participant was contacted by the same pharmacist across their three calls. Following a standardized communication guide, pharmacists evaluated and addressed participants' barriers to managing their asthma medications. The communication guide is based upon the Indian Health Services' patient-counseling model and the recognition, identification, and management technique for managing medication use problems.25 Pharmacists collaborated with participants to identify root cause(s) of and implement solutions to asthma-related problems.26
Pharmacists also reviewed participants' electronic health records and/or contacted their primary healthcare provider if they deemed it clinically necessary to help the participant resolve identified problems. Participants were referred to the appropriate healthcare provider (e.g., primary care provider, specialty provider, or urgent care/emergency room services provider) if severe asthma-related problems were identified. In addition, pharmacists used a series of questions to assess whether participants needed additional education regarding inhaler technique.27 Each encounter with intervention group participants was electronically documented. Pharmacists reviewed documentation before initiating subsequent contacts with the intervention group participants.
Training to provide the intervention was based upon self-efficacy theory.28 A patient–provider communication expert educated study pharmacists about the components of the interaction protocol (i.e., communication guide) developed for this project. The pharmacists reviewed mock encounters of a pharmacist using the communication guide during a patient consultation, practiced using the guide during role-playing, and received feedback regarding their role-playing. An established asthma educator and researcher provided an overview of asthma management. To ensure clinical consistency in intervention efforts, pharmacists were certified in the National Asthma Educator Certification Board Exam.
Pharmacists were evaluated during the intervention by a health communication scientist to examine their fidelity to the interaction protocol. Using the standardized counseling framework as a guide, the scientist reviewed and commented on the pharmacists' adherence to the protocol. In addition, the study team conducted weekly meetings to discuss issues that arose during the intervention period.
Qualitative Interviews
Interviews were conducted with a randomly selected sample of 15 intervention group participants after all 3-month post-intervention follow-up surveys were completed. All intervention group participants (n=49) were eligible for random selection regardless of their completion of the intervention. Previous intervention studies have used similar proportions of participants in sampling procedures for qualitative evaluations.29,30 All study participants who were selected agreed to participate. An interviewer used a standardized guide to conduct confidential, one-on-one telephone interviews. The interview guide contained questions about participants' experiences, likes and dislikes regarding the intervention, and management of asthma. All interviews were audio-recorded and transcribed.
Measures
The Asthma Control Test (ACT), a well-validated self-report instrument, was used to measure participants' control of asthma.31 The ACT consists of five items measured on a 5-point scale. Thus ACT scores can range from 5 (not controlled) to 25 (completely controlled). An ACT score equal to or greater than 19 is indicative of well-controlled asthma.32 Patient activation was measured with the validated 13-item Patient Activation Measure (PAM).33 The PAM assesses an individual's knowledge, skills, and confidence for self-management. PAM raw scores range from 13 to 52, with higher scores indicating greater activation. Medication utilization was operationalized as the use of LTC (yes/no) and low adherence to LTC (yes/no). Adherence to controller medications was assessed with the eight-item Morisky Medication Adherence Scale.34 Morisky Medication Adherence Scale scores can range from 0 to 8; a score below 6 is indicative of low adherence. Asthma control, patient activation, and medication utilization were assessed in both groups at baseline and 3 months post-intervention (6 months after baseline) by the research assistants. In addition, asthma control (ACT) was assessed in the intervention group at the beginning of all three telephone consultations by the pharmacists. Participants' demographic characteristics (age, gender, marital status, race/ethnicity, education, and smoking status) also were collected at baseline.
Analysis
Descriptive statistics were calculated to characterize study participants, including study accrual and dropout. Two research assistants independently reviewed the interview transcripts and identified statements describing participants' perceptions about the intervention. First, specific statements were abstracted directly from transcripts, and similar statements were classified into categories. The research assistants then examined relationships across categories for persistent themes. To reconcile discrepant classifications of statements into categories, a thorough review of positive and negative examples and discussion was conducted to obtain consensus.
Difference-in-difference modeling was used to compare intervention and control group differences in the change for asthma control and patient activation. Given the small sample size and potential impact of violating distributional assumptions, we estimated standard errors and 95% confidence intervals using a bias-corrected bootstrapping approach.35,36 Standard errors were adjusted for clustering within individuals by feasible generalized least squares estimators. Two-sample tests of proportions examined the differences (at follow-up) between the proportions of intervention and control group participants who were using at least one LTC medication and had low adherence.
Paired-samples t tests examined (1) differences in asthma control (mean ACT score) across all time points (baseline, intervention phone calls 1, 2, and 3, and follow-up) for the intervention group and (2) difference in asthma control (mean ACT score) from baseline to follow-up for the control group. In addition, two-sample tests of proportions were conducted to assess whether the intervention and control groups contained the same proportion of participants who were using at least one LTC medication and had low adherence from baseline to follow-up. All quantitative analyses were conducted using STATA version 11.0.37
Results
Ninety-eight participants were recruited out of the 126 patients approached (78% accrual). Table 1 presents the characteristics of participants by group. The mean age of participants was 44.6 years old (SD=15.8 years), 75% were female, and 91% were white. There were no significant differences between participants in the intervention and control groups at baseline. Eighty-three participants completed the study. Seven control and eight intervention group participants dropped out of the study (15% overall dropout). Of the 49 intervention group participants, 84% completed all three telephone encounters, 4% completed only two encounters, 8% completed only one encounter, and 4% failed to complete any encounters.
Table 1.
Baseline Characteristics (n=98)
| STUDY VARIABLE | OVERALL SAMPLE | INTERVENTION | CONTROL | DIFFERENCE:TEST STATISTIC |
|---|---|---|---|---|
| Age [mean (SD)] | 44.6 (15.8) | 45.4 (16.8) | 43.7 (14.0) | 0.59 |
| Gender [n (%)] | ||||
| Male | 23 (23.5) | 13 (26.5) | 10 (20.4) | 0.48 |
| Female | 75 (76.5) | 36 (73.5) | 39 (79.6) | |
| Race [n (%)] | Fisher's exact: 0.44 | |||
| White | 91 (92.9) | 47 (95.9) | 44 (89.8) | |
| Other | 7 (7.1) | 2 (4.1) | 5 (10.2) | |
| Education [n (%)] | 0.44 | |||
| Less than high school degree | 16 (16.3) | 6 (12.2) | 10 (20.4) | |
| High school degree only | 41 (41.8) | 20 (40.8) | 21 (42.9) | |
| Some college | 41 (41.8) | 23 (46.9) | 18 (36.7) | |
| Current smoker [n (%)] | ||||
| Yes | 30 (30.6) | 12 (24.5) | 18 (36.7) | 0.19 |
| No | 68 (69.4) | 37 (75.5) | 31 (63.3) | |
| Asthma Control Test score [mean (SD)] | 17.4 (4.4) | 17.1 (4.5) | 17.7 (4.2) | 0.52 |
| ACT score categories, pre-intervention [n (%)] | 0.58 | |||
| Poorly controlled | 30 (30.6) | 6 (12.2) | 14 (28.6) | |
| Somewhat controlled | 37 (37.8) | 20 (40.8) | 21 (42.9) | |
| Well controlled | 31 (31.6) | 23 (46.9) | 14 (28.6) | |
| Patient Activation Measure score (raw) [mean (SD)] | 42.8 (5.0) | 42.8 (5.0) | 42.7 (5.0) | 0.87 |
| Morisky Sum Score, long-term controllers [mean (SD)] | 6.1 (5.5) | 6.4 (6.0) | 5.9 (5.0) | 0.67 |
| Low adherence (yes) [n (%)] | 48 (36.8) | 22 (57.9) | 26 (68.4) | 0.34 |
Qualitative Feedback Regarding the Intervention
The majority of interviewees (11 out of 15) described their experience during the telephone interaction with the pharmacist as being positive and very helpful. Table 2 contains examples of participants' responses. Participants indicated that the pharmacists educated them about ways to improve self-management, afforded them opportunities to ask questions, and responded with immediate feedback. The majority of participants failed to indicate any dislikes about (12 out of 15) or desires to make changes (12 out of 15) to the intervention. In response to a question about dislikes, one participant stated, “Hanging up. Because I was learning something and it was nice to learn something every time.” Two participants mentioned time; however, this was not an obstacle to their participation.
Table 2.
Examples of Participants' Feedback During Interviews
| THEMES | EXAMPLES |
|---|---|
| Positive and very helpful | “I think that that was a very good experience. She was very helpful.” “I thought my experience was very good. I felt comfortable talking with her about my problems or how I was doing with my asthma.” “She was very conscious of my needs and very friendly.” |
| Improve self-management | “I think just understanding things better and finding easier ways for me to take what I need and still make sure I'm doing the right thing to manage it.” “It's better now. There are things that I didn't realize before that I do now. So, you know like about shaking it up and rinsing it out if I haven't used it in a while and things like that. I definitely learned some things from it.” “Well, it really helped out a lot because I learned some better ways to rinse my inhaler, how to use, how many seconds to hold my breath. It's really helped with my quality of breathing tremendously.” |
| Questions and immediate feedback | “Very informative and she let me ask questions and she took the time to answer so that I could understand. She would ask me if I understood what she was explaining and it was really nice.” “I like having that immediate feedback if I had any questions or comment about my asthma. She was there to answer it right away and if she didn't have an answer, she would find one and get back to me as soon as possible.” “Just the fact that they were open to looking at different things for me to take because I am like the person who does not want to be on the wrong medication or be like guinea pig. That we went the one direction I wanted to go.” |
| Time | “I don't know. Sometimes you know you have to do things, usually I told her so it was okay really, it worked out okay.” “Just that it was time consuming but you know that's okay that was no big deal.” |
Between-Group Comparisons
Findings from the difference-in-difference model with PAM as the dependent variable revealed a statistically significant positive effect of the intervention on patient activation at the 3-month post-intervention follow-up period (β=2.01, 95% confidence interval 0.15 to 3.91) (Table 3). Results failed to indicate a significant difference between the two groups at 3 months post-intervention for the ACT score. However, there was a trend indicating that the intervention group contained a smaller proportion of participants (26%) who indicated low adherence to LTC regimens in comparison with the control group (47%) at follow-up (p=0.07).
Table 3.
Overall Intervention Effect on Asthma Control (Asthma Control Test) and Patient Activation (Patients Activation Measure)
| VARIABLE | ACT | PAM |
|---|---|---|
| Group (intervention) | −0.57 (−2.37 to 1.08) | 0.16 (−1.77 to 2.15) |
| Time (follow-up) | 0.79 (−0.42 to 1.86) | −0.30 (−1.65 to 0.96) |
| Intervention×Time | 0.08 (−1.73 to 1.86) | 2.01 (0.15 to 3.91)a |
Models included group (intervention or control), time (follow-up), and an intervention×time interaction as independent variables, with the coefficient on intervention×time representing the intervention effect of interest. The numbers are coefficients (95% bias corrected confidence interval).
p<0.05.
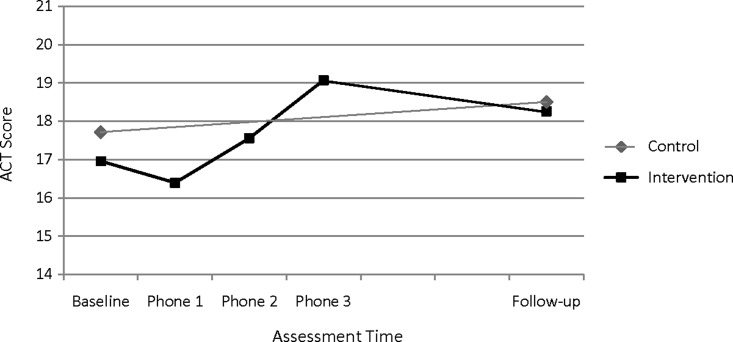
Within-Group Comparisons
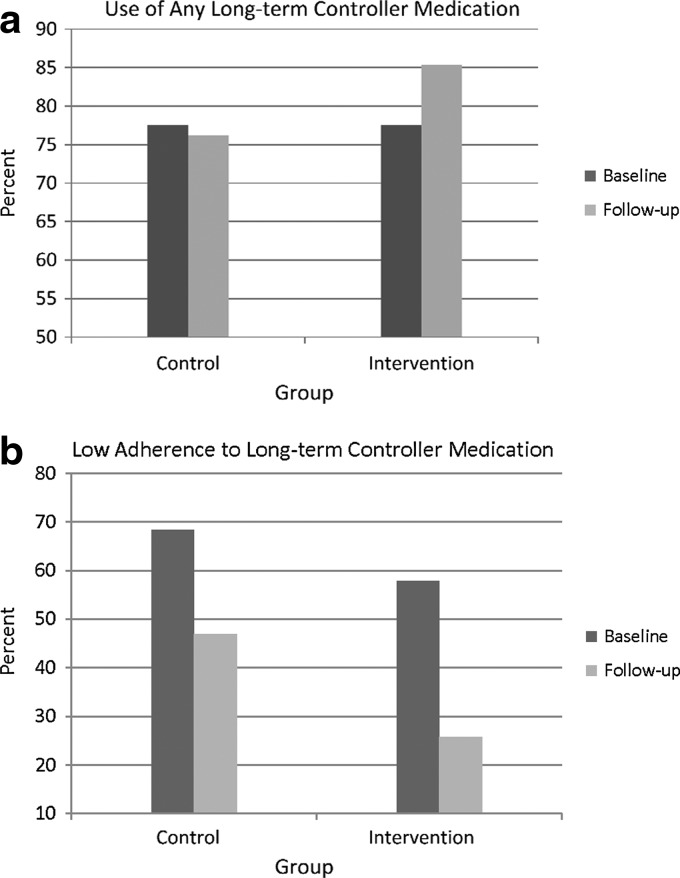
The intervention group's mean ACT scores increased during the telephone encounter period (Fig. 1). Findings showed a statistically significant improvement in mean ACT scores during the treatment period for the intervention group from baseline to phone assessment 3 (t=2.84, p<0.01). At the 3-month post-intervention follow-up, 85% of the intervention group was taking an LTC medication in contrast to 78% at baseline (Fig. 2a). In addition, a significantly lower percentage of intervention group participants had low adherence to LTC medications at the 3-month post-intervention follow-up compared with their baseline (26% vs. 58%, z=−2.78, p<0.01) (Fig. 2b).
Fig. 1.
Asthma control (mean Asthma Control Test [ACT] scores) during the study period for intervention and control groups.
Fig. 2.
Medication use in the intervention and control groups at baseline and follow-up: (a) use of any long-term controller medication and (b) low adherence to long-term controller medication.
Discussion
Given the challenges of providing quality care to low-income patients in rural, underserved areas, the telephonic pharmacist intervention offers a hopeful strategy. This pilot study provides evidence of the feasibility and acceptability of implementing the PARTE intervention in an underserved, rural population. Over 75% of individuals who were approached agreed to participate, and 85% of those participants completed the study, which included one call a month for 3 months for the intervention group. Participants reported positive attitudes and opinions regarding the intervention. Study findings show that this pharmacist care via telecommunications improved patients' activation in their care and offered preliminary trends, suggesting a positive effect on asthma control and the use of LTC medication in the intervention group.
Patient activation encompasses patient knowledge, skill, and confidence for self-management.33 The intervention in this study was based upon a framework that focuses on identifying and resolving problems by using communication strategies that acknowledge patient concerns and preferences and involves patients in decision-making processes. This patient-centered approach may have contributed to the intervention's success,16,38 consistent with earlier work that found positive associations between activation and self-management behaviors.39 By increasing patient activation, this intervention may foster improved self-management of asthma, including the use of inhaler medications and avoidance of environmental triggers. Study findings support this assertion by showing an improvement of adherence to LTC medications in the intervention group.
Pilot findings also suggest an improvement in asthma control within the intervention group during the course of telephone interactions with the pharmacists. This intervention found similar findings to previous research on non-telephonic interventions in which nurses and pharmacists (through face-to-face communication) use monitoring to improve outcomes. Van der Meer et al.40 found that weekly monitoring and subsequent treatment adjustment led to improved asthma control in patients with partly and uncontrolled asthma. Herborg et al.10 demonstrated that community pharmacists monitoring outcomes had beneficial effects on asthma symptom status and asthma-related quality of life. Study findings, along with previous research and clinical guidelines, suggest that periodically monitoring patients and addressing their needs play an important role in the improvement of asthma control.41
This study contains limitations that warrant mentioning. First, outcomes of interest were measured with patient-reported instruments. Although well-validated instruments were used in this study, there exists the possibility of social desirability bias or the Hawthorne effect influencing the results. Future studies should incorporate objective assessments such as forced expiratory volume in 1 s to evaluate the intervention in order to overcome such bias. The primary purpose of this pilot study was to assess the feasibility and acceptability of this intervention by underserved populations, which is well suited for small sample sizes. However, the economic and clinical impacts of this intervention need to be assessed in larger trials. Third, participants in this study received care from a federally qualified health center; this trial provided needed insights about the acceptability of the intervention specifically by this group. However, it would be useful in the future for a larger study to test this intervention in broader, more representative populations and settings given that the federally qualified health centers have been documented as providing exceptional chronic disease care.42–45 Finally, this study did not account for seasonal variations. Future research should evaluate this intervention over a longer duration of time to provide additional information regarding its impact on asthma outcomes during various allergenic conditions.
Conclusions
This study used a randomized controlled design to pilot test a telepharmacy intervention to improve underserved, rural patients' asthma control. Results provide evidence of the feasibility and acceptability of this intervention in the targeted population. Findings also showed beneficial effects on patients' involvement in care and trends of improvement in asthma control and medication use. The successful implementation and completion of this pilot study suggest that this telepharmacy intervention is well suited for a robust study to evaluate its impact in a larger sample of uncontrolled asthma patients, and thus a larger trial is needed to confirm these initial findings.
Acknowledgments
We would like to thank Xin Ruppel, Pharm.D., B.C.P.S., M.B.A., Mary Jo Knobloch, M.S., Jennifer L. Grimm, Pharm.D., Tonja L. Larson, Pharm.D., and Douglas D. Seubert for their contributions to this study. This project was supported by grant 1UL1RR025011 from the Clinical & Translational Science Award program of the National Center for Research Resources, National Institutes of Health.
Disclosure Statement
No competing financial interests exist.
References
- 1.Akinbami LJ. Moorman JE. Liu X. Asthma prevalence, healthcare use, and mortality: United States, 2005–2009. Natl Health Stat Report. 2011;(32):1–14. [PubMed] [Google Scholar]
- 2.Jackson JE. Doescher MP. Hart LG. A national study of lifetime asthma prevalence and trends in metro and non-metro counties, 2000–2003. 2007. http://depts.washington.edu/uwrhrc/uploads/RHRC_WP108_Jackson.pdf. [Sep 14;2010 ]. http://depts.washington.edu/uwrhrc/uploads/RHRC_WP108_Jackson.pdf
- 3.American Lung Association. Trends in asthma morbidity and mortality. www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf. [Apr 7;2011 ]. www.lungusa.org/finding-cures/our-research/trend-reports/asthma-trend-report.pdf
- 4.Mehuys E. Van Bortel L. De Bolle L. Van Tongelen I. Annemans L. Remon JP. Brusselle G. Effectiveness of pharmacist intervention for asthma control improvement. Eur Respir J. 2008;31:790–799. doi: 10.1183/09031936.00112007. [DOI] [PubMed] [Google Scholar]
- 5.Gillisen A. Patient's adherence in asthma. J Physiol Pharmacol. 2007;58(Suppl 5):205–222. [PubMed] [Google Scholar]
- 6.Smith JR. Mildenhall S. Noble M. Mugford M. Shepstone L. Harrison BD. Clinician-assessed poor compliance identifies adults with severe asthma who are at risk of adverse outcomes. J Asthma. 2005;42:437–445. doi: 10.1081/JAS-67949. [DOI] [PubMed] [Google Scholar]
- 7.Bender BG. Overcoming barriers to nonadherence in asthma treatment. J Allergy Clin Immunol. 2002;109(6 Suppl):S554–S559. doi: 10.1067/mai.2002.124570. [DOI] [PubMed] [Google Scholar]
- 8.Howell G. Nonadherence to medical therapy in asthma: Risk factors, barriers, and strategies for improving. J Asthma. 2008;45:723–729. doi: 10.1080/02770900802395512. [DOI] [PubMed] [Google Scholar]
- 9.Hibbard JH. Stockard J. Mahoney ER. Tusler M. Development of the Patient Activation Measure (PAM): Conceptualizing and measuring activation in patients and consumers. Health Serv Res. 2004;39:1005–1026. doi: 10.1111/j.1475-6773.2004.00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herborg H. Soendergaard B. Froekjaer B. Fonnesbaek L. Jorgensen T. Hepler CD. Grainger-Rousseau TJ. Ersboell BK. Improving drug therapy for patients with asthma—Part 1: Patient outcomes. J Am Pharm Assoc (Wash) 2001;41:539–550. doi: 10.1016/s1086-5802(16)31278-5. [DOI] [PubMed] [Google Scholar]
- 11.Herborg H. Soendergaard B. Jorgensen T. Fonnesbaek L. Hepler CD. Holst H. Froekjaer B. Improving drug therapy for patients with asthma—Part 2: Use of antiasthma medications. J Am Pharm Assoc (Wash) 2001;41:551–559. doi: 10.1016/s1086-5802(16)31279-7. [DOI] [PubMed] [Google Scholar]
- 12.Mangiapane S. Schulz M. Muhlig S. Ihle P. Schubert I. Waldmann HC. Community pharmacy-based pharmaceutical care for asthma patients. Ann Pharmacother. 2005;39:1817–1822. doi: 10.1345/aph.1G180. [DOI] [PubMed] [Google Scholar]
- 13.Valet RS. Perry TT. Hartert TV. Rural health disparities in asthma care and outcomes. J Allergy Clin Immunol. 2009;123:1220–1225. doi: 10.1016/j.jaci.2008.12.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ownby DR. Asthma in rural America. Ann Allergy Asthma Immunol. 2005;95(5 Suppl 1):S17–S22. doi: 10.1016/s1081-1206(10)61005-8. [DOI] [PubMed] [Google Scholar]
- 15.Withy K. Davis J. Followup after an emergency department visit for asthma: Urban/rural patterns. Ethn Dis. 2008;18(2 Suppl 2):S2-247–S2-251. [PMC free article] [PubMed] [Google Scholar]
- 16.Bender BG. Apter A. Bogen DK. Dickinson P. Fisher L. Wamboldt FS. Westfall JM. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. J Am Board Fam Med. 2010;23:159–165. doi: 10.3122/jabfm.2010.02.090112. [DOI] [PubMed] [Google Scholar]
- 17.Angaran DM. Telemedicine and telepharmacy: Current status and future implications. Am J Health Syst Pharm. 1999;56:1405–1426. doi: 10.1093/ajhp/56.14.1405. [DOI] [PubMed] [Google Scholar]
- 18.McLean S. Chandler D. Nurmatov U. Liu J. Pagliari C. Car J. Sheikh A. Telehealthcare for asthma. Cochrane Database Syst Rev. 2010;(10):CD007717. doi: 10.1002/14651858.CD007717.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Poulson LK. Nissen L. Coombes I. Pharmaceutical review using telemedicine—A before and after feasibility study. J Telemed Telecare. 2010;16:95–99. doi: 10.1258/jtt.2009.090716. [DOI] [PubMed] [Google Scholar]
- 20.Elliott RA. Barber N. Clifford S. Horne R. Hartley E. The cost effectiveness of a telephone-based pharmacy advisory service to improve adherence to newly prescribed medicines. Pharm World Sci. 2008;30:17–23. doi: 10.1007/s11096-007-9134-y. [DOI] [PubMed] [Google Scholar]
- 21.Clifton GD. Byer H. Heaton K. Haberman DJ. Gill H. Provision of pharmacy services to underserved populations via remote dispensing and two-way videoconferencing. Am J Health Syst Pharm. 2003;60:2577–2582. doi: 10.1093/ajhp/60.24.2577. [DOI] [PubMed] [Google Scholar]
- 22.Barbanel D. Eldridge S. Griffiths C. Can a self-management programme delivered by a community pharmacist improve asthma control? A randomised trial. Thorax. 2003;58:851–854. doi: 10.1136/thorax.58.10.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bynum A. Hopkins D. Thomas A. Copeland N. Irwin C. The effect of telepharmacy counseling on metered-dose inhaler technique among adolescents with asthma in rural Arkansas. Telemed J E Health. 2001;7:207–217. doi: 10.1089/153056201316970902. [DOI] [PubMed] [Google Scholar]
- 24.Young HN. Havican SN. Chewning BA. Sorkness CA. Ruppel X. Griesbach SA. Patient And phaRmacist Telephonic Encounters (PARTE) in an underserved rural population with asthma: Methods and rationale. Innov Pharm. 2011;2:1–10. doi: 10.24926/iip.v2i3.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gardner M. Boyce RW. Herrier RN. Pharmacist-patient consultation program PPCP—Unit I. Washington, DC: Indian Health Service, U.S. Public Health Service; 1997. [Google Scholar]
- 26.Weiss M. Britten N. What is concordance? Pharmaceut J. 2003;271:493. [Google Scholar]
- 27.Nelson P. Young HN. Knobloch MJ. Griesbach SA. Telephonic monitoring and optimization of inhaler technique. Telemed J E Health. 2011;17:734–740. doi: 10.1089/tmj.2011.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bandura A. Self-efficacy: The exercise of control. New York: Freeman; 1997. [Google Scholar]
- 29.Bird L. Arthur A. Cox K. "Did the trial kill the intervention?" Experiences from the development, implementation and evaluation of a complex intervention. BMC Med Res Methodol. 2011;11:24. doi: 10.1186/1471-2288-11-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Murtagh MJ. Thomson RG. May CR. Rapley T. Heaven BR. Graham RH. Kaner EF. Stobbart L. Eccles MP. Qualitative methods in a randomised controlled trial: The role of an integrated qualitative process evaluation in providing evidence to discontinue the intervention in one arm of a trial of a decision support tool. Qual Saf Health Care. 2007;16:224–229. doi: 10.1136/qshc.2006.018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nathan RA. Sorkness CA. Kosinski M. Schatz M. Li JT. Marcus P. Murray JJ. Pendergraft TB. Development of the Asthma Control Test: A survey for assessing asthma control. J Allergy Clin Immunol. 2004;113:59–65. doi: 10.1016/j.jaci.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 32.Schatz M. Sorkness CA. Li JT. Marcus P. Murray JJ. Nathan RA. Kosinski M. Pendergraft TB. Jhingran P. Asthma control test: Reliability, validity, and responsiveness in patients not previously followed by asthma specialists. J Allergy Clin Immunol. 2006;117:549–556. doi: 10.1016/j.jaci.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 33.Hibbard JH. Mahoney ER. Stockard J. Tusler M. Development and testing of a short form of the patient activation measure. Health Serv Res. 2005;40:1918–1930. doi: 10.1111/j.1475-6773.2005.00438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morisky DE. Ang A. Krousel-Wood M. Ward HJ. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens (Greenwich) 2008;10:348–354. doi: 10.1111/j.1751-7176.2008.07572.x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Barber JA. Thompson SG. Analysis of cost data in randomized trials: An application of the non-parametric bootstrap. Stat Med. 2000;19:3219–3236. doi: 10.1002/1097-0258(20001215)19:23<3219::aid-sim623>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 36.Efron B. Tibshirani RJ. An introduction to the bootstrap. New York: Chapman & Hall; 1993. [Google Scholar]
- 37.Stata statistical software: Release 11. College Station, TX: StataCorp LP; 2009. [Google Scholar]
- 38.Bender BG. Rankin A. Tran ZV. Wamboldt FS. Brief-interval telephone surveys of medication adherence and asthma symptoms in the Childhood Asthma Management Program Continuation Study. Ann Allergy Asthma Immunol. 2008;101:382–386. doi: 10.1016/S1081-1206(10)60314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hibbard JH. Mahoney ER. Stock R. Tusler M. Do increases in patient activation result in improved self-management behaviors? Health Serv Res. 2007;42:1443–1463. doi: 10.1111/j.1475-6773.2006.00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van der Meer V. van Stel HF. Bakker MJ. Roldaan AC. Assendelft WJ. Sterk PJ. Rabe KF. Sont JK. Weekly self-monitoring and treatment adjustment benefit patients with partly controlled and uncontrolled asthma: An analysis of the SMASHING study. Respir Res. 2010;11:74. doi: 10.1186/1465-9921-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Expert Panel Report 3 (EPR-3): Guidelines for the diagnosis and management of asthma—Summary report 2007. J Allergy Clin Immunol. 2007;120(5 Suppl):S94–S138. doi: 10.1016/j.jaci.2007.09.043. Erratum in J Allergy Clin Immunol 2008;121:1330. [DOI] [PubMed] [Google Scholar]
- 42.Rothkopf J. Brookler K. Wadhwa S. Sajovetz M. Medicaid patients seen at federally qualified health centers use hospital services less than those seen by private providers. Health Aff. 2011;30:1335–1342. doi: 10.1377/hlthaff.2011.0066. [DOI] [PubMed] [Google Scholar]
- 43.Probst JC. Laditka JN. Laditka SB. Association between community health center and rural health clinic presence and county-level hospitalization rates for ambulatory care sensitive conditions: An analysis across eight US states. BMC Health Serv Res. 2009;9:134. doi: 10.1186/1472-6963-9-134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang W. Mueller KJ. Chen LW. Conway K. The role of rural health clinics in hospitalization due to ambulatory care sensitive conditions: A study in Nebraska. J Rural Health. 2006;22:220–223. doi: 10.1111/j.1748-0361.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 45.Rust G. Baltrus P. Ye JL. Daniels E. Quarshie A. Boumbulian P. Strothers H. Presence of a community health center and uninsured emergency department visit rates in rural counties. J Rural Health. 2009;25:8–16. doi: 10.1111/j.1748-0361.2009.00193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]