Abstract
The pace of reproductive aging has been of considerable interest, especially in regard to the long postreproductive period in modern women. Here we use data for both sexes from a 37-year longitudinal study of a wild baboon population to place reproductive aging within a life history context for this species, a primate relative of humans that evolved in the same savannah habitat as humans did. We examine the patterns and pace of reproductive aging, including birth rates and reproductive hormones for both sexes, and compare reproductive aging to age-related changes in several other traits. Reproductive senescence occurs later in baboon females than males. Delayed senescence in females relative to males is also found in several other traits, such as dominance status and body condition, but not in molar wear or glucocorticoid profiles. Survival, health, and well-being are the product of risk factors in morphological, physiological, and behavioral traits that differ in rate of senescence and in dependence on social or ecological conditions; some will be very sensitive to differences in circumstances and others less so.
Keywords: reproductive aging, baboons, toothwear, body condition, steroid hormones, senescence
Over 30 years ago, Sarah Hrdy introduced what arguably stands as the first published lifespan perspective on female reproductive strategies in wild nonhuman primates.1–3 Prevailing wisdom at the time was that senescence did not occur in wild populations. In contrast, Hrdy posited that female nonhuman primates experience age-related reproductive decline and a postreproductive stage that presages the long postreproductive period of modern women (see Hawkes and Smith3a). Moreover, she suggested that within natural societies of nonhuman primates, such as those of the langur monkeys she studied in Asia, reproductive senescence is associated with fitness benefits.1–3
Thus, our study of reproductive aging in wild baboons has roots in Hrdy’s perspective on langur reproductive strategies, but relevant data for aging in natural populations were largely unavailable at that time. In fact, patterns of aging in wild animals, not just wild primates, still remain largely undescribed despite their importance to understanding fundamental aspects of life-history evolution, and despite early speculation on their importance in the evolutionary and behavioral ecology of humans and other species.
Using almost four decades of data from the Amboseli Baboon Research Project (ABRP), we are now in a position to evaluate reproductive aging in wild female primates and compare females’ reproductive time-course with that of males. In addition, we examine reproductive aging of both sexes in the context of aging patterns in several other traits, addressing the question of whether reproductive aging resembles or stands out from that of other traits. We begin with a general introduction to baboons and their relationships to humans, and then describe the Amboseli baboon population, the ABRP longitudinal life-history research program, and a brief overview of relevant methods.
Wild baboons as a model for understanding human aging
Baboons (genus Papio) are among the best studied of the cercopithecine primates.4 They are large, diurnal, semi-terrestrial monkeys that are highly selective and flexible foragers. Baboons have a close evolutionary relationship with humans (~94% sequence similarity genome-wide)5 and striking behavioral and ecological similarities as well.6 They have achieved a nearly continental distribution in Africa, occupying habitats ranging from moist evergreen forests to deserts, and from equatorial to temperate regions. Among primates, this positions baboons as second only to humans in geographical and environmental range. Their ability to cope with environmental extremes and with high levels of predictable and unpredictable environmental variability, even during demanding life stages, such as parental care and aging, is similarly shared with humans. Furthermore, baboons show little or no seasonality of reproduction in most habitats. In other words, baboons have both adapted to diverse habitats and, in major aspects of their life histories, including those associated with reproduction, have largely escaped from the seasonal constraints of these diverse habitats. This is a combination of traits that they share with humans but with few other primates. Like many human hunter-gatherer societies, most baboon (sub)species live in stable social groups of 20–100 members, including multiple adults and juveniles of both sexes. Baboons also possess highly differentiated social relationships, and a flexible mating and social system. These traits position baboons as a widespread “generalist” species, like humans.
In the wild, female baboons usually reach menarche early in their fifth year; during adulthood they have highly visible and regular sexual swellings that indicate ovarian cycle phase,7 and they exhibit skin-color changes on their hindquarters that indicate pregnancy, beginning by the end of the first trimester of their 6-month gestation. Infants are completely dependent on their mothers for almost a year, followed by a second year of increasing independence during which time their mothers usually conceive again. Males experience testicular enlargement and associated onset of sperm production in their sixth or seventh year, but do not become fully adult until they are about 8–10 years old, when they achieve an adult body mass nearly twice that of adult females. Males usually disperse from their group of birth as older subadults or young adults. Females and males do not form permanent mating bonds with each other. Instead, mating takes place in the context of mate-guarding episodes, typically called consortships. Demographic senescence in Amboseli is evident by about 15 years of age.8 In Amboseli and two other wild populations, the oldest reported individuals were 27-year-old females.8a Baboons and humans spend a similar proportion of their lifetime in the several major life stages (infancy, the juvenile period, adolescence, and adulthood), with each stage in baboons—and the total lifespan—lasting about one-third as many years as in humans.
The Amboseli ecosystem, baboon population, and baboon research project
Located at the northwestern base of Mt. Kilimanjaro, the Amboseli-Longido ecosystem is a semi-arid, short-grass savanna in a Pleistocene lakebed. This ecosystem exhibits both seasonal and long-term patterns of environmental change, ones that are typical, in type and magnitude, of the changes that characterized the East African environments in which both humans and baboons evolved.9,10
The ABRP, ongoing since 1971, includes detailed longitudinal data on demography, ontogeny, ecology, behavior, parentage, and steroid hormone profiles on known individuals that are part of the robust undisturbed Amboseli baboon population (see www.princeton.edu/~baboon and publications therein). At any given time, the ABRP monitors approximately 300 individually known animals of all ages. The data reported here were obtained solely on animals that move freely through their environment and subsist entirely on natural food sources found in the wild. We visually recognize all individuals in our five study groups; like humans, baboons have highly distinctive facial and morphological features that make them easily recognizable to experienced observers. All animals in study groups are well habituated to the presence of neutral observers.
Our various methods are described in the next section.
Methods
We employ several distinct research methodologies in collecting individual-based data, including the following for the results presented herein: (1) observational monitoring of demographic and behavioral events for all individuals in the study population, (2) genetic paternity analysis, (3) steroid hormone analysis, and (4) occasional darting to immobilize animals in order to take morphometric measurements and perform other procedures. Data are subsequently stored and accessed through our long-term database, BABASE (www.papio.biology.edu).
Field methods for data collection
Our long-term analyses of data from ABRP depend on continuity and consistency in the quality and intensity of data collection over the years. We achieve this consistency through a number of key resources and strategies, including the presence of long-term observers and a comprehensive set of written protocols for field, lab, and data management, which are publicly available online at the ABRP website (www.princeton.edu/~baboon). Some types of data have been obtained since 1971, e.g., demographic events for some individuals. Other types of data collection began at various times since then. In particular, fecal samples for genetic analysis of paternity are available since 1993 (these samples represent individuals born as early as 1968), fecal samples for hormone metabolite extraction since 2000, and morphological data from 1989–1991 and 2006–2008.
Our observational methods are well documented, and we outline them briefly here. Six days per week, we follow 1–2 social groups of baboons each day for six observation hours per day. Throughout the day, we collect a diverse range of observational data including those of particular relevance for evaluating aging in the traits reported here. We begin each day with a systematic group census to record births, deaths, immigrations, and emigrations. We also record the color of each female’s paracallosal skin, and the size and status (turgescent or deturgescent) of her sex skin.7,11,12 This information allows us to retrospectively determine, for each female on each day of the study, her reproductive condition (pregnant, cycling, lactating) and, if cycling, the day of her cycle relative to ovulation. Throughout the day, while collecting systematic behavioral samples, we collect freshly deposited fecal samples from all individuals; these provide our primary window into the physiology of aging and our primary source of DNA for paternity determination (used for measuring male birth rates).
Genetic paternity analysis
Fecal DNA occurs in low quantity and is often degraded. However, extensive work by a number of groups, including ours, has clearly shown that with careful controls the results of fecal DNA analysis are reliable and repeatable.13–17 We assign paternity based on exclusion and also through the use of the likelihood-based paternity assignment program CERVUS 2.0.18 With these methods we have unambiguously assigned paternity to approximately 300 individuals born in Amboseli (Alberts et al.17 and unpublished). Paternity assignments, which are comparable to the known maternities for females, provide the data for evaluating age-related patterns of male birth rates by associating each birth with the age of the infant’s father.
Fecal steroid analysis (glucocorticoids and reproductive steroid hormones)
Freshly deposited fecal samples from known individuals are collected in vials prefilled with 95% ethanol to approximate a volumetric ratio of 2:5 feces to ethanol. Samples are stored in Amboseli for less than 2 weeks in a charcoal refrigerator, then are transported first to the University of Nairobi for initial processing and then to Princeton University for hormone extraction and purification followed by assay using an 125I radioimmunoassay.19–21
Results and discussion
Age-related changes in reproduction: birth rates and steroid hormones
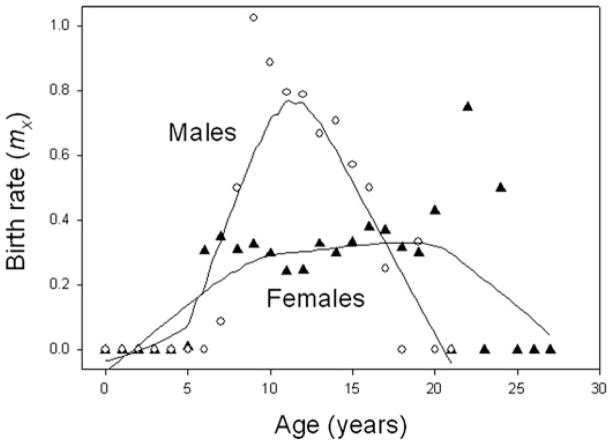
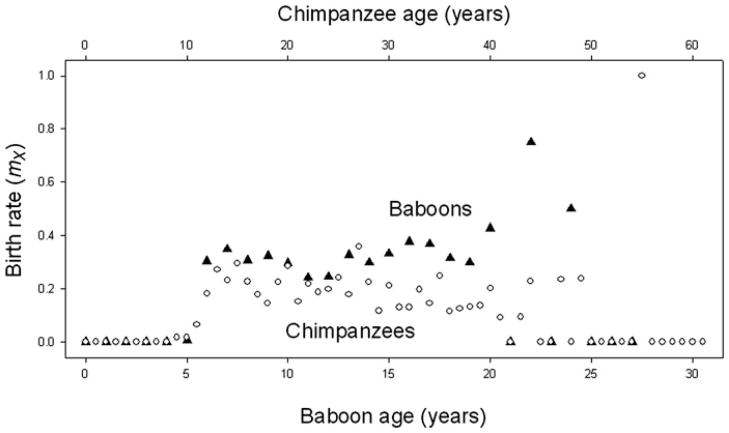
Baboon reproductive output declines with age, and the patterns of change differ from humans in potentially informative ways. In Amboseli, females continue to produce offspring in their early to mid-20s—equivalent to women in their 60s. In addition, birth rates start to decline only at approximately 18 years of age8 (Fig. 1, also Ref. 12), an age that is equivalent to the 50s in humans (relying again on the general observation that the various baboon life stages are about one-third as long as the equivalent in humans). The age-related pattern of birth rates in baboon females is essentially the same as that recently reported for wild populations of chimpanzees.22 Like the baboons, the chimpanzees exhibited declining birth rates only at relatively old ages and complete cessation of births occurred among only the very oldest individuals (Fig. 2).
Figure 1.
Age-related patterns of birth rates for Amboseli baboons. Female birth rates (filled triangles) are stable until late adulthood. In contrast, male birth rates (open circles; based on genetic analysis) exhibit a strongly age-related pattern, following a similar pattern to dominance rank,24 with a sharp peak early in adulthood followed by a relatively steep decline. Plotted with lowest curve, window = 0.5.
Figure 2.
Age-related patterns of birth rates for female Amboseli baboons and for chimpanzees—filled triangles for baboons, open circles for chimpanzees. Baboon data are as in Figure 1. Chimpanzee data are from Ref. 22; note that data for ages 53–61 years in the chimpanzee plot are from a single female. Data are plotted on a scale of two chimpanzee years to one for baboons.
As expected from the stable birth rates across most of adulthood for baboon females, female baboons also maintain relatively high estrogen concentrations into late adulthood (Fig. 3), although high variance characterizes the later age classes, consistent with findings of greater variance in reproductive measures among older women.23 Sample sizes for individual fecal estrogen (fE) profiles for baboon females do not yet provide adequate statistical power to evaluate longitudinal patterns across adulthood, but the available data indicate individual patterns very similar to the populational one. In addition, we are initiating studies to evaluate age changes in progesterone; a small preliminary populational data set revealed no age-related change in progesterone (fP) concentration or in the fE/fP ratio (P = 0.909 and 0.577, respectively).
Figure 3.
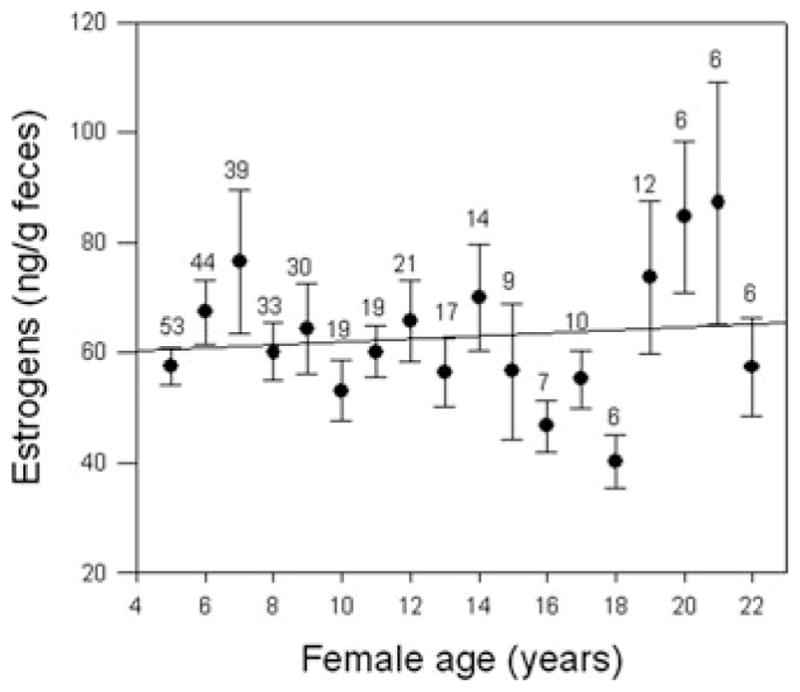
Estrogen concentrations for baboon females are stable into relatively old age, following a similar pattern to that for female birth rates. The plotted value and bars for each year represent the mean and SE across all cycling individuals (pregnant and lactating individuals were excluded). Within any year, each female is represented by only a single data point, which is calculated as the mean of all values obtained for that female during that year of age. The number of individuals contributing to the data for each age appears just above the error bar for that age.
Male baboons in Amboseli, in contrast to females, experience peak paternity in early adulthood (around 9 years of age), and a steady age-related decline in reproductive output begins immediately thereafter (Fig. 1).17 The decline in male birthrates occurs as male dominance rank declines because male reproductive output is highly influenced by competition with other males; high-ranking males generally obtain greater access to reproductive females than do low-ranking males.24
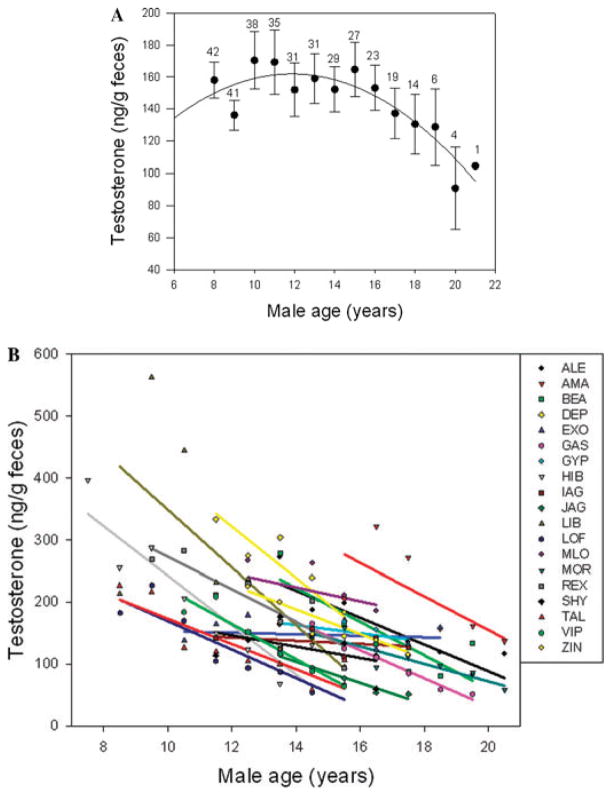
Adult male baboons also exhibit clear age-related decline in testosterone21,25 (fT) (Fig. 4A), although testosterone decreases a few years later and the decline proceeds more gradually than the changes in male birth rates. This decline in testosterone occurs when estrogens in cycling females remain high. This population-level pattern could result from a situation in which males with initially high testosterone experience high mortality risk, so that surviving males are disproportionately those with consistently low T, having exhibited little or no decrease in T concentrations during aging. However, initial examination of individual trajectories of T for Amboseli males (Fig. 4B), reveals that declining T concentrations with age are characteristic of 17 of the 19 individual males that we have observed. Declines in T with age are widely documented in men and are often associated with health risks.26,27 Interestingly, Feldman et al.26 found steeper declines in their longitudinal study than suggested by cross-sectional trends, perhaps suggesting that men with more steep declines are at higher mortality risk. Future investigations will evaluate predictors and sequelae of individual variability in both peak levels and rate of decline in baboon males.
Figure 4.
(A) Testosterone concentrations decline with age for baboon males in our sample, starting by mid-adulthood (see Fig. 3 for the contrasting pattern in females). Calculations and symbols as in Figure 3. (B) The population-level decline with age in testosterone concentrations can be explained by decline with age for individual adult male baboons across adulthood (at least 5 years of data for each male). Only 2 of 19 males did not exhibit a pattern of decline.
Preliminary analyses of a small data set (not graphed) indicate that estrogen (fE) concentrations in our population of male baboons increased (R2 = 0.396, P = 0.016) and fT to fE ratios decreased with age (R2 = 0.558, P = 0.002). High fE in males as in females may be protective against bone loss28 and cardiovascular disease.29 However, declines in T -to-E ratio are suggested by some studies of men30–33 and are thought to have adverse consequences for male longevity.
In summary, in both offspring production and fecal estrogen profiles, female reproductive patterns are age-related only during relatively old ages, and the pattern in offspring production is strikingly similar to that reported for wild chimpanzees. In contrast, male reproductive patterns are highly age-related in both offspring production and fecal testosterone. They exhibit a pattern of rapid rise in early adulthood followed shortly thereafter by steady decline.21 This decline parallels the well-known pattern of age-related dominance rank in male baboons.
Age-related patterns of senescence in other morphological and physiological traits
Age-related changes in body mass index (BMI)
In humans, having a low BMI is a known risk factor for mortality, particularly among the elderly,34 so that the relationship between BMI and mortality risk is U-shaped, with mortality risk increasing at both extremes.35,36 Studies of western humans have often focused on potential adverse effects of a high BMI (of being overweight or obese) because obesity is so prevalent in these societies. However, the adverse effects of obesity appear to be attenuated in old age, while the adverse effects of being underweight are exacerbated.36–38
In Amboseli, healthy baboons that subsist entirely on natural foods exhibit an extraordinarily low level of body fat, 1.9%, and they never approach obesity (although obesity does occur in baboons that visit refuse sites associated with tourist facilities, not the subjects of this study).39 Not only is obesity absent, we have frequently observed animals that appear to be exceptionally thin, particularly old animals (Fig. 5) or females that are lactating during dry seasons or droughts.
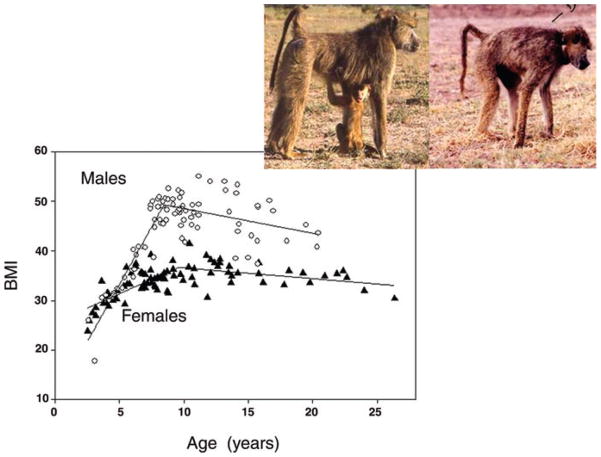
Figure 5.
BMI increases for females (filled triangles) during early years of adulthood (until about 10 years of age, approximately 4 years after their first birth) and then declines very gradually during aging. Male (open circles) BMI increases more dramatically until attainment of adulthood (age 8 years) and then begins a more rapid decline than that of female BMI. Plotted lines are for best-fit piecewise regression model, providing a somewhat better fit for males (adj R2 = 0.76) and an equally good fit (adj R2 = 0.51) for females than a quadratic model. Pictured is a female during early adulthood and the same female at age 27, the oldest age yet recorded for wild baboons.
We measured BMI in a subset of the Amboseli baboons that we briefly immobilized through darting. BMI exhibits clear age-related patterns and sex differences during adulthood (Fig. 5). The two sexes do not differ in BMI until the sixth year of life, when females experience their first conception and males enter into the subadult period that is characterized by an adolescent growth spurt not seen in females.40 This male growth spurt occurs more in weight than in long bones and results in BMI in young adult males that is almost 50% greater, and body mass approximately 100% greater, than that of same-aged females. Male BMI peaks at about the onset of adulthood (8 years of age) and declines steadily throughout adulthood. Females, in contrast, continue to experience gradual increase in BMI during the early years of adulthood, peaking at approximately 10 years of age (4 years after the average age at first birth). Female BMI then declines steadily but at a slower rate than in males, narrowing the sex difference. Very few males live into their 20s,8 and the limited data for males in the late teens suggest high variability and perhaps differential mortality for those of low BMI. Tooth wear is likely to be a major contributor to changes in BMI and is described below.
Age-related changes in tooth wear
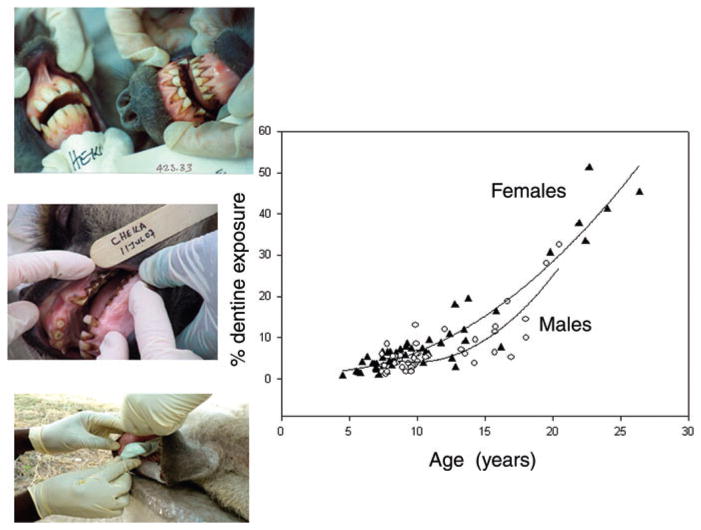
Tooth wear increases with age in many species (including several primates41–44). A landmark study in wild lemurs found that increased tooth wear in older mothers was associated with decreased survival of their infants,43 indicating a strong link between tooth wear and food processing ability. In Amboseli, we have previously documented that periodontal health decreases with age.45 Further, we have observed dramatic changes in tooth wear with age, using photographs taken during brief immobilizations (Fig. 6).
Figure 6.
Molar wear (measured as proportion of dentine exposure, PDE) is age-related in both sexes, as seen here for M1—the first molar. M1 wear tends to be as great in females (filled triangles) as in males (open circles) during aging. Quadratic models: adj R2 = 0.90 for females, adj R2 = 0.69 for males. Pictured is a comparison of a young adult female (7 years old) with her elderly mother (21 years old), also a 24-year-old female. Also shown is the molding technique used to obtain casts for measuring PDE.
We recently collected high quality tooth impressions on a subset of baboons that were briefly immobilized through darting. We measured tooth wear as the percentage of dentine exposure on the occlusal surface. Our analysis of tooth wear shows a strong signal of age in both sexes (Fig. 6 for the M1—the first molar).46,47,47a Unlike age-related patterns of change in BMI and reproduction, the age-related pattern in molar wear does not exhibit a male-biased aging pattern (Fig. 6). However, qualitative observations suggest that females experience similar aging patterns on their molars as on their other teeth, whereas males experience more major breaks and total loss of these other teeth; this will be the subject of forthcoming evaluation.
Age-related changes in glucocorticoids
Aging-associated hypercortisolism, manifested either in basal levels or in a stress response that is resistant to feedback mechanisms, has been documented in humans and in several studies of nonhuman primates.48,49 Chronically elevated glucocorticoid levels can jeopardize health and survival50,51 by decreasing reproductive hormones, suppressing immunity, promoting atherosclerosis and ulcers, decreasing muscle mass, and impairing growth and tissue repair.52–54 Although the incidence of cardiovascular diseases and ulcers is unknown for wild baboons, an increased risk of infectious diseases, a decrease in cutaneous wound healing, and loss of muscle mass are all likely to increase mortality risk as well as morbidity in natural populations.54
We documented a striking age-related change in adrenocortical axis function in Amboseli baboons, using a rare opportunity in which we collected blood from over 100 animals in 1989 and 1990.48 Males and females in our oldest age classes experienced both higher basal cortisol levels and increased resistance to downregulation. The single, cross-sectional measurements in that study provided the foundation for our recent ongoing studies of excreted glucocorticoids (fGC). Our initial analyses support our prior finding of an increase throughout adulthood in glucocorticoid concentrations for females (Fig. 7). Males, however, show no simple change with age in fGC in these longer-term analyses, and instead evidence more complex patterns, which are currently under investigation. Thus, glucocorticoids, like molar wear, exhibit age-related changes but not the strong male-biased sex differences in senescence that were evident in BMI, reproductive rates, and reproductive hormones.
Figure 7.
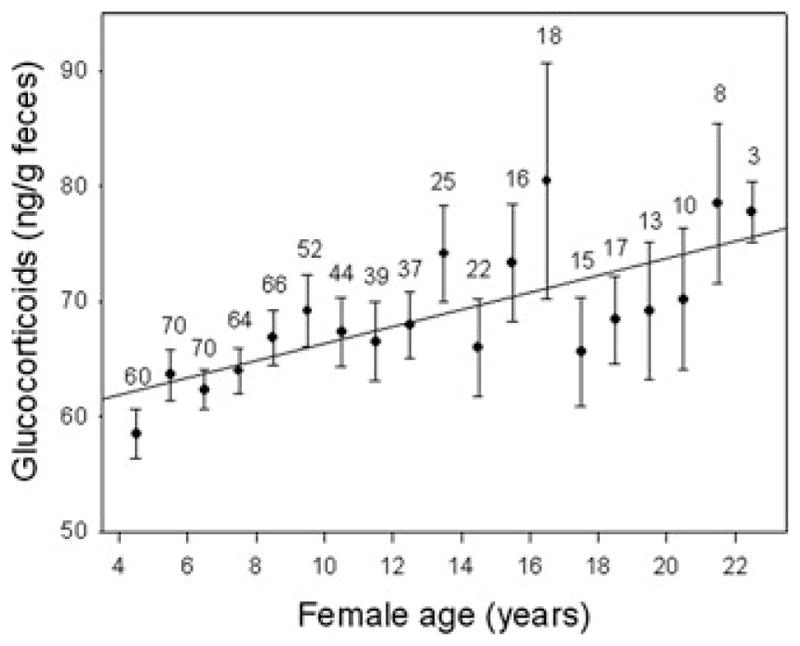
Glucocorticoid concentrations increase gradually with age for females. Calculations and symbols as in Figure 3.
Reproductive senescence in the wild: a comparison with other somatic systems and between the sexes
The signature of senescence in wild baboons is clear across diverse morphological, physiological, and behavioral systems, including reproduction of both females and males. However, the timing and pace of senescence differs across systems, in some cases between the sexes, and between baboons and humans, in multiple, informative ways. For example, reproductive senescence for female baboons is evident in birth rates and in estrogen levels only relatively late in life. Moreover, complete cessation of offspring production is experienced only by the very oldest females, those who reach their mid-20s, an age at which body condition as measured by BMI is very low and tooth wear is extensive. The age-related pattern of female baboon birth rates is the same as that in chimpanzee females. It stands in contrast to that observed in women, in which the timing of reproductive senescence far exceeds that of other organ systems, resulting in a long postreproductive period in humans (e.g., in Ref. 55 and sequala).
For baboon males, reproductive decline in both birth rates and testosterone concentrations parallel the early and rapid decline in male dominance status and in body condition (BMI). Thus, a potentially intertwined complex of traits—social, physiological, morphological, and offspring production—exhibit earlier and more rapid decline in males than in females. Recent comparisons of age-related patterns of androgens in men33 and in several closely related monkey taxa21 including Amboseli baboons, exhibit interesting predictability across taxa or subsets of human populations that can be related to levels of parental care or mating patterns. These findings suggest important directions for insights into plasticity of senescence patterns in men and nonhuman primate males across a range of taxa, as well as across ecological and social contexts.
Survival, overall health, and well-being reflect senescence and risk factors in many morphological, physiological, and behavioral traits. Each trait follows its own patterns of aging, either independently or in some cases with causative interrelationships. Age-related variability in these traits, in their causative relationships, and in their joint impact, will also exhibit varying degrees of dependence on social or ecological conditions; some will be very sensitive to differences in circumstances and others perhaps less so. The age-related pattern in androgen concentrations among men and among nonhuman primates is one example of a trait that is highly contingent upon social context.21,33 Another example of condition-dependent aging involves female reproduction in nonhuman primates. Some traits, such as age of menarche and fertility rates, exhibit strong response to differences in food availability; such responses are particularly evident where primates are provisioned by humans, either in captivity or in cases where the nonhuman populations live in close commensalism with humans and their foods. These situations provided most of the examples of postreproductive females first identified by Hrdy,2 and they constitute the majority of those reported recently across a broad range of primates.56 In addition, in these situations, we would expect that age-related senescence in body condition and tooth wear will be attenuated, as will demographic senescence. This may result in a greater prevalence of females experiencing a postreproductive period and perhaps, though not necessarily, an extended duration of the postreproductive phase if survival is enhanced more than fertility is (see Hawkes and Smith3a).
Understanding the evolutionary history, current diversity, and future potential of aging in humans and our closest relatives will require understanding the plasticities of various age-related traits and the contingencies among them. Nonhuman primate models from a range of ecological conditions will be essential for attaining this understanding, and critical for achieving a breadth of insights into aging.
Acknowledgments
Financial support for ABRP research was provided primarily by NSF IBN-0322613, NSF BSE-0323553, RO3 MH65294, NIA P30AG024361, and the Chicago Zoological Society. We thank the Kenya Wildlife Services, Institute of Primate Research, National Museums of Kenya, and members of the Amboseli-Longido pastoralist communities. Thanks go to the Amboseli field team who contributed to sample and data collection (R.S. Mututua, S. Sayialel, and J.K. Warutere) and to T. Wango for assistance in Nairobi. Thanks to T. Fenn, N. Learn, and L. Maryott for database assistance and to M. Emery-Thompson for the chimpanzee data in Figure 2. This research was approved by the IACUC at Princeton University (protocol #1689, 9 November 2007) and Duke University (#A1830-06-04), and adhered to all the laws and guidelines of Kenya (Kenya Research Permit MOEST 13/001/C351 Vol. II). We are grateful to two anonymous reviewers for helpful comments on an earlier version of the manuscript and to the NIA for supporting the Workshop on Reproductive Aging.
Footnotes
Conflicts of interest
The authors declare no conflicts of interest.
References
- 1.Hrdy SB. The Langurs of Abu: Female and Male Strategies of Reproduction. Harvard University Press; Cambridge, MA: 1977. [Google Scholar]
- 2.Hrdy SB. “Nepotists” and “altruists”: the behavior of old females among macaques and langur monkeys. In: Amuss PT, Harrell S, editors. Other Ways of Growing Old: Anthropological Perspectives. Stanford University Press; Stanford: 1981. pp. 59–76. [Google Scholar]
- 3.Hrdy SB. The Woman That Never Evolved. Harvard University Press; Cambridge: 1981. [Google Scholar]
- 3a.Hawkes K, Smith KR. Do women stop early? Similarities in fertility decline in humans and chimpanzees. Ann NY Acad Sci. 2010;1204:43–53. doi: 10.1111/j.1749-6632.2010.05527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Swedell L, Leigh SR. Reproduction and Fitness in Baboons. Springer; New York: 2006. [Google Scholar]
- 5.Rogers J, Hixson JE. Baboons as an animal model for genetic studies of common human disease. Am J Hum Genet. 1997;61:489–493. doi: 10.1086/515527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jolly CJ. Babooons, mandrills, and mangabeys: afropapionin socioecology in a phylogenetic perspective. In: Campbell CJ, et al., editors. Primates in Perspective. Oxford University Press; New York: 2007. pp. 240–251. [Google Scholar]
- 7.Gesquiere LR, Wango EO, Alberts SC, Altmann J. Mechanisms of sexual selection: sexual swellings and estrogen concentrations as fertility indicators and cues for male consort decisions in wild baboons. Horm Behav. 2007;51:114–125. doi: 10.1016/j.yhbeh.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 8.Alberts SC, Altmann J. Matrix models for primate life history analysis. In: Kappeler PM, Pereira ME, editors. Primate Life Histories and Socioecology. University of Chicago Press; Chicago: 2003. pp. 66–102. [Google Scholar]
- 8a.Bronikowski A, et al. The aging baboon: comparative demography and lifespan heritability in a nonhuman primate model system. Proc Natl Acad Sci USA. 2001;99:9591–9595. doi: 10.1073/pnas.142675599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Behrensmeyer AK. Climate change and human evolution. Science. 2006;311:476–478. doi: 10.1126/science.1116051. [DOI] [PubMed] [Google Scholar]
- 10.Potts R. Variability selection in hominid evolution. Evol Anthropol. 1998;7:81–96. [Google Scholar]
- 11.Beehner JC, et al. The endocrinology of pregnancy and fetal loss in wild baboons. Horm Behav. 2006;49:688–699. doi: 10.1016/j.yhbeh.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 12.Beehner JC, Onderdonk DA, Alberts SC, Altmann J. The ecology of conception and pregnancy failure in wild baboons. Behav Ecol. 2006;17:741–750. [Google Scholar]
- 13.Borries C, et al. DNA analyses support the hypothesis that infanticide is adaptive in langur monkeys. Proc R Soc Lond B Biol Sci. 1999;266:901–904. doi: 10.1098/rspb.1999.0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Constable J, Ashley MV, Goodall J, Pusey AE. Noninvasive paternity assignment in Gombe chimpanzees. Mol Ecol. 2001;10:1279–1300. doi: 10.1046/j.1365-294x.2001.01262.x. [DOI] [PubMed] [Google Scholar]
- 15.Morin PA, Chambers KE, Boesch C, Vigilant L. Quantitative polymerase chain reaction analysis of DNA from noninvasive samples for accurate microsatellite genotyping of wild chimpanzees (Pan troglodytes verus) Mol Ecol. 2001;10:1835–1844. doi: 10.1046/j.0962-1083.2001.01308.x. [DOI] [PubMed] [Google Scholar]
- 16.Vigilant L, Hofreiter M, Siedel H, Boesch C. Paternity and relatedness in wild chimpanzee communities. Proc Natl Acad Sci USA. 2001;98:12890–12895. doi: 10.1073/pnas.231320498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alberts SC, Buchan J, Altmann J. Sexual selection in wild baboons: from mating opportunities to paternity success. Anim Behav. 2006;72:1177–1196. [Google Scholar]
- 18.Marshall TC, Slate J, Kruuk LE, Pemberton JM. Statistical confidence for likelihood-based paternity inference in natural propulations. Mol Ecol. 1998;7:639–655. doi: 10.1046/j.1365-294x.1998.00374.x. [DOI] [PubMed] [Google Scholar]
- 19.Khan MZ, Altmann J, Isani SS, Yu J. A matter of time: evaluating the storage of fecal samples for steroid analysis. Gen Comp Endocrinol. 2002;128:57–64. doi: 10.1016/s0016-6480(02)00063-1. [DOI] [PubMed] [Google Scholar]
- 20.Lynch JW, et al. Concentrations of four fecal steroids in wild baboons: short-term storage conditions and consequences for data interpretation. Gen Comp Endocrinol. 2003;132:264–271. doi: 10.1016/s0016-6480(03)00093-5. [DOI] [PubMed] [Google Scholar]
- 21.Beehner J, et al. Testosterone related to age and life-history stages in male baboons and geladas. Horm Behav. 2009;56:472–480. doi: 10.1016/j.yhbeh.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson ME, et al. Aging and fertility patterns in wild chimpanzees provide insights into the evolution of menopause. Curr Biol. 2007;17:2150–2156. doi: 10.1016/j.cub.2007.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weinstein M, et al. Timing of menopause and patterns of menstrual bleeding. Am J Epidemiol. 2003;158:782–791. doi: 10.1093/aje/kwg223. [DOI] [PubMed] [Google Scholar]
- 24.Alberts SC, Watts HE, Altmann J. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Anim Behav. 2003;65:821–840. [Google Scholar]
- 25.Sapolsky R. Endocrine and behavioral correlates of drought in wild olive baboons (Papio anubis) Am J Primatol. 1986;11:217–227. doi: 10.1002/ajp.1350110303. [DOI] [PubMed] [Google Scholar]
- 26.Feldman HA, et al. Age trends in the level of serum testosterone and other hormones in middle-aged men: longitudinal results from the Massachusetts Male Aging Study. J Clin Endocrinol Metab. 2002;87:589–598. doi: 10.1210/jcem.87.2.8201. [DOI] [PubMed] [Google Scholar]
- 27.Wu FCW, von Eckardstein A. Androgens and coronary artery disease. Endocr Rev. 2003;24:183–217. doi: 10.1210/er.2001-0025. [DOI] [PubMed] [Google Scholar]
- 28.Kuchuk NO, et al. The association of sex hormone levels with quantitative ultrasound, bone mineral density, bone turnover and osteoporotic fractures in older men and women. Clin Endocrinol. 2007;67:295–303. doi: 10.1111/j.1365-2265.2007.02882.x. [DOI] [PubMed] [Google Scholar]
- 29.Arnlov J, et al. Endogenous sex hormones and cardiovascular disease incidence in men. Ann Intern Med. 2006;145:176–184. doi: 10.7326/0003-4819-145-3-200608010-00005. [DOI] [PubMed] [Google Scholar]
- 30.Vermeulen A, Kaufman JM, Giagulli VA. Influence of some biological indexes on sex hormone-binding globulin and androgen levels in aging or obese males. J Clin Endocrinol Metab. 1996;81:1821–1826. doi: 10.1210/jcem.81.5.8626841. [DOI] [PubMed] [Google Scholar]
- 31.Leifke E, et al. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol. 2000;53:689–695. doi: 10.1046/j.1365-2265.2000.01159.x. [DOI] [PubMed] [Google Scholar]
- 32.Khosla S, Melton LJ, Atkinson EJ, O’Fallon WM. Relationship of serum sex steroid levels to longitudinal changes in bone density in young versus elderly men. J Clin Endocrinol Metab. 2001;86:3555–3561. doi: 10.1210/jcem.86.8.7736. [DOI] [PubMed] [Google Scholar]
- 33.Muller M, et al. Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol. 2003;149:583–589. doi: 10.1530/eje.0.1490583. [DOI] [PubMed] [Google Scholar]
- 34.Wilson MMG. Bitter-sweet memories: truth and fiction. J Gerontol A Biol Sci Med Sci. 2001;56:M196–M199. doi: 10.1093/gerona/56.4.m196. [DOI] [PubMed] [Google Scholar]
- 35.Harris T, et al. Body-mass index and mortality among nonsmoking older persons—the Framingham-Heart-Study. JAMA. 1988;259:1520–1524. [PubMed] [Google Scholar]
- 36.Corrada MM, Kawas CH, Mozaffar F, Paganini-Hill A. Association of body mass index and weight change with all-cause mortality in the elderly. Am J Epidemiol. 2006;163:938–949. doi: 10.1093/aje/kwj114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stevens J, et al. The effect of age on the association between body-mass index and mortality. N Engl J Med. 1998;338:1–7. doi: 10.1056/NEJM199801013380101. [DOI] [PubMed] [Google Scholar]
- 38.Heiat A, Vaccarino V, Krumholz HM. An evidence-based assessment of federal guidelines for overweight and obesity as they apply to elderly persons. Arch Intern Med. 2001;161:1194–1203. doi: 10.1001/archinte.161.9.1194. [DOI] [PubMed] [Google Scholar]
- 39.Altmann J, et al. Body size and fatness of free-living baboons reflect food availability and activity levels. Am J Primatol. 1993;30:149–161. doi: 10.1002/ajp.1350300207. [DOI] [PubMed] [Google Scholar]
- 40.Altmann J, Alberts SC. Growth rates in a wild primate population: ecological influences and maternal effects. Behav Ecol Sociobiol. 2005;57:490–501. [Google Scholar]
- 41.Morbeck ME, Galloway A, Sumner DR. Getting old at Gombe: skeletal aging in wild-ranging chimpanzees. In: Erwin JM, Hof PR, editors. Aging in Nonhuman Primates. Karger; Basel: 2002. pp. 48–62. [Google Scholar]
- 42.Nichols KA, Zihlman AL. Skeletal and dental evidence of aging in captive western lowland gorillas: a preliminary report. In: Erwin JM, Hof PR, editors. Aging in Nonhuman Primates. Karger; Basel: 2002. pp. 22–31. [Google Scholar]
- 43.King SJ, et al. Dental senescence in a long-lived primate links infant survival to rainfall. Proc Natl Acad Sci USA. 2005;102:16579–16583. doi: 10.1073/pnas.0508377102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cuozzo FP, Sauther ML. Severe wear and tooth loss in wild ring-tailed lemurs (Lemur catta): a function of feeding ecology, dental structure, and individual life history. J Hum Evol. 2006;51:490–505. doi: 10.1016/j.jhevol.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Phillips-Conroy JE, et al. Periodontal health in free-ranging baboons of Ethiopia and Kenya. Am J Phys Anthropol. 1993;90:359–371. doi: 10.1002/ajpa.1330900310. [DOI] [PubMed] [Google Scholar]
- 46.Phillips-Conroy JE, Bergman T, Jolly CJ. Quantitative assessment of occlusal wear and age estimation in Ethiopian and Tanzanian baboons. In: Whitehead PF, Jolly CJ, editors. Old World Monkeys. Cambridge University Press; Cambridge, UK: 2000. pp. 321–340. [Google Scholar]
- 47.Kay RF, Cant JGH. Age assessment using cementum annulus counts and tooth wear in a free-ranging population of Macaca mulatta. Am J Primatol. 1988;15:1–15. doi: 10.1002/ajp.1350150103. [DOI] [PubMed] [Google Scholar]
- 47a.Galbany J, Altmann J, Pérez-Pérez A, Alberts SC. Age and individual foraging behavior predict tooth wear in Amboseli baboons. Amer J Phys Anthro. 2010 doi: 10.1002/ajpa.21368. (accepted pending revisions) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sapolsky RM, Altmann J. Incidence of hypercortisolism and dexamethasone resistance increases with age among wild baboons. Biol Psychiatry. 1991;30:1008–1016. doi: 10.1016/0006-3223(91)90121-2. [DOI] [PubMed] [Google Scholar]
- 49.Goncharova ND, Lapin BA. Effects of aging on hypothalamic-pituitary-adrenal system function in non-human primates. Mech Ageing Dev. 2002;123:1191–1201. doi: 10.1016/s0047-6374(02)00012-x. [DOI] [PubMed] [Google Scholar]
- 50.Sapolsky RM. Why Zebras Don’t Get Ulcers. W.H. Freeman and Company; New York: 1994. [Google Scholar]
- 51.Munck A, Guyre PM, Holbrook NJ. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- 52.Sapolsky RM. Social status and health in humans and other animals. Annu Rev Anthropol. 2004;33:393–418. [Google Scholar]
- 53.Sheffield-Moore M, Urban RJ. An overview of the endocrinology of skeletal muscle. Trends Endocrinol Metab. 2004;15:110–115. doi: 10.1016/j.tem.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Ashcroft GS, Mills SJ, Ashworth JJ. Ageing and wound healing. Biogerontology. 2002;3:337–345. doi: 10.1023/a:1021399228395. [DOI] [PubMed] [Google Scholar]
- 55.Hawkes K, et al. Grandmothering, menopause, and the evolution of human life histories. Proc Natl Acad Sci USA. 1998;95:1336–1339. doi: 10.1073/pnas.95.3.1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atsalis S, Margulis SW, Hof PR. Primate Reproductive Aging: Cross-Taxon Perspectives. S. Karger AG; Switzerland: 2008. [Google Scholar]