Abstract
Pancreatic adenocarcinoma upregulated factor (PAUF) is overproduced in certain types of cancer. However, little is known of the tumorigenic function of PAUF. In this study, we report the X-ray crystal structure of PAUF and reveal that PAUF is a mammalian lectin normally found in plant lectins. We also identify PAUF as an endogenous ligand of Toll-like receptor 2 (TLR2) and TLR4 by screening extracellular domain receptor pools.We further confirmed the specificity of the PAUF–TLR2 interaction. PAUF induces extracellular signal-regulated kinase (ERK) phosphorylation and activates the IKK-β-mediated TPL2/MEK/ERK signaling pathway through TLR2. In agreement with the result of TLR2-mediated ERK activation by PAUF, PAUF induces increased expression of the protumorigenic cytokines RANTES and MIF in THP-1 cells. However, PAUF does not fully activate Iκ-B-α signaling pathways in THP-1 cells, and fails to translocate the p65 subunit of the nuclear factor-κB (NF-κB) complex into the nucleus, resulting in no NF-κB activation. Surprisingly, we found that PAUF also associated with the CXC chemokine receptor (CXCR4)–TLR2 complex and inhibited CXCR4-dependent, TLR2-mediated NF-κB activation. Together, these findings suggest that the new cancer-associated ligand, PAUF, may activate TLR-mediated ERK signaling to produce the protumorigenic cytokines, but inhibits TLR-mediated NF-κB signaling, thereby facilitating tumor growth and escape from innate immune surveillance.
Keywords: PAUF, TLR, CXCR4, TPL2, ERK, NF-κB
Introduction
It is essential for cancer cells to express and secrete large amounts of molecules to escape immune surveillance and form a tumor (Folkman, 1990). Cancer cells also constitutively overexpress proteins that are expressed transiently or at very low levels in normal cells, thereby making them more visible to the immune effectors (Jemal et al., 2004). When first secreted into the circulating immune system, these proteins recruit immune effectors, including a combination of innate and adaptive immune cells, which is believed to be the most efficient way of eliminating cancer mass. Cancer cells also secrete various cytokines and chemokines, which can affect the immune and inflammatory responses in the microenvironment surrounding the tumor by autocrine or paracrine processes (Strieter et al., 2006). Certain inflammatory cytokines and chemokines suppress tumor growth and kill cancer cells, whereas others, including RANTES, MIF, SDF-1α and sICAM-1, stimulate tumor proliferation and angiogenesis (Singh et al., 2007). Furthermore, tumor cells can also release mediators, believed to contribute to tumor cell evasion of the immune response leading to enhanced tumor progression and cancer cell survival (Raman et al., 2007).
Toll-like receptors (TLRs) have a crucial role in innate and adaptive immune systems, particularly in the inflammatory response against various invading exogenous pathogens, as they recognize receptor-specific pathogen-associated molecular patterns of highly conserved pathogenic components of bacteria, viruses, fungi and parasites (Akira and Takeda, 2004). TLR-activated signaling pathways subsequently activate several signaling pathways, including the nuclear factor-κB (NF-κB), phospho-inositol-3 kinase-AKT and mitogen-associated protein kinase signaling pathways (Akira and Takeda, 2004). These signaling pathways are shared by the processes involved in tumorigenesis and tumor progression (Chen et al., 2008). Recently, Huang et al. (2005) showed that lipopolysaccharide (LPS)-induced activation of TLR4 in murine tumor cells promotes evasion from immune surveillance (Huang et al., 2005). In addition, the promotion of tumor growth by Listeria monocytogenes is mediated by L. monocytogenes-induced activation of TLR2 (Huang et al., 2007). In human epithelial ovarian cancer cells, there is a strong correlation between the ubiquitous expression of TLR4 and MyD88 and paclitaxel chemoresistance, leading to enhanced tumor survival that is mediated by the production of proinflammatory cytokines by activation of the TLR4 signaling pathway (Kelly et al., 2006).
Mammalian lectins function in many cellular processes, including cell adhesion, angiogenesis, metastasis, apoptosis, cell–cell interactions and recognition of pathogens (Liu and Rabinovich, 2005). Mammalian lectins contain conserved carbohydrate-recognition domains that are responsible for recognizing endogenous or exogenous carbohydrate structures (Liu and Rabinovich, 2005). Recently, galectin-3 has been shown to associate with TLR2, following phorbol 12-myristate 13-acetate (PMA) treatment in differentiated THP-1 cells (Jouault et al., 2006). Galectin-3 also suppresses the generation of tumor necrosis factor (TNF)-α-mediated LPS inflammation in galectin-3 knockout mice, which are more susceptible to endotoxin shock sepsis in an animal disease model (Bach et al., 2008). Together, these previous studies suggest that the functions of lectin and TLR may be closely related in the control of tumor progression by the innate immune system.
Previously, we identified pancreatic adenocarcinoma upregulated factor (PAUF) as a novel secreted protein with a putative hydrophobic 40-amino-acid signal peptide. PAUF, which is expressed only in primates, does not share homology with other proteins and is overexpressed in pancreatic cancers and in other cancer types (Kim et al., 2009). PAUF modulates the metastatic potential of pancreatic cancer cells; interestingly, it also results in upregulation of CXCR4 expression, which would likely increase cancer cell motility (Lee et al., 2010). Li et al. (2004) identified a clear link between HER2 and CXCR4, and demonstrated that CXCR4 overexpression has a central role in HER2-mediated metastasis, a function similar to its normal function as a regulator of cell migration. One provocative suggestion is that, when coassociated with bacterial pathogen-activated TLR2, CXCR4 might be a key receptor for immune evasion (Hajishengallis et al., 2008).
Although PAUF has been implicated in tumor progression and metastasis, little is known of the tumorigenic function of PAUF. In this study, we report the X-ray crystal structure of PAUF at 2.0 Å resolution. We also identify PAUF as a novel endogenous mammalian ligand of TLR2 and TLR4. PAUF activates the canonical signaling pathways of TLR2-tumor progression locus 2 (TPL2)/mitogen-activated ERK kinase (MEK)/extracellular signal-regulated kinase (ERK). However, PAUF fails to mediate TLR2-induced NF-κB activation. Importantly, PAUF can associate with the CXCR4–TLR2 complex, an association that activates CXCR4, and likely inhibits TLR2-mediated NF-κB activation in THP-1 cells. Finally, we propose that the secretion of PAUF by cancer cells is involved in selective cytokine production for cancer proliferation and metastasis.
Materials and methods
Cell culture and reagents
HEK293T cells, CHO cells and the human acute monocytic leukemia cell line, THP-1, from ATCC (Manassas, VA, USA) were grown in Dulbecco’s modified Eagle’s medium and RPMI medium, respectively, supplemented with 10% heat-inactivated bovine serum and 1% penicillin/streptomycin sulfate at 37 °C in a humidified 5% CO2 atmosphere. Opti-MEM media were obtained from Gibco BRL (Gaithersburg, MD, USA). LPS (E. coli serotype O55:B5), AMD3100 and tunicamycin were from Sigma (St Louis, MO, USA). We obtained antibodies including anti-Flag (Clone M2; Sigma), PD98059, Pam3Cys, SDF-1α and anti-β-tubulin (Calbiochem, Merck, Darmstat, Germany), anti-Histone H2B, anti-human p-ERK, p-AKT, p-Iκ-B-α and α-myc (Cell Signaling Technology, Beverly, MA, USA), anti-hemagglutinin (Covance, Berkeley CA, USA), anti-TLR2, anti-TLR4, anti-CXCR4, anti-mouse IgG2a, anti-p65, anti-phosphop65 and anti-β-actin for western blot or FACS analysis (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Neutralizing antibody of anti-TLR2 and anti-TLR4 for FACS analysis (eBioscience, San Diego, CA, USA), anti-mouse, rabbit and human IgG-horseradish peroxidase were from the Jackson Immuno Research Laboratories (West Grove, PA, USA). Anti-PAUF was generated in-house. NE-PER Nuclear and Cytoplasmic Extraction Reagents kits and Alexa 488 DyLight Antibody Labeling Kits were from Thermo Scientific (Rockford, IL, USA). Proteome Profiler Array Panel A (ARY005) for human cytokine measurement was from R&D Systems (Minneapolis, MN, USA).
Plasmid construction
The expression vectors pHA-mTLR2, -dominant mutant-mTLR2, -mTLR4, -mTLR6, pFlag-mCD14, -mMD2 were kindly provided by JY Lee (Gwangju Institute of Science and Technology, Gwangju, Korea). Human TLR1, 2, 3, 4, 5, 8, 9, and 10, CXCR4 and TPL2 cDNA were cloned from a human THP-1 monocyte cDNA library or purchased from OriGene (Rockville, MD, USA) by PCR methods, and then subcloned into pHA-hTLRs, pFlag-CXCR4, pcDNA3.1-TPL2-myc or pcDNA3.1-TPL2-Kinase Dead T290A (KD)-myc.
PAUF protein expression and purification
The PAUF gene, without the N-terminal signal sequence containing the His6 tag, was cloned into pET15b vector and overexpressed in E. coli BL21(DE3)*RIL. The methionine auxotroph E. coli strain B834 (DE3) was used to generate selenomethionine-substituted protein. After the induction of protein expression with 0.8 mm isopropyl β-d-1-thiogalactopyranoside, the cells were grown overnight at 15 °C. Collected cells were purified by standard affinity chromatography followed by Resource Q ion-exchange and HiLoad Superdex 75 gel filtration chromatography (GE Healthcare, Waukesha, WI, USA). Mammalian PAUF was also produced by the PAUF-CHO cell line stably expressing PAUF and then purified. Endotoxin contamination was undetectable by the Limulus Amebocyte Lysate assay (sensitive to 0.03 EU/ml; Charles River ENDOSAFE; E. coli O55:B5 10NG Lot number; EX53392; Charles River, Hollister, CA, USA).
Crystallization and structure determination
Crystals were grown by the sitting-drop vapor diffusion method at 22 °C by mixing 1 µl of protein solution and 1 µl of well solution (Adachi et al., 2003). For data collection, the crystals were flash-frozen by a liquid nitrogen stream in well solution supplemented with 20% trehalose as a cryoprotectant. Diffraction data were collected at the 4A beamline by the Pohang Accelerator Laboratory and were processed and scaled with the HKL2000 software package. The space group was P212121 with cell dimensions of a = 40.95 Å, b = 73.04 Å and c = 92.83 Å. There are two protein molecules in the asymmetric unit. Data statistics are summarized in Supplementary Table S1. For data statistics of native and SeMet data sets from PAUF X-ray crystallography, location of the four selenium atoms, and phasing and phase improvement were performed using the SOLVE and RESOLVE programs (Los Alamos National Laboratory, Los Alamos, NM, USA). The initial model was built using the automatic tracing procedure as implemented in ARP/wARP. Several cycles of manual rebuilding using the QUANTA software (Accelrys, San Diego, CA, USA) and refinement using the CNS program yielded final crystallographic R- and Rfree-values of 21.1 and 26.8%, respectively.
Western blot analysis and co-immunoprecipitation assay
The cells were lysed in buffer containing 20 mm Tris (pH 7.5), 150 mm NaCl, 1 mm EDTA, 1 mm EGTA, 0.5% Triton X-100, 1 mm sodium orthovanadate, 2.5 mm sodium pyrophosphate, 1 mm glycerophosphate, 1 mm phenylmethylsulfonyl fluoride, 1 µg/ml leupeptin and 1 µg/ml pepstatin. Proteins were visualized by using specific primary antibodies and horseradish peroxidase-conjugated as the secondary antibody, followed by color development using the ECL Plus detection kit (Amersham Biosciences, Piscataway, NJ, USA). For immunoprecipitation, 850 µg of extracted protein was incubated with 3 µg mouse anti-HA or –anti-Flag monoclonal antibody overnight at 4 °C on a rotating platform. Next day, 25 µl of 50% slurry of prewashed protein G-agarose beads (Calbiochem) was added to each sample and incubated at 4 °C for an additional 1 h. The samples were washed three times in lysis buffer and then subjected to western blot analysis.
Luciferase reporter assay
HEK293T or CHO cells were seeded into 12-well tissue culture plates (1×106 cells per well) and allowed to adhere at 37 °C overnight. The cells were then transiently transfected with TLR4/MD2/CD14/NF-κB firefly luciferase/pTK-Renilla luciferase or TLR2/TLR6/CD14/NF-κB firefly luciferase/pTK-Renilla luciferase using Effectene (Qiagen, Valencia, CA, USA). For cAMP-dependent luciferase assays, NF-κB firefly luciferase was substituted with cAMP response element luciferase. The amount of firefly- and Renilla luciferase in the cell lysates was assayed simultaneously using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA) with a Victor 1420 luminometer (Beckman Coulter, Fullerton, CA, USA).
Flow cytometry
For TLR2, TLR4 and CXCR4 surface binding and the neutralization assay by FACS analysis, we labeled PAUF, bovine serum albumin and anti-CXCR4 by using the Alexa 488 DyLight Antibody Labeling Kit (Thermo Scientific) following the manufacturer’s instructions. For the surface binding assay, freshly grown THP-1 cells at 1×106 cells per tube were collected and then incubated for 1 h at 4 °C with Alexa 488-PAUF in dose-dependent experiments. Alexa 488–BSA was used as a negative control. For the neutralization assay, cells subjected to the surface-binding assay were preincubated for 1 h at 4 °C with anti-isotype control antibody, anti-TLR2 or anti-TLR4 monoclonal antibodies in FACS buffer (phosphate-buffered saline containing 1% bovine serum albumin). The cells were then washed in FACS buffer before being analyzed on a FACSCalibur flow cytometer using Cell-Quest software (Becton Dickinson, San Jose, CA, USA).
Cytokine studies
Cytokine production was determined using the Human Cytokine Array panel A (R&D Systems) according to the manufacturer’s instructions. The intensity of the signals was quantified and normalized by densitometry using a digital imaging analysis system and MultiGauge 3.0 Image Analysis software (Fuji Film, Tokyo, Japan).
Statistical analysis
Data are presented as the mean±s.e. Statistical significance was evaluated by using the two-tailed Student’s t-test. A P-value <0.01 was considered significant.
Online supplemental materials
Supplementary Table 1 summarizes the statistics of the native and SeMet data sets from PAUF X-ray crystal analysis. Supplementary Table 2 shows the list of ECD receptors for screening. Supplementary Figure 1 shows a representative result for the PAUF and ECD receptor interaction. Supplementary Figure 2 shows PAUF interaction with TLR2, TLR5 and TLR6 in vivo and PAUF expression patterns in various cancer cell lines. Supplementary Figure 3 shows the time-dependent effects of PAUF-induced signaling pathway on ERK, JNK, p-38 and NFκB activation. Supplementary Figure 4 shows that PAUF signaling pathway was neutralized by an anti-TLR2 antibody and a dominant-negative mTLR2 mutant. Supplementary Figure 5 shows the CXCR4 expression in cells and effect of AMD3100 on PAUF- or Pam3Cys-induced NF-κB activation. Supplementary Figure 6 shows the antagonism of NF-κB-activation in THP-1 cells by PAUF and/or Pam3Cys treatment. Supplementary Figure 7 shows the original human cytokine protein array. Supplementary Figure 8 shows the effects of ERK signaling pathway on cytokine generation in THP-1 cells by PAUF and/or Pam3Cys treatment.
Results
Crystal structure of PAUF
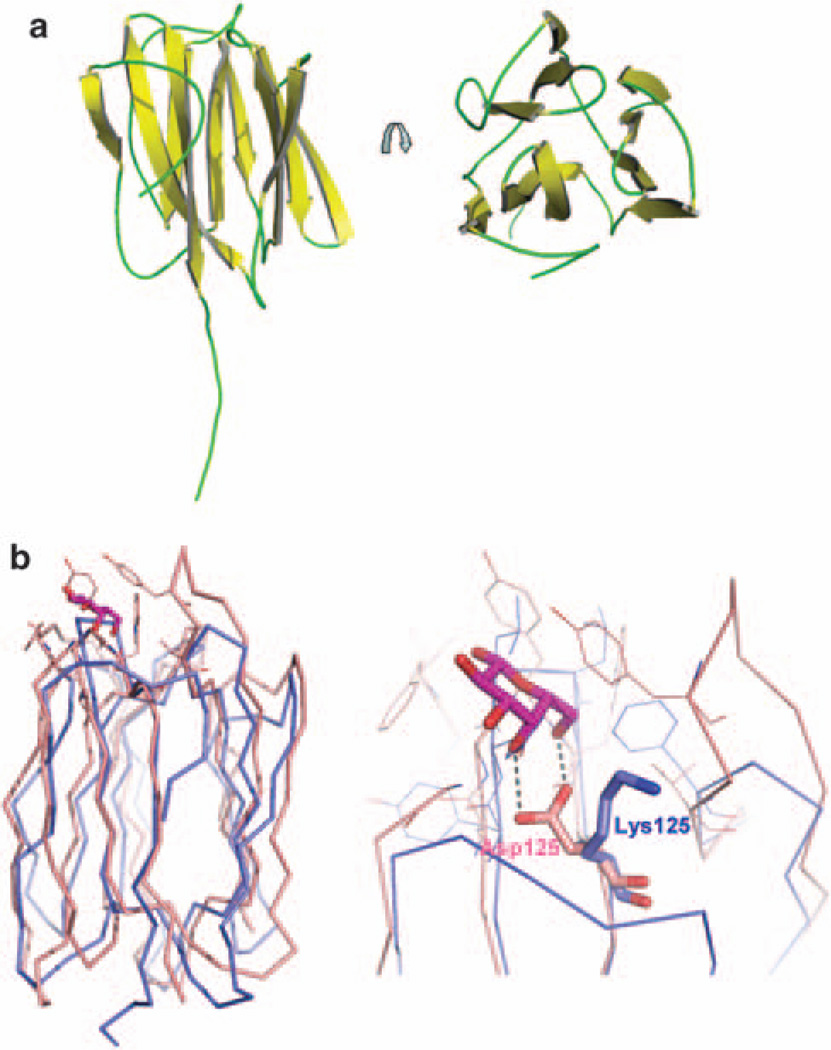
To investigate the physiological roles of PAUF, we determined the crystal structure of PAUF by the multiple wavelength anomalous diffraction method at 2.0Å resolution. There are two PAUF molecules in the asymmetric unit and each molecule is composed of 12 β-strands arranged in three β-sheets. Only one molecule has an additional extended tail as shown in Figure 1a. The tail starts at the residue Glu136 and ends at the residue Asn145. The C-terminal 11 residues are missing from both PAUF molecules in the asymmetric unit. The extended tail is 26 Å long and makes contact with symmetry-related molecules. Data statistics are shown in Supplementary Table S1.
Figure 1.
X-ray crystal structure of PAUF. Crystals were grown using the sitting-drop vapor diffusion method at 22 °C by mixing 1 µl of protein solution and 1 µl of well solution. The well solution contained 1.26m ammonium sulfate and 0.1M Na cacodylate, pH 6.5. Rod-shaped crystals were obtained within several days and data were collected as described in Materials and methods. (a) The overall structure of PAUF shows the β-prism motif and an extended tail. The β-strands are shown in yellow and the loops are in green. (b) The structure of PAUF (blue) is compared with the structure of a plant lectin, jacalin (1 UGW.pdb, pink). A galactose (purple) is bound in the carbohydrate recognition domain of jacalin through interactions with the conserved Asp and the loop between strands seven and eight. The conserved Asp of jacalin was replaced with Lys125 in PAUF. Figures were prepared by the PyMOL program.
A similarity search using the DALI server revealed that the structure of PAUF is similar to those of plant lectins such as agglutinin (Bourne et al., 1999), artocarpin (Jeyaprakash et al., 2004) and jacalin (Jeyaprakash et al., 2003). These lectins contain a characteristic structure known as the β-prism motif that has been shown to bind carbohydrates. The β-prism motif consists of three- or four-stranded β-sheets that form the faces of a triangular prism structure. The β-prism motif has also been reported in the structure of vitelline membrane outer layer protein I (Shimizu et al., 1994) and in the second domain of δ-endotoxin (Morse et al., 2001). In the lectin structures, the carbohydrates are recognized by the conserved Asp residue and the loop between strands seven and eight that form the side of carbohydrate binding pocket. It is of interest that a structural comparison between lectins and PAUF revealed that PAUF has Lys125 instead of the conserved Asp residue (Figure 1b). PAUF has no Asp residue nearby that can participate in carbohydrate binding. PAUF also lacks the long loop between strands seven and eight. Plant lectins form multimers for carbohydrate recognition (Barre et al., 2001), but purified PAUF crystallizes as a monomer. We next tested the ability of PAUF to bind to various carbohydrates (d-allose, d-glucose, l-glucose, d-mannose, l-mannose, d-mannose, l-galactose, d-galactose, d-fructose, d-psicose, d-sorbose and d-tagatose) in vitro. As expected, none of the monosaccharides bound to the PAUF protein (unpublished data). Taken together, these findings suggest that despite the structural similarities with plant lectins, PAUF may not be able to bind carbohydrates (Figure 1b).
PAUF is a newly identified endogenous mammalian ligand of TLR2 and TLR4
We commenced our investigations to identify the PAUF receptor based on the structural similarities of PAUF with lectins and the secretion of PAUF from cells. We first generated pools of secreted receptors covering the extracellular domain (ECD) of 135 single transmembrane receptors abundantly expressed in the surface membrane of hematopoietic cells (Supplementary Table 2). We then partially purified these secreted ECD receptors and incubated them with purified PAUF coated onto the wells of a 96-well microplate. After extensive washing, we measured the levels of receptor binding by ELISA. This screening procedure yielded a positive signal for the binding of PAUF to TLR2 only (Supplementary Figure 1a).
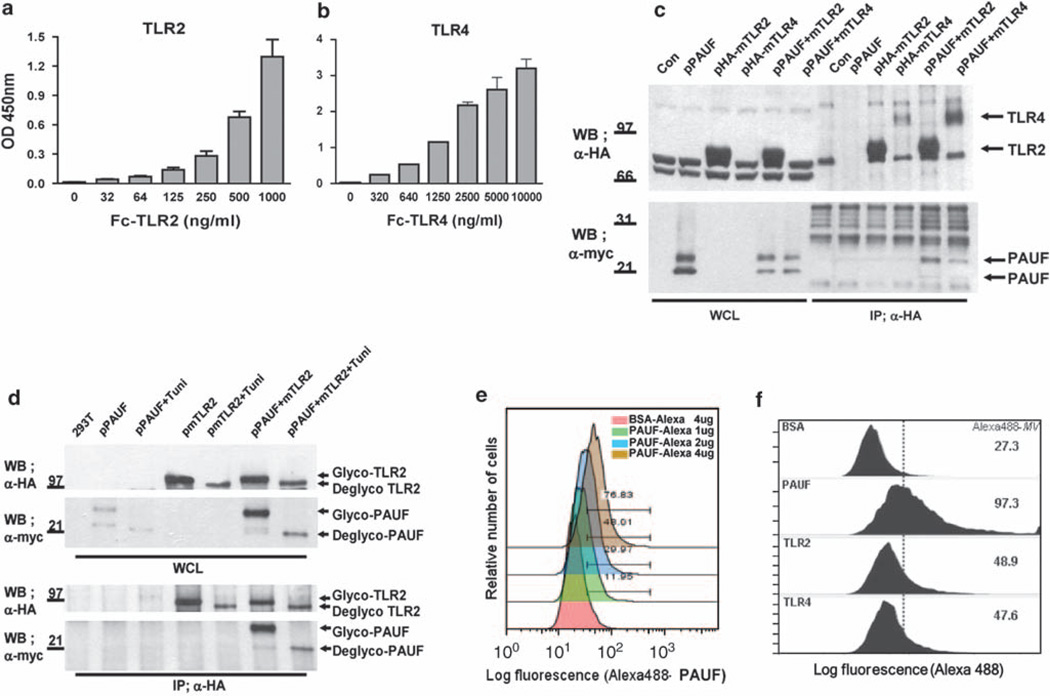
To confirm the specific interaction between PAUF and the receptors TLR2 and TLR4, which is from the same family receptor as TLR2, we performed dose-dependent experiments with the purified ECD of TLR2 and TLR4. ELISA revealed that the interaction between PAUF and both TLR2 and TLR4 was dose dependent, thus suggesting that PAUF is a ligand of both TLR2 and TLR4 (Figures 2a and b). We also confirmed the specific interaction between PAUF and both TLR2 and TLR4 in co-immunoprecipitation experiments using full-length TLR2 or TLR4 in human embryonic kidney (HEK)293T cells. The antihemagglutinin antibody co-immunoprecipitated a high portion of myc-tagged PAUF protein expressed from transfected cells (Figure 2c). These results suggest that the interaction between PAUF and both TLR2 and TLR4 is direct and occurs in the absence of other cofactors. There are a number of TLR family proteins in human cells, any one of which could conceivably bind PAUF. We tested these potential interactions using co-immunoprecipitation assays and found that PAUF associated with TLR2, 5 and 6, but not with TLR3, 8, 9 or 10 (Supplementary Figure 2), indicating that PAUF specifically binds to a subset of TLR family proteins.
Figure 2.
PAUF is a dual endogenous ligand of TLR2 and TLR4. (a, b) Dose-dependent binding of TLR2, TLR4 and PAUF in vitro. PAUF was coated 5 µg in ELISA plates and then treated with purified ECD-TLR2 and -TLR4 in a dose-dependent manner as indicated. Data represent the mean±s.e. of triplicate samples from single independent experiment. (a) and (b) are the ELISA-binding results for TLR2, TLR4 receptors and PAUF. (c) PAUF interacts with TLR2 and TLR4 in vivo. HEK293T cells were transiently transfected with the expression vectors pHA-mTLR2 and pHA-mTLR4, with or without pPAUF-myc. After immunoprecipitation with anti-HA antibody, the blots were probed with anti-HA and anti-myc as indicated. Data are representative blots one of three independent sets of experiments yielding similar results. (d) PAUF interacts with TLR2 independent of glycosylation. After co-transfection of the cells with pHA-TLR2 and pPAUF-myc, cells were left untreated or treated with tunicamycin (100 µm) for 6 h, and then cell extracts were prepared after another 24 h. After immunoprecipitation with anti-HA antibody, the blots were probed with anti-HA and anti-myc as indicated. Data are representative blots one of three independent sets of experiments yielding similar results. (e) Freshly grown THP-1 cells at 1×106 cells per tube were collected. Alexa 488-PAUF binds membrane receptors in THP-1 cells in a dose-dependent manner. Alexa 488-BSA used as a negative control. (f) The TLR2 and TLR4 antibodies neutralized the interactions between Alexa 488-PAUF and TLR2 or TLR4, respectively. THP-1 cells were incubated with the isotype control, TLR2 antibody (10 µg/ml) and TLR4 antibody (10 µg/ml) for 1 h before addition of Alexa 488-PAUF at 8 µg/ml. Cells were analyzed by flow cytometry. Alexa 488-MV is the median value from the histograms. Data are shown one of two independent sets of experiments yielding similar results (e, f).
PAUF directly binds to TLR2 at the amino-acid level
PAUF and TLR2 are N-glycosylated proteins. When TLR2 was co-immunoprecipitated with PAUF, the glycosylated form of PAUF protein was the dominantly co-immunoprecipitated form (Figure 2c). This result indicates that glycosylation might be a key process for the interaction between PAUF and TLR2. Thus, we tested whether N-linked glycosylation of PAUF and TLR2 has a role in their interaction. Treatment of co-transfected cells with tunicamycin, a glycosylation inhibitor, had no effect on the interaction between PAUF and TLR2, thus demonstrating that glycosylation is not required for the interaction between PAUF and TLR2 (Figure 2d). The slight decrease in the amount of tunicamycin-treated PAUF bound to TLR2 was caused by a reduction in the expression levels of PAUF and TLR2 under these conditions. This result is consistent with the interaction between TLR2 and TLR4 and the PAUF produced in Escherichia coli (Figures 2a and b), which did not contain the glycosylation form. Thus, it is most likely that PAUF and TLR2 interact directly at the amino-acid level.
PAUF binds to TLR2 and TLR4 on the surface membrane of THP-1 cells
THP-1 cells express both TLR2 and TLR4 on the surface membrane. Fluorescence-activated cell sorting (FACS) analysis showed that Alexa 488-labeled PAUF binds to THP-1 cells in a dose-dependent manner (Figure 2e). There was no binding of Alexa 488-labeled BSA, used as a negative control, to THP-1 cells. To confirm whether the binding of PAUF to the surface membrane of THP-1 cells is mediated by TLR2 and/or TLR4, we performed neutralization assays using anti-bodies to TLR2 and TLR4 to block the respective TLRs on THP-1 cell-surface membranes, a method that does not require permeabilization of the cell membrane or fixation. Preincubation of THP-1 cells with anti-TLR2 or anti-TLR4 antibodies resulted in a significant reduction in the level of binding of Alexa 488-labeled PAUF to TLR2 and TLR4, from the median value of 97.3–48.9, for anti-TLR2, and to 47.6, for anti-TLR4 (Figure 2f). Together, these results show that PAUF is a specific ligand of TLR2 and TLR4 on the surface membrane of THP-1 cells.
PAUF treatment increases phosphorylation of the ERK, JNK, p-38 but does not significantly induce the phosphorylation of Iκ-B-α in THP-1 cells
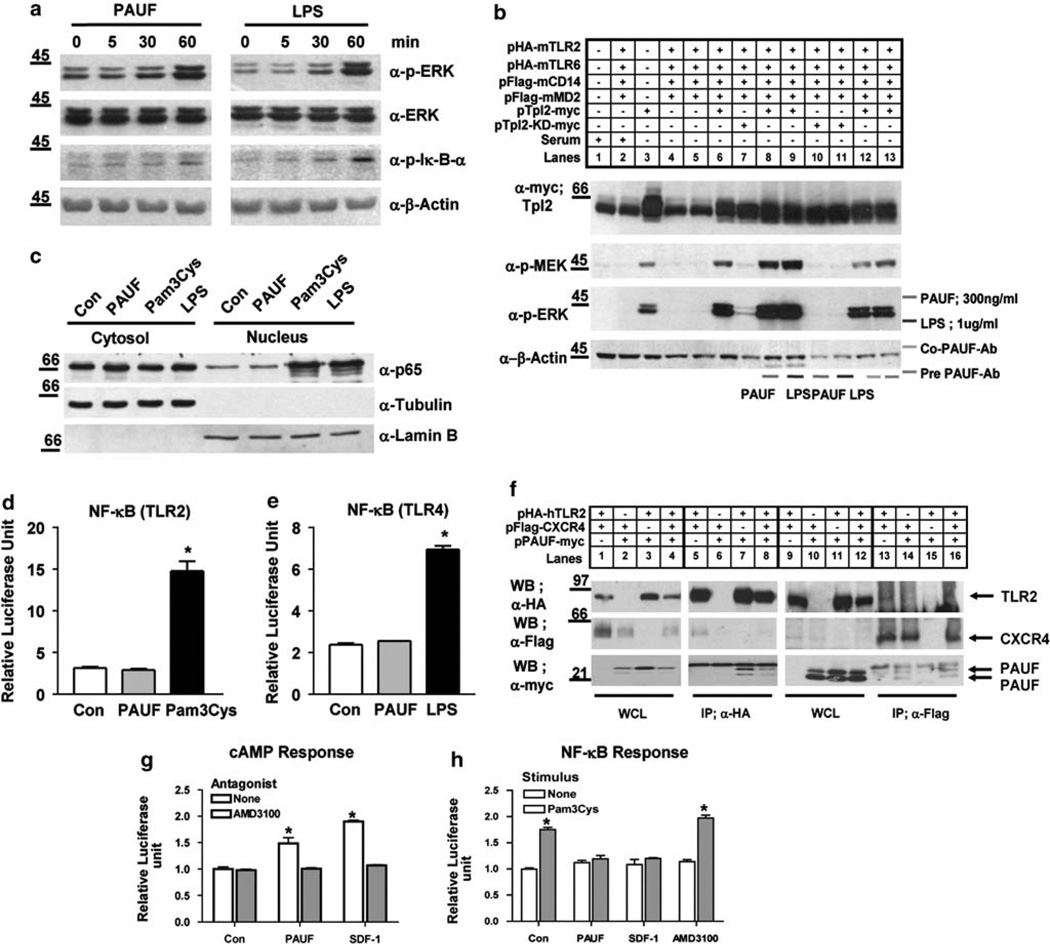
We next investigated whether PAUF activates the TLR signaling pathways by examining the effect of PAUF on phosphorylation-induced activation of endogenous signaling intermediates, including ERK, jun N-terminal kinase (JNK), p-38 and Iκ-B-α, in THP-1 cells. As shown in Figure 3a and Supplementary Figure 3, exposure of THP-1 cells to PAUF (100 ng/ml) for the indicated time periods strongly stimulated the phos-phorylation of ERK to levels comparable to LPS treatment, the positive control. Furthermore, by treatment with PAUF, THP-1 cells exhibited activation of JNK and p-38 (Supplementary Figure 3). The activation of JNK maintained an experimental time period, but p-38 was diminished at 120 min of exposure to PAUF. However, the phosphorylation of Iκ-B-α was not significantly increased by PAUF treatment. We also found that treatment with an anti-TLR2 antibody slightly inhibited both PAUF- and LPS-induced ERK phosphorylation (Supplementary Figure 4a). In addition, expression of a dominant-negative mTLR2 mutant also inhibited PAUF-induced ERK phosphorylation (Supplementary Figure 4b). These results suggest that PAUF-induced ERK activation depends on the TLR2 signaling pathway. However, after 60 min the level of phosphorylated Iκ-B-α was much less compared with the level induced by LPS treatment (Figure 3a). This result suggests that PAUF may activate the TLR-mediated ERK, JNK and p-38 signaling pathway, but not fully activate the TLR-mediated Iκ-B-α signaling pathway in THP-1 cells.
Figure 3.
Effects of PAUF and LPS on the signaling pathway in THP-1 cells and transiently transfected HEK293T cells. (a) THP-1 cells were stimulated time dependently with PAUF (100 ng/ml) and LPS (100 ng/ml) for 60 min. Cell lysates were subjected to western blot analysis. PAUF and LPS stimulation of endogenous phosphorylation of ERK and Iκ-B-α activation was assayed by using antibodies specific for phosphorylated proteins. Protein loading was controlled with reproved ERK or by including β-actin on the immunoblot. Data are representative blots one of three independent sets of experiments yielding similar results. (b) The effects of PAUF (300 ng/ml) and LPS (100 ng/ml) on MEK and ERK phosphorylation are dependent on TPL2 activity. HEK293T cells transiently transfected with the expression vectors pHA-mTLR2, pHA-mTLR6, pFlag-mCD14, pFlag-mMD2, pMyc-TPL2 or pMyc-TPL2-KD (kinase dead) were stimulated with PAUF or LPS for 30 min. PAUF was neutralized with the anti-PAUF antibody (3 µg/ ml) as indicated (lanes 12 and 13). Cell lysates were subjected to western blot analysis for the indicated proteins. PAUF- and LPS-induced activation of endogenous MEK and ERK was assayed using phospho-specific antibodies. Myc-TPL2 (WT) and kinase-dead myc-TPL2 (KD-TPL2) expression was also detected by western blot analysis. Protein loading was controlled by running β-actin on the immunoblot. Data are representative blots one of three independent sets of experiments yielding similar results. (c) THP-1 cells (2×106 cells per ml) in six-well plates were serum starved with Opti-MEM media for 20 h, and then incubated for 1 h in fresh culture medium with PAUF (1 µg/ml), Pam3Cys (100 nm) and LPS (1 µg/ml). Cytosolic and nucleus fractions were prepared with the NE-PER kit and subjected to western blot analysis. The activation of endogenous NF-κB was assayed using the p65 antibody. Protein loading and purity of fractions was controlled by including α-tubulin or α-lamin B on the immunoblot. Data are representative blots one of four independent sets of experiments yielding similar results. (d, e) For the NF-κB reporter assay, HEK293T cells (1×106 cells per well) were transiently transfected with TLR2/CD14/NF-κB firefly luciferase/pTK-Renilla luciferase or TLR4/MD2/CD14/NF-κB firefly luciferase/pTK-Renilla luciferase using Effectene transfection reagent (Qiagen). The cells were treated for 6 h as indicated with PAUF (1 µg/ml), Pam3Cys (30 nm) or LPS (1 µg/ml). Activation of NF-κB-firefly luciferase reporter activity was determined as the ratio of firefly luciferase to the activity of Renilla luciferase used as an internal control using the mean value of triplicate wells. Data represent the mean±s.e. (n = 3) from one of two independent sets of experiments. *P <0.01. (f) PAUF associated with TLR2 and CXCR4 in vivo. HEK293T cells were transiently transfected with the expression vectors, pHA-hTLR2 and pFlag-CXCR4 with or without pPAUF-myc. After immunoprecipitation of cell extracts with anti-HA and anti-Flag antibody, immunoprecipitates were electrophoresed and blots were probed with anti-HA, anti-Flag and anti-myc antibodies, as indicated. Data are representative blots one of three independent sets of experiments yielding similar results. (g) PAUF induced CXCR4-mediated elevation of cAMP. For the CRE reporter assay, CHO cells were transiently transfected with TLR2/TLR6/CXCR4/CD14/pTK-Renilla luciferase and CRE firefly luciferase. Cells were then stimulated with PAUF (200 ng/ml) and SDF-1α (200 ng/ml) with or without AMD3100 (1 µg/ml) for 6 h. (h) PAUF downregulated PAM3Cys-induced, TLR2-mediated NF-κB activation through association with CXCR4. CHO cells were transiently transfected as described in (g), above, substituting an NF-κB luciferase plasmid for the CRE reporter. The cells were then stimulated with PAUF (200 ng/ml), SDF-1α and AMD3100 (1 µg/ml) with or without Pam3Cys (20 nm) for 6 h. Data indicate mean±s.e. of three independent experiments in triplicates. *P <0.01 vs each control (g, h).
PAUF activates TPL2/MEK/ERK through the TLR2 signaling pathway in HEK293T cells
Our aforementioned results showed that PAUF could activate the ERK in THP-1 cells (Figure 3a). Thus, we further investigated whether PAUF stimulates the TLR2-mediated TPL2/MEK/ERK signaling pathway in HEK293T cells that do not express TLRs (Miettinen et al., 2008). We first examined the effect of PAUF on the TLR2/TPL2/MEK/ERK signaling pathway in HEK293T cells transiently transfected with TLR2. As expected, TLR2 expression did not induce MEK and ERK phosphorylation (Figure 3b, lanes 2, 4 and 5), but only TPL2 was able to induce the basal level of MEK and ERK phosphorylation (Figure 3b, lane 3). In contrast, MEK kinase was fully activated by TPL2 through TLR2 signaling pathways in HEK293T cells after PAUF and LPS treatment (Figure 3b, lanes 8 and 9). Notably, expression of kinase-dead TPL2 in HEK293T cells fully blocked PAUF or LPS induced-MEK and ERK phosphorylation (Figure 3b, lanes 10 and 11), suggesting a specific role for TLR2 in this signaling pathway. Treatment of HEK293T cells with anti-PAUF antibody and PAUF confirmed that PAUF-induced MEK and ERK phosphorylation is because of the intrinsic activity of PAUF mediated by the TLR2 signaling pathways and not because of endotoxin contamination (Figure 3b, lanes 12 and 13). Thus, PAUF- or LPS-induced MEK/ERK activation is dependent on TPL2 kinase activity mediated by IκB kinase (IKK)-β activation in HEK293T cells.
Failure of PAUF to translocate the p65 subunit of the NF-κB complex into the nucleus results in no NF-κB activation in THP-1 and HEK293T cells
Activation of the TLR2 and TLR4 signaling pathway ultimately leads to release of the p65 subunit from the NF-κB complex and its translocation into the nucleus. Still, unlike LPS, PAUF did not appear to induce the phosphorylation of Iκ-B-α fully, we further investigated whether PAUF induces changes in NF-κB activity in THP-1 cells. Nuclear extracts were prepared from THP-1 cells after PAUF, Pam3Cys and LPS treatment. Pam3Cys and LPS translocated the p65 subunit of the NF-κB complex into the nucleus, whereas PAUF did not (Figure 3c; Supplementary Figure 5b). Thus, the p65 subunit of the NF-κB complex is not characteristically activated after PAUF treatment. To confirm this result, we performed a TLR2- and TLR4-mediated NF-κB reporter assay using firefly luciferase reporter constructs for TLR2 or TLR4, including CD14, NF-κB and an internal control Renilla luciferase reporter plasmid. In case of TLR4 NF-κB reporter constructs, we added MD2 plasmid, which is a cofactor of TLR4 signaling activation. This NF-κB reporter assay in HEK293T cells showed a lack of NF-κB signaling pathway activation after PAUF treatment, but Pam3Cys and LPS, positive agonists of TLR2 and TLR4, respectively, markedly enhanced activation of the NF-κB signaling pathway (Figures 3d and e). Thus, unlike Pam3Cys and LPS, PAUF did not activate the NF-κB signaling pathway in THP-1 cells or in HEK293T cells overexpressing TLR2 or TLR4. This result strongly suggests that PAUF does not induce activate NF-κB activation in THP-1 and HEK293T cells, even though it is able to bind to TLR2 and TLR4.
PAUF induces association of TLR2 with CXCR4 and inhibits TLR2 activation
It is well established that activation of the TLR2 signaling pathway ultimately leads to NF-κB activation. However, although PAUF binds to TLR2 and induces TPL2/MEK/ERK activation, it unexpectedly did not promote NF-κB activation. We thus sought a molecular explanation for the lack of involvement of PAUF with the TLR2-mediated canonical signaling pathway. In the course of our studies, Hajishengallis et al. (2008) reported that CXCR4 associates with TLR2 and appears to inhibit TLR2-induced NF-κB activation in human monocytes and mouse macrophages after treatment with the surface fimbriae protein derived from Porphyronmonas gingivalis. Because CXCR4 expression is also upregulated by PAUF treatment (Lee et al., 2010), we tested whether PAUF inhibited TLR2-induced NF-κB activation by CXCR4. We first examined associations among PAUF, TLR2 and CXCR4 in co-immunoprecipitation experiments in HEK293T cells. We found that HA-tagged TLR2 co-immunoprecipitated with a portion of Flag-tagged CXCR4 (Figure 3f, lanes 5 and 8) and myc-tagged PAUF (Figure 3f, lanes 7 and 8). The Flag-tagged CXCR4 immunoprecipitates also contained HA-tagged TLR2 and myc-tagged PAUF (Figure 3f, lanes 13, 14 and 16). These results are consistent with the previous suggestion by Triantafilou et al. (2008) that the CXCR4 receptor is a component of the pattern–recognition receptor complex, and also strongly suggest that CXCR4 might be a TLR2 co-receptor.
To examine the functional consequences of PAUF, CXCR4 and TLR2 associations, we used a cyclin adenosine monophosphate (cAMP) and NF-κB reporter assay, which has been previously used to show that CXCR4-mediated activation of cAMP-dependent protein kinase (PKA) inhibits NF-κB activation (Hajishengallis et al., 2008). Both PAUF and SDF-1α, a CXCR4 agonist, induced an increase in cAMP-mediated luciferase activity, implying that cAMP was produced and PKA was activated (Figure 3g). This increased activity was abolished by the CXCR4 antagonist, AMD3100. We also did not see any translocation of p65 from cytosol to nucleus in THP-1 cells pretreated with AMD3100 with PAUF (Supplementary Figure 5b). These results indicate that PAUF is a potential activator of CXCR4 in HEK293T cells, but still did not induce translocation of p65 from cytosol to nucleus in THP-1 cells with inhibition of CXCR4 signaling pathway. To further explore the lack of TLR2-mediated NF-κB activation by PAUF, we examined NF-κB activation in transfected HEK293T and THP-1 cells (described in the legends for Figures 3h and Supplementary Figure 6) stimulated with PAUF. We found that both PAUF and SDF-1α inhibited Pam3Cys-induced NF-κB activation (Figure 3h). Consistent with this, PAUF partially attenuated Pam3Cys-induced nuclear translocation of p65 (Supplementary Figure 6). However, AMD3100 did not potentiate NF-κB activation mediated by Pam3Cys in HEK293T cells (Figure 3h). And also AMD3100 did not block Pam3Cys-induced nuclear translocation of p65 in THP-1 cells (Supplementary Figure 5b).
Thus, these results suggest that PAUF likely induces CXCR4-mediated cAMP production and PKA activation, which in turn partially inhibits TLR2-induced NF-κB activation. It is also possible that the PAUF–CXCR4 pathway allows cancer cells to escape from innate immune surveillance by inhibiting NF-κB activation.
PAUF activates the production of RANTES, MIF and IL-1RA cytokines, but reduces TNF-α levels
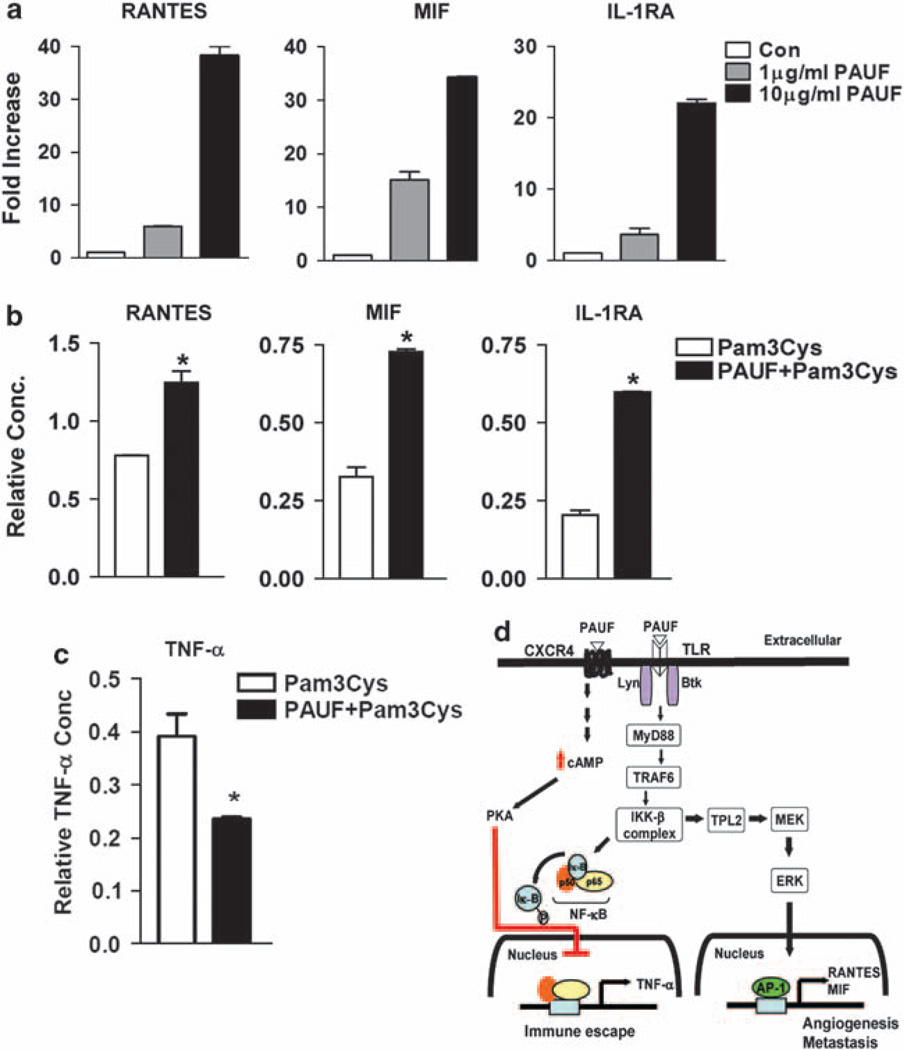
To explain the significance of PAUF-induced MEK/ERK activation, we then performed a functional cytokine array assay. Using a 34-cytokine-array panel, we examined cytokine secretion levels by assessing cell-free culture supernatants of THP-1 cells. Following stimulation of THP-1 cells with PAUF for 20 h, the levels of secreted RANTES, MIF and interleukin (IL)-1RA increased markedly in dose-dependent manner, whereas expression of other cytokine with TLR2-ligation to Pam3Cys, for example TNF-α, IL-1β, MCP-1, IL-8 and GRO-α did not increase following PAUF treatment of THP-1 cells (Figure 4a; Supplementary Figure 7). We next tested whether blockade of ERK affect the production of RANTES, MIF and IL-1RA induced by PAUF. Treatment of PD98059, the selective ERK signaling pathway inhibitor, slightly suppressed the generation of RANTES, MIF and IL-1RA induced by PAUF (Supplementary Figure 8). Consistent with our results (Figure 3), PAUF activates TLR2-TPL2/MEK/ERK signaling pathway and subsequently promotes the production of RANTES, MIF and IL-1RA.
Figure 4.
Human cytokine protein array after PAUF treatment in THP-1 cells. (a) After stimulated with PAUF as indicated, cell-free culture supernatants from THP-1 cells (2×106 cell per well) were collected. PAUF induced the secretion of RNATES, MIF and IL-1RA cytokines in a dose-dependent manner. Each array contains one duplicate negative control, to determine background absorbance and three duplicate positive controls against which all signals were normalized. The remaining signals represent the 34 cytokines/chemokines in duplicate. (b) After preincubation of THP-1 cells with PAUF (10 µg/ml) before treatment with Pam3Cys (10 nm), the secretion of RANTES, MIF and IL-1RA was synergistically increased. (c) TNF-α levels were significantly reduced by preincubation of THP-1 cells with PAUF (10 µg/ml) before treatment with Pam3Cys (10 nm). Data are mean intensity±s.e. of duplicate signals from one of two independent sets of cytokine array blots. *P <0.01 (b, c). (d) A proposed model that incorporates recently published data showing modulation of the NF-κB pathway through a TLR2/CXCR4-association mechanism. PAUF binds the CXCR4–TLR2 complex, suppressing TLR2-mediated NF-κB activation by inducing PKA activation.
As PAUF produced RANTES, MIF and IL-1RA cytokines, but not other cytokines induced by TLR2–NF-κB signaling pathway, we then tested whether preincubation of THP-1 cells with PAUF for 1 h before treatment with Pam3Cys, a selective TLR2 ligand, create an effect on the cytokine secretion profile compared with PAUF treatment alone. We found that PAUF and Pam3Cys synergistically increased the levels of RANTES, MIF and IL-1RA cytokines secreted by THP-1 cells, but there was no difference in the cytokine array, apart from these cytokines (Figure 4b). Notably, we also found that PAUF inhibits Pam3Cys-induced production of TNF-α, which is synthesized in an NF-κB-dependent manner (Figure 4c). In agreement with the results shown above (Figures 3d and e), TNF-α was the only chemokine whose secretion by THP-1 cells was remarkably reduced (~50%) at 20 h, indicating that PAUF suppresses TLR2-mediated TNF-α production. This reduced secretion is probably the result of CXCR4-mediated PKA activation, which negatively regulates Pam3Cys-induced TLR2 activation (Figure 4c).
Discussion
Previously we reported PAUF is overproduced in certain types of cancer and has an important role in the metastasis and progression of the disease (Kim et al., 2009; Lee et al., 2010). In this study, we provide a novel insight into the physiological roles of PAUF in tumor progression and metastasis. First, we report the X-ray crystal structure of PAUF at 2.0Å resolution and reveal that PAUF has a mammalian lectin family protein structure, which has many functions, ranging from cell adhesion, angiogenesis, metastasis, apoptosis and the cell–cell interactions to the recognition of pathogens. Interestingly, PAUF does not contain conserved carbohydrate-recognition domains, which are responsible for recognizing either endogenous or exogenous carbohydrate structures (Liu and Rabinovich, 2005). Recently, galectin-3, which has a galactose-binding CRD domains, has been shown to associate with TLR2 when applied to differentiated THP-1 cells after PMA treatment (Jouault et al., 2006). Also, galectin-3 suppresses the generation of TNF-α-mediated LPS inflammation in galectin-3 knockout mice, which have more susceptibility to endotoxin shock sepsis in animal disease model (Li et al., 2008). Thus, all these previous studies suggest that the lectin and TLR functions may be closely related to control the tumor progression and innate immune system.
Identification of PAUF receptors is critical to investigations of the function of this as yet uncharacterized secreted protein. To this end, we established innovative and versatile receptor identification methods for screening novel ligand–receptor interactions. We also validated this novel ligand–receptor screening strategy using the known ligand, vascular endothelial growth factor (VEGF), showing that VEGF yielded a strong positive signal with VEGFR1, but not with VEGFR3 or other receptors (Supplementary Figure 1b).
These screening strategy and co-immunoprecipitation assay with TLR2, TLR4 and PAUF results suggest that the interaction between PAUF and both TLR2 and TLR4 is direct and occurs in the absence of other cofactors. There are a number of TLR family proteins in human cells, any one of which could conceivably bind PAUF. We tested these potential interactions using co-immunoprecipitation assays and found that PAUF associated with TLR2, 4, 5 and 6, but not with TLR3, 8, 9 or 10 (Figure 2c; Supplementary Figure 2), indicating that PAUF specifically binds to a subset of TLR family proteins. To the best of our knowledge, PAUF is a mammalian ligand identified for the innate immune receptors TLR2 and TLR4, and appears to have a distinctive binding mode, despite not being fully characterized.
Banerjee et al. (2006) reported that a diverse range of TLRs use TPL2 to activate ERK in hemopoietic cells (Banerjee et al., 2006). The TLR signaling pathway activates the IKK-β complex, which subsequently phosphorylates Iκ-B-α, inhibitor of NF-κB complex, which in turn triggers NF-κB translocation into nucleus. IKK-β, which is a key kinase of phosphorylated TPL2, links inflammation and tumorigenesis in colitis-associated cancer (Greten et al., 2004; Cho et al., 2005). Consequently, TPL2 is liberated from NF-κB complex, a stabilizer of TPL2 activation, to generate an active pool of TPL2 that subsequently triggers activation of the MEK/ERK kinase cascade. Thus, IKK-β activates TPL2 by phosphorylating NF-κB complex and TPL2 (Cho et al., 2005).
Therefore, these results provide a supportive linkage between IKK-β and TPL2 activation in cells stimulated with PAUF or LPS bound to TLR2, and suggest that PAUF might have a role in cancer progression with TLRs (Raz and Lotan, 1987; Greten et al., 2004). PAUF activates the canonical signaling pathways of TLR2-TPL2/MEK/ERK. However, PAUF fails to mediate TLR2-induced NF-κB activation. Importantly, PAUF can associate with the CXCR4–TLR2 complex, an association that activates CXCR4 and likely inhibits TLR2-mediated NF-κB activation in THP-1 cells. Finally, we propose that the secretion of PAUF by cancer cells is involved in selective cytokine production for cancer proliferation and metastasis.
RANTES and MIF are involved in tumor progression, angiogenesis and metastasis (Azenshtein et al., 2002; Nishihira et al., 2003); and PAUF was originally identified in pancreatic cancer cells. Thus, the PAUF-induced cytokines, RANTES and MIF, might be major regulators of the angiogenic processes that facilitate tumor progression. In fact, it has been reported that PAUF has a potent metastatic effect in PANC-1 xenograft tumor animal models (Lee et al., 2010). IL-1RA, an antagonist of the IL-1R signaling pathway, functions by interfering with the binding of IL-1α and IL-1β to IL-1R (Ju et al., 1991). Therefore, the elevated level of IL-1RA induced by PAUF might lead to downregulation of IL-1R and TLR activation by inducing dimerization of these receptors through their TIR domains (Akira and Takeda, 2004). Collectively, our findings suggest that PAUF may facilitate cancer proliferation and metastasis by increasing the level of RANTES and MIF, and allow escape from innate immune surveillance by enhancing the activity of IL-1RA and decreasing TNF-α production.
In conclusion, our studies uncovered a new and exciting function of PAUF, which has the structure of a mammalian lectin. We identified PAUF as a ligand of TLR2 and TLR4, and provided evidence that PAUF activates the TLR2-mediated TPL2/MEK/ERK signaling pathway, resulting in increasing expression of AP-1 target genes. But PAUF does not appear to activate the TLR-mediated NF-κB signaling pathway. The lack of canonical NF-κB activation can be explained by our finding that PAUF associates with the CXCR4–TLR2 complex, likely inducing subsequent PKA activation (Figure 4d). The detailed molecular mechanisms underlying PAUF function remain to be clarified. One interesting question for future research is how PAUF, unlike Pam3Cys and LPS, activates TLR2/TPL/ERK signaling through binding to TLR2 and negatively regulates NF-κB activation through CXCR4 activation. It is possible that the CXCR4–TLR2 complex has different binding pockets for Pam3Cys and PAUF, thus allowing these different ligands to differentially activate downstream signaling pathways. Supportively, PAUF synergized the Pam3Cys-induced cytokine generation mediated by ERK activation related to AP-1 target genes, but antagonized the Pam3Cys-induced NF-κB activation through CXCR4 mediated by PKA activation in THP-1 cells. In fact, this possibility is supported by our findings that PAUF interacts with TLR2 at the amino-acid level and synergizes with Pam3Cys to induce production of RANTES, MIF and IL-1RA cytokines, irrespective of CXCR4 activation. Consistent with a previous report (Hajishengallis et al., 2008), PKA might function as a downstream effector of PAUF-CXCR4-TLR2; once activated, PKA could also inhibit TLR2-induced NF-κB activation. PAUF upregulates CXCR4 expression, and the increased cancer mobility induced by PAUF is attenuated by an anti-CXCR4 antibody or the CXCR4 inhibitor, AMD3100 (Lee et al., 2010). On the basis of these observations and our current findings, we propose that PAUF interacts with TLR2 and CXCR4, and simultaneously increases CXCR4 expression, thereby creating a microenvironment favorable for tumor progression and metastasis. Ultimately, establishing the molecular properties of PAUF and CXCR4 proteins in the microenvironment of cancers has the potential to lead to novel diagnostic and therapeutic clinical applications.
Finally, we have established and validated new, innovative ECD receptor pools for screening novel ligand–receptor interactions. We are currently expanding our ECD receptor pools to include 1000 receptors, creating a valuable resource for identifying ligand receptors.
Supplementary Material
Acknowledgements
This study was supported by grants from the National Creative Research Initiative Center Program, World Class University (WCU) program and the 21st Century Frontier Functional Human Genome Project of the Ministry of Education, Science and Technology in Korea. We thank Sang Yong Hong for technical support with the cloning work. We thank the staff of Beamline 4A at the Pohang Accelerator Laboratory, Korea, for assistance in data collection and Dr Hyun Kyu Song of Korea University for help with MAD data analysis.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
References
- Adachi H, Takano K, Morikawa M, Kanaya S, Yoshimura M, Mori Y, et al. Application of a two-liquid system to sitting-drop vapour-diffusion protein crystallization. Acta Crystallogr D Biol Crystallogr. 2003;59:194–196. doi: 10.1107/s0907444902019741. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Azenshtein E, Luboshits G, Shina S, Neumark E, Shahbazian D, Weil M, et al. The CC chemokine RANTES in breast carcinoma progression: regulation of expression and potential mechanisms of promalignant activity. Cancer Res. 2002;62:1093–1102. [PubMed] [Google Scholar]
- Bach JP, Rinn B, Meyer B, Dodel R, Bacher M. Role of MIF in inflammation and tumorigenesis. Oncology. 2008;75:127–133. doi: 10.1159/000155223. [DOI] [PubMed] [Google Scholar]
- Banerjee A, Gugasyan R, McMahon M, Gerondakis S. Diverse Toll-like receptors utilize Tpl2 to activate extracellular signal-regulated kinase (ERK) in hemopoietic cells. Proc Natl Acad Sci USA. 2006;103:3274–3279. doi: 10.1073/pnas.0511113103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barre A, Bourne Y, Van Damme EJ, Peumans WJ, Rouge P. Mannose-binding plant lectins: different structural scaffolds for a common sugar-recognition process. Biochimie. 2001;83:645–651. doi: 10.1016/s0300-9084(01)01315-3. [DOI] [PubMed] [Google Scholar]
- Bourne Y, Zamboni V, Barre A, Peumans WJ, Van Damme EJ, Rouge P. Helianthus tuberosus lectin reveals a widespread scaffold for mannose-binding lectins. Structure. 1999;7:1473–1482. doi: 10.1016/s0969-2126(00)88338-0. [DOI] [PubMed] [Google Scholar]
- Chen R, Alvero AB, Silasi DA, Steffensen KD, Mor G. Cancers take their Toll—the function and regulation of Toll-like receptors in cancer cells. Oncogene. 2008;27:225–233. doi: 10.1038/sj.onc.1210907. [DOI] [PubMed] [Google Scholar]
- Cho J, Melnick M, Solidakis GP, Tsichlis PN. Tpl2 (tumor progression locus 2) phosphorylation at Thr290 is induced by lipopolysaccharide via an Ikappa-B Kinase-beta-dependent pathway and is required for Tpl2 activation by external signals. J Biol Chem. 2005;280:20442–20448. doi: 10.1074/jbc.M413554200. [DOI] [PubMed] [Google Scholar]
- Folkman J. What is the evidence that tumors are angiogenesis dependent? J Natl Cancer Inst. 1990;82:4–6. doi: 10.1093/jnci/82.1.4. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, et al. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Hajishengallis G, Wang M, Liang S, Triantafilou M, Triantafilou K. Pathogen induction of CXCR4/TLR2 cross-talk impairs host defense function. Proc Natl Acad Sci USA. 2008;105:13532–13537. doi: 10.1073/pnas.0803852105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang B, Zhao J, Li H, He KL, Chen Y, Chen SH, et al. Toll-like receptors on tumor cells facilitate evasion of immune surveillance. Cancer Res. 2005;65:5009–5014. doi: 10.1158/0008-5472.CAN-05-0784. [DOI] [PubMed] [Google Scholar]
- Huang B, Zhao J, Shen S, Li H, He KL, Shen GX, et al. Listeria monocytogenes promotes tumor growth via tumor cell toll-like receptor 2 signaling. Cancer Res. 2007;67:4346–4352. doi: 10.1158/0008-5472.CAN-06-4067. [DOI] [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Katiyar S, Swaminathan CP, Sekar K, Surolia A, Vijayan M. Structural basis of the carbohydrate specificities of jacalin: an X-ray and modeling study. J Mol Biol. 2003;332:217–228. doi: 10.1016/s0022-2836(03)00901-x. [DOI] [PubMed] [Google Scholar]
- Jeyaprakash AA, Srivastav A, Surolia A, Vijayan M. Structural basis for the carbohydrate specificities of artocarpin: variation in the length of a loop as a strategy for generating ligand specificity. J Mol Biol. 2004;338:757–770. doi: 10.1016/j.jmb.2004.03.040. [DOI] [PubMed] [Google Scholar]
- Jouault T, El Abed-El Behi M, Martinez-Esparza M, Breuilh L, Trinel PA, Chamaillard M, et al. Specific recognition of Candida albicans by macrophages requires galectin-3 to discriminate Saccharomyces cerevisiae and needs association with TLR2 for signaling. J Immunol. 2006;177:4679–4687. doi: 10.4049/jimmunol.177.7.4679. [DOI] [PubMed] [Google Scholar]
- Ju G, Labriola-Tompkins E, Campen CA, Benjamin WR, Karas J, Plocinski J, et al. Conversion of the interleukin 1 receptor antagonist into an agonist by site-specific mutagenesis. Proc Natl Acad Sci USA. 1991;88:2658–2662. doi: 10.1073/pnas.88.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly MG, Alvero AB, Chen R, Silasi DA, Abrahams VM, Chan S, et al. TLR-4 signaling promotes tumor growth and paclitaxel chemoresistance in ovarian cancer. Cancer Res. 2006;66:3859–3868. doi: 10.1158/0008-5472.CAN-05-3948. [DOI] [PubMed] [Google Scholar]
- Kim SA, Lee Y, Jung DE, Park KH, Park JY, Gang J, et al. Pancreatic adenocarcinoma up-regulated factor (PAUF), a novel up-regulated secretory protein in pancreatic ductal adenocarcinoma. Cancer Sci. 2009;100:828–836. doi: 10.1111/j.1349-7006.2009.01106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y, Kim SJ, Park HD, Park EH, Huang SM, Jeon SB, et al. PAUF functions in the metastasis of human pancreatic cancer cells and upregulates CXCR4 expression. Oncogene. 2010;29:56–67. doi: 10.1038/onc.2009.298. [DOI] [PubMed] [Google Scholar]
- Li Y, Komai-Koma M, Gilchrist DS, Hsu DK, Liu FT, Springall T, et al. Galectin-3 is a negative regulator of lipopolysaccharide-mediated inflammation. J Immunol. 2008;181:2781–2789. doi: 10.4049/jimmunol.181.4.2781. [DOI] [PubMed] [Google Scholar]
- Li YM, Pan Y, Wei Y, Cheng X, Zhou BP, Tan M, et al. Upregulation of CXCR4 is essential for HER2-mediated tumor metastasis. Cancer Cell. 2004;6:459–469. doi: 10.1016/j.ccr.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- Miettinen M, Veckman V, Latvala S, Sareneva T, Matikainen S, Julkunen I. Live Lactobacillus rhamnosus and Streptococcus pyogenes differentially regulate Toll-like receptor (TLR) gene expression in human primary macrophages. J Leukoc Biol. 2008;84:1092–1100. doi: 10.1189/jlb.1206737. [DOI] [PubMed] [Google Scholar]
- Morse RJ, Yamamoto T, Stroud RM. Structure of Cry2Aa suggests an unexpected receptor binding epitope. Structure. 2001;9:409–417. doi: 10.1016/s0969-2126(01)00601-3. [DOI] [PubMed] [Google Scholar]
- Nishihira J, Ishibashi T, Fukushima T, Sun B, Sato Y, Todo S. Macrophage migration inhibitory factor (MIF): its potential role in tumor growth and tumor-associated angiogenesis. Ann NY Acad Sci. 2003;995:171–182. doi: 10.1111/j.1749-6632.2003.tb03220.x. [DOI] [PubMed] [Google Scholar]
- Raman D, Baugher PJ, Thu YM, Richmond A. Role of chemokines in tumor growth. Cancer Lett. 2007;256:137–165. doi: 10.1016/j.canlet.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raz A, Lotan R. Endogenous galactoside-binding lectins: a new class of functional tumor cell surface molecules related to metastasis. Cancer Metastasis Rev. 1987;6:433–452. doi: 10.1007/BF00144274. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Vassylyev DG, Kido S, Doi Y, Morikawa K. Crystal structure of vitelline membrane outer layer protein I (VMO-I): a folding motif with homologous Greek key structures related by an internal three-fold symmetry. EMBO J. 1994;13:1003–1010. doi: 10.1002/j.1460-2075.1994.tb06348.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sadanandam A, Singh RK. Chemokines in tumor angiogenesis and metastasis. Cancer Metastasis Rev. 2007;26:453–467. doi: 10.1007/s10555-007-9068-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strieter RM, Burdick MD, Mestas J, Gomperts B, Keane MP, Belperio JA. Cancer CXC chemokine networks and tumour angiogenesis. Eur J Cancer. 2006;42:768–778. doi: 10.1016/j.ejca.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Lepper PM, Briault CD, Ahmed MA, Dmochowski JM, Schumann C, et al. Chemokine receptor 4 (CXCR4) is part of the lipopolysaccharide ‘sensing apparatus’. Eur J Immunol. 2008;38:192–203. doi: 10.1002/eji.200636821. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.