Abstract
A field trial was performed under commercial feedlot conditions in western Canada to compare the efficacy of a new formulation of long-acting oxytetracycline (LA 30) to a standard long-acting oxytetracycline formulation (LA 20) and florfenicol (FLOR) for the treatment of undifferentiated fever (UF) in calves that received metaphylactic tilmicosin upon arrival at the feedlot. Seven hundred and ninety-seven recently weaned, auction market derived, crossbred, beef calves suffering from UF were allocated to 1 of 3 experimental groups as follows: LA 30, which received intramuscular long-acting oxytetracycline (300 mg/mL formulation) at the rate of 30 mg/kg body weight (BW) at the time of allocation; LA 20, which received intramuscular long-acting oxytetracycline (200 mg/mL formulation) at the rate of 20 mg/kg BW at the time of allocation; or FLOR, which received intramuscular florfenicol administered at the rate of 20 mg/kg BW at the time of allocation and again 48 hours later. Two hundred and sixty-six animals were allocated to the LA 30 group, 265 animals were allocated to the LA 20 group, and 266 animals were allocated to the FLOR group. The relative efficacy of the LA 30 group, as compared with the LA 20 and FLOR groups, was assessed by comparing relapse, chronicity, wastage, and mortality rates.
The overall mortality (RR = 0.50) rate in the LA 30 group was significantly (P < 0.05) lower than in the LA 20 group. However, the overall chronicity (RR = 2.56) and overall wastage (RR = 6.97) rates of the LA 30 group were significantly (P < 0.05) higher than in the LA 20 group. There were no significant (P ≥ 0.05) differences in UF relapse rates or cause specific mortality rates between the LA 30 and LA 20 groups. In the economic analysis, there was an advantage of $28.59 CDN per animal in the LA 30 group compared with the LA 20 group.
The overall chronicity (RR = 2.25) and overall wastage (RR = 2.80) rates of the LA 30 group were significantly (P < 0.05) higher than the FLOR group. There were no significant (P ≥ 0.05) differences in UF relapse rates, overall mortality rates, or cause specific mortality rates between the LA 30 and FLOR groups. In the economic analysis, there was an advantage of $12.90 CDN per animal in the LA 30 group compared with the FLOR group.
In summary, the results of this study indicate that it is more cost-effective to use a new formulation of long-acting oxytetracycline (300 mg/mL formulation administered at a rate of 30 mg/kg BW) than a standard long-acting oxytetracycline formulation (200 mg/mL formulation administered at a rate of 20 mg/kg BW) or florfenicol for the treatment of UF in feedlot calves that have previously received metaphylactic tilmicosin upon arrival at the feedlot.
Introduction
Undifferentiated fever (UF), also referred to as bovine respiratory disease (BRD) complex or shipping fever, continues to be one of the most economically significant health problem in calves entering beef feedlots (1,2,3,4,5,6,7,8). Management of this disease complex involves both metaphylactic and therapeutic administration of parenteral antimicrobials. Several studies have demonstrated the cost-effectiveness of administering metaphylactic tilmicosin (Micotil; Provel, Division Eli Lilly Canada, Guelph, Ontario) to feedlot calves due to reductions in BRD morbidity rates, BRD mortality rates, and, or, overall mortality rates and improvements in average daily gain and, or, feed efficiency as compared with no metaphylaxis (9,10,11,12,13,14,15). In calves that receive metaphylactic tilmicosin upon arrival at the feedlot, florfenicol (Nuflor; Schering Plough Animal Health, Division of Schering Canada, Pointe Claire, Quebec) has been shown to be more efficacious and cost-effective for the treatment of UF than tilmicosin (3). However, there is limited published research comparing the relative efficacy of other antimicrobials for the treatment of UF in feedlot calves receiving metaphylactic tilmicosin.
A new formulation of long-acting oxytetracycline (Tetradure LA-300; Merial Canada, Baie D'Urfé, Quebec) has been developed. The purpose of the investigation reported herein was to compare the therapeutic efficacy of the new formulation, administered at 30 mg/kg body weight (BW), with a standard formulation of long-acting oxytetracycline (Liquamycin LA-200; Animal Health Group, Pfizer Canada, London, Ontario), administered at 20 mg/kg BW, and florfenicol (Nuflor; Schering Plough Animal Health, Division of Schering Canada) for the treatment of UF in feedlot calves that received metaphylactic tilmicosin upon arrival at the feedlot.
Materials and methods
Trial facilities
The trial was conducted in a commercial feedlot near High River, Alberta, which has a capacity of 30 000 animals. The basic design of this feedlot is representative of standard design in western Canada. The animals were housed in open-air, dirt-floor pens, arranged side by side with central feed alleys and 20% porosity wood-fence windbreaks. There are 120 large pens in the feedlot, each of which has a capacity of approximately 250 animals.
There are 2 hospital facilities and an enclosed processing facility located in the feedlot. Each hospital facility has a hydraulic chute equipped with an individual animal scale, a chute-side computer for the collection of animal health data, and separation alleys to facilitate the return of animals to designated feedlot pens. Three open-air hospital pens are located adjacent to each hospital. The enclosed processing facility has a hydraulic chute equipped with an individual animal scale. There are 10 receiving pens located adjacent to the processing facility.
Trial animals
The animals utilized in the study were recently weaned crossbred beef calves, purchased from auction markets throughout western Canada. Approximately 75 animals per truck were transported to the feedlot, after assembly at the auction market. The animals allocated to the study were approximately 7 to 10 mo of age and weighed between 196 kg and 410 kg.
Upon arrival at the feedlot, the animals were moved through a hydraulic chute for a group of procedures known collectively as processing. All animals were weighed, ear tagged (to provide unique, individual animal identification), implanted with a zeranol growth implant (Ralgro; Schering Plough Animal Health, Division of Schering Canada), and vaccinated against infectious bovine rhinotracheitis (IBR) and parainfluenza-3 (PI3) viruses (Bovishield IBR-PI3; Animal Health Group, Pfizer Canada). In addition, each animal received a multivalent clostridial and Haemophilus somnus vaccine (Ultrabac 7/Somubac; Animal Health Group, Pfizer Canada), a Mannheimia (formerly Pasteurella) haemolytica bacterial extract (Presponse; Ayerst Veterinary Laboratories, Division of Wyeth-Ayerst Canada, Guelph, Ontario), topical ivermectin (Ivomec Pour-On; Merial Canada) at a rate of 1.0 mL/ 10 kg BW, and metaphylactic SC tilmicosin (Micotil; Provel, Division Eli Lilly Canada) at a rate of 10 mg/kg BW. Also, all bulls were castrated and all heifers were aborted with IM cloprostenol (Estrumate; Schering Plough Animal Health, Division of Schering Canada) at a dose of 1.5 mL per animal.
At approximately 70 d on feed for each pen, all animals were implanted with a growth implant (Synovex H, Synovex S, or Synovex Plus; Ayerst Veterinary Laboratories, Division of Wyeth-Ayerst Canada) and vaccinated against IBR and PI3 viruses (Bovishield IBR-PI3; Animal Health Group, Pfizer Canada).
Experimental design
Using mortality data from studies previously conducted by Jim, et al (unpublished observations), it was calculated that approximately 250 to 300 animals per experimental group would be necessary to detect relative differences in mortality of 75% or larger between the experimental groups, using a confidence of 95% (α = 0.05) and a power of 90% (β = 0.10).
Animals that arrived at the feedlot between September 30, 1997, and November 16, 1997, were candidates for the study. Subsequent to the processing event, animals were moved to designated feedlot pens. Each pen was checked once or twice daily by experienced animal health personnel for evidence of disease. Animals that were deemed “sick,” based on subjective criteria, such as general appearance and attitude, gauntness, reluctance to move, et cetera, were moved from the pen and presented to the hospital facility. In this study, the case definition for UF was an elevated rectal temperature (≥40.3∘C), a lack of abnormal clinical signs referable to organ systems other than the respiratory system, and no previous treatment history for any disease. At the time of allocation, the experimental animals were weighed and randomly assigned to 1 of 3 experimental groups as follows by using a computer-generated randomization table: LA 30, which received IM long-acting oxytetracycline (300 mg/mL formulation) at the rate of 30 mg/kg BW at allocation; LA 20, which received IM long-acting oxytetracycline (200 mg/mL formulation) at the rate of 20 mg/kg BW at allocation; or FLOR, which received IM florfenicol at the rate of 20 mg/kg BW at the time of allocation and again 48 h later.
Animals in the FLOR group were housed in the hospital facility during the treatment period and, following administration of the 2nd injection, were returned to their designated feedlot pens. Animals in the LA 30 and LA 20 groups were returned to their designated feedlot pens on the same day as allocation and treatment.
Subsequent to initial UF therapy, the experimental animals were observed once or twice daily by experienced animal health personnel for evidence of recurrent disease. The animal health personnel were blinded to the experimental status of each animal. Those animals that were deemed “sick,” based on subjective assessments of general appearance and attitude, gauntness, reluctance to move, et cetera, were moved from the pen and presented to the hospital facility. Subsequent to posttreatment intervals of 72 h, 72 h, and 48 h (2nd injection) in the LA 30, LA 20, and FLOR groups, respectively, first UF relapses were defined as animals with a lack of abnormal clinical signs referable to organ systems other than the respiratory system. Animals that were identified as first UF relapses prior to fulfillment of the appropriate posttreatment interval were returned to the pen without treatment. All first UF relapses were housed in the hospital facility for 2 d, treated with IM ceftiofur (Excenel; Pharmacia & Upjohn Animal Health, Division of Pharmacia & Upjohn, Orangeville, Ontario) at a dosage of 1 mg/kg BW once daily for 3 consecutive days, and returned to their original feedlot pen following the 3rd injection.
Second and third UF relapses were defined as those animals with a lack of abnormal clinical signs referable to organ systems other than the respiratory system. All second UF relapses were hospitalized, treated with IM trimethoprim-sulfadoxine (Trivetrin; Schering Canada) at a dosage of 2.66 mg trimethoprim + 13.33 mg sulfadoxine per kg BW once daily for 3 consecutive days, and returned to their original pen following the 3rd injection. All third UF relapses were hospitalized, treated with IM ampicillin-sulbactam (Synergistin, Animal Health Group, Pfizer Canada) at a dosage of 6.6 mg ampicillin + 3.3 mg sulbactam per kg BW once daily for 3 consecutive days, and returned to their original pen after the 3rd injection.
Three relapse treatment regimes (first UF relapse, second UF relapse, and third UF relapse) were the maximum permitted for all animals in the study. That is, once an animal was treated as a third UF relapse, no further therapy for UF occurred. Animals that were identified as sick subsequent to third UF relapse therapy were deemed to be “chronics.” Also, animals that were unsuitable to be returned to their designated feedlot pens, based on subjective appraisal of the attitude and appearance of each animal, were deemed to be “chronics.” Finally, all other diseases were treated as per a standard feedlot protocol provided by the consulting veterinarians. All treatment events, including treatment date, presumptive diagnosis, drug(s) used, and dose(s), were recorded on the chute-side computer system.
The animal health events of each animal were followed from allocation to slaughter. Animals that were deemed as chronics but did not die were defined as wastage. All animals that died during the study were necropsied by the attending feedlot veterinarians, and a diagnosis was made based on the findings of the gross postmortem examination.
Data collection and management
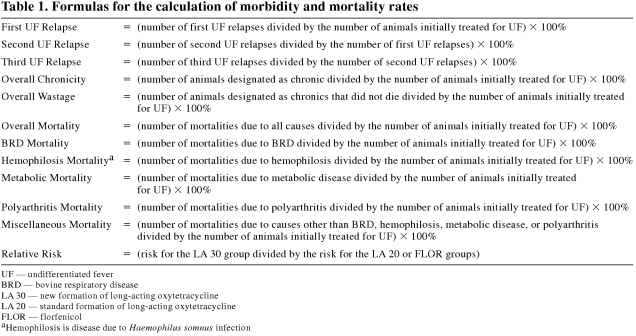
The computerized animal health data from allocation to slaughter were verified and summarized. From these data, risk rates for first UF relapse, second UF relapse, third UF relapse, overall chronicity, overall wastage, overall mortality (mortality due to all causes), BRD mortality (mortality due to bronchointerstitial pneumonia, chronic fibrinous pneumonia, chronic pleuritis, or fibrinous pneumonia), hemophilosis mortality (mortality due to myocarditis, pleuritis, or septicemia), metabolic mortality (mortality due to atypical interstitial pneumonia or bloat), polyarthritis mortality, and miscellaneous mortality (mortality due to enteritis, musculoskeletal injury, nervous disease, no gross lesions/unknown, peritonitis, ruptured urinary bladder, intestinal torsion, or viral pneumonia) were calculated for each experimental group (Table 1).
Table 1.
Statistical analysis
For the various animal health indices, relative risks (RR) and their 95% confidence intervals (95% CI) were calculated for the LA 30 group (relative to the LA 20 and FLOR groups) by using the precision-based technique (16). Fisher's exact two-tailed tests were calculated for each RR, using the method previously described for pair-wise comparisions from a 2 × 3 table (17). The Mantel-Haenszel procedure was used to investigate a possible confounding effect of feedlot pen (18).
Economic analysis
The relative cost-effectiveness of the experimental groups was calculated using a proprietary computer spreadsheet program (Microsoft Excel 97; Microsoft Corporation, Redmond, Washington, USA) that simulates all economic aspects of feedlot production, as previously described (3,4,19). The LA 30 group was independently compared with both the LA 20 and FLOR groups. The actual morbidity and mortality rates of each experimental group were included in the economic model when the rates were significantly (P < 0.05) different between the groups. When there were no significant (P ≥ 0.05) differences between the experimental groups, the morbidity and mortality rates of the LA 30 group were used for both experimental groups. All other factors were fixed in the economic simulations. The therapeutic costs used in the economic analysis were $5.00 CDN, $2.00 CDN, and $21.00 CDN for each LA 30-, LA 20-, and FLOR-treatment regime, respectively. The purchase price used in the analysis was $286.60 CDN per 100 kg ($130.00 CDN per 100 lb.) BW. The interest rate used in the analysis was 7.5% per annum. An economic value was not ascribed to animals designated as chronic. The value of chronics that did not die (wastage) was reduced by $100 CDN per animal as compared with other study animals. The value of a dead animal was $0.00 CDN. Feed consumed by animals prior to death was not estimated.
Results
Two hundred and sixty-six animals were allocated to the LA 30 group, 265 animals were allocated to the LA 20 group, and 266 animals were allocated to the FLOR group. There were no adverse reactions in any of the experimental groups. In the statistical analyses, there was no evidence to support a confounding effect of feedlot pen.
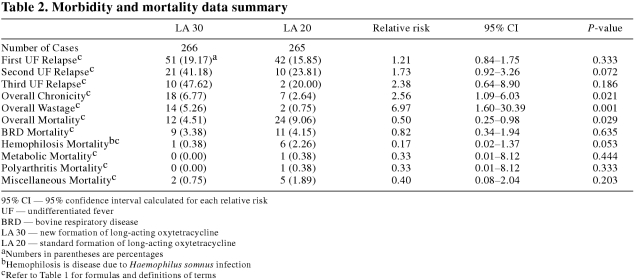
The morbidity and mortality variables for the LA 30 and LA 20 groups are summarized in Table 2. The overall mortality (RR = 0.50) rate of the LA 30 group was significantly (P < 0.05) lower than that of the LA 20 group. However, the overall chronicity (RR = 2.56) and overall wastage (RR = 6.97) rates of the LA 30 group were significantly (P < 0.05) higher than those of the LA 20 group. There were no significant (P ≥ 0.05) differences in first, second, or third UF relapse rates or BRD, hemophilosis, metabolic, polyarthritis, or miscellaneous mortality rates between the LA 30 and LA 20 groups. In the economic analysis, there was an advantage of $28.59 CDN per animal in the LA 30 group. This advantage in the LA 30 group was composed of $3.07 CDN and $4.60 CDN per animal disadvantages due to a higher antimicrobial cost and a higher wastage rate, respectively, along with a $36.26 CDN per animal advantage due to a lower overall mortality rate.
Table 2.
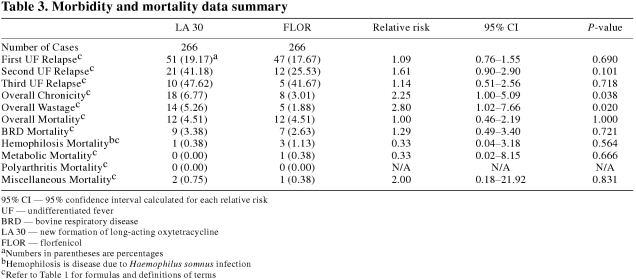
The morbidity and mortality variables for the LA 30 and FLOR groups are summarized in Table 3. The overall chronicity (RR = 2.25) and overall wastage (RR = 2.80) rates of the LA 30 group were significantly (P < 0.05) higher than those of the FLOR group. There were no significant (P ≥ 0.05) differences in first, second, or third UF relapse rates or overall, BRD, hemophilosis, metabolic, polyarthritis, or miscellaneous mortality rates between the LA 30 and FLOR groups. In the economic analysis, there was an advantage of $12.90 CDN per animal in the LA 30 group. This advantage in the LA 30 group was composed of a $16.35 CDN per animal advantage due to a lower antimicrobial cost along with a $3.45 CDN per animal disadvantage due to a higher wastage rate.
Table 3.
Discussion
The results of this study demonstrate that the new formulation of long-acting oxytetracycline (300 mg/mL formulation) administered at the rate of 30 mg/kg BW is more effective than a standard formulation of long- acting oxytetracycline (200 mg/mL formulation) administered at the rate of 20 mg/kg BW for the treatment of UF in feedlot calves in western Canada due to a lower mortality rate. The results of this study also demonstrate that this new formulation (300 mg/mL formulation) of long-acting oxytetracycline is comparable with florfenicol for the treatment of UF in feedlot calves in western Canada due to similar mortality rates. It is important to note that all calves in this study received metaphylactic tilmicosin upon arrival at the feedlot, which is a standard cost-effective, management procedure utilized by feedlots in western Canada.
In this study, the new formulation of long-acting oxytetracycline (300 mg/mL formulation) administered at a rate of 30 mg/kg BW was associated with higher chronicity and wastage rates when used for the treatment of UF in feedlot calves than either a standard formulation of long-acting oxytetracycline (200 mg/mL formulation) administered at a rate of 20 mg/kg BW or florfenicol administered at a rate of 20 mg/kg BW at the time of allocation and again 48 h later. The term wastage, which has been previously defined and discussed (3), refers to animals initially treated for UF that subsequently were deemed to be chronics but did not go on to die or be condemned at slaughter. In this economic analysis, the value realized for these animals was reduced by $100 CDN per animal as compared with other study animals. With respect to the difference in wastage rates between the LA 30 and LA 20 groups, every $100 CDN per animal increase in the cost of wastage results in a $4.61 CDN per animal reduction in the LA 30 group economic advantage. With respect to the difference in wastage rates between the LA 30 and FLOR groups, every $100 CDN per animal increase in the cost of wastage results in a $3.46 CDN per animal reduction in the LA 30 group economic advantage.
In the economic analyses, a value was not ascribed to chronicity because additional therapy was not administered at the time of designation. In addition, the ultimate impact of chronicity was incorporated into the economic analysis as wastage or mortality. Moreover, animals designated as chronic that “recovered” and returned to their original feedlot pen were subsequently marketed with their penmates at no discount to full market value.
The formulation and dosage rate of long-acting oxytetracycline (300 mg/mL formulation administered at a rate of 30 mg/kg BW) used in the LA 30 group are different than the standard formulation and rate of long-acting oxytetracycline (200 mg/mL formulation administered at a rate of 20 mg/kg BW) used for the treatment of UF in the LA 20 group. The differences in formulation and rate may account for the improved efficacy of long-acting oxytetracycline used in the LA 30 group. However, it cannot be determined from the results of this study whether the improved therapeutic efficacy of long-acting oxytetracycline observed in the LA 30 group was due to the formulation, the rate, a combination of both formulation and dosage rate, or other unidentified factors.
In summary, the results of this study indicate that it is more cost-effective to use a new formulation of long-acting oxytetracycline (300 mg/mL formulation administered at a rate of 30 mg/kg BW) than a standard long-acting oxytetracycline formulation (200 mg/mL formulation administered at a rate of 20 mg/kg BW) or florfenicol for the treatment of UF in feedlot calves that have previously received metaphylactic tilmicosin upon arrival at the feedlot.
Footnotes
Acknowledgments
The authors thank the management and staff of Western Feedlots Limited, High River, Alberta for their assistance and cooperation in conducting this study. CVJ
1. This project was wholly supported by research grants from Merial Canada Incorporated and Merial Limited, Iselin, New Jersey and was conducted for licensing purposes under the provisions of Investigational New Drug Submission #950371.
2. As of August 8, 2002, the long-acting oxytetracycline product used in this study was not approved for use in Canada for the application used in this study (therapeutic use at a rate of 30 mg/kg BW IM).
3. The withdrawal period prior to slaughter assigned by the Veterinary Drugs Directorate (VDD) (formerly the Bureau of Veterinary Drugs) for the long-acting oxytetracycline product used in this study, at 30 mg/kg BW IM, was 56 days (INDS 950371). Subsequently, the VDD assigned a withdrawal period prior to slaughter for this product, at 30mg/kg BW IM, of 28 days (ESC 973054 and ESC 983072).
4. In this study, tissue reactions at the injection sites were not evaluated.
Address all correspondence and reprint requests to Dr. Oliver C. Schunicht.
References
- 1.Booker CW, Jim GK, Guichon PT, Schunicht OC, Thorlakson BE, Lockwood PW. Evaluation of florfenicol for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1997;38:555–560. [PMC free article] [PubMed]
- 2.Booker CW, Guichon PT, Jim GK, Schunicht OC, Harland RJ, Morley PS. Seroepidemiology of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1999;40:40–48. [PMC free article] [PubMed]
- 3.Jim GK, Booker CW, Guichon PT, et al. A comparison of florfenicol and tilmicosin for the treatment of undifferentiated fever in feedlot calves in western Canada. Can Vet J 1999;40:179–184. [PMC free article] [PubMed]
- 4.Guichon PT, Jim GK, Booker CW, Schunicht OC, Wildman BK, Brown JR. Relative cost-effectiveness of treatment of feedlot calves with ivermectin versus treatment with a combination of fenbendazole, permethrin, and fenthion. J Am Vet Med Assoc 2000;216:1965–1969. [DOI] [PubMed]
- 5.National Agriculture Statistics Service. Cattle and Calves Death Loss. Washington, DC: US Dept Agric, 1996.
- 6.National Agriculture Statistics Service. Cattle and Calves Death Loss. Washington, DC: US Dept Agric, 1992.
- 7.Van Donkersgoed J, Janzen ED, Harland RJ. Epidemiologic features of calf mortality due to hemophilosis in a large feedlot. Can Vet J 1990;31:821–825. [PMC free article] [PubMed]
- 8.Kelly AP, Janzen ED. A review of morbidity and mortality rates and disease occurrence in North American feedlot cattle. Can Vet J 1986;27:496–500. [PMC free article] [PubMed]
- 9.Guthrie CA, Laudert SB, Zimmerman AG. Metaphylaxis for undifferentiated bovine respiratory disease. Compend Contin Educ Pract Vet 2000;22:S62–S67.
- 10.Vogel GJ, Laudert SB, Zimmerman A, Guthrie CA, Mechor GD, Moore GM. Effects of tilmicosin on acute undifferentiated respiratory tract disease in newly arrived feedlot cattle. J Am Vet Med Assoc 1998;212:1919–1924. [PubMed]
- 11.Galyean ML, Gunter SA, Malcolm-Callis KJ. Effects of arrival medication with tilmicosin phosphate on health and performance of newly received beef cattle. J Anim Sci 1995;73:1219–1226. [DOI] [PubMed]
- 12.Schumann FJ, Janzen ED, McKinnon JJ. Prophylactic tilmicosin medication of feedlot calves at arrival. Can Vet J 1990;31:285–288. [PMC free article] [PubMed]
- 13.Schumann FJ, Janzen ED, McKinnon JJ. Prophylactic medication of feedlot calves with tilmicosin. Vet Rec 1991;128:278–280. [DOI] [PubMed]
- 14.Morck DW, Merrill JD, Thorlakson BE, Olson ME, Tonkinson LV, Costerton JW. Prophylactic efficacy of tilmicosin for bovine respiratory tract disease. J Am Vet Med Assoc 1993;202:273–277. [PubMed]
- 15.Merrill JK, Jim GK, Guichon PT, Booker CW. A comparison of the prophylactic use of tilmicosin injection and long-acting oxytetracycline injection on morbidity, mortality, and performance of feedlot calves. J Anim Sci 1994;72(Suppl 1):143.
- 16.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research. Principles and Quantitative Methods. Belmont, California: Wadsworth, 1982:351–355.
- 17.Hirji KF, Vollset SE. Algorithm AS 293: Computing exact distributions for several ordered 2 × K tables. Appl Stat 1994; 43:541–548.
- 18.Kleinbaum DG, Kupper LL, Morgenstern H. Epidemiologic Research. Principles and Quantitative Methods. Belmont, California: Wadsworth, 1982:356–363.
- 19.Schunicht OC, Guichon PT, Booker CW, et al. Comparative cost-effectiveness of ivermectin versus topical organophosphate in feedlot yearlings. Can Vet J 2000;41:220–224. [PMC free article] [PubMed]