Abstract
A marked increase in leptospirosis in dogs was observed in 2000, part of an increasing trend observed in previous years in Ontario. The highest frequency of seropositive cases occurred from September to December 2000, with the peak in November. Large breed dogs were particularly affected. Clinical and clinicopathological data for 31 dogs admitted between 1998 and 2000 to the Ontario Veterinary College Veterinary Teaching Hospital were analyzed. Major clinical presenting features were acute onset of anorexia, depression, fever, and vomiting. Ninety percent of dogs, on admission, showed biochemical evidence of injury to several organs, notably combinations in the order of kidney, muscle, pancreas, and liver. Almost all dogs showed increased serum urea and creatinine levels, and the majority had increased total creatine kinase, bilirubin, alkaline phosphatase, and leukocytosis with neutrophilia. One-third were thrombocytopenic. Of dogs with liver-related abnormalities, most had evidence of cholestasis, with or without hepatocellular damage. Based on serologic studies, in the year 2000, the major serovar involved was autumnalis, but bratislava, grippotyphosa, and pomona were also implicated. The microscopic agglutination test often gave a confusing pattern of reactivities to the serovars that were tested. The high reactivity to serovar autumnalis may represent an erroneous or “paradoxical” reaction typical of early leptospiral serology. The year 2000 was the warmest in Ontario in each of the 4 fall months (September–December) of the previous decade, as well as being the third wettest in the fall period in the last decade. The increase in canine leptospirosis, therefore, may, in part, reflect climate change. The number of positive cases declined in 2001 by about one-third of those in 2000, but the number of submissions of sera for diagnosis increased markedly over previous years. Further work is required to isolate and to identify definitively serovars involved in resurgent canine leptospirosis and the common sources for dogs.
Introduction
Leptospirosis has increased in dogs in the United States (1,2,3,4,5,6,7), Québec (8,9), and Ontario (10,11,12,13) in the last few years. The serovars mainly involved in canine leptospirosis are no longer canicola and icterohaemorrhagiae, as reported before the 1970s (14,15); they now include grippotyphosa and pomona as the most common serovars (1,2,3,4,5,6,7,8,9,10,11,12,13), although bratislava (16) and possibly autumnalis (12) are sometimes the infecting serovars. The reason for the increase of leptospirosis in dogs and the change in the serovars involved may be the increased and endemic infection of urban wildlife (notably raccoons, skunks) with leptospirosis, combined with increased numbers of urban wildlife and an increasing index of suspicion by veterinarians, thus promoting serological testing, as well as successful control by vaccination of the previously important serovars.
Although canine leptospirosis is recognized to have been increasing in Ontario in the last few years (10,11,12,13), the fall of 2000 saw a marked rise in the number of cases. A major factor was probably the wet and exceptionally warm late summer and fall, which provided conditions that were ideal for the transmission of Leptospira from wildlife. The purpose of this paper is to describe some epidemiological features of the canine infection in Ontario, based on the year 2000; the major clinical and clinicopathological features of recent cases of the disease in dogs presented to the Ontario Veterinary College's Veterinary Teaching Hospital (VTH); and the characteristic histopathological changes observed. The role of urban raccoons and skunks as likely major reservoirs of infection is discussed as being among the aspects of the infection that require better understanding.
Materials and methods
Serological findings
All cases submitted to the Animal Health Laboratory (AHL), University of Guelph, for serological diagnosis of canine leptospirosis from January 1998 to December 2001 were retrieved from the computerized database. The microscopic agglutination test (MAT) had been carried out in the AHL under standard test conditions against the following serovars: autumnalis, bratislava, canicola, grippotyphosa, icterohaemorrhagiae, and pomona. For seroepidemiological purposes, titers ≤ 40 were regarded as negative, from 80 to 160 as suspicious, and ≥ 320 as positive for the particular serovar tested. Breed of dog and date of serum submission were recorded. Differences between seropositive and nonseropositive canine cases by serovar and year were analyzed by Pearson's chi-square and Fisher's exact test.
Clinical and clinicopathological findings
Clinical and clinicopathological data for 31 dogs admitted in 1998, 1999, and 2000 to the VTH, with an eventual diagnosis of leptospirosis were analyzed. Criteria for diagnosis were a MAT of ≥ 320 to one or more serovars, and a clinical illness compatible with leptospirosis with no alternative diagnosis. Clinical signs on presentation were recorded. Parameters of clinicopathological change were assessed by standard methods; the data recorded were those of the initial samples taken at the time of admission.
Histopathological findings
The microscopic changes observed in formalin-fixed, hematoxylin and eosin stained samples submitted as kidney biopsy specimens from 23 live dogs or assorted tissues (kidney, liver) from 7 dogs that had died were recorded. The majority of samples for which a histopathologic examination was performed were from those submitted to a private histopathology diagnostic service.
Meteorological data
Mean temperature and rainfall data for the fall months of the previous decade at the Waterloo Regional airport, about 15 km from the Ontario Veterinary College, were obtained from the Ontario Climate Centre, Environment Canada.
Results
Serological findings
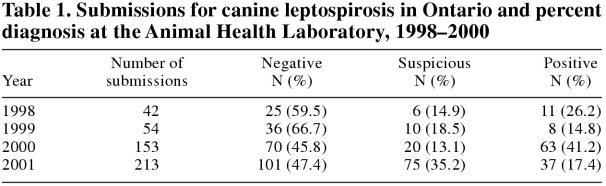
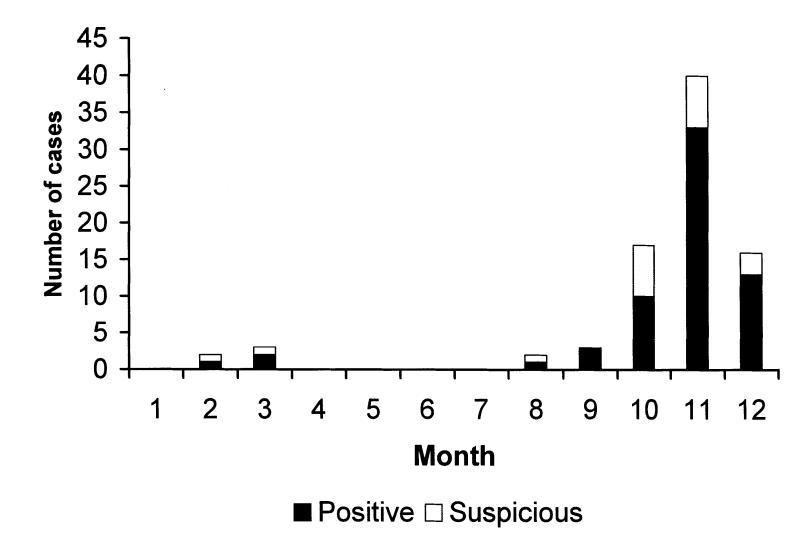
There was a marked increase in the diagnosis of canine leptospirosis in Ontario in 2000. The overall submission rate of canine sera for the serological diagnosis of canine leptospirosis increased 3.7 times in 2000 compared with 1998 (Table 1), and the proportion of seropositive cases in 2000 was 2.8 times those in 1999 and 1.6 times those in 1998 (Table 1, Figure 1). In 2000, the seropositive cases occurred in both spring and fall, but the highest submission rate and frequency of seropositive cases occurred from the beginning of September to the end of December 2000, with the peak in November (Figure 2). The numbers of positive or suspicious cases declined by about one-third in 2001, although the total number of sera submitted for diagnosis increased by about one-third. In 2001, in contrast to previous years, there were seropositive cases every month, with the largest numbers of cases occurring from the beginning of October to the end of January (data not shown).
Table 1.
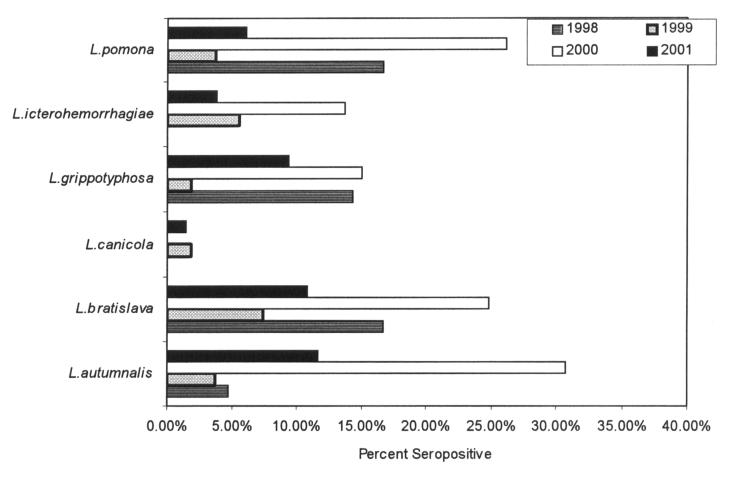
Figure 1. Leptospiral microscopic agglutination test, percentage seropositive canine sera, by year. Since dogs commonly reacted to several serovars, percent adds to more than 100.
Figure 2. Number of leptospiral microscopic agglutination test seropositive and suspicious canine sera by month, year 2000.
Typically, a seropositive dog exhibited positive MAT to a range of the serovars tested. In about one-third of the cases, the MAT for a single serovar was ≥ 2 dilutions greater than that of each of the other serovars, but in the other two-thirds, there were < 2 dilutions between titers of each serovar tested. Only 25 (31%) of 80 sera had a MAT in which 1 serovar had a titer ≥ 2 dilutions more than any of the other serovars, a proportion that was not different between years. Eighteen of 24 sera from January 1998 to December 2000 had serovar autumnalis showing titers ≥ 2 dilutions more than any of the other serovars. There was a significant increase in the proportion of seropositive to seronegative sera in 2000 compared with 1998 for serovars autumnalis (P = 0.0006) and icterohaemorrhagiae (P = 0.009), and in 2000 compared with 1999 for serovars autumnalis (P = 0.0001), bratislava (P = 0.006), grippotyphosa (P = 0.006), and pomona (P = 0.004).
The most common breed of dog that was seropositive was “mixed breed” (65 of 167 serologically positive or suspicious). The following breeds occurred ≥ 6 times: Labrador or golden retriever (n = 12); miniature schnauzer (n = 11); Doberman pinscher (n = 10); German shepherd (n = 9); Alaskan malamute (n = 6); bichon frise (n = 6).
Clinical and clinicopathological findings
Dogs were presented with nonspecific signs of lethargy (90%), inappetance (81%), dehydration (52%), and weight loss of variable severity (29%). Other signs at presentation included vomiting (81%), abdominal or lumbar pain (65%), polyuria/polydipsia (42%), tachypnea (35%), stiff gait suggestive of arthralgia or myalgia (35%), icterus (29%), and lymphadenopathy (19%). Four dogs were pyrexic; renomegaly was detected in 3 dogs; and either petechiation, oculonasal discharge, or ascites was observed in 1 dog each.
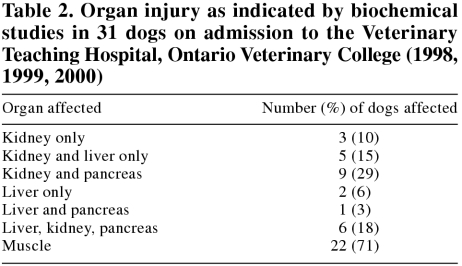
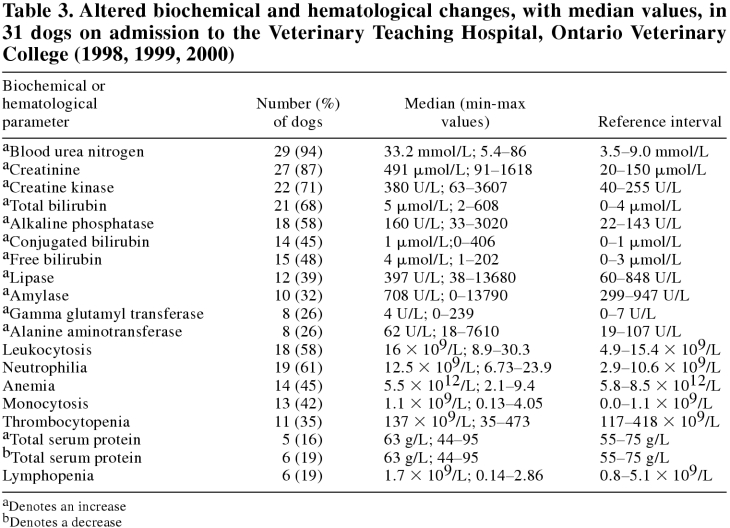
Ninety percent of dogs on admission showed biochemical evidence of injury to several organs, notably combinations in the order of kidney, muscle, pancreas, and liver (Table 2). Electrolyte and mineral imbalances were observed in 87%; acid-base disturbances in 29%; and altered total protein, albumin, or globulins in 71%. Almost all dogs on admission showed increased urea and creatinine, and the majority had increased total creatine kinase, bilirubin, and alkaline phosphatase, as well as leukocytosis with neutrophilia (Table 3). Thrombocytopenia was recorded in one-third of dogs. Of dogs with liver-related abnormalities, 32% had evidence of cholestasis only, 26% of both hepatocellular and cholestatic disease, and none had evidence of hepatocellular damage only.
Table 2.
Table 3.
Histopathological findings
The repeatable and significant microscopic lesions were restricted to kidney and liver. In those cases for which histologic evaluation of both organs was possible, lesions were always present in both. In the kidney, there was widespread acute renal tubular necrosis, characterized by cortical tubular flattening, increased basophilia, and the appearance of granular casts within tubular lumens. There was patchy-to-diffuse interstitial edema, and, in about half the cases, there was a mild, multifocal-to-diffuse lymphocytic interstitial inflammation. In those cases judged, on the basis of histologic criteria, to have a slightly longer clinical course, the interstitial edema was converted to immature fibrosis and the magnitude of the lymphocytic infiltrate increased.
In the liver, the lesions were usually very subtle, with diffuse margination of neutrophils (sometimes intermingled with lymphocytes) along the sinusoids, accompanied by hypertrophy of Kupffer cells. The overall impression was one of a diffusely “busy” liver, typical of the hepatic reaction to bacteremia. The hypercellularity was accompanied in about half the cases by widespread but subtle single cell hepatocellular necrosis, and by an increased number of mitotic figures within hepatocytes. In some livers; presumably with older lesions, there was a diffuse interstitial lymphocytic hepatitis, a marked increase in mitotic figures, and other evidence of attempted hepatic regeneration; such as anisokaryosis and binucleation, and some degree of lobular collapse that probably represented a sequel to extensive single cell necrosis.
Meteoreological data
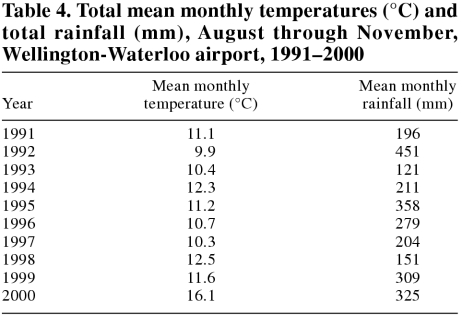
Compared with the previous decade, the year 2000 had the warmest August, September, October, and November, the 2nd highest rainfall in October, and the 3rd highest rainfall in August, September, and November. Mean monthly temperatures and rainfall for August through November are shown in Table 4. The mean temperature for December 2000 (-8.7°C) was the coldest December in the decade.
Table 4.
Discussion
There has been a major change in serovars involved in canine leptospirosis in the United States and Canada, from canicola and, to a lesser extent, icterohaemorrhagiae in the 1950s and 1960s (14,15) to grippotyphosa and pomona, and, to a lesser extent, bratislava (1,2,3,4,5,6,7,8,9,10,11,12,13,16). The decline to relative insignificance of canicola and icterohaemorrhagiae is likely the result of vaccination of dogs against these 2 serovars, especially in the 1970s. A recent study of the prevalence of leptospirosis in dogs in the United States and Canada from 1970 through 1998 has shown that canine leptospirosis has resurged from the early 1990s to reach the levels last seen in the early 1970s, after which time infection caused by serovars canicola and icterohaemorrrhagiae was controlled by vaccination (17).
Although leptospirosis has increased markedly in dogs in recent years, there is evidence that the “new” serovars have caused clinical leptospirosis in dogs for many years, and it may be that leptospirosis has been under-diagnosed during the last 2 decades (3,10). Nevertheless, in the late 1980s and early 1990s, only 1 or 2 canine cases a year were being diagnosed serologically in Ontario by the forerunner of the AHL. The large number of cases observed in 2000 was unprecedented and seems to have been the result of the conjunction of the apparent general increase in leptospirosis in urban wildlife reservoirs, discussed below; the unusually warm late summer and fall conditions; and the increased submission rate to the AHL. Although the large number of cases in 2000 was probably related to the warmest and 3rd wettest fall of the 1991 to 2000 decade, the general pattern of increased cases of canine leptospirosis in recent years cannot be attributed to fall climatic factors (Table 4). The peak incidence of disease in November was later than the peak in October reported for canine leptospirosis from 1980 to 1995 in New York State (3), again probably because of the unusual length of warmth of the fall months. One intriguing feature was the small rise in cases in February and March, usually times of extreme cold in Ontario, which would seem to preclude the spread of this fastidious and environmentally sensitive organism. A small “spring rise” was also identified among New York State cases (3).
Most serological studies in the United States and Canada implicate serovars grippotyphosa and pomona as the main serovars currently causing canine leptospirosis (1,2,3,4,5,6,7,8,9,10,11,12,13), based on the predominant MAT, but our study identified serovar autumnalis as a possible important serovar, which emerged particularly in 2000. Identification of the infecting serovar based on the MAT response early in infection is, however, problematic because of the paradoxical effects observed in the serological response to early leptospirosis (18). A “paradoxical reaction” occurs when the MAT shows highest reactivity to a serovar(s) other than the infecting serovar; with time, however, the major infecting serovar often gives the highest titer of the serovars tested (8,18). Nevertheless, the high seroprevalence of autumnalis, especially in 2000, is a striking finding that supports the need for isolation and identification of the causative serovars, rather than relying on serological studies to determine the infecting serovar. Serovar autumnalis has been included among those tested for in MAT ever since work, many years ago, had identified it, only on the basis of serologic testing, as being present in dogs in Toronto (19). Tests of canine sera for the serovar autumnalis are sometimes not done in the United States (2,4), so that this serovar may have been missed. However, it seems possible that reactivity to serovar autumnalis represents a “paradoxical” cross-reactivity between this serovar and others, notably pomona. Such paradoxical cross-reactivity is well recognized in the early serological response to leptospiral infection and was readily seen in the broad cross-reactivities observed. For example, Kingscote (20) consistently isolated serovar pomona from the kidneys of red foxes in southwestern Ontario with severe lesions of interstitial nephritis; antibodies to serovar autumnalis were present in each of these foxes at titers equal to, and usually exceeding, those of pomona. Others have observed similar paradoxical reactions between autumnalis and pomona (21). This suggestion that early serological response of dogs with leptospirosis to autumnalis represents a paradoxical reaction supported by the failure to isolate autumnalis from dogs in the United States or Canada (7,10,16).
No attempt was made to determine vaccination histories for dogs. Anecdotal evidence is that most dogs in Ontario were not vaccinated against canicola and icterohaemorrhagiae in the period described, because of adverse effects of these vaccines and the lack of evidence of infection caused by these serovars; a grippotyphosa and pomona vaccine was licenced for use in Canada in 2001, but it was not used in Ontario, other than in special instances, before this time. Figure 1 shows the low proportion of dogs that were positive for serovar canicola, a vaccination serovar. The coincidence of diagnostic titers with typical clinical signs of leptospirosis supports the diagnosis made in this study.
The most common clinical presentations of leptospirosis in the dogs in this study were nonspecific signs including lethargy, inappetance, vomiting and abdominal pain, findings that are similar to those in other reports of canine leptospirosis (2,3,4,5,8,10,13). The stiff gait and lumbar pain suggestive of myalgia (or arthralgia) observed in about one-third of affected dogs was supported by biochemical evidence of muscle damage in over two-thirds. Clinical evidence of myalgia may be a subtle but important clue to leptospirosis in a dog. Polymyositis has been described as the main clinical manifestation of leptospirosis in a dog (22). Clinicopathological changes were typical of those described by others, although with more evidence of increased pancreatic enzymes, and almost invariably involved renal disease. Increases in amylase and lipase may have been indicative of concurrent pancreatitis, localized infarcts, or decreased renal metabolism. Only 3 animals had liver rather than kidney disease. Although liver disease without kidney disease is uncommon, it may be the only manifestation of canine leptospirosis (23). The decrease in thrombocytes in only one-third of affected dogs is less than sometimes reported, and might reflect delayed admission of these animals to the referral hospital.
The most striking feature of the histologic changes was the contrast between the subtlety of histologic lesions and the reported severity of clinical signs. In the first few cases examined, the kidney was described as being “almost normal” and skepticism was expressed that these subtle changes of tubular basophilia and modest tubular flattening were enough to explain 5 or 6 d of unrelenting renal failure. Subtle though the renal lesions were, the liver changes were often even more so, presenting an even greater contrast to the severity of clinical disease and biochemical abnormalities. Even in dogs with profound clinical and biochemical evidence of liver disease, the changes could be easily missed on routine histologic screening. While the slight diffuse sinusoidal hypercellularity is seen in other bacteremic diseases and in livers reacting, for example, to a breakdown in the intestinal barrier, the presence of widely scattered single cell necrosis was a distinctive feature that separated leptospirosis from other inflammatory liver diseases. Single cell necrosis is also observed in idiosyncratic drug reactions, but in those instances, the sinusoidal neutrophilia and Kupffer cell hypertrophy, typical of leptospirosis, are absent.
The most common breed of dog affected was “mixed breed”, while other breeds commonly represented included large breed dogs. Others have reported large, herding, hound, or working male dogs as predominant in cases of canine leptospirosis (2,3,4,6,17), presumptively associated with a tendency to spend more time outside, but the numbers of miniature schnauzers and bichon frises reported here indicate that canine leptospirosis is not restricted to active, “sporty” types of dogs.
There is evidence that leptospiral infection is being spread by raccoons in Ontario and Quebec (2,24,25). Raccoons are plentiful in urban areas of Ontario, with numbers that, in places, may reach even as high as 100/km2 (1 per 10m2), far exceeding numbers observed in forests or farmland surrounding these areas (26). Forested-parks and residential areas support the highest densities, because of the availability of nesting trees, water, and food in such city environments. The most commonly identified sources of serovar grippotyphosa in the United States are field voles and raccoons (1). Skunks may also be a reservoir, but they are probably more often a source of serovar pomona (27). However, this serovar-host reservoir association is not complete (28,29). Interstitial nephritis, in some cases with visible leptospires, is common in raccoons in the United States (30). There is evidence from a seroprevalence study of raccoons in Illinois that grippotyphosa infection increased over a 4-year period from 28% in 1992 to 65% in 1993 (31), suggesting that leptospiral infection might have been spreading in raccoons in the years shortly before the resurgence of canine leptospirosis. By contrast to raccoons, skunk numbers in urban environments are lower (6 to 12/km2), more similar to those observed in rural habitats (32). To reduce possible transmission of infection from raccoons, dog owners should be warned against leaving food and water outside for their dogs, particularly during the late summer and the fall of the year. In addition, canine leptospiral vaccines containing serovars grippotyphosa and pomona have recently become available in Canada and provide effective protection against infection.
In summary, canine leptospirosis has become increasingly common in recent years in southwestern Ontario, but it surged dramatically in the year 2000, suggesting that urban leptospirosis may become one of the adverse effects of climate change. Additional work is required to define the serovars involved directly though isolation, rather than indirectly through serology, and to confirm the relative importance of raccoons, skunks, or other wildlife reservoirs in maintaining the infection in urban environments. The importance of leptospirosis as a zoonotic infection from dogs (33,34) emphasizes the need for veterinarians to consider recommending vaccination as a likely effective and relatively inexpensive way of controlling the infection in dogs.
Footnotes
Acknowledgment
The authors thank Dr. R.C. Rosatte, Ontario Ministry of Natural Resources, for data relating to raccoon and skunk populations in Ontario. CVJ
Address correspondence to Dr. John F. Prescott; email prescott@uoguelph.ca.
Reprints will not be available from the authors.
References
- 1.Bolin CA. Diagnosis of leptospirosis: a reemerging disease of companion animals. Sem Vet Med Surg (Small Anim) 1996;11: 166–171. [DOI] [PubMed]
- 2.Adin CA, Cowgill LD. Treatment and outcome of dogs with leptospirosis: 36 cases (1990–1998). J Am Vet Med Assoc 2000; 216:371–375. [DOI] [PubMed]
- 3.Birnbaum N, Barr SC, Center SA, Schermerhorn T, Randolph JF, Simpson KW. Naturally acquired leptospirosis in 36 dogs: serological and clinicopathological features J Small Anim Pract 1998;39:231–236. [DOI] [PubMed]
- 4.Rentko VT, Clark N, Ross LA, et al. Canine leptospirosis: A retrospective study of 17 cases. J Vet Intern Med 1992;6:235–244. [DOI] [PubMed]
- 5.Anonymous. Leptospirosis cases rising in United States. J Am Vet Med Assoc 1998;212:472. [PubMed]
- 6.Harkin KR, Gartrell CL. Canine leptospirosis in New Jersey and Michigan: 17 cases (1990–1995). J Am Anim Hosp Assoc 1996; 32:495–501. [DOI] [PubMed]
- 7.Brown CA, Roberts AW, Miller MA, et al. Leptospira interrogans serovar grippotyphosa infection in dogs. J Am Vet Med Assoc 1996;209:1265–7. [PubMed]
- 8.Kalin M, Devaux C, DiFruscia R, Lemay S, Higgins R. Three cases of canine leptospirosis in Quebec. Can Vet J 1999;40:187–191. [PMC free article] [PubMed]
- 9.Ribotta M, Fortin M. Higgins R, Beadin S. Canine leptospirosis: serology. Can Vet J 2000;41:494–495. [PMC free article] [PubMed]
- 10.Prescott JF, Ferrier RL, Nicholson VM, Johnston KM, Hoff B. Is canine leptospirosis underdiagnosed in southern Ontario? A case report and serological study. Can Vet J 1991:481–486. [PMC free article] [PubMed]
- 11.Hrinivich K, Prescott JF. Leptospirosis in two unrelated dogs. Can Vet J 1997;38:509–510. [PMC free article] [PubMed]
- 12.Gillick A. Leptospirosis emerging in Ontario. College of Veterinarians of Ontario “Update”. 1999;Jan/Feb:10–12.
- 13.Prescott JF, Key D, Osuch M. Leptospirosis in dogs. Can Vet J 1999;40:430–431. [PMC free article] [PubMed]
- 14.Alexander AD, Gleiser CA, Malnati P, et al. Observations on the prevalence of leptospirosis in canine populations of the United States. Am J Hyg 1957;65:43–56. [DOI] [PubMed]
- 15.Hubbert WT, Shotts EB. Leptospirosis in kennel dogs. J Am Vet Med Assoc 1966;148:1152–1159. [PubMed]
- 16.Nielsen JN, Cochran GK, Cassells JA, et al. Leptospira interrogans serovar bratislava in two dogs. J Am Vet Med Assoc 1991;199: 351–352. [PubMed]
- 17.Ward MP, Glickman LT, Guptill LF. Prevalence of and risk factors for leptospirosis among dogs in the United States and Canada: 677 cases (197–1998). J Am Vet Med Assoc 2002;220:53–58. [DOI] [PubMed]
- 18.Faine S, Adler B, Bolin C, Perolat P. Leptospira and Leptospirosis, 2nd ed. Melbourne, Australia: MedSci, 2000.
- 19.Kingscote B, Tittiger F. Serological survey of dogs from Toronto for leptospiral antibodies. Can Vet J 1976;17:192–193. [PMC free article] [PubMed]
- 20.Kingscote BF. Leptospirosis in red foxes in Ontario. J Wildlife Dis 1986;22:475–478. [DOI] [PubMed]
- 21.Clark LG, Kresse JI, Marshak RR, Hollister CJ. Leptospira pomona infection in an eastern red fox (Vulpes fulva fulva). Nature (Lond) 1960;188:1040–1041. [DOI] [PubMed]
- 22.Poncelet L, Fontaine M, Balligand M. Polymyositis associated with Leptospira australis infection in a dog. Vet Rec 1991;129:40. [DOI] [PubMed]
- 23.Bishop L, Strandberg JD, Adams RJ, Brownstein DG, Patterson R. Chronic active hepatitis in dogs associated with leptospires. Am J Vet Res 1979;40:839–844. [PubMed]
- 24.Warshawsky B, Lindsay R, Artsob H. Leptospirosis confirmed in three trappers in the Middlesex-London heath unit area. Publ Health Epidemiol Rep Ontario 1998;9:181–182.
- 25.Mikaelian I, Higgins R, Lequient M, et al. Leptospirosis in raccoons in Quebec: 2 case reports and seroprevalence in a recreational area. Can Vet J 1997;38:440–442. [PMC free article] [PubMed]
- 26.Rosatte RC. Management of raccoons (Procyon lotor) in Ontario, Canada: Do human interventions and disease have signficant impact on raccoon populations? Mammalia 2000;64:369–390.
- 27.McGowan JE, Karstad L. Field and laboratory studies of skunks, raccoons and groundhogs as reservoirs of Leptospira pomona. Can Vet J 1965;6:243–252. [PMC free article] [PubMed]
- 28.Abdulla PK, Karstad LH, Fish NA. Investigation of leptospirosis in wildlife in Ontario. Can J Pub Hlth 1962;53:445–451. [PubMed]
- 29.Alexander AD, Flyger V, Herman YF, McConnell SJ, Rothstein N, Yager RH. Survey of wild mammals in a Chesapeake Bay area for selected zoonoses. J Wildlife Dis 1972;8:119–126. [DOI] [PubMed]
- 30.Hamir AN, Hanlon CA, Niezgoda M, Rupprecht CE. The prevalence of interstitial nephritis and leptospirosis in 283 raccoons (Procyon lotor) from 5 different sites in the United States. Can Vet J 2001;42:869–871. [PMC free article] [PubMed]
- 31.Mitchell MA, Hungerford LL, Nixon C, et al. Serologic survey for selected infectious disease agents in raccoons from Illinois. J Wildlife Dis 1999;35:347–355. [DOI] [PubMed]
- 32.Broadfoot JD, Rosatte RC, O'Leary DT. Raccoon and skunk population models for urban disease control planning in Ontario, Canada. Ecol Applicat 2001;11:295–303.
- 33.Barkin RM, Glosser JW. Leptospirosis-an epidemic in children. Am J Epidemiol 1973;98:184–191. [DOI] [PubMed]
- 34.Wong ML, Kaplan S, Dunkle LM, Stechenberg BW, Feigin RD. Leptospirosis: A childhood disease. J Pediatrics 1977;90:532–537. [DOI] [PubMed]