Since the original discovery of the cardioprotective effects of ischemic preconditioning in 19861, one of the unsolved mysteries of the phenomenon is the mechanism that is responsible for the energy sparing effect. It was recognized very early that during the sustained period of ischemia, ATP utilization was much slower in the preconditioned myocardium than in the non-preconditioned myocardium2, 3. This was recognized as a slower rate of decline in tissue ATP during ischemia, but the effect is even more pronounced if the total rate of ATP utilization is considered, which includes the ATP generated from anaerobic glycolysis during ischemia as well as the creatine phosphate and ATP present in the tissue at the start of ischemia. As shown in Figure 1, this energy sparing effect is present in both dog and rat myocardium. The energy sparing effect is apparent within the first 5 minutes of ischemia (Figure 2). The slower rate of ATP utilization results in less stimulation of anaerobic glycolysis, attenuates the fall in intracellular pH, and minimizes the ionic alterations that are characteristic of ischemia3. However, it is unlikely that ATP preservation is a major factor in the protective effect, because there is loss of ATP during the preconditioning protocol, and therefore less ATP reserves in the tissue at the start of the sustained period of ischemia; despite slower ATP utilization, ATP depletion occurs after a similar duration of ischemia in preconditioned and non-preconditioned myocardium2, 3. In fact, the onset of contracture, which is a marker of severe ATP depletion, occurs more rapidly with some protocols of preconditioning in rodent hearts, which are none-the-less protected from ischemic injury3, 4. Therefore, if the energy-sparing effect of preconditioning is important for the protective effect, it is likely through an indirect mechanism.
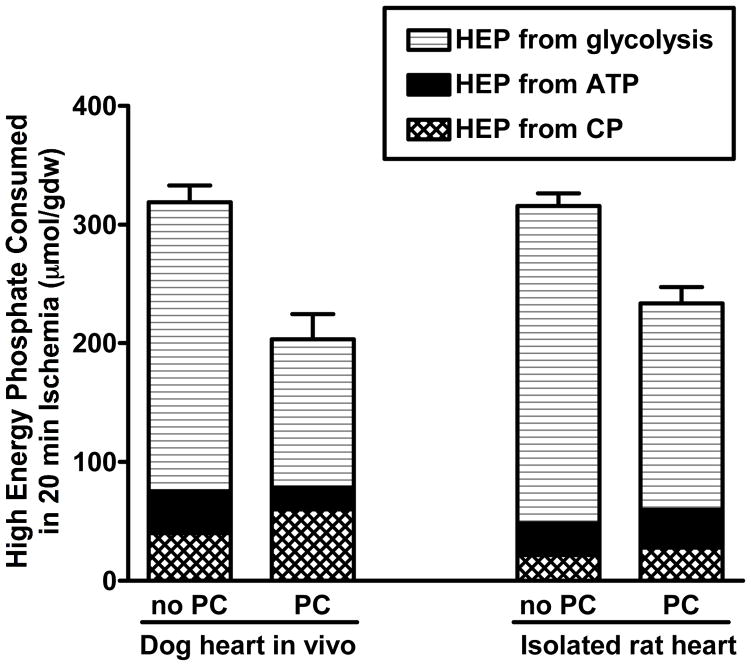
Figure 1.
Total amount of high-energy phosphate consumed in severely or totally ischemic myocardium during 20 minutes of ischemia. The total amount of high energy phosphate consumed is equal to the amount of ATP produced by anaerobic glycolysis and the amount of high energy phosphate that is derived from tissue reserves of ATP and creatine phosphate. For this calculation, it was assumed that the flux through anaerobic glycolysis is equal to the change in tissue lactate and that the lactate came from glycogen, which yield 1.5 high-energy phosphates per lactate. Creatine phosphate breakdown yields 1 high energy phosphate, ATP yields 2, and ADP yields 1. This calculation is described in more detail in Murry et al1, and the data for the dog heart is taken from the same source. Data for the isolated rat heart was obtained from analysis of tissue extracts, using the same assays as for the dog heart. For both the dog and rat experiments, preconditioning consisted of 4 cycles of 5 minutes of ischemia and 5 minutes of reperfusion.
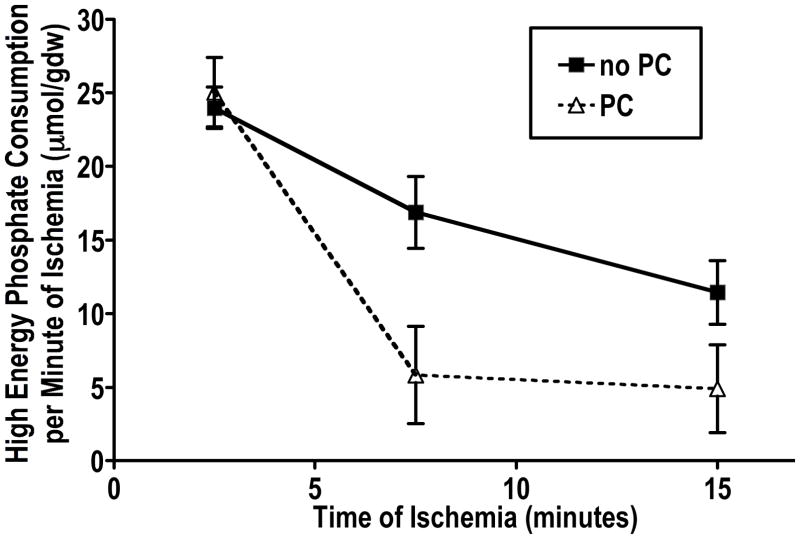
Figure 2.
The data are obtained from Murry et al1, as described in Figure 1. The value at each time point is obtained from the changes in lactate, ATP, ADP, and creatine phosphate. The value for 2.5 minutes reflects the difference from time 0 to 5 minutes of ischemia. The value for 7.5 minutes reflects the difference from 5 to 10 minutes of ischemia, and the value for 15 minutes reflects the difference from 10 to 20 minutes of ischemia. The data are replotted from Figure 12 of Murry et al1.
We have found two cardioprotective interventions that target the mitochondria and are associated with an energy-conserving effect. These two interventions may help to illuminate the basis for the energy conserving effect. The first intervention is overexpression of Bcl-25 and the second intervention is treatment with glycogen synthase kinase (GSK) inhibitors6. Both of these interventions are associated with a reduction in the amount of cell death that occurs during a defined period of ischemia, and both of these interventions are associated with an alteration in mitochondrial transport that may account for the energy-conserving effect. The protective effect of GSK inhibitors is seen whether the inhibitor is administered prior to ischemia or at the onset of reperfusion and is observed in both isolated heart models and in vivo7–9. Studies of mice with cardiac-specific overexpression of Bcl-25 showed that ATP consumption during the first few minutes of ischemia was slower than in wild-type hearts, and the fall in intracellular pH was less in the Bcl-2 overexpressor hearts. Previous work has shown that reversal of the mitochondrial F1F0ATPase is responsible for approximately 50% of ATP consumption during ischemia and that slowing this process slows the rate of anaerobic glycolysis10–12, resulting in less intracellular acidification. To determine whether Bcl-2 overexpression slows mitochondrial ATP hydrolysis under de-energized conditions, we studied the effects of Bcl-2 on isolated mitochondria, de-energized with the uncoupler dinitrophenol, and we found that ATP hydrolysis by the mitochondria was markedly slowed in mitochondria with high levels of Bcl-2, either from Bcl-2 overexpressor hearts where the Bcl-2 remained associated with the mitochondria, or from wild-type hearts where recombinant Bcl-2 was added to the mitochondria. We hypothesized that this might be due to inhibition of anion transport through VDAC under de-energized conditions, and we performed co-immunoprecipitation experiments to show that Bcl-2 binds to VDAC. We also found that the amount of Bcl-2 that binds to VDAC is increased during ischemia5.
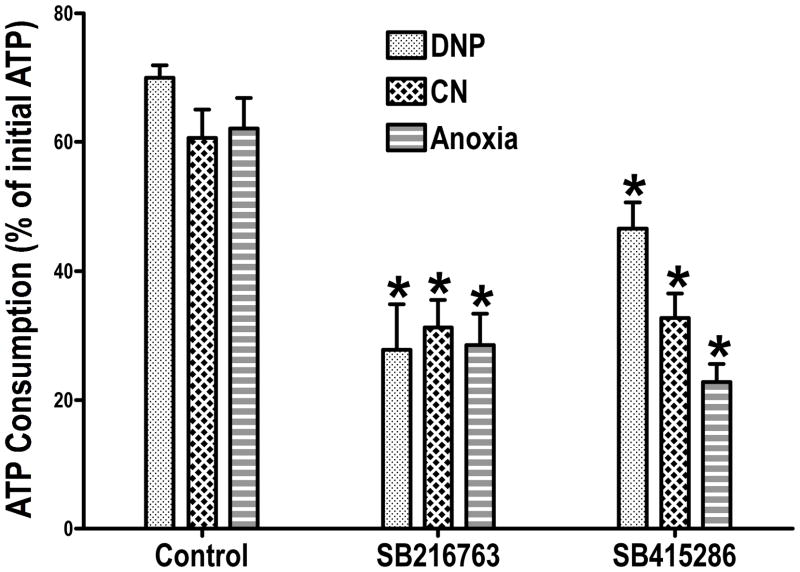
Since other cardioprotective interventions also have an energy conserving effect13, 14, it is possible that Bcl-2 overexpression is only one of several possible mechanisms that can affect mitochondrial ATP consumption under de-energized conditions. To investigate this possibility, we studied another intervention, which may be more closely linked to ischemic preconditioning, which is GSK inhibition. We6, 7 and others8, 15 have shown that GSK inhibition occurs during ischemic preconditioning and various forms of pharmacological preconditioning. Although recent studies with genetically altered mice have questioned the requirement for GSK inhibition in cardioprotection16, the bulk of data support a role for GSK7, 8, 15, 17, 18 and there are questions about possible protective adaptations in the studies with the genetically-altered mice17. We wanted to determine if the protection we observed with GSK inhibitors was associated with a mitochondrial energy-conserving effect similar to the effect of Bcl-2 overexpression. We used two different GSK inhibitors and pretreated isolated perfused rat hearts with the inhibitors6. We chose concentrations of inhibitors that attenuated ischemic injury. We isolated mitochondria from control hearts and hearts treated with GSK inhibitors, and we studied mitochondrial ATP consumption under de-energized conditions. We used the same de-energization protocol that we used for the Bcl-2 experiments (the uncoupler dinitrophenol), and we used two other protocols that would be more analogous to ischemic conditions. These other protocols involved treating the mitochondria with cyanide to block respiration and making the mitochondria anoxic by having them consume the oxygen in a sealed oxygraph chamber. These protocols prevent mitochondrial respiration but do not immediately de-energize the mitochondria, and therefore the duration of treatment was extended to allow the mitochondria to become de-energized. As shown in Figure 3, regardless of the protocol, we found that ATP hydrolysis by the isolated mitochondria was slower in the mitochondria from hearts treated with GSK inhibitors than in control mitochondria.
Figure 3.
The data are replotted from Das et al, Figure 25. For the dinitrophenol (DNP) group, ATP consumption was measured after 5 minutes of incubation. For the cyanide (CN) group, ATP consumption was measured after 20 minutes of incubation. For the anoxia group, ATP consumption was measured after 60 minutes of incubation. Details are in Das et al5.
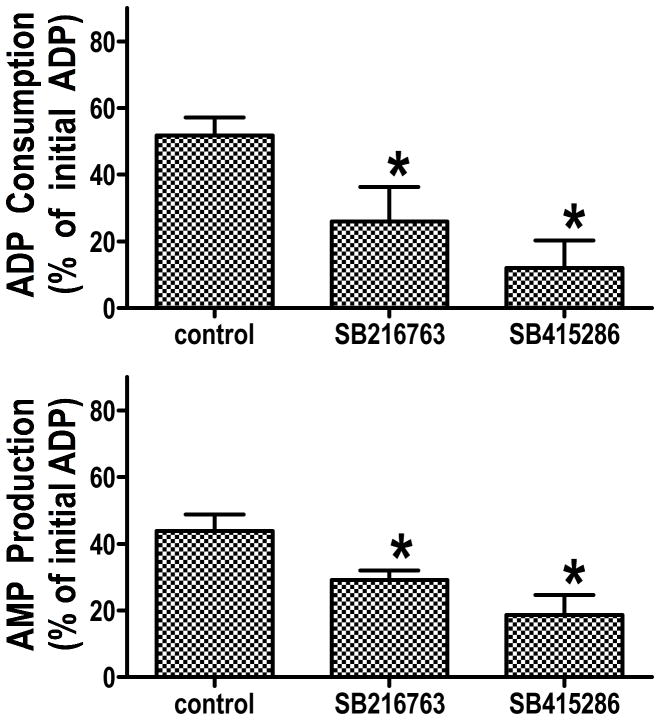
The finding that Bcl-2 and GSK inhibitors slow ATP hydrolysis by de-energized mitochondria does not clarify the site of the inhibitory effect. The assay is based on addition of ATP to the medium, and the measurement of the amount of ATP remaining at the end of incubation. Inhibition of ATP transport through VDAC (the voltage-dependent anion channel in the outer mitochondrial membrane), inhibition of ATP transport through ANT (the adenine nucleotide translocator in the inner mitochondrial membrane), or inhibition of the F1F0ATPase in the mitochondrial matrix could account for the energy-conserving effect of these cardioprotective interventions. To clarify the site of the inhibitory effect, we sonicated the mitochondria to disrupt the membranes and expose the F1F0ATPase, and we found that GSK inhibitors do not affect ATP hydrolysis in the sonicated mitochondrial fragments6, indicating that the GSK inhibitors are not affecting F1F0ATPase activity. It is also possible that ATP hydrolysis does not involve the physiologic adenine nucleotide transporters, but rather involves the mitochondrial permeability transition pore (mPTP), and several investigators have proposed that inhibition of the mPTP is an important element of many cardioprotective interventions15, 19–22. To evaluate this, we treated the mitochondria with cyclosporin A, at a concentration which inhibits calcium-induced swelling, and we saw no effect of cyclosporin A on ATP hydrolysis in either control mitochondria or mitochondria treated with a GSK inhibitor. Finally, we used an assay that would distinguish between transport across the outer mitochondrial membrane through VDAC versus transport across the inner mitochondrial membrane through ANT. As shown in Figure 4, this assay takes advantage of the enzyme adenylate kinase which is located in the intermembrane space. Isolated mitochondria are treated with cyanide to block respiration and de-energize the mitochondria, and oligomycin was added to block the F1F0ATPase. After 20 minutes of incubation to allow the mitochondria to become de-energized, ADP was added to the medium. ADP can enter the intermembrane space through VDAC, where it can be converted by adenylate kinase to ATP and AMP, which can then exit the mitochondrial outer membrane through VDAC. This assay measures the rate of adenine nucleotide transport through VDAC and the rate of conversion of ADP to ATP and AMP by adenylate kinase. We measured both the amount of ADP remaining in the medium and the amount of AMP that was present in the medium 10 minutes after ADP addition. Figure 5 shows that ADP consumption and AMP production were reduced in the GSK inhibitor treated mitochondria to a similar extent. To be certain that the GSK inhibitors were not altering adenylate kinase activity, mitochondria were sonicated and the same assay was performed. Under these conditions, there was no effect of GSK inhibitors6, and the rate of AMP production in the control and GSK inhibitor treated mitochondrial fragments was comparable to the control intact mitochondria.
Figure 4.
Diagramatic scheme of the assay for VDAC transport of adenine nucleotides. ADP is added to the medium and the amount of ADP consumed and the amount of AMP produced is measured. More details are available in Das et al5.
Figure 5.
The data are obtained from Das et al5. Cyanide and oligomycin were added for 20 minutes, and then ADP was added and the mitochondria were incubated for an additional 10 minutes. At the end of the incubation, the mitochondria were pelleted and the amount of ADP remaining in the medium and the amount of AMP in the medium was measured. ADP consumption is the difference between the amount of ADP added and the amount of ADP remaining at the end of incubation.
These data suggest that GSK inhibition alters anion transport through the outer mitochondrial membrane under de-energized conditions. Since GSK is a kinase, it is possible that the mechanism of the inhibitory effect on transport is related to a phosphorylation event. As an initial screen for phosphorylation differences between control and GSK inhibitor-treated myocardium, whole tissue extracts were prepared and proteins were separated and evaluated by western blotting using an antibody that recognizes proteins that are phosphorylated at Akt sites. We found that this antibody identified a protein at ~32 kD that was highly phosphorylated in control myocardium and there was virtually no detectable phosphorylated protein at this molecular weight in myocardium treated with GSK inhibitors6. Using 2D gels, we found that this protein stained with antibodies to VDAC, and we used mass spectrometry to confirm that VDAC was present in the spots. Thus it appears that VDAC is phosphorylated by a GSK dependent mechanism; GSK-3beta is normally active and VDAC is normally present in the phosphorylated form. In the presence of GSK inhibitors, VDAC is primarily in the nonphosphorylated form.
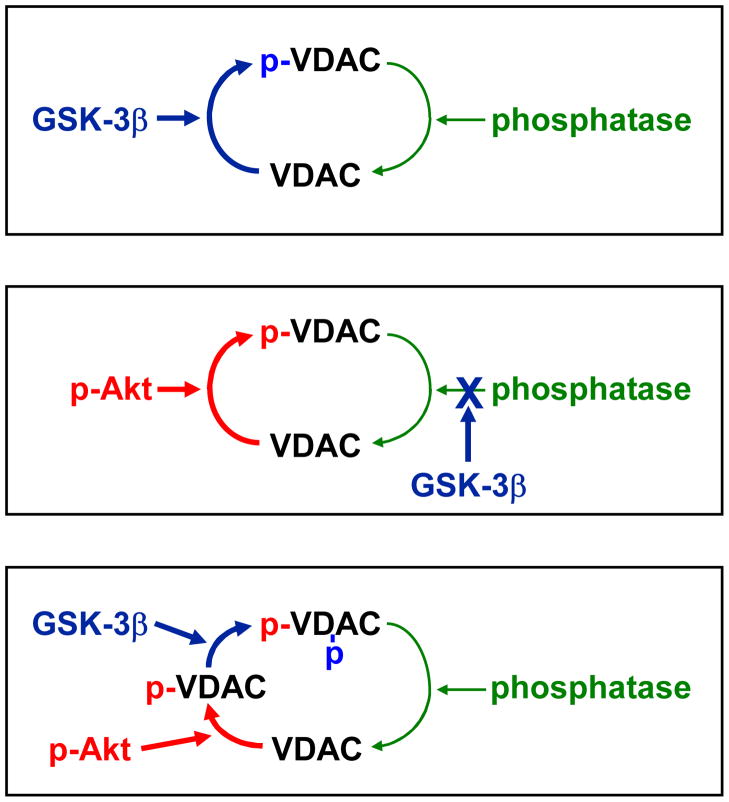
Figure 6 shows several possible mechanisms that could explain these findings. The data would be consistent with direct phosphorylation of VDAC by GSK-3beta. GSK-3beta is normally active and therefore VDAC would be phosphorylated at baseline. When GSK is inhibited either by phosphorylation such as during preconditioning or by use of inhibitors, VDAC phosphorylation stops and the phosphatase dephosphorylates VDAC. An alternative mechanism would be that Akt phosphorylates VDAC and GSK regulates the phosphatase that dephosphorylates VDAC. There is a basal level of Akt activity, which could be sufficient to maintain VDAC in a phosphorylated state if the phosphatase activity was inhibited by GSK. In this case, inhibition of GSK, by either phosphorylation or pharmacological inhibitors, removes the inhibitory effect of GSK on the phosphatase that dephosphorylates VDAC, phosphatase activity now exceeds Akt activity, and VDAC is primarily in the dephosphorylated state. The third possibility is that there could be more than 1 phosphorylation site. This is an attractive concept since GSK-3beta is known to prefer primed substrates23. If Akt is responsible for the initial phosphorylation and GSK-3beta was responsible for the second phosphorylation, then it would be expected that the basal level of Akt activity and the high basal GSK-3beta activity would result in VDAC in the phosphorylated state at baseline. It could be that the second phosphorylation is necessary to protect the initial phosphorylation from phosphatase activity. When GSK is inhibited, then the phosphatase would be able to dephosphorylate VDAC. Although the third mechanism is the most attractive, the data do not distinguish between these possibilities. To assess whether Akt or GSK-3beta can phosphorylate VDAC, we partially purified VDAC and assessed whether recombinant active Akt or GSK-3beta could phosphorylate VDAC, and we found evidence that both kinases were capable of phosphorylating the isolated protein6. Whether either or both kinases phosphorylate VDAC in situ remains to be determined.
Figure 6.
Possible mechanisms of GSK regulation of VDAC phosphorylation.
Finally, it was intriguing that Bcl-2 overexpression and GSK inhibition have a very similar effect on mitochondrial adenine nucleotide metabolism under de-energized conditions, which raises the possibility that there could be synergism between Bcl-2 and GSK-regulated VDAC phosphorylation. One possibility would be that Bcl-2 binding to VDAC under de-energized conditions alters anion transport through VDAC, and the critical level of Bcl-2 binding might be achieved either by overexpressing Bcl-2 or by increasing the affinity of VDAC for Bcl-2. Although it is possible that VDAC phosphorylation status directly alters anion transport, it is also possible that phosphorylation alters the binding between Bcl-2 and VDAC. For example, in non-cardiac cells, inhibition of GSK results in decreased phosphorylation of VDAC, which leads to decreased binding of hexokinase to VDAC and reduced cell death24. To address the possibility that VDAC phosphorylation affects Bcl-2 binding, we looked for evidence that binding of Bcl-2 to VDAC would be increased under conditions where VDAC would be dephosphorylated6. We found evidence that Bcl-2 translocated to the mitochondria in hearts treated with GSK inhibitors, and we found that the amount of Bcl-2 that co-immunoprecipitates with VDAC is increased in the presence of a GSK inhibitor. These results are consistent with the hypothesis that increased Bcl-2 binding to VDAC may be involved in the effects of GSK inhibitors on adenine nucleotide transport through VDAC under de-energized conditions, but proof that this is the most important mechanism will require more experimentation.
The finding that the cardioprotective effects of both Bcl-2 overexpression and GSK inhibitors are associated with altered adenine nucleotide transport across the outer mitochondrial membrane, through VDAC, suggests that outer mitochondrial membrane transport may be an important site of metabolic regulation under de-energized conditions, which may contribute to the protective effects of these interventions. The finding that this mechanism is present in several different cardioprotective interventions suggests the possibility that this may be a general mechanism of cardioprotection, but this hypothesis remains to be proven.
References
- 1.Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74(5):1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 2.Murry CE, Richard VJ, Reimer KA, Jennings RB. Ischemic preconditioning slows energy metabolism and delays ultrastructural damage during a sustained ischemic episode. Circ Res. 1990;66(4):913–931. doi: 10.1161/01.res.66.4.913. [DOI] [PubMed] [Google Scholar]
- 3.Steenbergen C, Perlman ME, London RE, Murphy E. Mechanism of preconditioning. Ionic alterations. Circ Res. 1993;72(1):112–125. doi: 10.1161/01.res.72.1.112. [DOI] [PubMed] [Google Scholar]
- 4.Asimakis GK, Inners-McBride K, Medellin G, Conti VR. Ischemic preconditioning attenuates acidosis and postischemic dysfunction in isolated rat heart. Am J Physiol. 1992;263(3 Pt 2):H887–894. doi: 10.1152/ajpheart.1992.263.3.H887. [DOI] [PubMed] [Google Scholar]
- 5.Imahashi K, Schneider MD, Steenbergen C, Murphy E. Transgenic expression of Bcl-2 modulates energy metabolism, prevents cytosolic acidification during ischemia, and reduces ischemia/reperfusion injury. Circ Res. 2004;95(7):734–741. doi: 10.1161/01.RES.0000143898.67182.4c. [DOI] [PubMed] [Google Scholar]
- 6.Das S, Wong R, Rajapakse N, Murphy E, Steenbergen C. Glycogen synthase kinase 3 inhibition slows mitochondrial adenine nucleotide transport and regulates voltage-dependent anion channel phosphorylation. Circ Res. 2008;103(9):983–991. doi: 10.1161/CIRCRESAHA.108.178970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tong H, Imahashi K, Steenbergen C, Murphy E. Phosphorylation of glycogen synthase kinase-3beta during preconditioning through a phosphatidylinositol-3-kinase--dependent pathway is cardioprotective. Circ Res. 2002;90(4):377–379. doi: 10.1161/01.res.0000012567.95445.55. [DOI] [PubMed] [Google Scholar]
- 8.Gross ER, Hsu AK, Gross GJ. Opioid-induced cardioprotection occurs via glycogen synthase kinase beta inhibition during reperfusion in intact rat hearts. Circ Res. 2004;94(7):960–966. doi: 10.1161/01.RES.0000122392.33172.09. [DOI] [PubMed] [Google Scholar]
- 9.Gross ER, Hsu AK, Gross GJ. GSK3beta inhibition and K(ATP) channel opening mediate acute opioid-induced cardioprotection at reperfusion. Basic Res Cardiol. 2007;102(4):341–349. doi: 10.1007/s00395-007-0651-6. [DOI] [PubMed] [Google Scholar]
- 10.Jennings RB, Reimer KA, Steenbergen C. Effect of inhibition of the mitochondrial ATPase on net myocardial ATP in total ischemia. J Mol Cell Cardiol. 1991;23(12):1383–1395. doi: 10.1016/0022-2828(91)90185-o. [DOI] [PubMed] [Google Scholar]
- 11.Di Lisa F, Blank PS, Colonna R, Gambassi G, Silverman HS, Stern MD, Hansford RG. Mitochondrial membrane potential in single living adult rat cardiac myocytes exposed to anoxia or metabolic inhibition. The Journal of physiology. 1995;486 (Pt 1):1–13. doi: 10.1113/jphysiol.1995.sp020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grover GJ, Atwal KS, Sleph PG, Wang FL, Monshizadegan H, Monticello T, Green DW. Excessive ATP hydrolysis in ischemic myocardium by mitochondrial F1F0-ATPase: effect of selective pharmacological inhibition of mitochondrial ATPase hydrolase activity. Am J Physiol Heart Circ Physiol. 2004;287(4):H1747–1755. doi: 10.1152/ajpheart.01019.2003. [DOI] [PubMed] [Google Scholar]
- 13.Forbes RA, Steenbergen C, Murphy E. Diazoxide-induced cardioprotection requires signaling through a redox-sensitive mechanism. Circ Res. 2001;88(8):802–809. doi: 10.1161/hh0801.089342. [DOI] [PubMed] [Google Scholar]
- 14.Dos Santos P, Kowaltowski AJ, Laclau MN, Seetharaman S, Paucek P, Boudina S, Thambo JB, Tariosse L, Garlid KD. Mechanisms by which opening the mitochondrial ATP- sensitive K(+) channel protects the ischemic heart. Am J Physiol Heart Circ Physiol. 2002;283(1):H284–295. doi: 10.1152/ajpheart.00034.2002. [DOI] [PubMed] [Google Scholar]
- 15.Juhaszova M, Zorov DB, Kim SH, Pepe S, Fu Q, Fishbein KW, Ziman BD, Wang S, Ytrehus K, Antos CL, Olson EN, Sollott SJ. Glycogen synthase kinase-3beta mediates convergence of protection signaling to inhibit the mitochondrial permeability transition pore. J Clin Invest. 2004;113(11):1535–1549. doi: 10.1172/JCI19906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nishino Y, Webb IG, Davidson SM, Ahmed AI, Clark JE, Jacquet S, Shah AM, Miura T, Yellon DM, Avkiran M, Marber MS. Glycogen synthase kinase-3 inactivation is not required for ischemic preconditioning or postconditioning in the mouse. Circ Res. 2008;103(3):307–314. doi: 10.1161/CIRCRESAHA.107.169953. [DOI] [PubMed] [Google Scholar]
- 17.Murphy E, Steenbergen C. Does inhibition of glycogen synthase kinase protect in mice? Circ Res. 2008;103(3):226–228. doi: 10.1161/CIRCRESAHA.108.181602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomez L, Thibault H, Gharib A, Dumont JM, Vuagniaux G, Scalfaro P, Derumeaux G, Ovize M. Inhibition of mitochondrial permeability transition improves functional recovery and reduces mortality following acute myocardial infarction in mice. Am J Physiol Heart Circ Physiol. 2007;293(3):H1654–1661. doi: 10.1152/ajpheart.01378.2006. [DOI] [PubMed] [Google Scholar]
- 19.Javadov SA, Clarke S, Das M, Griffiths EJ, Lim KH, Halestrap AP. Ischaemic preconditioning inhibits opening of mitochondrial permeability transition pores in the reperfused rat heart. The Journal of physiology. 2003;549(Pt 2):513–524. doi: 10.1113/jphysiol.2003.034231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hausenloy DJ, Yellon DM, Mani-Babu S, Duchen MR. Preconditioning protects by inhibiting the mitochondrial permeability transition. Am J Physiol Heart Circ Physiol. 2004;287(2):H841–849. doi: 10.1152/ajpheart.00678.2003. [DOI] [PubMed] [Google Scholar]
- 21.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol. 2008;103(3):274–284. doi: 10.1007/s00395-007-0691-y. [DOI] [PubMed] [Google Scholar]
- 22.Ruiz-Meana M, Abellan A, Miro-Casas E, Garcia-Dorado D. Opening of mitochondrial permeability transition pore induces hypercontracture in Ca2+ overloaded cardiac myocytes. Basic Res Cardiol. 2007;102(6):542–552. doi: 10.1007/s00395-007-0675-y. [DOI] [PubMed] [Google Scholar]
- 23.Murphy E, Steenbergen C. Inhibition of GSK-3beta as a target for cardioprotection: the importance of timing, location, duration and degree of inhibition. Expert Opin Ther Targets. 2005;9(3):447–456. doi: 10.1517/14728222.9.3.447. [DOI] [PubMed] [Google Scholar]
- 24.Pastorino JG, Hoek JB, Shulga N. Activation of glycogen synthase kinase 3beta disrupts the binding of hexokinase II to mitochondria by phosphorylating voltage-dependent anion channel and potentiates chemotherapy-induced cytotoxicity. Cancer Res. 2005;65(22):10545–10554. doi: 10.1158/0008-5472.CAN-05-1925. [DOI] [PubMed] [Google Scholar]