Abstract
Ultrasound is recognized as a very accurate first line diagnostic tool when a patient presents with a symptomatic lower extremity suggesting acute venous thrombus. Advantages of ultrasound are that it does not involve using radiation or contrast and can be performed portably, generally with no complications. In many patients, other abnormalities such as Baker's cysts, arterial aneurysms, or hematomas can be detected and can explain the symptoms. Some limitations are that ultrasound is dependent on the skills of the sonographer and is very limited in visualization of iliac and abdominal veins. We are currently investigating computed tomography (CT) venography, which appears to be an accurate alternative, particularly for obese patients or when pelvic or abdominal thrombus is suspected.
Found in both outpatients and inpatients and affecting over 20 million individuals annually in the United States, acute deep venous thrombosis (DVT) is of great clinical concern. Predisposing factors are venous stasis from inactivity or congestive heart failure, pelvic or lower extremity surgery, pregnancy, obesity, coagulopathy, paralysis, or malignancy (1). The prompt diagnosis of DVT is critical because of its association with acute pulmonary embolism, which occurs in up to 50% of cases of untreated DVT and carries a 30% mortality rate. However, the clinical accuracy of diagnosing DVT and its location is generally poor (2).
Approximately 90% of pulmonary emboli arise from the lower extremities (3). Thrombosis in the calf may cause symptoms in the thigh and vice versa (4). Patients with bilateral leg symptoms in the absence of significant risk factors should first be assessed for cardiac disease or chronic peripheral vascular disease. If significant risk factors exist, ultrasound is warranted.
Differential Diagnosis
The full differential diagnosis should be considered in possible cases of DVT including Baker's cyst, cellulitis, lymphedema, chronic venous insufficiency, superficial thrombophlebitis, popliteal venous or arterial aneurysm, enlarged lymph nodes compressing the veins, heterotopic ossification, hematoma, and muscle tears (5). Duplex Doppler compression ultrasound is the current study of choice for the diagnosis of suspected DVT. CT venography, a promising new modality we are beginning to investigate at Ochsner Clinic Foundation, may be particularly useful in detecting DVT above the inguinal ligament and in the obese when acquisition of images is technically more challenging.
Diagnosis And Imaging
Full diagnostic capabilities for the ultrasound evaluation and diagnosis include Doppler spectral analysis, color flow Doppler imaging (CFDI), transducer compression, and high resolution B-Mode imaging. Normal ultrasound findings include unidirectional flow, compressibility of the vein, and a lumen free of internal echoes (Figure 1). In order to demonstrate the compressibility of a normal vein, minimal external compression is needed with the transducer in the transverse position (Figure 2). Unidirectional flow is best demonstrated with CFDI. Doppler spectral analysis is beneficial in evaluating venous flow, which normally changes during the respiratory cycle termed respiratory phasicity (Figure 3). Increased intra-abdominal pressure during inspiration causes a decrease in venous flow, while in expiration the visualized flow increases. In the normal venous system, with distal mechanical compression flow augmentation is seen, which becomes particularly useful to prove patency in the smaller calf vessels.
Figure 1.
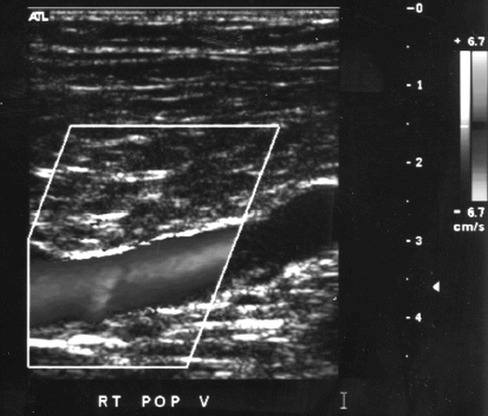
Normal unidirectional flow seen with color flow Doppler imaging in a popliteal vein
Figure 2.
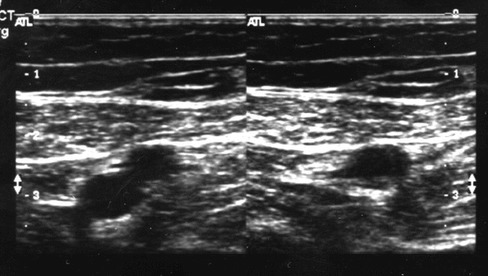
Transverse gray scale images demonstrate a normal superficial femoral artery (superficial) and vein (deep) non compressed (left}, and after compression, with collapse of the deep vein (right}
Figure 3.
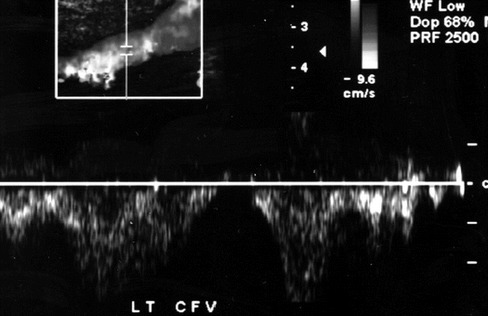
Doppler spectral analysis of a normal common femoral vein demonstrating changes in flow during the respiratory cycle termed respiratory phasicity
For the average person, a 5 MHz linear transducer is the scan head of choice. Often, transducers are changed during an examination depending upon the depth of the vessel and the patient's body habitus. For larger patients, a more penetrating transducer of 2.5 MHz or 3.75 MHz can be used with the shortcoming of slightly reduced resolution. The vessels routinely visualized are the common femoral veins; greater saphenous veins; superficial femoral veins; popliteal veins; calf veins including the anterior tibial, posterior tibial, and peroneal veins; and, if possible, the iliac veins. Sonographers should carefully study the femoral veins, being cautious not to miss duplicated vessels. In a recent report, Screaton et al (6) and Cronan (7) demonstrated that multiple femoral veins were present in 177 (46%) of 381 venograms, a much higher rate than the generally accepted frequency of duplication of 20%–25%. Despite this increased frequency, the false negative detection rate was not significantly different.
Examination Protocol
The traditional protocol described in the American College of Radiology (ACR) Standard for Performance of the Peripheral Venous Ultrasound examination (adopted in 1993) calls for careful examination of the full length of the common femoral vein, superficial femoral vein, and popliteal veins (8). Images with and without compression and using CFDI to detect directional flow are all useful. Doppler spectral analysis helps in assessing phasicity and augmentation responses and is particularly helpful as a secondary means of evaluating the patency of the iliac veins. Although the greater saphenous vein is not included in the deep venous system, its origin is often visualized because of the risk of saphenous thrombophlebitis extending into the deep systems.
Some debate has surfaced recently in regards to use of a limited examination, which reduces the time needed to perform a study from 30–40 minutes to approximately 20 minutes. Pezzullo et al reported in a prospective study of 53 symptomatic patients and a retrospective study in 155 symptomatic patients that a limited compression examination of the common femoral and popliteal veins depicts each case of DVT that was detected with the more traditional complete examination. This protocol reduced the examination time by 54% (9). There can be a need for visualizing all three major deep veins, as an isolated thrombus may exist in any of the three major deep veins.
Visualization of calf veins is not required by ACR standards because of the generally accepted principle that no significant thrombus (capable of causing pulmonary embolism) arises from these veins. A calf vein thrombus will resolve spontaneously 80% of the time but occasionally can extend into the proximal deep venous system. In patients whose symptoms persist, a repeat scan may be indicated. On repeat examinations, 2% of patients show abnormalities due to extension of calf vein thrombus (10).
Traditionally, both lower extremities are routinely studied. However, more recent data by Cronan report the likelihood of finding clots in an asymptomatic leg between 0% and 1%, which questions the routine evaluation of an asymptomatic leg (11).
A classic acute venous thrombus appears hypoechoic with no flow seen on CFDI (Figure 4). Acute DVT can be demonstrated as an intraluminal defect or color void. Acute DVT expands the venous lumen, is noncompressible, and does not demonstrate augmentation. As an indirect sign, the distal vein demonstrates nonvariable venous signals or loss of phasicity (Figure 5). The latter finding is particularly important when the iliac veins are not well visualized and can only be secondarily evaluated by interrogating the common femoral veins.
Figure 4.
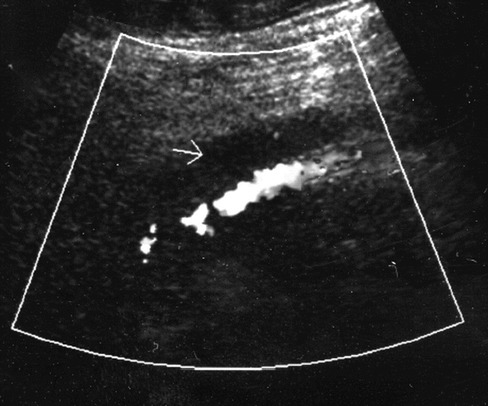
Acute venous thrombus with expanded hypoechoic vein with no flow seen on color flow Doppler imaging
Figure 5.
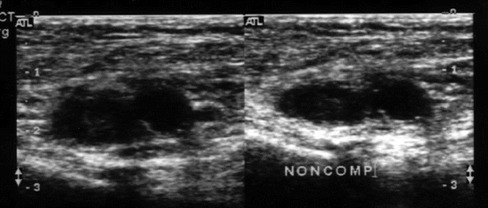
Transverse gray scale images of the common femoral vessels with acute deep venous thrombosis. The image on the left is without compression and the image on the right demonstrates the non-compressible vein and adjacent artery
Accuracy
Ultrasound diagnosis is universally accepted as being highly accurate. Studies by Cronan indicate 95% sensitivity and 98% specificity for compression ultrasound (2). With the addition of CFDI, phasicity, and augmentation responses, ultrasound has a well-established record of accuracy.
Chronic venous thrombus can be difficult to diagnose and there is occasional difficulty differentiating them from an acute exacerbation. Cronan has reported that 6 months after acute DVT, 48% of veins will still have demonstrable abnormalities on ultrasound examination (2). Of the abnormal veins, 14% remained occluded while even more veins demonstrated residual clots as organized thrombus material along the vein wall. Some findings that can help distinguish chronic from acute thrombus include the fact that chronic thrombus does not expand the venous lumen and is more echogenic than acute thrombus. CFDI is useful in detecting flow within the lumen and also in visualizing collateral vessels, which suggests a chronic etiology.
Another occasional difficulty involves false positives resulting from proximal obstructions caused by extrinsic masses (leading to limited compressibility of the venous structures) or venous distension secondary to congestive heart failure. Technical difficulty involving obese patients or patients with edematous extremities can lead to false negative results. Other pitfalls include the nonvisualization of a small nonocclusive thrombi less than 3 cm, which the sonographer may overlook. Duplicated venous structures and collateral vessels can also lead to misdiagnoses. The iliac veins are often difficult to visualize, but careful attention to the spectral analysis of the distal iliac and femoral veins with awareness of the respiratory phasicity can give secondary clues to the correct diagnosis.
Use of CT Venography
There is some indicatation that the effectiveness of CT venography in the diagnosis of DVT may equal ultrasound and may actually be better in some circumstances (Figure 6). Loud et al recently studied CT venography of the lower extremities by capturing the venous phase of the contrast after completion of CT pulmonary angiography (12). In 308 patients, direct comparison with venous sonography revealed a 97% sensitivity and 100% specificity for the detection of femeropopliteal DVT. Additionally, in 17% of patients with DVT, the CT venogram defined pelvic or abdominal thrombi, which are often difficult or impossible to assess with conventional sonography (12). This possible alternative to ultrasound may be particularly useful in selected situations such as in very obese patients or when pelvic or abdominal thrombus identification is desired. An advantage of CT is that it is less dependent on the individual skills of technologists compared with ultrasound. We will be studying this application at Ochsner in the future.
Figure 6.
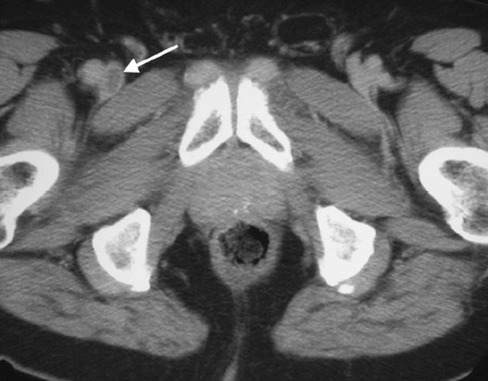
Computed tomography venogram demonstrating acute deep venous thrombus in the right common femoral vein
Summary
At this time, diagnostic ultrasound is the procedure of choice for evaluating patients for the risk of lower extremity DVT. Performance of the examination requires technical expertise and good diagnostic acumen. Established protocols for performing an adequate examination are well documented. In the future, CT venography may be used as another study of choice, particularly since it is less technically dependent than ultrasound and appears to be highly accurate.

Dr. DiVittorio is a fellow in Ochsner's Department of Radiology
References
- Foley W. D. Extremity venous disease. In: Foley WD (editor). Color Doppler Flow Imaging. Reading, MA: Andover Press, 1991:129–151. [Google Scholar]
- Cronan J. J. Venous thromboembolic disease: the role of US. Radiology. 1993;186:619–630. doi: 10.1148/radiology.186.3.8430164. [DOI] [PubMed] [Google Scholar]
- Needleman L., Polak J., Bettmann M. A. Suspected lower extremity deep vein thrombosis. American College of Radiology. ACR Appropriateness Criteria for Imaging and Treatment Decisions. Reston, VA: American College of Radiology, 1998:49–53. [PubMed] [Google Scholar]
- Hirsh J., Hull R. D. Venous thromboembolism: natural history, diagnosis and management. In: Diagnosis of Venous Thrombosis. Boca Raton, FL: CRC Press, 1987; 23–28. [Google Scholar]
- Bluth E. I. Leg swelling with pain or edema. In: Bluth EI, Arger PH, Benson CB, et al. editors. Ultrasound: A Practical Approach to Clinical Problems. New York: Thieme, 2000:534–545. [Google Scholar]
- Screaton N. J., Gillard J. H., Berman L. H., Kemp P. M. Duplicated superficial femoral veins: a source of error in the sonographic investigation of deep vein thrombosis (editorial) Radiology. 1998;206:397–401. doi: 10.1148/radiology.206.2.9457192. [DOI] [PubMed] [Google Scholar]
- Cronan J. J. Venous duplex US of the lower extremities: effect of duplicated femoral veins. Radiology. 1998;206:308–309. doi: 10.1148/radiology.206.2.9457179. [DOI] [PubMed] [Google Scholar]
- American College of Radiology (2000) ACR Standards for Performance of the Peripheral Venous Ultrasound Examinations. Reston, VA. [Google Scholar]
- Pezzullo J. A., Perkins A. B., Cronan J. J. Symptomatic deep venous thrombosis: diagnosis with limited compression US. Radiology. 1996;198:67–70. doi: 10.1148/radiology.198.1.8539408. [DOI] [PubMed] [Google Scholar]
- Ginsberg J. S. Management of venous thromboembolism. N Engl J Med. 1996;335:1816–1828. doi: 10.1056/NEJM199612123352407. [DOI] [PubMed] [Google Scholar]
- Cronan J. J. Controversies in venous ultrasound. Semin Ultrasound, CT, MR. 1997;18:33–38. doi: 10.1016/s0887-2171(97)90036-6. [DOI] [PubMed] [Google Scholar]
- Loud P. A., Katz D. S., Bruce D. A. Deep venous thrombosis with suspected pulmonary embolism: detection with combined CT venography and pulmonary angiography. Radiology. 2001;219:498–502. doi: 10.1148/radiology.219.2.r01ma26498. [DOI] [PubMed] [Google Scholar]


