Abstract
Data suggest that the activation of immune responses and the release of inflammatory cytokines may play a role in the pathophysiology of major depression. One mechanism by which cytokines may contribute to depression is through their effects on the glucocorticoid receptor (GR). Altered GR function in depression has been demonstrated by neuroendocrine challenge tests that reliably reveal reduced GR sensitivity as manifested by nonsuppression of cortisol following dexamethasone administration in vivo and lack of immune suppression following administration of glucocorticoids in vitro. Relevant to the GR, cytokines have been shown to decrease GR expression, block translocation of the GR from cytoplasm to nucleus, and disrupt GR-DNA binding through nuclear protein-protein interactions. In addition, cytokines have been shown to increase the expression of the relatively inert GR beta isoform. Specific cytokine signaling molecules that have been shown to be involved in the disruption of GR activity include p38 mitogen-activated protein kinase, which is associated with reduced GR translocation, and signal transducer and activator of transcription (STAT)5, which binds to GR in the nucleus. Nuclear factor-κB (NF-κB) also has been shown to lead to GR suppression through mutually inhibitory GR-NF-κB nuclear interactions. Interestingly, several antidepressants have been shown to enhance GR function, as has activation of protein kinase A (PKA). Antidepressants and PKA activation have also been found to inhibit inflammatory cytokines and their signaling pathways, suggesting that drugs that target both inflammatory responses and the GR may have special efficacy in the treatment of depression.
Keywords: glucocorticoids, cytokines, mood, cell signaling, inflammation, glucocorticoid, NF-κB, p38 MAPK, glucocorticoid insensitivity
Introduction
There has been increasing appreciation for the potential role of inflammation in the pathophysiology of a number of disorders, including cardiovascular disease, diabetes, and cancer. Indeed, in all of these diseases, inflammatory markers have been found to predict disease development and outcome, and in each case, the mechanisms by which inflammation contributes to pathology have been elaborated.1–3 For example, inflammatory processes have been shown to play a central role in arterial plaque formation, thereby contributing to cardiovascular disease.4 In diabetes, inflammatory signaling pathways have been shown to antagonize signaling through the insulin receptor.5 In cancer, there is increasing data to indicate that activation of nuclear factor-κB (NF-κB), as well as other inflammatory mediators, plays an important role in neoplastic transformation and chemotherapy resistance.3
Given the recognition that inflammation may serve as a common mechanism for many illnesses, there has been growing interest in the notion that inflammation and activation of the innate immune response may play a role in neuropsychiatric disorders, including major depression. Patients with major depression have been found to exhibit increased biomarkers of inflammation in both the peripheral blood and cerebrospinal fluid (CSF).6 However, major depression is a disorder that is characterized by hyperactivity of the hypothalamic-pituitary-adrenal (HPA) axis and increased release of glucocorticoids, which are some of the most potent anti-inflammatory hormones in the body.7–9 Nevertheless, closer scrutiny reveals that in many depressed patients there is evidence of glucocorticoid resistance as manifested, for example, by dexamethasone (DEX) nonsuppression.7,8 Thus, although glucocorticoids (i.e., cortisol) may be elevated in patients with major depression, they appear to fail to inhibit inflammatory responses by virtue of insufficient glucocorticoid signaling.10 Mounting data indicate that insufficient glucocorticoid signaling in major depression and potentially other clinical disorders may be a consequence of the impact of innate immune cytokines on the receptors for glucocorticoid hormones.11 These effects of innate immune cytokines on glucocorticoid receptors (GRs) may represent a contributing pathophysiologic pathway to the development of major depression and are the topic of this review.
Inflammation and Major Depression
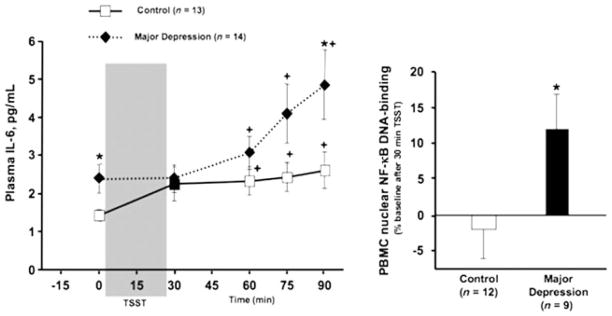
There are many reasons to believe that the activation of innate immune inflammatory responses may play a role in the pathophysiology of major depression. As noted above, patients with major depression have been found to exhibit increased biomarkers of inflammation in both the periphery and the brain.12 Specifically, increased concentrations of innate immune cytokines, including interleukin (IL)-1, IL-6 and tumor necrosis factor (TNF)-α, as well as their soluble receptors, have been found in the peripheral blood and/or CSF of depressed patients.6 In addition, increases in acute-phase proteins, such as C-reactive protein (CRP), chemokines, and adhesion molecules, have been described.6 Compared to nondepressed, healthy control subjects, patients with major depression also exhibit increased stress-induced innate immune responses, including increased NF-κB-DNA binding and plasma IL-6 following exposure to a public-speaking and mental-arithmetic stressor (Fig. 1).13 Of note, depressed patients with increased early life stress may be especially likely to exhibit evidence of increased baseline and stress-induced innate immune system activation.13–15
Figure 1.
Baseline and stress-induced inflammatory responses in patients with major depression. Patients with major depression and a history of increased early life stress displayed elevated baseline and acute psychosocial stress-induced circulating concentrations of interleukin (IL)-6, as well as enhanced psychosocial stress-induced nuclear factor-κB (NF-κB)-DNA binding in peripheral blood mononuclear cells. Left panel: Circulating concentrations of IL-6 before and 30, 60, 75, and 90 min after the start of the Trier Social Stressor Test (TSST) in healthy controls vs. patients with current major depression and increased history of early life stress. * vs. Control group at the given timepoint, P ≤ 0.05; + vs. 0 min within the same group P < 0.025. Right panel: Percent change in nuclear NF-κB-DNA binding from before to 30 min after the start of the TSST (ΔNF-κB) in a subset of the same participants. * vs. control group, P ≤ 0.05. (Reprinted with permission from Pace, T. W. W., Mletzko, T.C., Alagbe, O., Musselman, D.L., Nemeroff, C.B., Miller, A.H., 2006. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. American Journal of Psychiatry 163, 1630–1633, Copyright 2006 American Psychiatric Publishing, Inc.13)
Aside from evidence of increased inflammatory markers in depressed patients, data from laboratory animals and humans indicate that the administration of innate immune cytokines, including interferon (IFN)-α, leads to multiple behavioral changes that overlap with major depression, including depressed mood, anhedonia, fatigue, psychomotor slowing, disrupted sleep, cognitive impairment, anxiety, and suicidal ideation.6,16 From an evolutionary standpoint, it has been suggested that these behavioral accoutrements of an activated innate immune response in part subserve the reallocation of energy resources to fight infection and heal wounds, while at the same time maintaining vigilance (anxiety, insomnia) against further injury.6,17
Regarding the mechanisms by which cytokines may influence behavior, there is a growing body of research that has begun to clarify how cytokine signals access the brain and ultimately interact with pathophysiologic pathways relevant to mood regulation. For example, several routes by which cytokine signals reach the brain have been described, including (1) passage through leaky regions in the blood–brain barrier, (2) active transport via saturable transport molecules, and (3) binding to receptors on visceral afferent nerve fibers, which in turn relay cytokine signals to the brain through the nucleus of the solitary tract.16,18,19 Once cytokine signals reach the brain, they are further transduced and amplified in part through activation of inflammatory intermediaries, such as NF-κB.20 There is also a cytokine network within the brain that includes glial elements, such as astrocytes and microglia, which produce cytokines and chemokines, and multiple cell types, including neurons, which express cytokine receptors.16 Of note, inhibition of the activation of microglia, a primary source of cytokines in the brain, by the pharmacologic agent, minocycline, has been shown to block the development of behavioral changes following peripheral activation of the innate immune response in mice.21,22
Regarding the mechanisms by which innate immune cytokines alter behavior, data indicate that cytokines can influence virtually every pathophysiologic pathway relevant to depression, including neurotransmitter metabolism, neuroendocrine function, synaptic plasticity and regional brain activity. For example, cytokine-induced activation of the enzyme, indolamine 2,3 dioxygenase (IDO), which metabolizes tryptophan to kynurenine and quinolinic acid, is believed to contribute to reduced availability of serotonin.16,23,24 Indeed, decreased tryptophan, the primary precursor of serotonin, and increased kynurenine in the peripheral blood have been associated with the development of depression in patients administered the innate immune cytokine, IFN-α.25 In addition, blockade of IDO has been shown to inhibit the development of cytokine-induced behavioral changes in mice.21 Activation of IDO and the subsequent conversion of kynurenine to kynurenic acid in astrocytes may also influence the release of glutamate, and by extension, dopamine, whose release is regulated in part by glutamatergic activity.26 Monoamine metabolism can also be influenced by cytokine signaling pathways. Mitogen activated protein kinase (MAPK) pathways, including p38 and extracellular signal-regulated kinases (ERK) 1/2, have been found to influence the expression and/or activity of the membrane reuptake pumps for both serotonin and dopamine.27–29 Moreover, activated p38 in peripheral blood mononuclear cells has been associated with decreased CSF concentrations of the serotonin metabolite, 5-hydroxyindoleactectic acid, in juvenile rhesus monkeys, which were maternally abused or rejected as infants.30
Regarding neuroendocrine pathways, innate immune cytokines are potent stimulators of the neuroregulatory peptide corticotropin-releasing factor (CRF).31 A number of studies have found evidence of increased CRF activity in patients with major depression, including increased CSF concentrations of CRF and increased CRF mRNA and protein in the hypothalamus of postmortem brain samples from depressed patients.7,10,32 In addition, CRF has been shown to have powerful effects on multiple behaviors, including fear/anxiety, sleep, locomotor activity and food intake.32 Cytokines have also been associated with alterations in mood-relevant nerve growth factors, including brain-derived neurotrophic factor (BDNF). For example, IL-1 has been shown in a number of studies to mediate stress-induced decreases in central nervous system BDNF, as well as neurogenesis.33–35 Finally, several studies have shown that cytokines can influence regional brain activity in the basal ganglia and frontal cortex, including increased activity in the dorsal anterior cingulate cortex (dACC).36,37 The dACC has been referred to as a “neural alarm” system,38 and increased activity in this brain region during innate immune system activation may underlie the evolutionarily derived need for vigilance that the vulnerable, wounded and/or infected animal may require for survival.
It should be noted that depressed patients who fail to respond to antidepressants are more likely to exhibit increased inflammatory markers prior to treatment, and reduced inflammation during antidepressant treatment has been associated with responsiveness to therapy in some studies.6,39
Glucocorticoid Resistance in Major Depression
Although data supporting a potential role for innate immune system activation and inflammation in the development of depression are compelling, a fairly cursory review of the literature on the neurobiology of depression immediately reveals a problem with this hypothesis. One of the more reproducible findings in biological psychiatry is increased activation of the HPA axis and elevated circulating concentrations of the glucocorticoid cortisol in patients with major depression.7,8,10 Relevant to the inflammation hypothesis of depression, cortisol is one of the most potent anti-inflammatory hormones in the body.9 Therefore, the co-existence of increased inflammation and glucocorticoid excess presents a conundrum that warrants further consideration. One possibility is that although circulating concentrations of glucocorticoids may be high (or normal), signaling through the GR may be low.10
There is considerable evidence that glucocorticoid signaling is decreased in patients with major depression. Probably the most reliable finding in this regard is nonsuppression of ACTH or cortisol secretion following administration of the synthetic glucocorticoid, DEX in the presence or absence of CRF during the DEX suppression test (DST) or DEX-CRF test, respectively.10 Rates of DEX nonsuppression in depressed patients range from 25% to 80% depending on a number of factors, including age, sex, severity of depression, and the type of test employed (the DEX-CRF test is believed to be more sensitive than the DST).10 Of note, similar to inflammatory markers, both the DST and the DEX-CRF test have been shown to predict clinical response to antidepressants, and in the case of the DEX-CRF test, there is evidence that impaired glucocorticoid responsiveness represents a genetically based risk factor for the development of depression.10 Reduced sensitivity to glucocorticoids in depressed patients is also evident in immune cells stimulated in vitro and in the skin following topical application of glucocorticoids.7,11,40 These latter findings indicate that decreased glucocorticoid responsiveness in depressed patients is not solely a function of altered in vivo pharmacokinetic properties of glucocorticoids (including DEX) in depressed patients. Moreover, taken together, these data indicate that reduced glucocorticoid signaling is apparent in a number of cell types and tissues, including neuroendocrine, immune and dermal elements.
Cytokine Effects on GR Function
Given the coexistence of glucocorticoid resistance and increased inflammation in patients with major depression, there has been speculation that cytokine effects on the GR may be an important link in this association. There is a rich database demonstrating that cytokines can influence GR function at multiple levels, including GR translocation from cytoplasm to nucleus, GR protein-protein interactions, GR binding to its DNA response element, and induction of GR isoforms that have reduced capacity to bind ligand and access to hormone.11
Based on early findings that chronic administration of endotoxin was associated with the development of DEX nonsuppression in rats41 (a finding that has recently been repeated using herpes simplex-1 infection42), in our laboratory we examined the impact of relevant endotoxin- and virally induced cytokines, including IL-1, on GR function in vitro. Using a mouse L929 fibroblast cell line, administration of IL-1-α was found to induce a significant decrease in DEX-induced GR translocation from cytoplasm to nucleus that was associated with decreased GR-DNA binding and decreased DEX-induced activation of a reporter gene construct with multiple glucocorticoid response elements (GRE) upstream of its promoter region.43 Effects were reversed by co-administration of IL-1 receptor antagonist (IL-1ra). Subsequent studies examining the signal transduction mechanisms responsible for these effects revealed that IL-1-α induction of p38 MAPK was involved, given that antisense oligonucleotides targeting p38 were able to reverse the effects of IL-1-α on DEX-induced, GR-mediated gene transcription.44 These results are consistent with a number of studies that have shown that cytokine induction of p38 MAPK can directly influence GR function, including GR nuclear translocation, in part through GR phosphorylation (Table 1, Fig. 2). Of note, IL-1-α treatment was also associated with significant upregulation of GR protein that was primarily apparent in the cytosolic compartment.43 One possibility is that IL-1–induced decreases in GR shuttling from cytoplasm to nucleus may lead to reduced autoregulation of receptor expression and thus a compensatory GR upregulation.
Table 1.
Cytokine Effects on Glucocorticoid Receptor (GR) Function
| Author | Cytokine | GR Effect | Signal Transduction Mechanism |
|---|---|---|---|
| Pariante et al.43 | IL-1 alpha | ↓ Dex-induced GR translocation, ↓ GR-mediated gene transcription | Not examined |
| Wang et al.44 | IL-1 alpha | ↓ GR-mediated gene transcription, ↓ GR-GRE binding | Activated p38 signal pathway |
| Raddatz et al.45 | IL-1 beta | ↓ Dex-induced GR translocation and GR-mediated gene transcription | Activated NF-kappa B signal pathway, protein-protein interaction |
| Goleva et al.46 | IL-2 | ↓ Dex-induced GR translocation | Activated STAT5 and p38 signal pathway, protein-protein interaction |
| Biola et al.48 | IL-2 | ↓ GR-mediated gene transcription | Activated STAT5 signal pathway |
| Irusen et al.49 | IL-2/IL-4 | ↓ GR nuclear ligand-binding affinity | Activated p38 signal pathway resulting in GR phosphorylation |
| Spahn et al.50 | IL-13 | ↓ GR ligand-binding affinity | Activated p38 signal pathway resulting in GR phosphorylation |
| Xu et al.47 | IL-15 and IL-4 | ↓ Dex-induced GR translocation | Activated p38 signal pathway resulting in GR phosphorylation |
| Hu et al., submitted | IFN-alpha | ↓ GR-mediated gene transcription, ↓ GR-GRE binding | Activated p38 MAPK, STAT5 signal pathway, GR-STAT5 protein-protein interaction |
| Kino et al.51 | TNF-alpha | ↓ GR-mediated gene transcription | protein-protein interaction |
| Zhang et al.52 | IL-6 | Synergistic effect on glucocorticoid signaling | Activated STAT3 forms a transactivating/signaling complex |
| Hu F et al.53 | Cox2 inhibitor | ↑ Dex-induced GR translocation ↑ GR-mediated gene transcription |
Inhibition of p38 activity |
| Wang et al.54 | JNK inhibitor | ↑ Dex-induced GR translocation ↑ GR-GRE binding |
Inhibition of JNK activity |
| Szatzmary et al.55 | TNF-alpha | ↓ Dex-induced GR-mediated gene transcription | Activated p38 MAPK (MKK6-dependent) pathway that targets GR ligand-binding domain; activated JNK (MKK7-dependent) |
| Onda et al.56 | TNF-alpha | ↓ Prednisolone-induced GR translocation and GR-mediated gene transcription | Activated MEK-1/ERK pathway (both GR-mediated transcription and GR translocation) |
| Tliba et al.57 | TNF-alpha and IFN-beta or IFN-gamma | ↓ Dex-induced GR-mediated gene transcription | Activated interferon regulatory factor 1 pathway |
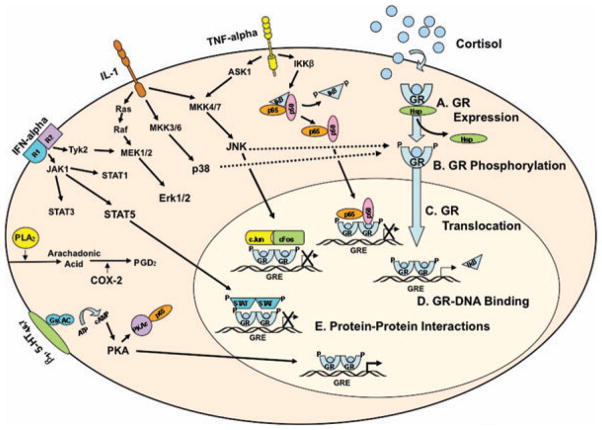
Figure 2.
Interactions between cytokine and glucocorticoid receptor (GR) signaling pathways. Selected cytokines and their signal transduction pathways are depicted in a simplified fashion to illustrate representative interactions between cytokine and GR signaling events. Cortisol binds to GR, resulting in dissociation of heat shock protein (HSP) complexes and subsequent phosphorylation. GR then translocates to the nucleus, where it dimerizes and either interacts with other transcription factors or binds to glucocorticoid response elements (GREs) upstream of GR-regulated genes (e.g., inhibitor κ-B or IκB). TNF-α binds to its receptor and results in activation of IκB kinase β (IKKβ), which phosphorylates IκB, allowing NF-κB (shown here as p65 and p50 Rel subunits) to translocate to the nucleus. Through protein-protein interactions, activated NF-κB associates with GR, thus interfering with GR-DNA binding. IL-1 binds to its receptor, initiating (a) mitogen activated protein kinase (MAPK) kinase (MKK)4/7, which culminates in activation of c-Jun amino-terminal kinase (JNK), (b) MKK3/6, which culminates in activation of p38, and (c) Ras, which results in activation of the extracellular signal-related kinase (Erk)1/2. Of note, MKK4/7 activation of JNK can also occur through TNF-α receptor binding. As depicted by the dotted lines, both p38 and JNK can phosphorylate key GR residues, thereby disrupting nuclear translocation of GR. Interferon (IFN)-α binds to its receptor resulting in Janus kinase (Jak) phosphorylation, represented as Jak1 and tyrosine kinase (Tyk)2. Jak1 phosphorylates signal transducers and activators of transcription (STAT) proteins, including STAT1, STAT3, and STAT5. Tyk2 can also activate elements of the Ras signaling pathway, resulting in activation of Erk1/2. Activated STATs translocate to the nucleus, where they can interact with GR through protein-protein interactions, thereby interfering with GR-DNA binding. Phospholipids are hydrolyzed by phospholipase A2 (PLA2) to form arachidonic acid, which is metabolized by cyclooxygenase (COX) 2 to produce prostaglandin D2 (PGD2). Stimulation of serotonergic receptors 4, 6, or 7 (5-HT4, 6, 7) and beta adrenergic receptors (beta1) induces a conformational change in G stimulatory (Gs) protein, which then activates adenylyl cyclase (AC). AC, in turn, converts adenosine triphosphate (ATP) to cyclic adenosine monophosphate (cAMP). cAMP then induces a conformational change in protein kinase A (PKA), which translocates to the nucleus, where it is able to enhance GR-DNA binding. In addition, the catalytic subunit of PKA (PKAc) interacts with p65, thereby inhibiting NF-κB nuclear translocation. (Reprinted with permission from Pace, T. W. W., Hu, F., & Miller, A. H. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain, Behavior, and Immunity 21, 9–19, Copyright 2007 Elsevier.11) (In color in Annals online.)
The potential clinical relevance of these effects of IL-1 on the GR has recently been elaborated in an elegant series of studies conducted in mice exposed to social disruption stress. Social disruption stress in mice has been shown to lead to glucocorticoid resistance in splenic monocytes that is associated with increased susceptibility to the lethal effects of inflammatory stimuli, including endotoxic shock and pulmonary virus infection.58,59 Interestingly, the mechanism of glucocorticoid resistance in socially disrupted mice involves decreased GR translocation from cytoplasm to nucleus,60 an effect that is eliminated (along with glucocorticoid resistance) in gene-targeted mice in which the IL-1 receptor gene has been knocked out.61 Taken together, these data provide powerful confirmatory evidence that stress (a common precipitant of neuropsychiatric disorders, including depression) can lead to reduced GR function that is characterized by alterations in GR translocation and is mediated by stress-induced innate immune cytokines, such as IL-1.
In addition to IL-1 effects on the GR through activation of p38 MAPK pathways, cytokine effects on GR function through activation of other cytokine signaling pathways have received considerable attention, including c-Jun amino-terminal kinases (JNK1/2/3), ERK-1/2, signal transducers and activators of transcription (STATs), and NF-κB (Table 1, Fig. 2). Activation of JNK results in phosphorylation of c-Jun, which in turn dimerizes with c-Fos to form AP-1, which has been shown to interact with the GR through protein-protein interactions (Fig. 2).62 Interestingly, activation of JNK has also been shown to result in increased nuclear export of GR.63 Studies in our lab have shown that JNK may play an important role in GR regulation under resting (unstimulated) conditions through mutually inhibitory protein-protein interactions.48 Pharmacologic inhibition of JNK signaling significantly increased DEX-induced GR-GRE binding, as well as DEX-induced reporter gene activity in mouse L929 fibroblasts and mouse hippocampal HT22 cells. Comparable effects were observed after treatment of cells with antisense oligonucleotides directed against JNK. Changes in nuclear translocation of GR were not associated with JNK inhibition. Of note, both JNK and p38 signaling pathways have been implicated in the inhibitory effects of TNF-α on GR function.47
Activation of ERK signaling pathways has also been shown to influence the GR. For example, ERK activation has been found to mediate super-antigen–induced corticosteroid resistance in human T cells.64 In addition, inhibition of prednisolone-induced GR translocation, as well as GR-mediated gene transcription in human epidermal cells by TNF-α, is dependent on ERK activation.46 Inhibition of GR function by activation of ERK is believed to be in part related to phosphorylation of GR cofactors.65 Regarding Janus kinase (Jak)-STAT pathways, Jak-STAT activation has also been found to decrease GR function through effects on various cofactors including CREB binding protein (CBP) as well as through direct GR-STAT protein-protein interactions.66 Interactions between Jak-STAT and GR signaling pathways have been most studied in the context of protein-protein interactions between STAT5 and GR. Studies using immunoprecipitation have demonstrated that STAT5 and GR form complexes in the nucleus that ultimately disrupt GR-DNA binding (Fig. 2). Additional mechanisms that may be involved in STAT5-induced inhibition of GR function are believed to include disruption of GR translocation.56 Finally, there is a rich literature detailing interactions between GR and NF-κB. NF-κB has been found to directly interact with GR in the nucleus through protein-protein interactions, causing mutual repression of both GR and NF-κB function.67 GR and NF-κB have also been shown to compete with one another for the coactivators steroid receptor coactivator-1 (SRC-1) and CBP in the nucleus.68 Indeed, NF-κB–mediated repression of GR activity is inhibited by overexpression of CBP (and vice versa), and GR activity is also inhibited by SRC-1 over-expression.68
Other inflammatory mediators that contribute to GR regulation include cyclooxyegnase (COX)-2. For example, the COX-2 inhibitor nimesulide has been shown to induce GR-DNA binding, GR-mediated gene transcription and GR phosphorylation in cultured human osteoarthritic synovial fibroblast cells.69 However, these observations are somewhat confounded because nimesulide also inhibits phosphodiesterase (PDE) type IV, and PDE IV inhibitors, like rolipram, have been shown to enhance GR function (see below).70,71 Nevertheless, studies from our group have found that treatment of rat PC12 cells with the COX-1 and COX-2 inhibitor, ibuprofen, or the selective COX-2 inhibitor, celecoxib, significantly increased GR-mediated gene transcription. However, the same studies found that the selective COX-1 inhibitor, valerylsalicylic acid, had no effects on GR function.49 Because ibuprofen and other nonsteroidal anti-inflammatory drugs have been shown to inhibit p38 MAPK activity in Jurkat T cells,72,73 we administered anisomycin, a potent activator of p38, along with celecoxib to rat PC12 cells. Anisomycin reversed the celecoxib-induced enhancement of GR-mediated gene transcription in a dose-dependent manner, indicating the effects of COX-2 inhibitors on GR may be related to inhibition of p38.49 Recent data also indicate that anisomycin is a potent activator of JNK signaling pathways,73 which in turn have been shown to inhibit GR function (see above). Taken together, these results suggest that MAPK signaling pathways (including both p38 and JNK) may be involved in the effects of COX-2 inhibition on GR function.
Aside from the effects of cytokine signaling pathways on GR function, there are several other pathways by which cytokines can influence glucocorticoid signaling. One of these is the induction of the beta isoform of the GR. There are two isoforms of the GR in humans, human (h)GR-α and hGR-β.74 hGR-α contains the full set of 12 α helices required for ligand binding, while hGR-β is unable to bind cortisol due a modified 11th and absent 12th alpha helix. In addition, hGR-β has been found to limit the activity of hGR-α as a transcription factor.74 Interestingly, treatment of HeLaS3 and CEMC7 cells with either TNF-α or IL-1 was shown to increase hGR β expression two-fold, while only increasing hGR-α 1.5-fold.75 Given the dominant negative effects of hGR-β on hGR-α function, the relative expression of hGR-α and hGR-β is believed to contribute to conditions of glucocorticoid resistance in disorders, such as glucocorticoid-resistant asthma, leukemia, ulcerative colitis, and rheumatoid arthritis.74 Nevertheless, a role for hGR-β in major depression has yet to be established. One report found that while expression of hGR-α is decreased in patients with major depression, -β expression is unchanged.76 Of note, an equivalent of hGR-β has yet to be described in other species, including mice and rats.77,78 Interestingly, hGR-β has recently been found to bind the GR antagonist, RU38486, and is transcriptionally active (even in the absence of ligand, similar to GR-α), raising questions whether the effects of RU38486 in neuropsychiatric disorders in humans may be mediated in part through effects on hGR-β.79 (See also Revollo and Cidlowski.79a)
Other pathways by which cytokines can influence GR signaling include their capacity to regulate 11 beta-hydroxysteroid dehydrogenase (11β-HSD), an enzyme which plays a role in end organ metabolism of glucocorticoids. Several innate immune cytokines have been shown to increase the activity of 11β-HSD type 2, which inactivates cortisol, thereby increasing the availability of glucocorticoids.80 Innate immune cytokines also can alter the concentration of corticosteroid binding globulin (CBG), which binds the majority of circulating cortisol. Only free (unbound) cortisol is capable of passively diffusing across the cell membrane to interact with the GR. Cytokines, including IL-6, have been shown to inhibit CBG production and cytokine-induced decreases in CBG are believed to play a role in the decreased levels of CBG seen in septic shock and burn patients.81,82 Finally, innate immune cytokines, including TNF-α, are capable of influencing activity of the p-glycoprotein (multidrug resistance) pump, which pumps the glucocorticoids, cortisol and DEX, out of the cell, while having no effect on corticosterone or RU38486.83 Chronic, low dose TNF-α has been found to increase the expression and activity of the p-glycoprotein pump, while higher doses and acute administration of this cytokine-inhibited pump activity.84
Relationship between Inflammation and GR Signaling in Major Depression and Stress
Given the potential effects of cytokines on GR signaling, several studies have examined the relationship between inflammatory biomarkers and glucocorticoid resistance in patients with major depression. Results from these largely cross-sectional studies have been mixed with some studies reporting a limited association between glucocorticoid resistance and increased markers of inflammation, while others have reported a clear association between these variables. Indeed, one of the earliest studies in this regard showed a significant correlation between post-DEX concentrations of cortisol and the production of IL-1 by peripheral blood mononuclear cells.85 In addition, reduced skin sensitivity to topical glucocorticoid administration in depressed patients was found to significantly correlate with increased blood concentrations of TNF-α.40 In contrast, another study found that cytokine levels in depressed patients with evidence of glucocorticoid resistance and glucocorticoid excess were low, suggesting that glucocorticoid sensitivity in depressed subjects is maintained or possibly increased during depressive illness.86 Increased in vitro sensitivity of innate immune responses to glucocorticoids was also found in depressed patients prior to a laboratory stressor, however, following stressor administration, depressed patients became significantly less sensitive to DEX in vitro, whereas controls became significantly more sensitive.87 Of note, in a study examining the neuroendocrine and immune responses to lipopolysaccharide (LPS) following DEX administration, subjects with evidence of glucocorticoid resistance (as manifested by increased ACTH and cortisol responses) exhibited increased cytokine (IL-6 and TNF-α) responses regardless of whether they were depressed or not, indicating that the relationship between HPA axis sensitivity to glucocorticoids and responsiveness of the innate immune system may be unrelated to diagnosis.88
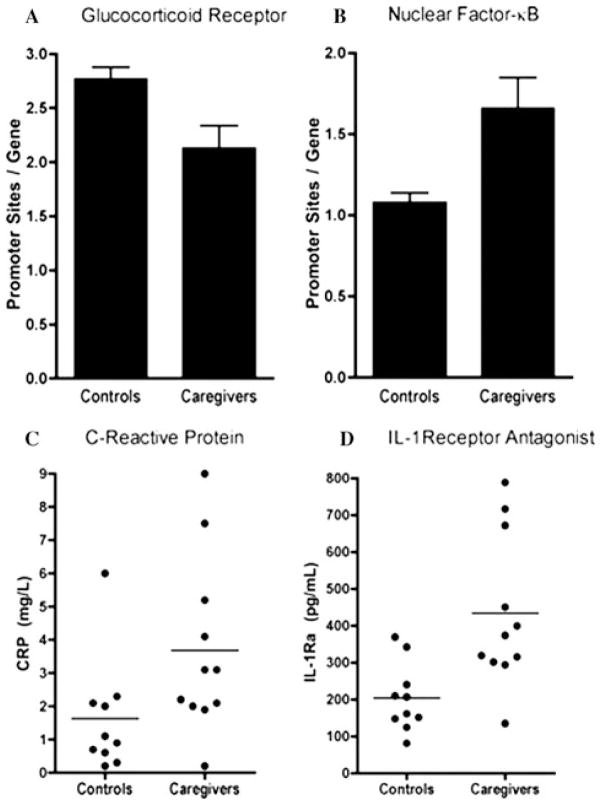
More clear evidence of a reciprocal relationship between inflammatory and glucocorticoid signaling pathways has recently been observed in a study of individuals exposed to the chronic stress of caring for a patient with brain cancer versus nonstressed, healthy control subjects.89 Compared to controls, peripheral blood monocytes isolated from subjects exposed to chronic caregiving stress exhibited significant increases in expression of genes containing promoter response elements for NF-κB and significant decreases in genes containing promoter elements for the GR (Fig. 3). Chronic caregivers also exhibited significant increases in peripheral blood IL-1ra, as well as increases in CRP, the mean of which was in the high-risk (high-inflammation) category for cardiovascular disease established by the Centers for Disease Control and Prevention and the American Heart Association (>3 mg/L).90 Salivary cortisol levels collected over 3 consecutive days were no different between the groups. These data, which capitalize on the advantages of state-of-the-art microarray and bioinformatics technology, provide compelling evidence that in the context of stress, the balance between the neuroendocrine and immune systems is clearly tipped toward increased inflammatory signaling at the expense of a decrease in GR signaling. Whether such a relationship occurs in the context of depression remains to be established.
Figure 3.
Glucocorticoid and inflammatory signaling during chronic stress. Compared to controls, peripheral blood monocytes isolated from subjects exposed to chronic caregiving stress exhibited significant increases in expression of genes containing promoter response elements for NF-κB (A) and significant decreases in genes containing promoter elements for the GR (B). Chronic caregivers also exhibited significant increases in plasma concentrations of IL-1ra (C), as well as increased plasma concentrations of C-reactive protein (D). (Reprinted with permission from Miller, G. E., Chen, E., Sze, J., Marin, T., Arevalo, J.M., Doll, R., Ma, R., and Cole, S.W. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-κB signaling. Biological Psychiatry. 64(4), 266–272, Copyright 2008 Elsevier.89)
IFN-α as a Model System to Study HPA-Immune Interactions in Depression
To further unravel the cause-and-effect relationships between cytokine exposure and the development of alterations in HPA axis function and glucocorticoid signaling, a number of investigators have seized the opportunity of patients undergoing treatment with IFN-α. IFN-α has both antiviral and antiproliferative properties and, therefore, is used clinically for the treatment of infectious diseases and cancers, including malignant melanoma and renal cell carcinoma.91 Of note, IFN-α is the only FDA-approved drug for the treatment of hepatitis C, which is believed to afflict 4–5 million individuals in the United States.91 Aside from being an innate immune cytokine itself, IFN-α is a potent inducer of other innate immune cytokines, including most notably IL-6 and to a lesser extent IL-1 and TNF-α and their soluble receptors.92,93 In addition, IFN-α has been shown, like other innate immune cytokines, to stimulate the release of CRF in the rat hypothalamus and amygdala and increase the release of CRF protein from rat hypothalamic explants.94,95 Moreover, IFN-α acutely activates the HPA axis in humans, stimulating dramatic increases in ACTH and cortisol within several hours.95,96 Of importance to the relationship of these IFN-α–induced neuroendocrine and immune changes with behavior, IFN-α causes marked depressive symptoms in a high percentage of patients, depending on the dose and route of administration. Prominent symptoms include depressed mood, anhedonia, fatigue, psychomotor slowing, anxiety, and insomnia.97 Indeed, in a study examining symptom criteria for major depression, 45% of patients receiving high dose IFN-α for cancer treatment met criteria for major depression and a third of patients dropped out of IFN-α treatment due to behavioral toxicity.98
Studies evaluating IFN-α and the neuroendocrine system have examined the impact of both acute and chronic IFN-α–induced neuroendocrine changes on mood. In terms of acute administration, patients who developed major depression during IFN-α treatment were found to exhibit significantly higher ACTH and cortisol responses to the first injection of IFN-α compared to individuals who did not develop depression.96 No differences were found in IL-6 responses between those who became depressed and those who did not. Of note, the degree of HPA axis responsiveness to IFN-α in terms of both ACTH and cortisol was found to correlate with the severity of depressive symptoms during IFN-α therapy. Interestingly, consistent with subsequent studies examining cortisol secretion during chronic IFN-α administration, no relationship was found between depressive symptoms and cortisol responses to IFN-α after several weeks of treatment.96 These data were interpreted to suggest that exaggerated HPA axis responses to the first injection of IFN-α in patients who developed depression during IFN-α therapy were likely secondary to sensitized neuroendocrine responses, possibly as a function of sensitized CRF pathways, which in turn represent a vulnerability to IFN-α–induced behavioral change.
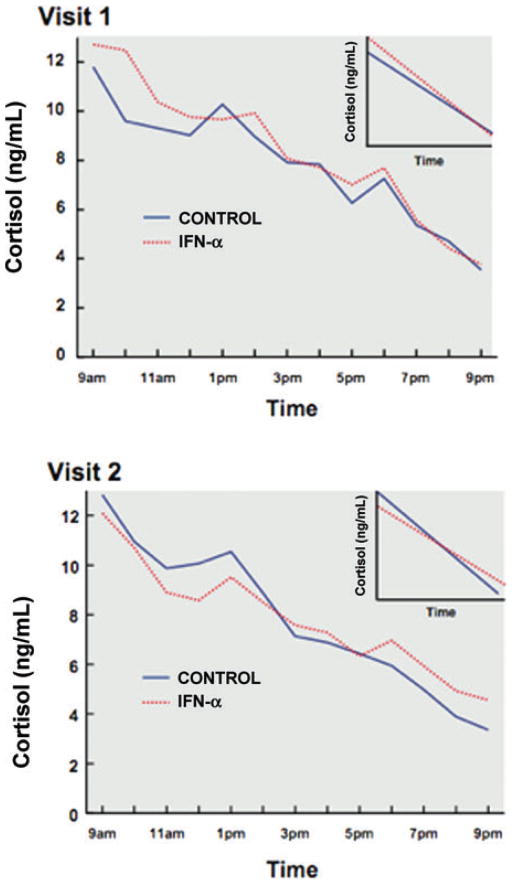
Recently, work in our laboratory has assumed a more detailed evaluation of the long-term consequences of IFN-α on HPA axis function. Indeed, in a study of patients with hepatitis C, we examined a sample of subjects who were either awaiting IFN-α treatment or received 12 weeks of IFN-α therapy in combination with ribavirin, a nucleoside analogue that interferes with viral replication.93 Subjects were studied under controlled conditions in an inpatient research facility at base line and then after 12 weeks. Interestingly, patients who received IFN-α treatment exhibited significant flattening of the cortisol curve compared to non-IFN-α–treated controls (Fig. 4).93 In addition, flattening of the cortisol curve was found to significantly correlate with the development of depressive symptoms, as well as fatigue, in these subjects. Flattening of the cortisol curve has been reported in a number of medical conditions, including cardiovascular disease and cancer, and has been associated with a worse outcome in these disorders.99,100 Moreover, flattening of the cortisol curve has been associated with fatigue in breast cancer survivors.101 Of note, the degree of difference between cortisol slopes in IFN-α–treated versus nontreated subjects was similar to those differences reported in the literature of various clinical populations.93 The occurrence of flattening of the cortisol slope in this longitudinal study suggests that flattening of the cortisol curve in the context of various medical illnesses may be a consequence of chronic exposure to innate immune cytokines by virtue of disease-associated inflammation.
Figure 4.
Diurnal cortisol secretion during treatment with IFN-α. Patients who received interferon (IFN)-α treatment for 12 weeks exhibited significant flattening of the cortisol curve compared to non-IFN-α-treated controls. Mean raw cortisol values from 9 a.m. to 9 p.m. in controls (blue/solid line) versus subjects treated with IFN-α plus ribavirin (red/dashed line) at Visit 1 and Visit 2. Cortisol slopes from 9 a.m. to 9 p.m. in controls (blue/solid line) versus subjects treated with IFN-α plus ribavirin (red/dashed line) at Visit 1 and Visit 2 are also depicted in inserts in each graph. Compared to control subjects, IFN-α/ribavirin-treated patients exhibited a significantly flatter cortisol slope (P < 0.05) and significantly higher evening cortisol values (P < 0.05). (Reprinted with permission from Raison, C.L., Borisov, A.S., Woolwine, B.J., Massung, B., Vogt, G., and Miller, A.H. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Molecular Psychiatry. 3 June 2008, doi:10.1038/mp.2008.58, Copyright 2008 Nature Publishing Group.93) (In color in Annals online.)
To further explore changes in diurnal cortisol secretion that may have accounted for the flattened cortisol curve during IFN-α treatment, we examined the relative contribution of a decrease in morning cortisol values versus an increase in evening cortisol values.93 Statistical analyses revealed that cortisol values in the evening were significantly higher in IFN-α–treated subjects compared to controls, and like the flattened cortisol curve, increases in evening cortisol concentrations over the course of the study were associated with increased symptoms of depression and fatigue. These data suggest that feedback inhibition of cortisol secretion late in the day may be disrupted as a function of IFN-α treatment and may be secondary to inhibitory effects of IFN-α on GR function. Relevant to the potential role of the GR in these effects, IFN-α is well known to activate Jak-STAT as well as p38 MAPK signaling pathways, both of which have been implicated in the disruption of GR function (see above). Future studies are required to further elucidate the relative interplay of neuroendocrine and immune factors that contribute to behavioral changes in IFN-α–treated patients. Nevertheless, the data provide a clear indication that chronic exposure to innate immune cytokines leads to changes in neuroendocrine function in vivo that “map onto” changes in relevant clinical populations and bespeak involvement of alterations in glucocorticoid sensitivity to feedback inhibition, potentially mediated by cytokine effects on the GR.
Treatment Implications
Given the role of glucocorticoids in regulating innate immune responses and the capacity of innate immune cytokines to disrupt HPA axis and GR function, as well as behavior, therapeutic strategies aimed at restoring the balance between the neuroendocrine and immune systems may be relevant for the treatment of behavioral disorders, such as depression. Clearly, anti-inflammatory therapies targeting innate immune cytokines and their signaling pathways are an obvious approach in this regard. Nevertheless, strategies that target inflammation, as well as GR function, may be of special relevance. Standard antidepressants appear to possess some of this dual mechanism of action, as do drugs that target protein kinase A (PKA) signaling pathways.
Antidepressants
A variety of antidepressants have been shown to enhance GR translocation and function both in vivo and in vitro.7 Early studies revealed that the antidepressant desipramine is capable of increasing both DEX-induced GR-mediated gene transcription and GR translocation (even in the absence of DEX).102 Other antidepressants, including clomipramine, fluoxetine, paroxetine, and citalopram, are also known to exert these effects on GR.103 The effects of antidepressants on GR function have been shown to be related in part to their inhibitory effects on the p-glycoprotein pump, which would make more hormone available to activate GR.104 However, other signal transduction pathways induced by antidepressant, such as cAMP and PKA, may be involved (see below). Of relevance to interactions between cytokines and GR, data also indicate that antidepressants exhibit the capacity to inhibit cytokine production both in vitro and in vivo.105 For example, amitriptyline decreased LPS-stimulated release of IL-1-β and TNF-α from a mixed glial culture.106 Nevertheless, the extent to which the effects of antidepressants on cytokines are related to their actions on GR is yet to be established.
PKA
A number of studies have shown that the PKA signaling pathway plays an essential role in GR function. GR and the catalytic subunit of PKA have been found to associate in a ligand-independent manner both in vivo and in vitro.107 Moreover, PKA has been shown to phosphorylate GR independent of the presence of relevant chaperone proteins (i.e., hsp90), and the transformational state of GR and phosphorylation of GR by PKA can be inhibited by H-8, a PKA inhibitor.108 PKA agonists, including forskolin and 8-Br-cAMP, increase GR mRNA stability and GR mRNA levels and enhance GR transcription and function.109,110 Treatment of human lung fibroblasts and vascular smooth muscle cells with the β2 adrenergic receptor agonists, salbutamol or salmeterol, was found to initiate GR translocation from cytoplasm to nucleus, increase GR-DNA binding, and increase GR-mediated gene transcription.111 In the same study, addition of cAMP alone was also shown to induce GR-GRE binding and a PKA-inhibiting peptide reduced this effect. PKA also activates GR function in a ligand-independent manner.111 Studies conducted in our laboratory also support a role for the cAMP-PKA pathway in GR regulation. We found that the PDE type IV inhibitor, rolipram, which antagonizes the breakdown of cAMP, significantly enhanced GR-mediated gene transcription in LMCAT mouse fibroblast cells and rat C6 glioma cells, both in the presence and absence of DEX. This effect was independent of the p-glycoprotein pump.71 In addition to direct effects on GR function, increased PKA activity has also been found to reverse GR resistance. Although DEX (up to 1 uM) fails to cause cell death in glucocorticoid-resistant lymphoid cells (CEM-C1), when combined with forskolin DEX (1 uM) causes 90% cell death.112 The GR antagonist RU486 blocks this effect, demonstrating its dependence on the GR. In further support of the role of cAMP-dependent PKA pathways in GR function, cAMP-resistant cell lines have been shown to give rise to a significantly higher frequency of glucocorticoid-resistant cell variants.113
In addition to its effects on the GR, cAMP and PKA signaling pathways also interact with cytokine signaling pathways, including NF-κB, MAPK and Jak-STAT pathways.114–116 Indeed, NF-kB transcription is blocked by increased PKA activity through interaction of the catalytic subunit of PKA with p65, which stops p65 transactivation114 (Fig. 2). Elevated PKA also inhibits MAPK pathways by phosphorylation of serine residues on raf-1, which leads to a reduced affinity of raf for Ras in a number of cell types.117 In addition, the PKA activator forskolin inhibits MAPK-induced raf-1 translocation118 and inhibits T cell activation through the downregulation of MAPK pathways.119 Finally, cAMP has been found to inhibit IL-6–induced DNA binding of STAT1 and STAT3.116 Taken together, these data suggest that drugs that can activate cAMP-PKA pathways, such as PDE type IV inhibitors, may represent an intriguing therapeutic strategy in reversing glucocorticoid resistance. Pharmacologic therapies targeting PKA may represent a “double hit” on mechanisms driving glucocorticoid resistance, given that PKA pathways can both enhance GR function and inhibit inflammatory signaling.71
The potential role of PKA signaling pathways in enhancing GR function and inhibiting inflammatory signaling pathways may be especially relevant in light of the fact that depressed patients have been found to exhibit reduced G protein function in mononuclear cells.120 In addition, cultured fibroblasts collected from depressed patients have been shown to have reduced cAMP-dependent protein kinase activity.121 Cyclic AMP/PKA signal transduction pathways have also been shown to be reduced in postmortem brain tissue from depressed patients. Studies investigating the mechanisms by which antidepressants exert their effects suggest that cAMP and PKA pathways act as mediators of the psychotropic effects of these agents (e.g., Nibuya et al.122). Therefore, it is possible that disruption in the cAMP/PKA pathway in major depression is linked to cytokine-induced glucocorticoid resistance. As such, antidepressants and other pharmacologic agents that enhance PKA signaling may overcome GR alterations via a direct effect on crosstalk between these pathways.
COX-2 Inhibitors
As noted above, COX-2 inhibitors have been shown to enhance GR function (DEX-induced GR-mediated reporter gene activity and GR-GRE binding), and these effects can be blocked by administration of anisomycin. Anisomycin activates both p38 and JNK signaling pathways. These studies suggest that COX-2 inhibitors may serve to enhance GR function and potentially reverse glucocorticoid resistance through inhibition of p38 and/or JNK. Of note, COX-2 inhibitors were found to augment the effects of the antidepressant reboxetine in treating patients with major depression.123
Summary
In summary, there is evidence of both glucocorticoid resistance and increased inflammatory markers, including elevated innate immune cytokines, in patients with major depression. In addition, data indicate that cytokines and their signaling pathways can significantly decrease GR function and glucocorticoid signaling both in vitro and in vivo. Taken together, these findings support the hypothesis that increased innate immune cytokines may contribute to glucocorticoid resistance in major depression. Moreover, given the role of glucocorticoids in restraining innate immune responses and the capacity of innate immune cytokines to influence behavior, the effects of cytokines on glucocorticoid signaling and the brain may in turn contribute to the pathophysiology of depression in some depressed patients. Future studies are needed to further examine the relative balance of glucocorticoid and innate immune signaling in major depression, and consideration should be given to therapeutic strategies that both augment GR function and/or inhibit inflammatory pathways for the treatment of patients with major depression and increased inflammation.
Acknowledgments
Supported by PHS Grants R03MH079870 (T.W.W.P.), K05MH069124 (A.H.M.), R01HL073921 (A.H.M.), R01MH075102 (A.H.M.), and NCRR M01RR00039 (Emory University Hospital General Clinical Research Center).
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Ridker P. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- 2.Pradhan A, Ridker P. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–834. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- 3.Aggarwal B, et al. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–1621. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 4.Bisoendial R, Kastelein J, Stroes E. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195:e10–18. doi: 10.1016/j.atherosclerosis.2007.04.053. [DOI] [PubMed] [Google Scholar]
- 5.Bouzakri K, Zierath J. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem. 2007;282:7783–7789. doi: 10.1074/jbc.M608602200. [DOI] [PubMed] [Google Scholar]
- 6.Raison C, Capuron L, Miller A. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pariante C, Miller A. Glucocorticoid receptors in major depression: relevance to pathophysiology and treatment. Biol Psychiatry. 2001;49:391–404. doi: 10.1016/s0006-3223(00)01088-x. [DOI] [PubMed] [Google Scholar]
- 8.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 9.Rhen T, Cidlowski J. Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med. 2005;353:1711–1723. doi: 10.1056/NEJMra050541. [DOI] [PubMed] [Google Scholar]
- 10.Raison C, Miller A. When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry. 2003;160:1554–1565. doi: 10.1176/appi.ajp.160.9.1554. [DOI] [PubMed] [Google Scholar]
- 11.Pace TW, Hu F, Miller A. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- 13.Pace TW, et al. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–1633. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- 14.Danese A, et al. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Danese A, et al. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA. 2007;104:1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dantzer R, et al. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maier S, Watkins L. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- 18.Quan N, Banks W. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 19.Ericsson A, Kovacs K, Sawchenko P. A functional anatomical analysis of central pathways subserving the effects of interleukin-1 on stress-related neuroendocrine neurons. J Neurosci. 1994;14:897–913. doi: 10.1523/JNEUROSCI.14-02-00897.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadjar A, et al. Inactivation of the cerebral NFkappaB pathway inhibits interleukin-1beta-induced sickness behavior and c-Fos expression in various brain nuclei. Neuropsychopharmacology. 2005;30:1492–1499. doi: 10.1038/sj.npp.1300755. [DOI] [PubMed] [Google Scholar]
- 21.O’Connor J, et al. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2009;14:511–522. doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry C, et al. Minocycline attenuates lipopolysaccharide (LPS)-induced neuroinflammation, sickness behavior, and anhedonia. J Neuroinflammation. 2008;5:15. doi: 10.1186/1742-2094-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller N, Schwarz M. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- 24.Wichers M, et al. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–544. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- 25.Capuron L, et al. Interferon-alpha-induced changes in tryptophan metabolism. Relationship to depression and paroxetine treatment. Biol Psychiatry. 2003;54:906–914. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- 27.Zhu C, Blakely R, Hewlett W. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–2131. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- 28.Zhu C, et al. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–15658. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- 29.Moron J, et al. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8488. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sanchez M, et al. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–897. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- 31.Besedovsky H, del Rey A. Immune-neuroendocrine interactions: facts and hypotheses. Endocr Rev. 1996;17:64–102. doi: 10.1210/edrv-17-1-64. [DOI] [PubMed] [Google Scholar]
- 32.Owens M, Nemeroff C. The role of corticotropin-releasing factor in the pathophysiology of affective and anxiety disorders: laboratory and clinical studies. Ciba Found Symp. 1993;172:296–308. doi: 10.1002/9780470514368.ch15. discussion 308–316. [DOI] [PubMed] [Google Scholar]
- 33.Barrientos R, et al. BDNF mRNA expression in rat hippocampus following contextual learning is blocked by intrahippocampal IL-1beta administration. J Neuroimmunol. 2004;155:119–126. doi: 10.1016/j.jneuroim.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 34.Ben Menachem-Zidon O, et al. Intrahippocampal transplantation of transgenic neural precursor cells overexpressing interleukin-1 receptor antagonist blocks chronic isolation-induced impairment in memory and neurogenesis. Neuropsychopharmacology. 2008;33:2251–2262. doi: 10.1038/sj.npp.1301606. [DOI] [PubMed] [Google Scholar]
- 35.Koo J, Duman R. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc Natl Acad Sci USA. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Capuron L, et al. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–196. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Capuron L, et al. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–2392. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- 38.Eisenberger N, Lieberman M. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 39.Lanquillon S, et al. Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology. 2000;22:370–379. doi: 10.1016/S0893-133X(99)00134-7. [DOI] [PubMed] [Google Scholar]
- 40.Fitzgerald P, et al. Cutaneous glucocorticoid receptor sensitivity and pro-inflammatory cytokine levels in antidepressant-resistant depression. Psychol Med. 2006;36:37–43. doi: 10.1017/S003329170500632X. [DOI] [PubMed] [Google Scholar]
- 41.Weidenfeld J, Yirmiya R. Effects of bacterial endotoxin on the glucocorticoid feedback regulation of adrenocortical response to stress. Neuroimmunomodulation. 1996;3:352–357. doi: 10.1159/000097295. [DOI] [PubMed] [Google Scholar]
- 42.Bener D, et al. Glucocorticoid resistance following herpes simplex-1 infection: role of hippocampal glucocorticoid receptors. Neuroendocrinology. 2007;85:207–215. doi: 10.1159/000102976. [DOI] [PubMed] [Google Scholar]
- 43.Pariante C, et al. The proinflammatory cytokine, interleukin-1α, reduces glucocorticoid receptor translocation and function. Endocrinology. 1999;140:4359–4366. doi: 10.1210/endo.140.9.6986. [DOI] [PubMed] [Google Scholar]
- 44.Wang X, Wu H, Miller A. Interleukin 1α (IL-1α) induced activation of p38 mitogen-activated protein kinase inhibits glucocorticoid receptor function. Mol Psychiatry. 2004;9:65–75. doi: 10.1038/sj.mp.4001339. [DOI] [PubMed] [Google Scholar]
- 45.Raddatz D, et al. Glucocorticoid receptor signaling in the intestinal epithelial cell lines IEC-6 and Caco-2: evidence of inhibition by interleukin-1β. Int J Colorectal Disease. 2001;16:377–383. doi: 10.1007/s003840100331. [DOI] [PubMed] [Google Scholar]
- 46.Goleva E, Kisich K, Leung D. A role for STAT5 in the pathogenesis of IL-2-induced glucocorticoid resistance. J Immunol. 2002;169:5934–5940. doi: 10.4049/jimmunol.169.10.5934. [DOI] [PubMed] [Google Scholar]
- 47.Xu Q, et al. CD56+ cells induce steroid resistance in B cells exposed to IL-15. J Immunol. 2004;172:7110–7115. doi: 10.4049/jimmunol.172.11.7110. [DOI] [PubMed] [Google Scholar]
- 48.Biola A, et al. Interleukin-2 inhibits glucocorticoid receptor transcriptional activity through a mechanism involving STAT5 (signal transducer and activator of transcription 5) but not AP-1. Mol Endocrinol. 2001;15:1062–1076. doi: 10.1210/mend.15.7.0657. [DOI] [PubMed] [Google Scholar]
- 49.Irusen E, et al. p38 Mitogen-activated protein kinase-induced glucocorticoid receptor phosphorylation reduces its activity: role in steroid-insensitive asthma. J Allergy Clin Immunol. 2002;109:649–657. doi: 10.1067/mai.2002.122465. [DOI] [PubMed] [Google Scholar]
- 50.Spahn J, et al. A novel action of IL-13: induction of diminished monocyte glucocorticoid receptor-binding affinity. J Immunol. 1996;157:2654–2659. [PubMed] [Google Scholar]
- 51.Kino T, Chrousos G. Tumor necrosis factor alpha receptor- and Fas-associated FLASH inhibit transcriptional activity of the glucocorticoid receptor by binding to and interfering with its interaction with p160 type nuclear receptor coactivators. J Biol Chem. 2003;278:3023–3029. doi: 10.1074/jbc.M209234200. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Z, et al. STAT3 acts as a co-activator of glucocorticoid receptor signaling. J Biol Chem. 1997;272:30607–30610. doi: 10.1074/jbc.272.49.30607. [DOI] [PubMed] [Google Scholar]
- 53.Hu F, et al. Inhibition of COX-2 by celecoxib enhances glucocorticoid receptor function. Mol Psychiatry. 2005;10:426–428. doi: 10.1038/sj.mp.4001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang X, et al. Inhibition of Jun N-terminal kinase (JNK) enhances glucocorticoid receptor-mediated function in mouse hippocampal HT22 cells. Neuropsychopharmacology. 2005;30:242–249. doi: 10.1038/sj.npp.1300606. [DOI] [PubMed] [Google Scholar]
- 55.Szatmary Z, Garabedian M, Vilcek J. Inhibition of glucocorticoid receptor-mediated transcriptional activation by p38 mitogen-activated protein (MAP) kinase. J Biol Chem. 2004;279:43708–43715. doi: 10.1074/jbc.M406568200. [DOI] [PubMed] [Google Scholar]
- 56.Onda K, et al. Mitogen-activated protein kinase kinase 1/extracellular signal-regulated kinase (MEK-1/ERK) inhibitors sensitize reduced glucocorticoid response mediated by TNFα in human epidermal keratinocytes (HaCaT) Biochem Biophys Res Commun. 2006;351:266–272. doi: 10.1016/j.bbrc.2006.10.032. [DOI] [PubMed] [Google Scholar]
- 57.Tliba O, et al. Cytokines induce an early steroid resistance in airway smooth muscle cells: novel role of interferon regulatory factor-1. Am J Respir Cell Mol Biol. 2008;38:463–472. doi: 10.1165/rcmb.2007-0226OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quan N, et al. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115:36–45. doi: 10.1016/s0165-5728(01)00273-9. [DOI] [PubMed] [Google Scholar]
- 59.Sheridan J, et al. Social disruption, immunity, and susceptibility to viral infection. Role of glucocorticoid insensitivity and NGF. Ann N Y Acad Sci. 2000;917:894–905. doi: 10.1111/j.1749-6632.2000.tb05455.x. [DOI] [PubMed] [Google Scholar]
- 60.Quan N, et al. Molecular mechanisms of glucocorticoid resistance in splenocytes of socially stressed male mice. J Neuroimmunol. 2003;137:51–58. doi: 10.1016/s0165-5728(03)00042-0. [DOI] [PubMed] [Google Scholar]
- 61.Engler H, et al. Interleukin-1 receptor type 1-deficient mice fail to develop social stress-associated glucocorticoid resistance in the spleen. Psychoneuroendocrinology. 2008;33:108–117. doi: 10.1016/j.psyneuen.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smoak K, Cidlowski J. Mechanisms of glucocorticoid receptor signaling during inflammation. Mech Ageing Dev. 2004;125:697–706. doi: 10.1016/j.mad.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 63.Itoh M, et al. Nuclear export of glucocorticoid receptor is enhanced by c-Jun N-terminal kinase-mediated phosphorylation. Mol Endocrinol. 2002;16:2382–2392. doi: 10.1210/me.2002-0144. [DOI] [PubMed] [Google Scholar]
- 64.Li L, et al. Superantigen-induced corticosteroid resistance of human T cells occurs through activation of the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase (MEK-ERK) pathway. J Allergy Clin Immunol. 2004;114:1059–1069. doi: 10.1016/j.jaci.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 65.Rogatsky I, Logan S, Garabedian M. Antagonism of glucocorticoid receptor transcriptional activation by the c-Jun N-terminal kinase. Proc Natl Acad Sci USA. 1998;95:2050–2055. doi: 10.1073/pnas.95.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rogatsky I, Ivashkiv L. Glucocoritcoid modulation of cytokine signaling. Tissue Antigens. 2006;68:1–12. doi: 10.1111/j.1399-0039.2006.00599.x. [DOI] [PubMed] [Google Scholar]
- 67.McKay L, Cidlowski J. Molecular control of immune/inflammatory responses: interactions between nuclear factor-kappa B and steroid receptor-signaling pathways. Endocr Rev. 1999;20:435–459. doi: 10.1210/edrv.20.4.0375. [DOI] [PubMed] [Google Scholar]
- 68.Sheppard K, et al. Nuclear integration of glucocorticoid receptor and nuclear factor-kappaB signaling by CREB-binding protein and steroid receptor coactivator-1. J Biol Chem. 1998;273:29291–29294. doi: 10.1074/jbc.273.45.29291. [DOI] [PubMed] [Google Scholar]
- 69.Di Battista J, et al. Enhancement of phosphorylation and transcriptional activity of the glucocorticoid receptor in human synovial fibroblasts by nimesulide, a preferential cyclooxygenase 2 inhibitor. Arthritis Rheum. 1999;42:157–166. doi: 10.1002/1529-0131(199901)42:1<157::AID-ANR19>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 70.Bevilacqua M, et al. Nimesulide decreases superoxide production by inhibiting phosphodiesterase type IV. Eur J Pharmacol. 1994;268:415–423. doi: 10.1016/0922-4106(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 71.Miller A, Vogt G, Pearce B. The phosphodiesterase type 4 inhibitor, rolipram, enhances glucocorticoid receptor function. Neuropsychopharmacology. 2002;27:939–948. doi: 10.1016/S0893-133X(02)00381-0. [DOI] [PubMed] [Google Scholar]
- 72.Paccani S, et al. Nonsteroidal anti-inflammatory drugs suppress T-cell activation by inhibiting p38 MAPK induction. J Biol Chem. 2002;277:1509–1513. doi: 10.1074/jbc.M110676200. [DOI] [PubMed] [Google Scholar]
- 73.Paccani S, et al. Nonsteroidal anti-inflammatory drugs inhibit a Fyn-dependent pathway coupled to Rac and stress kinase activation in TCR signaling. Blood. 2005;105:2042–2048. doi: 10.1182/blood-2004-04-1299. [DOI] [PubMed] [Google Scholar]
- 74.Lewis-Tuffin L, Cidlowski J. The physiology of human glucocorticoid receptor beta (hGR-beta) and glucocorticoid resistance. Ann N Y Acad Sci. 2006;1069:1–9. doi: 10.1196/annals.1351.001. [DOI] [PubMed] [Google Scholar]
- 75.Webster J, et al. Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. PNAS. 2001;98:6865–6870. doi: 10.1073/pnas.121455098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matsubara T, et al. Reduced glucocorticoid receptor alpha expression in mood disorder patients and first-degree relatives. Biol Psychiatry. 2006;59:689–695. doi: 10.1016/j.biopsych.2005.09.026. [DOI] [PubMed] [Google Scholar]
- 77.Otto C, Reichardt H, Schutz G. Absence of glucocorticoid receptor-beta in mice. J Biol Chem. 1997;272:26665–26668. doi: 10.1074/jbc.272.42.26665. [DOI] [PubMed] [Google Scholar]
- 78.De Kloet E, et al. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 79.Lewis-Tuffin L, et al. Human glucocorticoid receptor beta binds RU-486 and is transcriptionally active. Mol Cell Biol. 2007;27:2266–2282. doi: 10.1128/MCB.01439-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79a.Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann N Y Acad Sci. Glucocorticods and Mood: Clinical Manifestations, Risk Factors, and Molecular Mechanisms. 2009 doi: 10.1111/j.1749-6632.2009.04986.x. In press. [DOI] [PubMed] [Google Scholar]
- 80.Kossintseva I, et al. Proinflammatory cytokines inhibit human placental 11beta-hydroxysteroid dehydrogenase type 2 activity through Ca2+ and cAMP pathways. Am J Physiol Endocrinol Metab. 2006;290:E282–288. doi: 10.1152/ajpendo.00328.2005. [DOI] [PubMed] [Google Scholar]
- 81.Bernier J, et al. Decreased corticosteroid-binding globulin in burn patients: relationship with interleukin-6 and fat in nutritional support. Crit Care Med. 1998;26:452–460. doi: 10.1097/00003246-199803000-00014. [DOI] [PubMed] [Google Scholar]
- 82.Emptoz-Bonneton A, et al. Corticosteroid-binding globulin synthesis regulation by cytokines and glucocorticoids in human hepatoblastoma-derived (HepG2) cells. J Clin Endocrinol Metab. 1997;82:3758–3762. doi: 10.1210/jcem.82.11.4362. [DOI] [PubMed] [Google Scholar]
- 83.Pariante C. The role of multi-drug resistance p-glycoprotein in glucocorticoid function: studies in animals and relevance in humans. Eur J Pharmacol. 2008;583:263–271. doi: 10.1016/j.ejphar.2007.11.067. [DOI] [PubMed] [Google Scholar]
- 84.Bauer B, Hartz A, Miller D. Tumor necrosis factor alpha and endothelin-1 increase P-glycoprotein expression and transport activity at the blood-brain barrier. Mol Pharmacol. 2007;71:667–675. doi: 10.1124/mol.106.029512. [DOI] [PubMed] [Google Scholar]
- 85.Maes M, et al. Interleukin-1 beta: a putative mediator of HPA axis hyperactivity in major depression? Am J Psychiatry. 1993;150:1189–1193. doi: 10.1176/ajp.150.8.1189. [DOI] [PubMed] [Google Scholar]
- 86.Schuld A, et al. Hypothalamo-pituitary-adrenal function in patients with depressive disorders is correlated with baseline cytokine levels, but not with cytokine responses to hydrocortisone. J Psychiatr Res. 2003;37:463–470. doi: 10.1016/s0022-3956(03)00054-2. [DOI] [PubMed] [Google Scholar]
- 87.Miller G, et al. Clinical depression and regulation of the inflammatory response during acute stress. Psychosom Med. 2005;67:679–687. doi: 10.1097/01.psy.0000174172.82428.ce. [DOI] [PubMed] [Google Scholar]
- 88.Vedder H, et al. Immune-endocrine host response to endotoxin in major depression. J Psychiatr Res. 2007;41:280–289. doi: 10.1016/j.jpsychires.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 89.Miller G, et al. A functional genomic finger-print of chronic stress in humans: Blunted glucocorticoid and increased NF-kappaB signaling. Biol Psychiatry. 2008;64(4):266–272. doi: 10.1016/j.biopsych.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pearson T, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 91.Raison C, et al. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005;19:105–123. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Taylor J, Grossberg S. The effects of interferon-alpha on the production and action of other cytokines. Semin Oncol. 1998;25:23–29. [PubMed] [Google Scholar]
- 93.Raison C, et al. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2008 Jun 3; doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Raber J, Koob G, Bloom F. Interferon-alpha and transforming growth factor-beta 1 regulate corticotropin-releasing factor release from the amygdala: comparison with the hypothalamic response. Neurochem Int. 1997;30:455–463. doi: 10.1016/s0197-0186(96)00082-4. [DOI] [PubMed] [Google Scholar]
- 95.Gisslinger H, et al. Interferon-alpha stimulates the hypothalamic-pituitary-adrenal axis in vivo and in vitro. Neuroendocrinology. 1993;57:489–495. doi: 10.1159/000126396. [DOI] [PubMed] [Google Scholar]
- 96.Capuron L, et al. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003;160:1342–1345. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- 97.Capuron L, et al. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002;26:643–652. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- 98.Musselman D, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–966. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 99.Sephton S, et al. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 100.Matthews K, et al. Diurnal cortisol decline is related to coronary calcification: CARDIA study. Psychosom Med. 2006;68:657–661. doi: 10.1097/01.psy.0000244071.42939.0e. [DOI] [PubMed] [Google Scholar]
- 101.Bower J, et al. Diurnal cortisol rhythm and fatigue in breast cancer survivors. Psychoneuroendocrinology. 2005;30:92–100. doi: 10.1016/j.psyneuen.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 102.Pariante C, et al. Steroid-independent translocation of the glucocorticoid receptor by the antidepressant desipramine. Mol Pharmacol. 1997;52:571–581. doi: 10.1124/mol.52.4.571. [DOI] [PubMed] [Google Scholar]
- 103.Pariante C, et al. Antidepressants enhance glucocorticoid receptor function in vitro by modulating the membrane steroid transporters. Br J Pharmacol. 2001;134:1335–1343. doi: 10.1038/sj.bjp.0704368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yau J, et al. The antidepressant desipramine requires the ABCB1 (Mdr1)-type p-glycoprotein to upregulate the glucocorticoid receptor in mice. Neuropsychopharmacology. 2007;32:2520–2529. doi: 10.1038/sj.npp.1301389. [DOI] [PubMed] [Google Scholar]
- 105.Kenis G, Maes M. Effects of antidepressants on the production of cytokines. Int J Neuropsychopharmacol. 2002;5:401–412. doi: 10.1017/S1461145702003164. [DOI] [PubMed] [Google Scholar]
- 106.Obuchowicz E, et al. Amitriptyline and nortriptyline inhibit interleukin-1 release by rat mixed glial and microglial cell cultures. Int J Neuropsychopharmacol. 2006;9:27–35. doi: 10.1017/S146114570500547X. [DOI] [PubMed] [Google Scholar]
- 107.Doucas V, et al. Cytoplasmic catalytic subunit of protein kinase A mediates cross-repression by NF-kappa B and the glucocorticoid receptor. Proc Natl Acad Sci USA. 2000;97:11893–11898. doi: 10.1073/pnas.220413297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Haske T, Nakao M, Moudgil V. Phosphorylation of immunopurified rat liver glucocorticoid receptor by the catalytic subunit of cAMP-dependent protein kinase. Mol Cell Biochem. 1994;132:163–171. doi: 10.1007/BF00926925. [DOI] [PubMed] [Google Scholar]
- 109.Penuelas I, et al. cAMP activates transcription of the human glucocorticoid receptor gene promoter. J Steroid Biochem Mol Biol. 1998;67:89–94. doi: 10.1016/s0960-0760(98)00097-1. [DOI] [PubMed] [Google Scholar]
- 110.Dong Y, et al. The mechanism of cAMP-induced glucocorticoid receptor expression. Correlation to cellular glucocorticoid response. J Biol Chem. 1989;264:13679–13683. [PubMed] [Google Scholar]
- 111.Eickelberg O, et al. Ligand-independent activation of the glucocorticoid receptor by beta2-adrenergic receptor agonists in primary human lung fibroblasts and vascular smooth muscle cells. J Biol Chem. 1999;274:1005–1010. doi: 10.1074/jbc.274.2.1005. [DOI] [PubMed] [Google Scholar]
- 112.Medh R, et al. Resistance of human leukemic CEM-C1 cells is overcome by synergism between glucocorticoid and protein kinase A pathways: correlation with c-Myc suppression. Cancer Res. 1998;58:3684–3693. [PubMed] [Google Scholar]
- 113.Gruol D, et al. Isolation of new types of dexamethasone-resistant variants from a cAMP-resistant lymphoma. J Steroid Biochem. 1986;24:255–258. doi: 10.1016/0022-4731(86)90060-9. [DOI] [PubMed] [Google Scholar]
- 114.Takahashi E, et al. Expression of c-fos, rather than c-jun or glucocorticoid-receptor mRNA, correlates with decreased glucocorticoid response of peripheral blood mononuclear cells in asthma. Int Immunopharmacol. 2002;2:1419–1427. doi: 10.1016/s1567-5769(02)00083-8. [DOI] [PubMed] [Google Scholar]
- 115.Saxena M, et al. Crosstalk between cAMP-dependent kinase and MAP kinase through a protein tyrosine phosphatase. Nat Cell Biol. 1999;1:305–311. doi: 10.1038/13024. [DOI] [PubMed] [Google Scholar]
- 116.Sengupta T, Schmitt E, Ivashkiv L. Inhibition of cytokines and JAK-STAT activation by distinct signaling pathways. Proc Natl Acad Sci USA. 1996;93:9499–9504. doi: 10.1073/pnas.93.18.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hafner S, et al. Mechanism of inhibition of Raf-1 by protein kinase A. Mol Cell Biol. 1994;14:6696–6703. doi: 10.1128/mcb.14.10.6696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melck D, et al. Involvement of the cAMP/protein kinase A pathway and of mitogen-activated protein kinase in the anti-proliferative effects of anandamide in human breast cancer cells. FEBS Lett. 1999;463:235–240. doi: 10.1016/s0014-5793(99)01639-7. [DOI] [PubMed] [Google Scholar]
- 119.Tamir A, Granot Y, Isakov N. Inhibition of T lymphocyte activation by cAMP is associated with down-regulation of two parallel mitogen-activated protein kinase pathways, the extracellular signal-related kinase and c-Jun N-terminal kinase. J Immunol. 1996;157:1514–1522. [PubMed] [Google Scholar]
- 120.Avissar S, et al. Reduced G protein functions and immunoreactive levels in mononuclear leukocytes of patients with depression. Am J Psychiatry. 1997;154:211–217. doi: 10.1176/ajp.154.2.211. [DOI] [PubMed] [Google Scholar]
- 121.Shelton R, Mainer D, Sulser F. cAMP-dependent protein kinase activity in major depression. Am J Psychiatry. 1996;153:1037–1042. doi: 10.1176/ajp.153.8.1037. [DOI] [PubMed] [Google Scholar]
- 122.Nibuya M, Nestler E, Duman R. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J Neurosci. 1996;16:2365–2372. doi: 10.1523/JNEUROSCI.16-07-02365.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muller N, et al. The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry. 2006;11:680–684. doi: 10.1038/sj.mp.4001805. [DOI] [PubMed] [Google Scholar]