Abstract
Interleukin (IL)-33 is a recently described pro-inflammatory cytokine. Here we demonstrate IL-33 as a regulator of functional osteoclasts (OCs) from human CD14+ monocytes. IL-33 stimulates formation of tartrate-resistant acid phosphatase (TRAP)+ multinuclear OCs from monocytes. This action was suppressed by anti-ST2 antibody, suggesting that IL-33 acts through its receptor ST2, but not by the receptor activator of NF-κB ligand (RANKL) decoy, osteoprotegerin, or anti-RANKL antibody. IL-33 stimulated activating phosphorylations of signaling molecules in monocytes that are critical for OC development. These included Syk, phospholipase Cγ2, Gab2, MAP kinases, TAK-1, and NF-κB. IL-33 also enhanced expression of OC differentiation factors including TNF-α receptor-associated factor 6 (TRAF6), nuclear factor of activated T cells cytoplasmic 1, c-Fos, c-Src, cathepsin K, and calcitonin receptor. IL-33 eventually induced bone resorption. This study suggests that the osteoclastogenic property of IL-33 is mediated through TRAF6 as well as the immunoreceptor tyrosine-based activation motif-dependent Syk/PLCγ pathway in human CD14+ monocytes.
Keywords: Interleukin-33, Osteoclasts, Differentiation, Human CD14+ monocytes, Bone resorption
Introduction
Enhanced osteoclastogenesis and bone resorption underlie osteopenic diseases including rheumatoid arthritis (RA) [1, 2]. Bone erosion in inflammatory bone diseases is critically dependent on multinucleated osteoclasts (OCs), which arise from hematopoietic myeloid progenitors [3] and can be derived from human CD14+ monocytes [4, 5]. Osteoclastogenesis is classically regulated by macrophage colony-stimulating factor (M-CSF) [6] and the receptor activator of NF-κB ligand (RANKL) [7]. M-CSF is critical for the proliferation and survival of the OC precursor cells whereas RANKL orchestrates the whole process of OC differentiation and action [8, 9]. M-CSF, through its receptor c-Fms, activates Akt and ERK1/2 to ensure proliferation and survival of the OC lineage [10]. The interaction of RANKL with its receptor, RANK, results in engagement of TNF receptor-associated factor 6 (TRAF6) and downstream activation of the mitogen-activated protein (MAP) kinases, the nuclear factor κB (NF-κB), and activator protein-1 (AP-1) [8, 9]. However, productive RANK signaling also requires co-option of the adaptor protein, DAP12, and the Fc-receptor common γ subunit (FcRγ) after phosphorylation of their immunoreceptor tyrosine-based activation motifs (ITAMs) by c-Src kinase [11]. This interaction results in cooperative signaling through the activation of Syk tyrosine kinase and ultimately the activation of nuclear factor of activated T cells cytoplasmic 1 (NFATc1).
The recently described cytokine interleukin (IL)-33 is now thought to participate in a variety of inflammatory diseases. Early studies revealed that it is expressed in intestine of patients with Crohn’s disease, blood vessels of inflamed tonsils, and synovium of patients with RA and that this expression appeared to correlate with the severity of the inflammatory condition [12]. The administration of IL-33 to mice results in substantial pathological changes indicative of Th2 responses including eosinophilia in various organs, epithelial hyperplasia, augmented production of Th2-related cytokines, elevated serum IgA and IgE levels, and mucus secretion [13]. Recent studies suggest that IL-33 may also act as a proinflammatory cytokine in asthma, septic shock, fibroproliferative diseases, collagen vascular diseases, pleural malignancy, and cardiovascular diseases [14]. However, it is still unknown whether IL-33 has the critical role in osteoclastogenesis.
IL-33 acts through ST2, which was initially recognized as an orphan receptor associated with inflammatory and autoimmune diseases [13], is now known to be a member of Toll-like/IL-1 receptor family [14, 15]. Like other members of this family, ST2 contains a Toll/IL-1 receptor (TIR) domain that permits recruitment of TIR domain-containing adaptor protein, myeloid differentiating factor 88 [13, 14, 16] and, in turn, IL-1 receptor-associated kinases (IRAKs) and TRAF6. The engagement of TRAF6 leads to activation of the MAP kinases, ERK1/2 [13, 17], Jun N-terminal kinase (JNK), and p38, as well as NF-κB pathway [18–20].
The transmembrane form of ST2 (as opposed to the soluble form, sST2) is expressed predominantly on mast cells and Th2 cells. Indeed, functional studies of ST2 indicate a role in inflammatory disorders that are associated with these two types of cells [14]. Recently, it was also reported that IL-33 exacerbates antigen-induced arthritis by activating mast cells [21] and inhibition of IL-33 signaling attenuates the severity of bone erosion in an experimental arthritis animal model [22]. These observations suggested to us that IL-33, directly or indirectly, alters bone homeostasis through increased OC differentiation and activity.
Here we report that IL-33, acting specifically through ST2, regulates the formation of OCs from human peripheral blood CD14+ monocytes and bone resorption. Our studies reveal novel features of IL-33-mediated signaling in CD14+ monocytes which are the source of OC progenitors. They also reveal that although IL-33 acts independently of RANKL in human CD14+ monocytes, it shares many of the signaling and functional attributes of RANKL.
Materials and methods
Reagents
Reagents were obtained as follows: recombinant human IL-33, recombinant human RANKL (sRANKL), neutralizing antibodies against RANKL and ST2, and osteoprotegerin (OPG) from R&D Systems Inc. (Minneapolis, MN); recombinant human M-CSF from Sigma (St. Louis, MO); recombinant mouse IL-33 from PeproTech Inc. (Rock Hill, NJ); antibodies against TRAF6, NFATc1, Syk, cathepsin K, and IkB-α from Santa Cruz (Santa Cruz, CA), calcitonin receptor from Thermo Fisher Scientific (Rockford, IL), c-Src from Millipore (London, UK), and the phosphorylated forms of Syk (Tyr525/526), TAK-1 (Thr184/187), Akt (Thr308), ERK1/2 (Thr202/Tyr204), p38 (Thr180/Tyr182), JNK (Thr183/Tyr185), NF-κB (Ser536) and c-Jun (Ser63) from Cell Signaling (Beverly, MA); Piceatannol, U73122, SB203580, SP600125, and Bay11-7082 from Merck KGaA (Darmstadt, Germany); and media and reagents for cell culture from Gibco-BRL (Grand Island, NY).
Isolation and culture of CD14+ monocytes
Human peripheral blood mononuclear cells (PBMCs) were isolated from blood of healthy volunteers by centrifugation over Ficoll/Paque Plus (GE Healthcare, Uppsala, Sweden) and CD14+ monocytes were isolated from the PBMCs by using anti-CD14-conjugated microbeads as previously reported [23]. To isolate human CD14+ monocytes, human PBMCs (1 × 107 cells) were incubated with human anti-CD14 conjugated micro-beads (Miltenyi Biotech, Sunnyvale, CA) for 15 min at 4°C. The CD14+ cells were suspended in solution A (1× PBS solution containing 0.5% BSA and 2 mM EDTA), deposited by centrifugation, resuspended in 500 μl of solution A, and separated on LS columns (Miltenyi Biotech, Sunnyvale, CA). The isolated CD14+ cells were incubated in α-MEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin–streptomycin, and 10 ng/ml M-CSF. The cell preparations contained >95% CD14+ monocytes as determined by FACS analysis (Becton-Dickinson, Oxnard, NJ). Informed consent was obtained from all volunteers and the protocol was approved prior to the study according to the institutional guidelines.
Differentiation of OCs from CD14+ monocytes
Human CD14+ monocytes (1 × 105 cells/well in 96-well cluster plate) were incubated in α-MEM supplemented with 10% FBS, 2 mM l-glutamine, 100 U/ml penicillin–streptomycin, and 10 ng/ml of M-CSF. Where stated, IL-33 (50 ng/ml or as indicated elsewhere) and/or sRANKL (20 ng/ml) were added to cultures. The medium was replenished every 3 days for 20 days or as indicated. The cells were examined after staining for tartrate-resistant acid phosphatase (TRAP) using a Leukocyte acid phosphatase kit (Sigma, St. Louis, MO). TRAP+ cells that contained more than three nuclei were counted as OCs.
Determination of bone resorption
CD14+ Monocytes (2 × 105 cells/well) were cultured for 20 days on Osteologic disks (BD Biosciences, Bedford, MA) in supplemented α-MEM with M-CSF (10 ng/ml) and, where indicated, IL-33 or sRANKL. The media were replaced every 3 days for 20 days. On day 20, cells were removed by 6% NaOCl and the areas of resorption pits were measured using Multi Gauge V3.1 software (Fujifilm, Tokyo, Japan).
Immunoblotting analysis
The CD14+ monocytes (3 × 106 cells/dish) were stimulated by 50 ng/ml IL-33 or 20 ng/ml sRANKL for 15 min or as indicated in supplemented α-MEM containing 10 ng/ml of M-CSF. The cells were washed with 1× PBS twice and lysed in 50 μl of lysis buffer (20 mM HEPES, pH 7.5, 150 mM NaCl, 1% Nonidet P40, 10% glycerol, 60 mM octyl-β-glucoside, 10 mM NaF, 1 mM Na3VO4, 1 mM phenylmethylsulfonyl fluoride, 2.5 mM nitrophenylphosphate, 0.7 μg/ml pepstatin, and a protease inhibitor cocktail tablet). The whole-cell lysates were subjected to immunoblot analysis using specific antibodies. Immunoreactive proteins were detected with HRP-coupled secondary antibodies and enhanced chemiluminescence according to the manufacturer’s protocol (Amersham Biosciences, Piscataway, NJ).
Electrophoretic mobility-shift assay
CD14+ monocytes (5 × 106 cells/30-mm dish) were cultured in the supplemented α-MEM containing 10 ng/ml of M-CSF for 3 days. Cells were then stimulated or not with 50 ng/ml of IL-33. Nuclear extracts were prepared by using an EMSA kit according to the manufacturer’s instructions (Panomics, Freemont, CA). Electrophoretic mobility shift assays (EMSA) were carried out using EMSA “Gel-Shift” (Panomics, Freemont, CA) according to the manufacturer’s instructions. Nuclear extracts were incubated with a biotin-labeled oligonucleotide containing the consensus binding sequence for NF-κB (5′-AGTTGAGGGGACTTTCCCAGGC-3′) for 30 min at 15°C, and reaction products were separated in a 6.0% non-denaturing polyacrylamide gel. After transfer to Biodyne B membrane (Pall Corporation, Ann Arbor, MI) for 30 min at 300 mA, biotinylated DNA was detected by use of horseradish peroxidase-based chemiluminescence.
Analysis of ST-2 expression on CD14+ monocytes by flow cytometry
Isolated CD14+ monocytes were washed and incubated with either anti-ST2 antibody or isotype-matched control antibody for 30 min at 4°C and subsequently stained with FITC-conjugated rat anti-goat antibody (BD Biosciences, San Jose, CA) for 30 min. Multicolor analysis was performed in a FACSCalibur flow cytometer (Becton–Dickinson, Franklin Lakes, NJ) and data were analyzed using FlowJo software (Tree Star Inc., Ashland, OR).
RNA extraction and reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was isolated from human CD14+ monocytes or mouse BMMs using easy-spin™ (iNtRON, Korea). The first-strand cDNA was synthesized with ImProm-II™ Reverse Transcription System (Promega, Madison, WI) according to the manufacturer’s protocol. PCR amplification was carried out as follows: cDNAs were denatured for 5 min at 94°C. The amplification involved denaturation at 94°C for 30 s, annealing at 60°C for 30 s, and extension at 72°C for 1 min with 30 cycles (for ST2) or 20 cycles (for β-actin). The primer sequences used in PCR analysis were (forward) 5′-GGCTTGAGAAGGCACACCGT-3′ and (reverse) 5′-GGGAGTGGGGGAGGACGAAC-3′ for human ST2; (forward) 5′-TGCGTACATCATTTACCCTCGGGTC-3′ and (reverse) 5′-GCCACTCAACGGAGCCGCAA-3′ for mouse ST2; (forward) 5′-ATCATGTTTGAGACCTTCAACAC-3′ and (reverse) 5′-CAGGAGGAGCAATGATCTTG-3′ for β-actin.
Statistical analysis
The results were expressed as mean ± SEM of values from three independent experiments or as indicated. Statistical analysis was performed using one-way analysis of variance and Dunnett’s test.
Results
IL-33 stimulates OC formation from human CD14+ monocytes via ST2
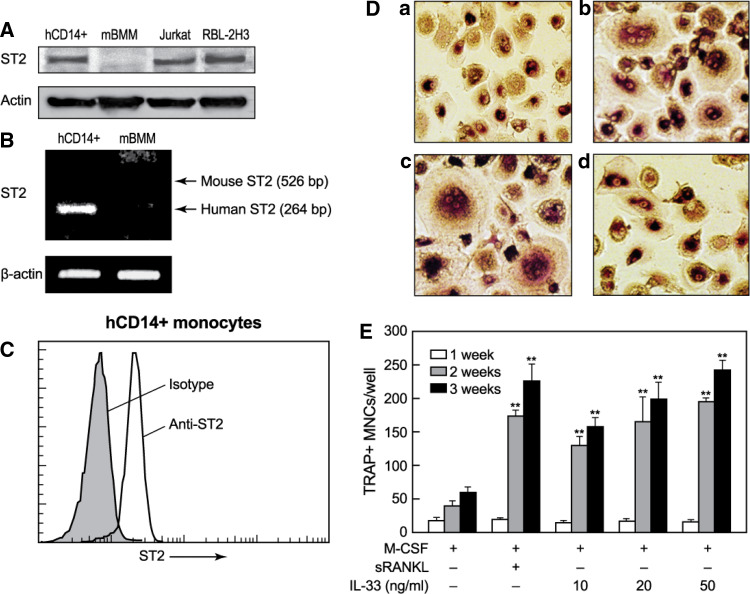
We initially determined whether the isolated human CD14+ monocytes express the ST2 receptor for IL-33. As shown in Fig. 1a and b, the expression level of ST2 in human CD14+ monocytes was measurable but lower than that in RBL-2H3 mast cells although ST2 protein and mRNA was not detectable in mouse BMMs (Fig. 1a, b). More than 90% of human CD14+ monocytes expressed ST2 receptor at their cell surface (Fig. 1c). To examine whether IL-33 regulated OC differentiation, we next determined if IL-33 stimulated OC differentiation from human PBMCs and CD14+ monocytes isolated from them. All cultures contained M-CSF to ensure monocyte survival. IL-33 stimulated formation of TRAP+ multinuclear OCs from both PBMC (data not shown) and CD14+ monocytes (Fig. 1d). The formation of OCs was apparent after 2 weeks and the number of identifiable OCs was dependent on the concentration of IL-33. At 20 ng/ml, IL-33 exhibited a similar time dependency and potency as 20 ng/ml sRANKL (Fig. 1e). Another set of experiments demonstrated that IL-33 and sRANKL were indeed equi-potent and that the effects of both in combination were additive (Fig. 2a).
Fig. 1.
IL-33 induces formation of OCs in cultures of human CD14+ monocytes. a The equal amount of whole-cell lysates obtained from human CD14+ monocytes (hCD14+), mouse bone marrow-derived macrophages (mBMM), Jurkat T cells, and RBL-2H3 mast cells was separated by SDS-PAGE and subjected to Western-blot analysis by using anti-ST2 antibody. b For measurement of ST2 mRNA expression, total RNA was isolated, and mRNA expression of ST2 in human CD14+ monocytes or mBMMs were analyzed by RT-PCR using specific primers as described in the “Material and methods”. c The isolated CD14+ monocytes were incubated with either anti-ST2 antibody or isotype-matched control antibody and subsequently stained with FITC-conjugated rat anti-goat antibody. Cells were analyzed by a FACSCalibur flow cytometer. d, e Human blood CD14+ monocytes were cultured in the presence of M-CSF (10 ng/ml) and subjected to the following treatments. d Cells were fixed and stained for TRAP 20 days after culture with M-CSF alone (panel a) or M-CSF in combination with 20 ng/ml sRANKL (panel b), 50 ng/ml IL-33 (panel c), or IL-33 along with 5 μg/ml anti-ST2 antibody (panel d). e TRAP+ multinuclear cells (3 or more nuclei per cell) were counted 1, 2, or 3 weeks after culture with M-CSF, alone or in combination with sRANKL (20 ng/ml) or the indicated concentration of IL-33. a–d Representative images from at least three independent experiments are shown. Values are the mean ± SEM of values from three independent experiments; *p < 0.05 and **p < 0.01 indicate significant differences from corresponding values for M-CSF treatment alone
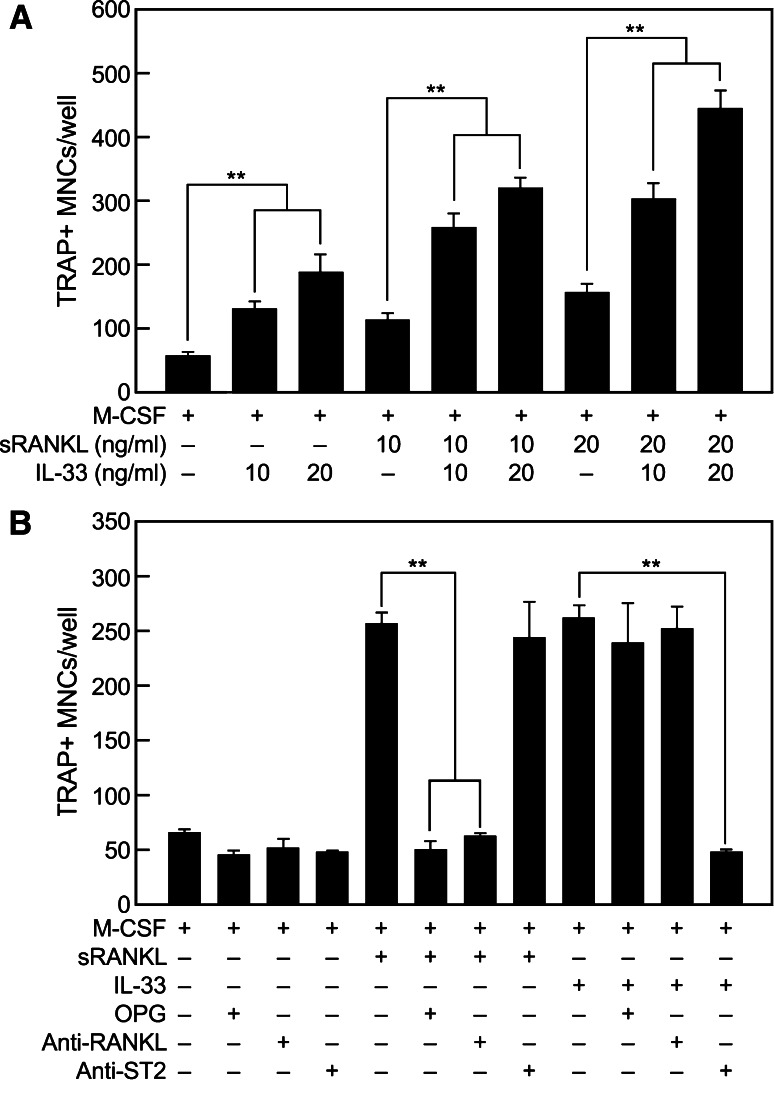
Fig. 2.
IL-33-induced OC differentiation is enhanced by sRANKL and is blocked by the treatment of antibody against ST2 but not by OPG or anti-RANKL antibody. a Human CD14+ monocytes were cultured for 20 days in the presence of the designated concentrations of IL-33 or sRANKL and 10 ng/ml M-CSF, individually or in combination as noted. b The monocytes were cultured for 20 days in M-CSF (10 ng/ml), alone or in combination with sRANKL (20 ng/ml) or IL-33 (50 ng/ml). In addition, some cultures contained OPG (0.25 μg/ml), anti-RANKL antibody (1 μg/ml) or anti-ST-2 antibody (5 μg/ml) as indicated. a, b Multinuclear TRAP+ OCs were counted and values shown are the mean ± SEM of values from three independent experiments; *p < 0.05 and **p < 0.01
We next investigated whether the action of IL-33 is dependent or not on RANKL. Induction of OC formation by sRANKL in cultures was, as expected, suppressed by pretreatment of cells with the decoy receptor, OPG [24], or anti-RANKL antibody but not by anti-ST2 antibody (Fig. 2b). Conversely, the induction of OC formation by IL-33 was inhibited by anti-ST2 antibody but not by OPG or anti-RANKL antibody (Fig. 2b).
IL-33 stimulates critical intracellular signaling molecules for OC differentiation
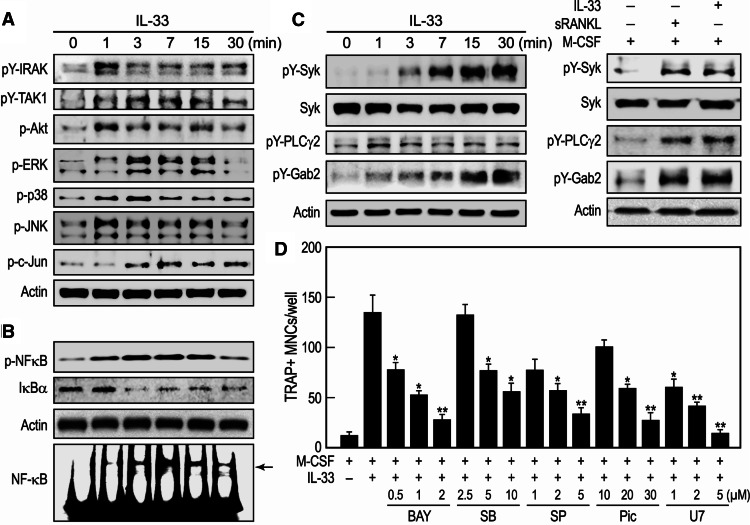
Addition of IL-33 to human CD14+ monocytes in the presence of M-CSF resulted in activation of early IL-33-mediated signaling events as indicated by the phosphorylation of IRAK, TAK-1, and Akt (Fig. 3a). Downstream signaling events were also apparent from the phosphorylation of all three MAP kinases (i.e., ERK1/2, p38, and JNK) and NF-κB (Fig. 3a, b). Activation of NF-κB was also apparent from the phosphorylation of p65 subunit of NF-κB, a rapid decline in levels of the inhibitor of κB (IκB)-the inhibitory component of the NF-κB kinase complex (Fig. 3b), and enhanced association of NF-κB with cellular DNA (Fig. 3b, gel shift assay). The AP-1 component and substrate of JNK, c-Jun, was also phosphorylated (Fig. 3a). In addition to the above events, IL-33 caused sustained phosphorylation of Syk, phospholipase (PL) Cγ2, and Gab2 (Fig. 3c) and to the same extent as sRANKL (right panels, Fig. 3c). To further investigate the correlation between activating signaling molecules by IL-33 and differentiation of OCs, we used specific inhibitors against NF-κB (Bay11-7082), p38 (SB203580), JNK (SP600125), Syk (Piceatannol), and PLCγ (U73122) at doses that were not cytotoxic. The differentiation of OCs was significantly suppressed by all inhibitors in a dose-dependent manner (Fig. 3d).
Fig. 3.
IL-33 activates signaling molecules in CD14+ monocytes that are associated with OC differentiation. a–c Human CD14+ monocytes were incubated with 10 ng/ml M-CSF along with 50 ng/ml IL-33 for the indicated times or, for the bottom panels of c, at the time of optimal phosphorylation. Immunoblots were prepared from whole-cell lysates and probed with antibodies against the designated proteins or their phosphorylated cognates. Representative immunoblot images from three independent experiments are shown. b Monocytes were similarly stimulated for detection of DNA binding of NF-κB by EMSA as described in “Materials and methods”. d CD14+ monocytes were cultured for 20 days in the presence of 10 ng/ml M-CSF and 50 ng/ml IL-33, with or without inhibitors: Bay11-7082 (BAY), SB203580 (SB), SP600125 (SP), piceatannol (Pic), or U73122 (U7), and then the cells were stained for TRAP. TRAP+ multinuclear cells (three or more nuclei per cell) were counted and values are the mean ± SEM of values from three independent experiments; *p < 0.05 and **p < 0.01 indicate significant differences from the value by M-CSF + IL-33 treatment without inhibitors
IL-33 enhances expression of factors critical for development of functional OCs
IL-33-treated CD14+ monocytes also exhibited increased expression of several markers of OC maturation over the course of 20 days as compared to control samples that contained M-CSF alone (Fig. 4a, b). These markers included TRAF6, NFATc1, c-Fos, and Syk, which are known to regulate differentiation of RANKL-stimulated monocytes into mature OCs [8, 9]. Expression of other critical factors for bone resorption, including c-Src and cathepsin K, were also enhanced by IL-33 (Fig. 4a). The effects of IL-33 were comparable with those of sRANKL with respect to the increased expression of TRAF6, cathepsin K, NFATc1, c-Src, c-Fos, and Syk (Fig. 4c). The expression of another marker of mature OCs, the calcitonin receptor, was also increased by IL-33 (Fig. 4d). Interestingly, sRANKL increased expression of ST2 as does IL-33 (Fig. 4b).
Fig. 4.
IL-33 stimulates the expression of markers of mature OC. Human CD14+ monocytes were cultured in the presence of 10 ng/ml M-CSF, alone or in combination with 50 ng/ml IL-33 or 20 ng/ml sRANKL for the number of days noted (a) or for 20 days (b, d). Immunoblots were prepared from whole-cell lysates and probed with antibodies against the designated proteins. Representative images are shown (a, b, d) and band densities (fold increase over M-CSF alone) (c) from three independent experiments are expressed as the mean ± SEM of values; *p < 0.05 and **p < 0.01 indicate significant differences from value for M-CSF treatment alone. CTR calcitonin receptor
Bone resorption is also stimulated by IL-33 through ST2
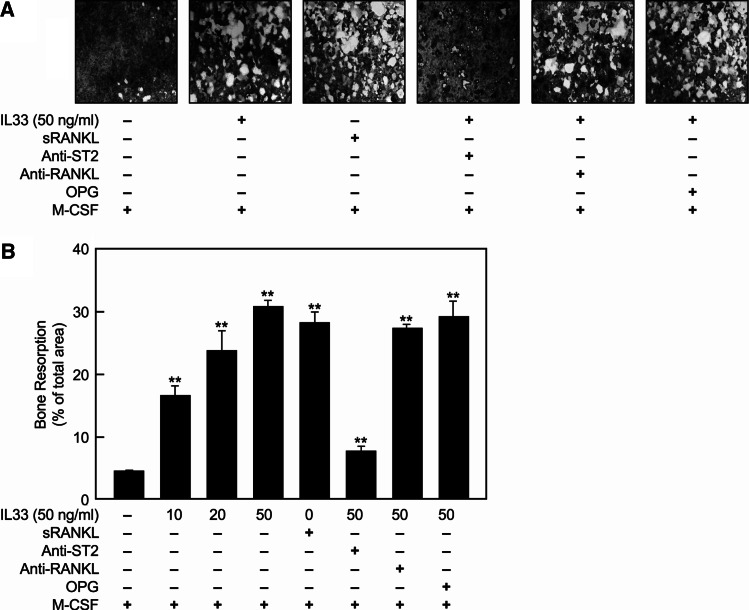
The effect of IL-33 on bone resorption was examined using Osteologic disks (BD Biosciences, San Jose, CA) to evaluate the actions of IL-33 on bone tissue. Formation of lacunae was significantly enhanced by IL-33 in a dose-dependent manner (Fig. 5). This action was largely suppressed by the presence of anti-ST2 antibody but not by OPG or anti-RANKL antibody treatment (Fig. 5).
Fig. 5.
IL-33 induces bone resorption through the ST2 receptor independently of RANKL/RANK. Human CD14+ monocytes were cultured in M-CSF (10 ng/ml) on Osteologic disks for 20 days with varying concentrations of IL-33 as indicated or 20 ng/ml sRANKL with or without anti-RANKL (1 μg/ml), anti-ST2 antibody (5 μg/ml), or OPG (0.25 μg/ml). The resorption lacunae were examined by microscope after removal of cells as described in “Materials and methods”. a Representative images are shown from three independent experiments. b The percentage of the area of resorption lacunae (i.e., bright areas in a) was quantified and expressed as the mean ± SEM value from three independent experiments; *p < 0.05 and **p < 0.01 indicate significant differences from value for M-CSF treatment alone
Discussion
Osteoclasts are multinucleated cells formed by fusion of mononuclear phagocyte precursors and are the cells responsible for bone resorption in RA and other bone-related diseases [4, 5]. It is generally accepted that RANKL is essential for osteoclast formation and function [2] as well as heightened OC activity in RA [25]. Patients with active RA have higher levels of RANKL than do healthy adults or patients with inactive RA [26]. However, RANKL-independent factors such as TNF-α [27], LIGHT [28], IL-8 [29], and secreted osteoclastogenic factor of activated T cells [30] were recently reported as additional factors promoting OC differentiation although their effects and mechanisms of action have yet to be determined in vivo.
IL-33 was recently identified as a ligand for the orphan IL-1 family receptor T1/ST2 and is mainly expressed in smooth-muscle cells, epithelial cells, fibroblasts, keratinocytes, dendritic cells, and activated macrophages [13]. It has been suggested previously that IL-33 has a proinflammatory function in arthritis and that IL-33 and ST2 are detectable in the synovium of patients with RA [12]. The presence of IL-33 may enable stimulation of synovial tissue-resident cells and thus maintain the inflammatory state. In collagen-induced arthritis animals, disabling IL-33 function by soluble ST2 administration [21], ST2 gene deletion [31], or use of blocking ST2-specific antibody [22] resulted in decreased severity of disease. Adoptive cell transfer experiments showed that the inflammatory symptoms produced in the collagen model are mediated primarily through release of mast cell-derived cytokines [21]. However, the direct effect of IL-33 on OC differentiation and function had not been previously determined.
In this study, we demonstrate that IL-33 was equally potent in stimulating OC differentiation from human CD14+ monocytes as sRANKL (Fig. 1e). Furthermore, the combined treatment of IL-33 and sRANKL led to additive formation of OCs (Fig. 2a), raising the possibility that these two stimulants (IL-33 + sRANKL) act in concert under certain physiological conditions. The IL-33 effect was inhibited by anti-ST2 antibody, but not by OPG or anti-RANKL antibody (Fig. 2b), suggesting that IL-33 induces the formation of OCs through ST2 receptors independently of RANKL/RANK in the cells. Studies with IL-1 have demonstrated that IL-1 itself cannot induce OC differentiation but can do so after ectopic overexpression of the IL-1RI in mBMMs in a RANKL/RANK-independent manner [32]. In addition, IL-1 did not induce the necessary expression of NFATc1 for osteoclastogenesis. In contrast, in our hands, IL-33 strongly stimulated expression of c-Fos and NFATc1 in CD14+ monocytes (Fig. 4a). Although IL-1/IL-1R and IL-33/ST2 pathways share some pathways in common, it is plausible that additional mechanisms exist for OC differentiation that are unique to IL-33/ST2 activation.
We should note that we were unable to detect significant expression of the ST2 receptor in mBMMs and CD68+ cells in mouse synovial tissues (data not shown), as compared to human monocytes, mast cells, and T cells (Fig. 1a, b) leaving the possibility that IL-33 may not exert the same osteoclastogenic activity in mBMMs. However, IL-33 could hypothetically stimulate OC differentiation indirectly through induction of RANKL in synovial cells and T cells in vivo by mast cell-derived cytokines such as IL-1, IL-6, and TNF-α. Consistent with this hypothesis, the level of RANKL mRNA in mouse joints is suppressed by administration of anti-ST2 antibody [22], suggesting that the IL-33/ST2 signal could increase the RANKL expression in the joints. As reported here, we find that expression of ST2 is also stimulated by RANKL in CD14+ monocytes (Fig. 4a). This and previous results strongly suggest that IL-33 and RANKL can act cooperatively to stimulate osteoclastogenesis in vivo.
The RANKL/RANK complex recruits the adaptor molecule TRAF6, which is a critical adaptor protein for differentiation and function of OCs. The complex of RANK and TRAF6 leads to activation of MAP kinases and costimulatory signaling molecules, via DAP12 and FcRγ, including Syk and PLCγ [9]. In this study, IL-33 was found to stimulate TRAF6 expression and activate many of the same signaling molecules as RANKL including the TRAF6-mediated as well as the DAP12 and FcRγ-dependent costimulatory signals that are thought to be critical for the OC differentiation and bone-resorbing effects. These included JNK, p38 MAP kinase (Fig. 3a), and the transcription factors AP-1 and NF-κB (Fig. 3b). Although it is unclear whether RANKL acts via IRAK, IL-33 does activate IRAK which, in turn, activates the NF-κB pathway [13]. Therefore IRAK may participate in the regulation of OC differentiation for which NF-κB is essential. The activation of Syk, PLCγ2, and Gab2 by IL-33 has not been previously reported but as noted previously, the activation of Syk [33] and PLCγ2 [34, 35] by RANKL occurs through recruitment of DAP12 and FcRγ [11]. We have yet to investigate whether an analogous mechanism exists for IL-33.
M-CSF is known to be a critical factor for the survival of OC progenitor cells by activating Akt and ERK1/2 [10]. We find that IL-33 also potentiates the activation of both these molecules in the presence of M-CSF (Fig. 3a). This result suggests that M-CSF and IL-33 act cooperatively in regulating survival for differentiation of CD14+ monocytes to OCs.
The expressions of typical OC markers such as TRAF6, NFATc1, c-Fos, cathepsin K, the calcitonin receptor, and c-Src were significantly increased by a stimulation of IL-33 (Fig. 4), thus providing additional evidence that IL-33 stimulated differentiation of functional OCs. The fact that IL-33 is as effective as RANKL in inducing expression of NFATc1 (Fig. 4b, c) is particularly notable in that ectopic overexpression of NFATc1 in bone marrow-derived monocytes is sufficient by itself to promote OC differentiation in RANKL-deficient monocytes [36].
Our results are consistent with the notion that IL-33 can stimulate the formation of functional OCs (Figs. 2b, 5) without RANK/RANKL in human CD14+ monocytes. Interestingly, the expression of Syk is increased by the treatment with either IL-33 or sRANKL (Fig. 4a, b). Syk plays an essential role in cytoskeletal rearrangement and bone-resorption capacity in RANKL-stimulated OCs [33, 37]. Other IL-33-inducible markers that were indicative of progression to functional OCs included c-Src, which is thought to be the kinase responsible for phosphorylating DAP12 and FcRγ [33] and cathepsin K, a potent collagenase and papain-like protease that can cause bone resorption [37]. Of note, IL-33 also induced expression of its own receptor, ST2. The enhanced expression of ST2, along with ST2-related signaling molecules such as Syk and TRAF6, could signify a progression in sensitivity to IL-33 during differentiation and maturation of OCs (Fig. 4).
Although IL-33 and RANKL operate independently and through different receptors, both utilize common signaling pathways. The parallel increase in c-Src and Syk during the period of IL-33-driven OC differentiation (Fig. 4) might be another example of common pathways as both molecules are reported to form a signaling complex with αvβ3 integrin, which enables the phosphorylation of Syk by c-Src in RANKL-stimulated cells [38]. This ternary complex in along with DAP12 and FcRγ constitute a critical signaling component for the bone-resorbing capacity of OCs.
In summary, our results identify IL-33 as a novel regulator of differentiation of functional OCs that acts independently of RANKL/RANK in human CD14+ monocytes, through canonical IL1/ST2-mediated signaling pathways. Nevertheless, IL-33 and RANKL engage similar signaling pathways. Of particular note, IL-33 also stimulates the same pathway that is normally engaged by RANKL through the RANK co-receptor-adaptor complex, DAP12/FcRγ, and that enables activation of NFATc1 via Syk and PLCγ2. In addition to the similarities in signaling events, IL-33 appears to induce the same functional outputs as RANKL in stimulating OC differentiation and bone resorption. These results point to the possible participation of IL-33 in patients with RA where markedly elevated levels of IL-33 are present in joint synovial tissues [21, 22]. The ability of IL-33 to stimulate OC formation and bone resorption could have therapeutic implications in inflammatory bone diseases.
Acknowledgments
This work was supported by the Konkuk University and partly by the Korea Science and Engineering Foundation (KOSEF) grant funded by the Korea government (MEST) (No. 2009-0077125) and the grant of the Korean Ministry of Education, Science and Technology (The Regional Core Research Program/Chungbuk BIT Research-Oriented University Consortium). Dr. Michael A. Beaven was supported by the Intramural Program of the National Heart, Lung, and Blood Institute, National Institutes of Health.
Footnotes
S. H. Mun, N. Y. Ko, and H.S. Kim equally contributed to this work.
References
- 1.McInnes IB, Schett G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat Rev Immunol. 2007;7:429–442. doi: 10.1038/nri2094. [DOI] [PubMed] [Google Scholar]
- 2.Takayanagi H. Osteoimmunology and the effects of the immune system on bone. Nat Rev Rheumatol. 2009;5:667–676. doi: 10.1038/nrrheum.2009.217. [DOI] [PubMed] [Google Scholar]
- 3.Udagawa N, Takahashi N, Akatsu T, Tanaka H, Sasaki T, Nishihara T, Koga T, Martin TJ, Suda T. Origin of osteoclasts: mature monocytes and macrophages are capable of differentiating into osteoclasts under a suitable microenvironment prepared by bone marrow-derived stromal cells. Proc Natl Acad Sci USA. 1990;87:7260–7264. doi: 10.1073/pnas.87.18.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Massey HM, Flanagan AM. Human osteoclasts derive from CD14-positive monocytes. Br J Haematol. 1999;106:167–170. doi: 10.1046/j.1365-2141.1999.01491.x. [DOI] [PubMed] [Google Scholar]
- 5.Shalhoub V, Elliott G, Chiu L, Manoukian R, Kelley M, Hawkins N, Davy E, Shimamoto G, Beck J, Kaufman SA. Characterization of osteoclast precursors in human blood. Br J Haematol. 2000;111:501–512. doi: 10.1046/j.1365-2141.2000.02379.x. [DOI] [PubMed] [Google Scholar]
- 6.Lagasse E, Weissman IL. Enforced expression of Bcl-2 in monocytes rescues macrophages and partially reverses osteopetrosis in op/op mice. Cell. 1997;89:1021–1031. doi: 10.1016/S0092-8674(00)80290-1. [DOI] [PubMed] [Google Scholar]
- 7.Kong YY, Yoshida H, Sarosi I, Tan HL, Timms E, Capparelli C, Morony S, Oliveira-dos-Santos AJ, Van G, Itie A, Khoo W, Wakeham A, Dunstan CR, Lacey DL, Mak TW, Boyle WJ, Penninger JM. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 8.Walsh MC, Kim N, Kadono Y, Rho J, Lee SY, Lorenzo J, Choi Y. Osteoimmunology: interplay between the immune system and bone metabolism. Annu Rev Immunol. 2006;24:33–63. doi: 10.1146/annurev.immunol.24.021605.090646. [DOI] [PubMed] [Google Scholar]
- 9.Takayanagi H. Osteoimmunology: shared mechanisms and crosstalk between the immune and bone systems. Nature. 2007;7:292–304. doi: 10.1038/nri2062. [DOI] [PubMed] [Google Scholar]
- 10.Gingery A, Bradley E, Shaw A, Oursler MJ. Phosphatidylinositol 3-kinase coordinately activates the MEK/ERK and AKT/NFkappaB pathways to maintain osteoclast survival. J Cell Biochem. 2003;89:165–179. doi: 10.1002/jcb.10503. [DOI] [PubMed] [Google Scholar]
- 11.Koga T, Inui M, Inoue K, Kim S, Suematsu A, Kobayashi E, Iwata T, Ohnishi H, Matozaki T, Kodama T, Taniguchi T, Takayanagi H, Takai T. Costimulatory signals mediated by the ITAM motif cooperate with RANKL for bone homeostasis. Nature. 2004;428:758–763. doi: 10.1038/nature02444. [DOI] [PubMed] [Google Scholar]
- 12.Carriere V, Roussel L, Ortega N, Lacorre DA, Americh L, Aguilar L, Bouche G, Girard JP. IL-33, the IL-1-like cytokine ligand for ST2 receptor, is a chromatin-associated nuclear factor in vivo. Proc Natl Acad Sci USA. 2007;104:282–287. doi: 10.1073/pnas.0606854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmitz J, Owyang A, Oldham E, Song Y, Murphy E, McClanahan TK, Zurawski G, Moshrefi M, Qin J, Li X, Gorman DM, Bazan JF, Kastelein RA. IL-33, an interleukin-1-like cytokine that signals via the IL-1 receptor-related protein ST2 and induces T helper type 2-associated cytokines. Immunity. 2005;23:479–490. doi: 10.1016/j.immuni.2005.09.015. [DOI] [PubMed] [Google Scholar]
- 14.Kakkar R, Lee RT. The IL-33/ST-2 pathway: therapeutic target and novel biomarker. Nat Rev Drug Discov. 2008;7:827–840. doi: 10.1038/nrd2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chackerian AA, Oldham ER, Murphy EE, Schmitz J, Pflanz S, Kastelein RA. IL-1 receptor accessory protein and ST2 comprise the IL-33 receptor complex. J Immunol. 2007;179:2551–2555. doi: 10.4049/jimmunol.179.4.2551. [DOI] [PubMed] [Google Scholar]
- 16.Gadina M, Jefferies CA. IL-33: a sheep in wolf’s clothing? Sci STKE. 2007;390:pe31. doi: 10.1126/stke.3902007pe31. [DOI] [PubMed] [Google Scholar]
- 17.Iikura M, Suto H, Kajiwara N, Oboki K, Ohno T, Okayama Y, Saito H, Galli SJ, Nakae S. IL-33 can promote survival, adhesion and cytokine production in human mast cells. Lab Investig. 2007;87:971–978. doi: 10.1038/labinvest.3700663. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya-Tsuji J, Kishimoto K, Hiyama A, Inoue J, Cao Z, Matsumoto K. The kinase TAK1 can activate the NIK-IkB as well as the MAP kinase cascade in the IL-1 signaling pathway. Nature. 1999;398:252–256. doi: 10.1038/18465. [DOI] [PubMed] [Google Scholar]
- 19.Jiang Z, Ninomiya-Tsuji J, Qian Y, Matsumoto K, Li X. Interleukin-1 (IL-1) receptor-associated kinase-dependent IL-1-induced signaling complexes phosphorylate TAK1 and TAB 2 at the plasma membrane and activate TAK1 in the cytosol. Mol Cell Biol. 2002;22:7158–7167. doi: 10.1128/MCB.22.20.7158-7167.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shim JH, Xiao C, Paschal AE, Bailey ST, Rao P, Hayden MS, Lee KY, Bussey C, Steckel M, Tanaka N, Yamada G, Akira S, Matsumoto K, Ghosh S. TAK1, but not TAB 1 or TAB 2, plays an essential role in multiple signaling pathways in vivo. Genes Dev. 2005;19:2668–2681. doi: 10.1101/gad.1360605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu D, Jiang HR, Kewin P, Li Y, Mu R, Fraser AR, Pitman N, Kurowska-Stolarska M, McKenzie AN, McInnes IB, Liew FY. IL-33 exacerbates antigen-induced arthritis by activating mast cells. Proc Natl Acad Sci USA. 2008;105:10913–10918. doi: 10.1073/pnas.0801898105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Palmer G, Talabot-Ayer D, Lamacchia C, Toy D, Seemayer CA, Viatte S, Finckh A, Smith DE, Gabay C. Inhibition of interleukin-33 signaling attenuates the severity of experimental arthritis. Arthritis Rheum. 2009;60:738–749. doi: 10.1002/art.24305. [DOI] [PubMed] [Google Scholar]
- 23.Lee CK, Lee EY, Chung SM, Mun SH, Yoo B, Moon HB. Effects of disease-modifying antirheumatic drugs and antiinflammatory cytokines on human osteoclastogenesis through interaction with receptor activator of nuclear factor kappaB, osteoprotegerin, and receptor activator of nuclear factor kappaB ligand. Arthritis Rheum. 2004;50:3831–3843. doi: 10.1002/art.20637. [DOI] [PubMed] [Google Scholar]
- 24.Simonet WS, Lacey DL, Dunstan CR, Kelley M, Chang MS, Lüthy R, Nguyen HQ, Wooden S, Bennett L, Boone T, Shimamoto G, DeRose M, Elliott R, Colombero A, Tan HL, Trail G, Sullivan J, Davy E, Bucay N, Renshaw-Gegg L, Hughes TM, Hill D, Pattison W, Campbell P, Sander S, Van G, Tarpley J, Derby P, Lee R, Boyle WJ. Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell. 1997;89:309–319. doi: 10.1016/S0092-8674(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 25.Hofbauer LC, Schoppet M. Clinical implications of the osteoprotegerin/RANKL/RANK system for bone and vascular diseases. JAMA. 2004;292:490–495. doi: 10.1001/jama.292.4.490. [DOI] [PubMed] [Google Scholar]
- 26.Crotti TN, Smith MD, Weedon H, Ahern MJ, Findlay DM, Kraan M, Tak PP, Haynes DR. Receptor activator NF-B ligand (RANKL) expression in synovial tissue from patients with rheumatoid arthritis, spondyloarthropathy, osteoarthritis, and from normal patients: semiquantitative and quantitative analysis. Ann Rheum Dis. 2002;61:1047–1054. doi: 10.1136/ard.61.12.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi K, Takahashi N, Jimi E, Udagawa N, Takami M, Kotake S, Nakagawa N, Kinosaki M, Yamaguchi K, Shima N, Yasuda H, Morinaga T, Higashio K, Martin TJ, Suda T. Tumor necrosis factor alpha stimulates osteoclast differentiation by a mechanism independent of the ODF/RANKL-RANK interaction. J Exp Med. 2000;191:275–286. doi: 10.1084/jem.191.2.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Edwards JR, Sun SG, Locklin R, Shipman CM, Adamopoulos IE, Athanasou NA, Sabokbar A. LIGHT (TNFSF14), a novel mediator of bone resorption, is elevated in rheumatoid arthritis. Arthritis Rheum. 2006;54:1451–1462. doi: 10.1002/art.21821. [DOI] [PubMed] [Google Scholar]
- 29.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/S8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 30.Rifas L, Weitzmann MN. A novel T cell cytokine, secreted osteoclastogenic factor of activated T cells, induces osteoclast formation in a RANKL-Independent manner. Arthritis Rheum. 2009;60:3324–3335. doi: 10.1002/art.24877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu D, Chan WL, Leung BP, Huang F, Wheeler R, Piedrafita D, Robinson JH, Liew FY. Selective expression of a stable cell surface molecule on type 2 but not type 1 helper T cells. J Exp Med. 1998;187:787–794. doi: 10.1084/jem.187.5.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JH, Jin HM, Kim K, Song I, Youn BU, Matsuo K, Kim N. The mechanism of osteoclast differentiation induced by IL-1. J Immunol. 2009;183:1862–1870. doi: 10.4049/jimmunol.0803007. [DOI] [PubMed] [Google Scholar]
- 33.Mócsai A, Humphrey MB, Van Ziffle JA, Hu Y, Burghardt A, Spusta SC, Majumdar S, Lanier LL, Lowell CA, Nakamura MC. The immunomodulatory adapter proteins DAP12 and Fc receptor gamma-chain (FcRgamma) regulate development of functional osteoclasts through the Syk tyrosine kinase. Proc Natl Acad Sci USA. 2004;101:6158–6163. doi: 10.1073/pnas.0401602101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao D, Epple H, Uthgenannt B, Novack DV, Faccio R. PLCgamma2 regulates osteoclastogenesis via its interaction with ITAM proteins and GAB2. J Clin Investig. 2006;116:2869–2879. doi: 10.1172/JCI28775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shinohara M, Koga T, Okamoto K, Sakaguchi S, Arai K, Yasuda H, Takai T, Kodama T, Morio T, Geha RS, Kitamura D, Kurosaki T, Ellmeier W, Takayanagi H. Tyrosine kinases Btk and Tec regulate osteoclast differentiation by linking RANK and ITAM signals. Cell. 2008;132:794–806. doi: 10.1016/j.cell.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 36.Takayanagi H, Kim S, Koga T, Nishina H, Isshiki M, Yoshida H, Saiura A, Isobe M, Yokochi T, Inoue J, Wagner EF, Mak TW, Kodama T, Taniguchi T. Induction and activation of the transcription factor NFATc1 (NFAT2) integrate RANKL signaling in terminal differentiation of osteoclasts. Dev Cell. 2002;3:889–901. doi: 10.1016/S1534-5807(02)00369-6. [DOI] [PubMed] [Google Scholar]
- 37.Zou W, Kitaura H, Reeve J, Long F, Tybulewicz VL, Shattil SJ, Ginsberg MH, Ross FP, Teitelbaum SL. Syk, c-Src, the alphavbeta3 integrin, and ITAM immunoreceptors, in concert, regulate osteoclastic bone resorption. J Cell Biol. 2007;176:877–888. doi: 10.1083/jcb.200611083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao Q, Jia Y, Xiao Y. Cathepsin K: a therapeutic target for bone diseases. Biochem Biophys Res Commun. 2009;380:721–723. doi: 10.1016/j.bbrc.2009.01.139. [DOI] [PubMed] [Google Scholar]