Abstract
The mammalian Golgi complex is a highly dynamic organelle consisting of stacks of flattened cisternae with associated coated vesicles and membrane tubules that contribute to cargo import and export, intra-cisternal trafficking, and overall Golgi architecture. At the morphological level, all of these structures are continuously remodeled to carry out these trafficking functions. Recent advances have shown that continual phospholipid remodeling by phospholipase A (PLA) and lysophospholipid acyltransferase (LPAT) enzymes, which deacylate and reacylate Golgi phospholipids, respectively, contributes to this morphological remodeling. Here we review the identification and characterization of four cytoplasmic PLA enzymes and one integral membrane LPAT that participate in the dynamic functional organization of the Golgi complex, and how some of these enzymes are integrated to determine the relative abundance of COPI vesicle and membrane tubule formation.
Keywords: Golgi complex, Lands cycle, cytoplasmic PLA2, Lysophospholipid acyltransferases, COPI coated vesicles, membrane tubules
Introduction
The mammalian Golgi complex is an iconic structure consisting of stacks of flattened membrane cisternae that are dynamically modified by the formation of coated vesicles and membrane tubules [1–5]. In addition, the cisternal stacks are often bridged together by membrane tubules into large ribbon-like structures, often forming a single interconnected organelle [6, 7]. The coated vesicles and membrane tubules mediate cargo import and export events, which by their combined activities help to shape the architecture and polarity of the Golgi complex, and contribute to bidirectional trafficking of cargo across the cisternal stacks. Although numerous structural, scaffold, fusion, and tethering proteins, e.g., COPI and AP-1 clathrin coats, GRASP, SNARE proteins, etc., have been shown to be crucial elements that regulate the functional organization of the Golgi complex, many fundamental questions about how the Golgi works remain unanswered, e.g., how secretory proteins move from the cis to the trans side of the Golgi stack [8]. Our lack of understanding is primarily due to the integrated and dynamic nature of the Golgi membranes, which makes it difficult to alter only a single aspect of Golgi trafficking without affecting every other. Recently, the dynamic functional organization of the mammalian Golgi complex has been reported to involve continual phospholipid remodeling by the opposing actions of phospholipase A (PLA) and lysophospholipid acyltransferase (LPAT) enzymes, a process in which acyl chains are continually removed and reincorporated (Fig. 1) [9, 10]. In other words, dynamic remodeling of Golgi membrane phospholipids is thought to play a role in shaping the structure of the Golgi complex itself. This review will focus on how these recently identified enzymes contribute to the dynamic nature of the mammalian Golgi complex.
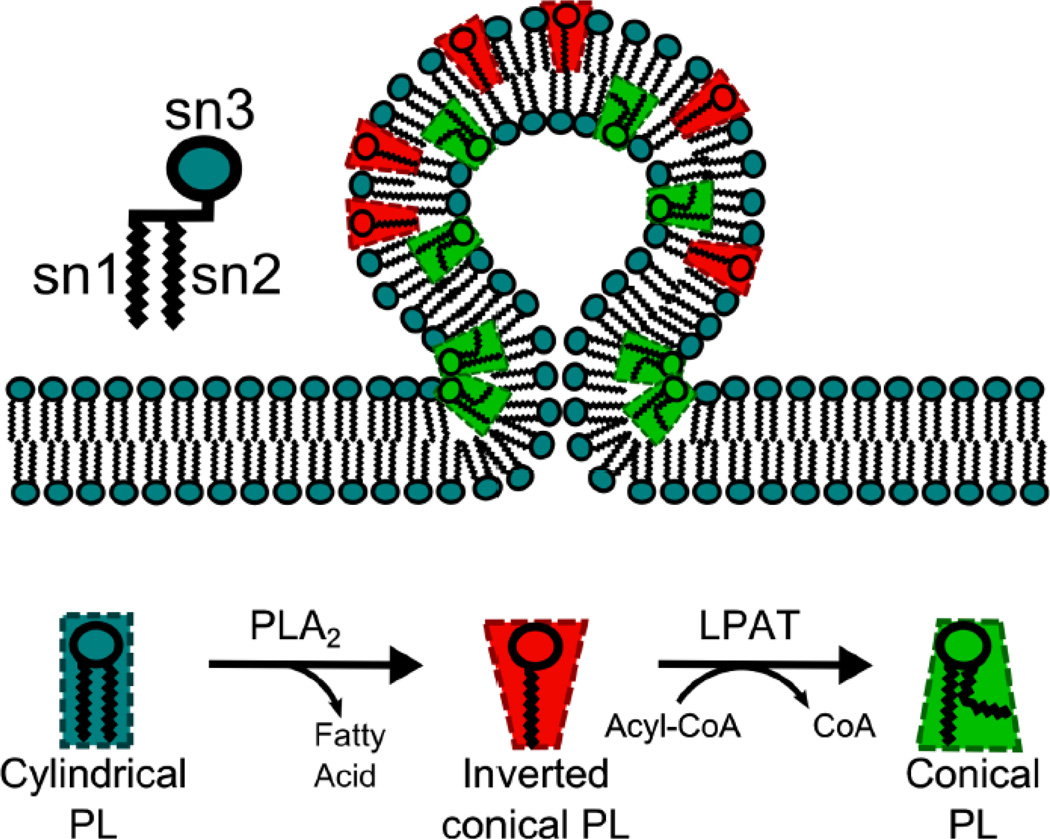
Figure 1.
A simple model of Lands cycle phospholipid remodeling by PLA2 and LPAT enzymes and how their phospholipid products can generate membrane curvature based on their physical shapes.
In the 1960’s Lands and colleagues established that after their synthesis, glycerol phospholipid acyl chains turnover in a cycle of deacylation and reacylation catalyzed by the opposing actions of PLA and LPAT enzymes, respectively, in what is now called the Lands cycle (Fig. 1) [11, 12]. The turnover of acyl chains serves to remodel phospholipids and thus alter their functional properties, although the full extent of the Lands cycle role in cells is unclear. Nevertheless, several functions of the Lands cycle have been established including the incorporation and release of arachidonic acid (AA) for the production of eicosanoids and prostaglandins [13], production of platelet activating factor (PAF) [14–16], and the incorporation of host-derived fatty acids into membrane phospholipids in Giardia and other intracellular parasites [17]. Here we describe recent studies which suggest that Lands cycle phospholipid remodeling might regulate the functional organization of the Golgi complex.
The mammalian Golgi complex, including the ER-Golgi-Intermediate Compartment (ERGIC) and trans-Golgi Network (TGN), produces various types of membrane cargo transporters such as COPI and AP-1 clathrin coated vesicles, regulated secretory granules, and constitutive secretory vesicles, whose function and biogenesis have been extensively studied (Fig. 2) [18–22]. For example, COPI coated vesicles mediate retrograde trafficking from the Golgi complex and ERGIC, whereas AP-1 coated vesicles mediate the sorting, packaging, and transport of newly synthesized lysosomal enzymes from the TGN to endosomes [19, 22, 23]. Membrane tubules of uniform diameter (60–80 nm) have also been observed to emanate from different regions of the Golgi complex (Fig. 2), but in contrast to coated vesicles, much less is known about their functions, and indeed, even their existence or prevalence under normal conditions is a matter of debate [4, 8, 24]. For example, although the ERGIC and TGN exhibit extensive membrane tubulation, the cisternal membranes of the Golgi stack proper generally have few tubules. When viewed by 3-D tomographic reconstructions, membrane tubules are observed to link cisternal stacks into intact ribbons, but they are often not prominently seen in static images to extend out into the cytoplasm [4, 25–28]. Rather, Golgi membrane tubules are most readily observed when coated vesicle formation is inhibited or when GFP-tagged proteins are overexpressed for live cell imaging experiments [29–33], leading to concerns that they are only seen under unusual conditions. However, recent studies have revived the idea that COPI (and perhaps AP-1) coated vesicles are intimately linked to the formation of membrane tubules [34], and that membrane tubules can perform trafficking functions independent of coated vesicles, both of which utilize Golgi-associated PLA2 and LPAT remodeling enzymes [9].
Figure 2.
Membrane trafficking pathways from and within the mammalian Golgi complex. Several studies have recently reported that four cytoplasmic PLA enzymes are associated with the Golgi complex and regulate trafficking in various ways. As shown in Step 1, the cytoplasmic PLA2 enzyme PAFAHIb induces membrane tubules from the Golgi complex that contribute to the formation of an intact ribbon structure. In addition, PAFAHIb also appears to influence anterograde trafficking from the TGN (Step 2). A second cytoplasmic enzyme, cPLA2α, mediates the formation of intra-cisternal membrane tubules that appear to facilitate anterograde transport when the Golgi complex receives a large bolus of secretory cargo (Step 3). In addition, cPLA2 may also negatively regulate COPI coated vesicle formation (Fig. 2, Step 4), perhaps by shifting the balance toward membrane tubule formation. A third cytoplasmic PLA2, PLA2G6, was shown to mediate the formation of membrane tubules from the ERGIC, which may be important for connecting regions involved in COPI vesicle budding (Step 5). The final PLA enzyme recently found associated with ERGIC and Golgi membranes is iPLA1γ, which appears to contribute to anterograde transport through the Golgi complex by an unknown mechanism.
The relationship between Golgi complex coated vesicles and membrane tubules has puzzled cell biologists since the discovery of the remarkable properties of brefeldin A (BFA) [30, 33, 35–37]. BFA is an inhibitor of guanine nucleotide exchange factors (GEFs) for certain ADP-ribosylation factor (Arf) GTPases [38–40], which prevents the recruitment of COPI coatomer and AP-1 subunits to Golgi and TGN membranes and the formation of COPI and AP-1 coated vesicles [38, 41, 42]. As a consequence, Golgi and TGN membranes form extensive numbers of membrane tubules [30, 33, 36, 37, 43]. Interestingly, BFA-induced membrane tubules move in the same directions as the coated vesicles that would normally form, i.e., retrograde back to the ER and anterograde from the TGN to endosomes. These studies suggested that BFA-induced membrane tubules are exaggerated forms of membrane protrusions that would normally be used to produce coated vesicles [35]. These original observations on the effects of BFA were followed by a period when little progress was made on understanding the exact nature of Golgi membrane tubules. More recently, however, several studies have shown that RNAi knockdown of Golgi Arfs [44], some of their GEFs such as GBF1 [45], and AP-1-associated GGA1 (Golgi-localized, γ ear-containing, ADP ribosylation factor-binding) [46] also induced membrane tubule formation. These results are consistent with original ideas about the BFA effect, which are that membrane tubules reflect an inherent capacity of Golgi membranes to generate curvature, which if not used to make COPI or AP-1 coated vesicles, will continue to form abnormally long membrane tubules [35, 47]. Thus, the Golgi complex has an underlying COPI- and AP-1-independent mechanism capable of initiating membrane curvature, which recent studies report include PLA2 enzymes that associate with the cytoplasmic side of Golgi membranes.
Although COPI vesicle formation has been extensively examined, there is no clear consensus on the exact molecular mechanisms [22, 48]. A variety of in vitro reconstitution studies have shown that COPI vesicles can be produced on artificial liposomes with purified coatomer subunits and Arf1-GTP [19, 48–50], indicating that these components represent the minimal machinery required for COPI vesicles biogenesis. In particular, Arf1-GTP, which is required for recruiting the coatomer subunits for vesicle budding, has also been reported to be sufficient to mediate the fission step for release of a free vesicle [50]. Arf1 could induce membrane fission by virtue of its energetically favorable interaction with positively curved membranes and its unfavorable interaction with the negatively curved bud neck, which makes resolution of the fission event energetically attractive [48]. In addition to this minimal system, a variety of other proteins have been reported to participate in COPI vesicle fission. For example, incubation with ArfGAP1, phospholipase D (PLD), and brefeldin A-ribosylated substrate (BARS) have been shown stimulate the completion of membrane fission [51–55]. It is not clear how ArfGAP1 promotes budding or if ArfGAP1 is a stoichiometric component of the budded vesicle because it was recently reported that in vitro COPI vesiculation in the presence of GTP but lacking Arf GEFs is inhibited by enzymatic concentrations of ArfGAP1 [56]. The Arf1-GTP stimulated action of PLD has also been reported to contribute to vesicle fission by hydrolyzing phosphatidylcholine (PC) to phosphatidic acid (PA), which could aid in producing negative curvature and recruiting BARS at the bud neck [57–59]. Although BARS and PLD may not be required in the minimal machinery for COPI vesicle formation, they could enhance the efficiency of COPI budding in vivo (discussed further below).
PLA and LPAT Enzymes Remodel Phospholipids and the Golgi Complex
PLA enzymes cleave acyl chains from phospholipids to yield a lysophospholipid (LPL) and a free fatty acid (Fig. 1). PLA enzymes are subdivided by their specificity to the sn-1 (PLA1) and sn-2 (PLA2) position of phospholipids; some cross-specificity has been observed [60]. Nine PLA1 enzymes have been identified in mammals, six of which are extracellular proteins of the pancreatic lipase family; the remaining are intracellular lipases unrelated to pancreatic lipase [61]. The mammalian PLA2 superfamily comprises 14 groups of enzymes (Group I, II, etc.), which are distinguished by their cellular locations, substrate specificities, and Ca2+ dependence [13]. The sn-2 position of phospholipids in mammalian cells can be enriched in AA, which plays critical roles in many signal transduction pathways ranging from inflammation to mitogenesis [62]. Indeed, the diverse PLA2 superfamily plays important roles in myriad physiological processes such as reproduction, inflammation, heart disease, Alzheimer’s disease, and cancer [63–65]. Although most current literature primarily cite PLA2 enzymes for their roles in inflammation, support for other functions is provided by recent studies showing that cytoplasmic PLA2 enzymes regulate membrane trafficking.
The first indication that cytoplasmic PLA2 enzymes play a role in vesicle and membrane tubule biogenesis came from studies of pharmacological PLA2 inhibitors in cultured mammalian cells [9, 66, 67]. PLA2 inhibitors block intra-Golgi protein trafficking in cell-free reconstitution systems, thus implicating the requirement for PLA2 activity at the Golgi complex [68]. More specifically, cytoplasmic PLA2 enzymes appear to play critical roles in forming membrane tubules, as inhibitory PLA2 drugs block BFA-induced membrane tubulation and trafficking in these organelles [67, 69]. Conversely, PLA2 stimulating peptides melittin and a peptide from a mammalian protein containing a melittin homology doman, PLA2 activating protein peptide (PLAAp), enhance cytosol-dependent Golgi membrane tubulation in vitro [70], possibly by embedding within the hydrocarbon region of membranes to facilitate PLA2 activity. A potential link between the catalytic lipase activities of PLA2 enzymes and membrane tubulation is supported by a hypothesis that the conversion of cylindrically-shaped phospholipids to conicallyshaped products in local regions of a single leaflet of a lipid bilayer forces the membrane to adopt a curved structure, thus facilitating the formation of membrane tubules (Fig. 1) [66]. Indeed, diverse lipid components alone can induce membrane curvature as observed in fluorescent, giant unilamellar vesicles [71].
In the context of the Lands cycle, LPATs oppose the action of PLA enzymes (Fig. 1). LPATs catalyze the transfer of a fatty acid from an acyl-CoA donor to a LPL, most commonly at the sn-2 position, to yield a phospholipid. Mammalian LPATs comprise two gene families of transmembrane proteins, 1-acylglycerol-3-phosphate acyltransferase (AGPAT) and membrane bound O-acyltransferases (MBOAT) [72–74]. The catalytic activities and substrate specificities of their gene products are not entirely characterized; however, 14 or more members are expected to have more than nominal LPAT activity. These enzymes are also categorized, and sometimes named, according to substrate preferences, e.g., enzymes that produce PA from lysophosphatidic acid (LPA) are referred to as LPAATs; enzymes that produce PC are referred to as LPCATs [75]. Most LPATs are ER or mitochondria localized; AGPAT3/LPAAT3 is exceptional and also localizes to the Golgi complex [76].
The individual activities of PLA and LPAT enzymes to remodel membrane lipids has been extensively studied in terms of lipid homeostasis, inflammation, and signal transduction. Emerging evidence, from multiple independent studies, support an additional role for lipid remodeling in membrane trafficking of the Golgi complex, as well as other organelles. The exact molecular mechanisms responsible for LPAT mediated trafficking are still unknown. One possibility is that the interconversion of phospholipids and LPLs confers negative and positive curvature respectively, directly to membranes. This curvature promotes the formation of tubular and/or vesicular membrane carriers (Figs. 3 and 4) [66, 71, 77]. Another possibility is that compartment-specific PLA and LPAT activity produces a concentration gradient of lipid species that recruit effector proteins, such as COPI and clathrin coats (discussed further below). This recruitment model has many parallels to the extensively studied phosphoinositide phosphate (PIP) remodeling system [78]. It should be noted that lipid curvature and effector recruitment models are not mutually exclusive.
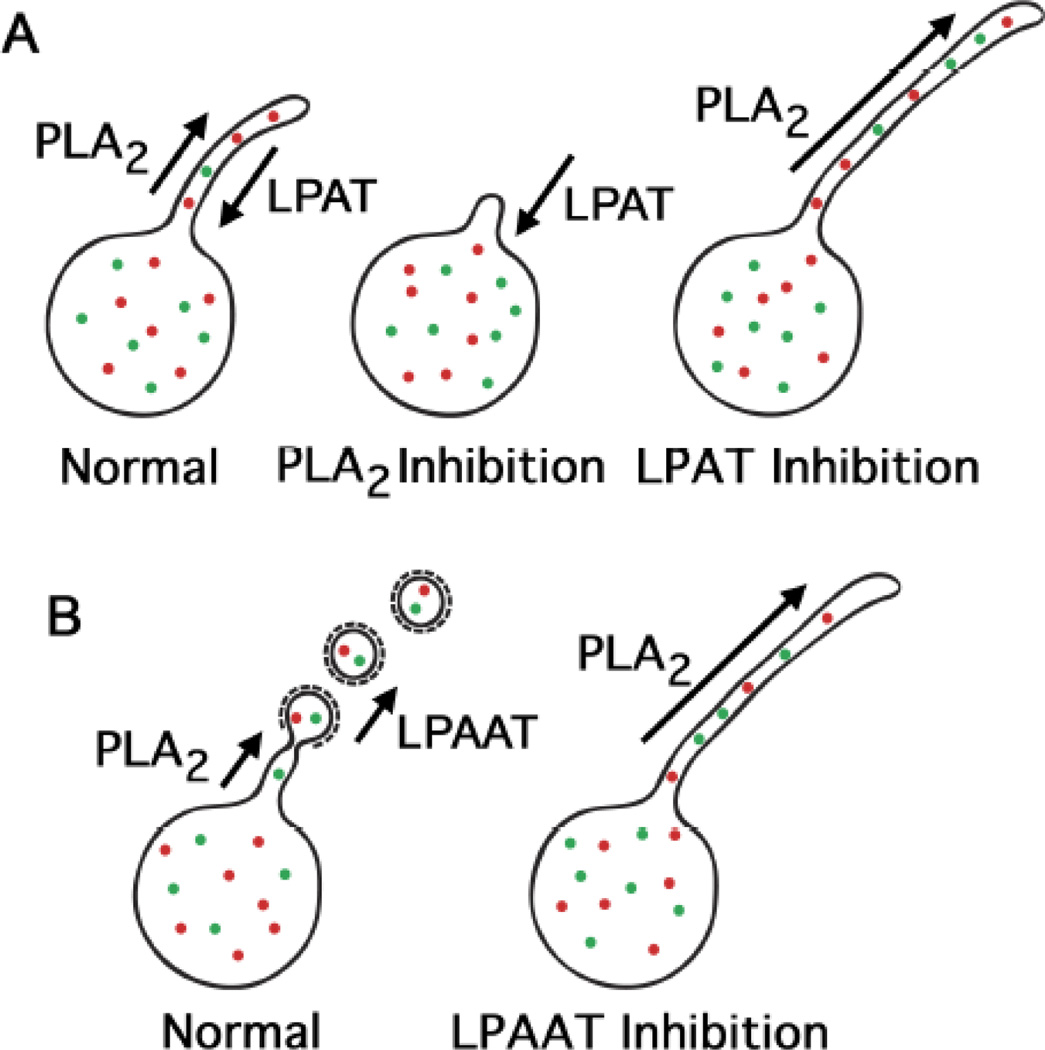
Figure 3.
Possible routes for the individual and concerted actions of PLA2 and LPAT enzymes to influence the formation of membrane tubules and coated vesicles. (A) Membrane tubules could be generated and regulated by the opposing actions of PLA2 and LPAT enzymes. Hydrolysis of membrane phospholipids by cytoplasmic PLA2 enzymes could generate positive curvature inducing LPLs, ultimately resulting membrane tubule formation. The LPLs could be reacylated by LPAT enzymes back to phospholipids, thus negatively regulating the formation of membrane tubules. (B) The hydrolytic activity of cytoplasmic PLA2 enzymes could generate membrane tubules that are consumed through coated vesicle budding, which is facilitated by LPAAT activity for membrane fission. In this generic model, the coated vesicles could be either COPI vesicles budding from the ERGIC or AP-1 clathrin coated vesicles budding from the TGN. The consequences of inhibition by PLA2 and LPAT antagonists are shown, which have been supported by several studies (9, 66).
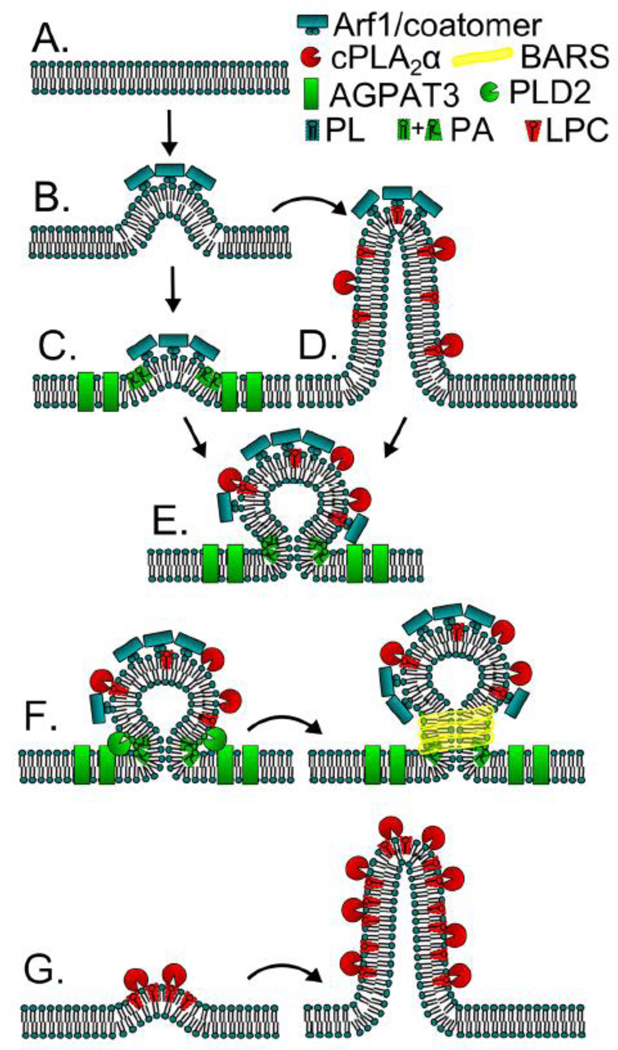
Figure 4.
Models for cPLA2α and AGPAT3/LPAAT3 activities contributing to COPI vesicle and Golgi membrane tubule formation. (A) Flat membrane that will be modified by curve producing proteins; (B) Arf1 and coatomer binding deforms membranes, initiating the first steps of vesiculation or tubulation; (B to C) AGPAT3/LPAAT3 activity produces unsaturated PA, a lipid that resists positive curvature; (B to D) cPLA2α activity produces LPC, a positive curvature stabilizing and tubule inducing lipid; (C and D to E) LPC stabilizes positive curvature of the bud and unsaturated PA stabilizes negative curvature at the bud neck; (F) The concerted PA production activities of AGPAT3/LPAAT3 and PLD2 at the bud neck help to recruit BARS and aide vesicle fission; (G) PLA2 activity can induce membrane curvature and tubulation in the absence of Arf1 and coatomer.
The localized synthesis, remodeling, and selective partitioning of phospholipids, as well as cholesterol, sphingolipids, and plasmalogens throughout the secretory system create tremendous potential for membrane heterogeneity and complexity [79]. The maintenance of a gradient of lipid species between membrane compartments is crucial to and assisted by the selective partitioning of protein and lipid cargoes into anterograde- and retrograde-bound vesicles and membrane tubules [80]. Curved membranes tend to exclude sphingolipids and are enriched in phospholipids. For instance, in vitro reconstitution of COPI budding has shown that phase transition of phospholipid enriched domains is prevented at bud sites [49]. Phospholipid transfer proteins, flippases, PIPs, and, DAG also play important roles in the dynamic organization of the Golgi complex but are beyond the scope of this review [55, 78, 81–87].
Adding to this cast of phospholipid remodelers, the past two years have seen the identification of four specific cytoplasmic PLA and one integral membrane LPAT enzyme that play roles in the dynamic functional organization of the Golgi complex (Table). Although these enzymes can individually catalyze changes in membrane phospholipids, it has not yet been directly demonstrated that any pair of PLA and LPAT enzymes are functionally coupled in a Lands cycle of phospholipid remodeling. In any case, it is likely that these discoveries herald many more reports of PLA and LPAT enzyme involvement in Golgi membrane trafficking, and other compartments of the secretory and endocytic pathways.
Table 1.
of Golgi-Remodeling Enzymes.
| Class | Gene Name | Features |
|---|---|---|
| PLA Enzymes | PAFAHIb | Has two Ca2+-independent PLA2 subunits, potentially links membrane tubules to microtubules, affects Golgi structure and trafficking, also regulates endocytic recycling |
| cPLA2α | Ca2+-dependent PLA2, maintains Golgi structure, provides intra-Golgi connections | |
| PLA2G6/iPLA2-β | A cytoplasmic PLA2 associated with the ERGIC and regulates it structure/function | |
| iPLA1γ | A cytoplasmic PLA1 enzyme located to cis/medial Golgi cisternal and influences anterograde trafficking | |
| LPATs | AGPAT3/LPAAT3 | An integral membrane LPAT of both the ER and Golgi complex that regulates both Golgi structure and trafficking |
PAFAHIb
One of the first PLA2 members discovered to be directly involved in membrane tubulation and trafficking are the two members of Group VIII PLA2s, also known as platelet activating factor acetylhydrolase (PAFAH) Ib 2 and 3. These two PLA2 enzymes, called α1 (gene name PAFAHIb2) and α2 (gene name PAFAHIb3), are Ca2+-independent and may form either homo- or hetero-dimers to form a catalytic pocket for the phospholipid substrate (Table) [88]. Both subunits within the dimer harbor a conserved GXSXG sequence and contribute critical residues to the catalytic pocket, where the loss of the serine is sufficient to abolish the lipase activity [89]. The catalytic dimers also associate with a non-catalytic, regulatory β-subunit also known as Lis1 (gene name PAFAHIb1), which is the causative gene for a neurodegenerative disorder called Miller-Dieker lissencephaly [90]. Lis1 is better known for its role in regulating dynein-mediated, microtubule-dependent transport [91, 92]. Lis1 appears to inhibit minus-end directed movement of dynein, and with the help of kinesin, the Lis1/dynein complex undergoes anterograde transport to the plus-end of microtubules, where the dynein-associated proteins, Nde1 or Ndel1, bind to the Lis1/dynein complex and mediate cargo transport back towards the minus ends of microtubules [93]. Nde1 and Ndel1 thus permit the compact accumulation of Lis1 in a juxtanuclear region where the minus ends of microtubules converge near centrosomes and indeed, mouse cells lacking Ndel1 show a cytoplasmic dispersed distribution of Lis1 [94]. Thus, Lis1 has dual, but not completely separate, roles in Golgi membrane dynamics via PAFAHIb-dependent phospholipid modification and dynein-mediated, microtubule-dependent transport.
Interestingly, the various possible combinations of catalytic dimers between α1 and α2 exhibit different substrate specificities and are differentially regulated by Lis1 [95]. Differences between α1 and α2 are punctuated by the observation that in rats, α1 is only expressed in developing, neonatal neurons and α2 is conversely expressed in both neo- and post-natal stages and is not limited to neural tissues [96].
All three members of the PAFAHIb group contribute to the maintenance of Golgi architecture (Fig. 2, steps 1 and 2) [97]. This was first shown when biochemical fractionation of bovine brain cytosol identified all three subunits as cofractionating with PLA2 activity and Golgi membrane tubulation in an in vitro reconstitution assay. Importantly, purified catalytically active, but not inactive α1 and α2 can induce Golgi membrane tubulation in vitro, and both α1 and α2 partially localize to the Golgi cisternae and the TGN in vivo. PAFAHIb α1 and α2 recruitment to Golgi membranes may be PIP dependent, as α1 appears to have a strong affinity for PI(4)P in protein-lipid overlay assays [98], and PI(4)P is known to be enriched within Golgi membranes [78]. Knockdown and over-expression studies demonstrated that both Lis1 binding and the PLA2 activities of α1 and α2 are important for regulating Golgi structure and function. For example, knockdown of α1 and α2 caused the Golgi ribbon to fragment into separated mini-stacks, which could result from both loss of PLA2 activity that is required to generate membrane tubules and Lis1-regulated, dynein-mediated movement of Golgi mini-stacks to the cell center [99, 100]. This idea is supported by the finding that Golgi mini-stacks produced following Lis1 knockdown are still able to generate membrane tubules, whereas mini-stacks in α1/α2 knockdown cells cannot [97]. Likewise, the loss of either Nde1 or Ndel1 also leads to the dispersal of the Golgi complex [101]. Although it is tempting to hypothesize that a compact, juxtanuclear Golgi structure can be achieved by α1/α2 establishing a link between Golgi membrane tubules and Lis1/dynein/Nde1/Ndel1, other reports demonstrate that α1/α2 directly compete with Ndel1 and dynein for Lis1 binding (Fig. 5) [102]. Indeed, Lis1 undergoes stark, conformational changes when it switches from a complex with a α2/α2 homodimer to one with Ndel1 [103].
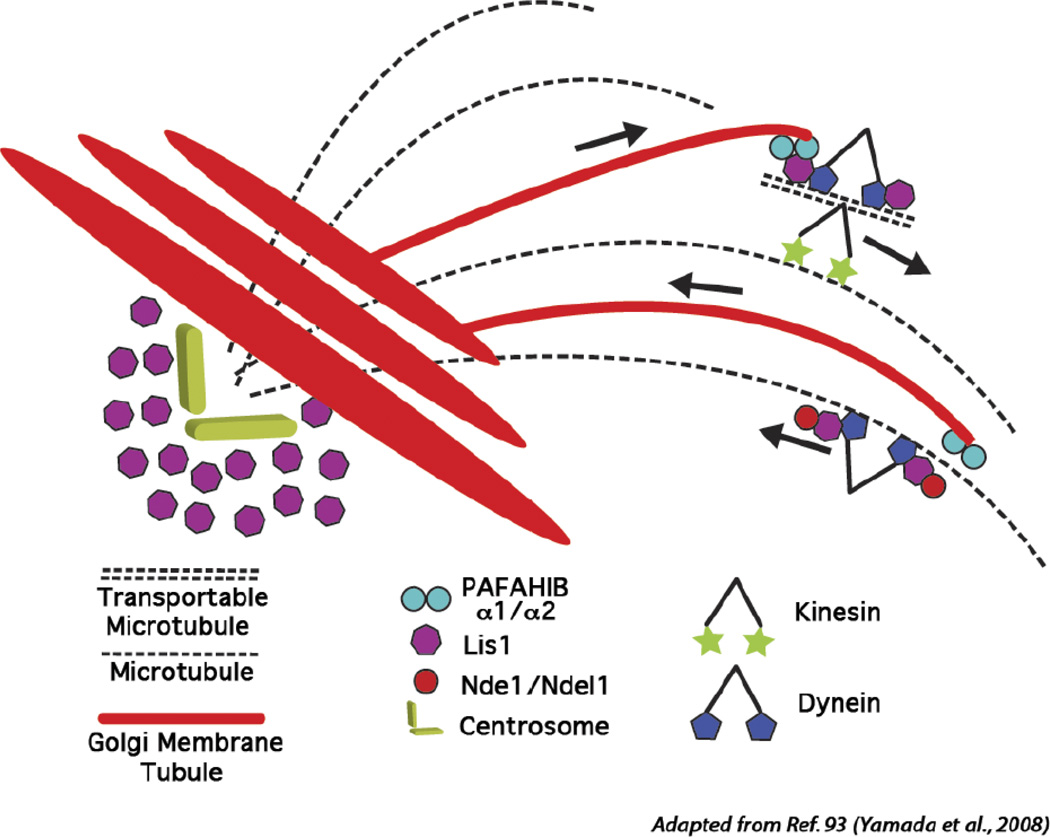
Figure 5.
Model integrating membrane curvature produced by the PLA2 activity of PAFAHIb α1 and α2 with Lis1-mediated dynein transport along microtubules. PAFAHIb initiates outward membrane curvature to generate a membrane tubule, which can be pulled/extended along microtubules (MT) by Lis1 and Ndel interactions with dynein. Dynein may be able to carry the membrane tubule towards the minus end of microtubules, facilitating the convergence of the Golgi stack and positioning at the microtubule organizing center (MTOC, minus end of the microtubules).
In addition to regulating membrane tubulation from the Golgi complex, PAFAHIb has been shown to have similar activity on early endosomes and the endocytic recycling compartment [98]. Moreover, PAFAHIb contributes to efficient endocytic recycling of transferrin and transferrin receptors. Thus, PAFAHIb functions at multiple intracellular compartments [9].
The discovery that PAFAHIb regulates Golgi structure and function is somewhat surprising given that it has a very strong substrate preference for PAF, which has an acetyl group in the sn-2 position and is best known as a potent extracellular mediator of platelet activation and inflammation [104, 105]. How then could PAFAHIb be involved in Golgi dynamics? First, PAFAHIb is a cytoplasmic enzyme, which due to its location is unavailable to hydrolyze extracellular PAF, and, moreover, mice knockout studies show that it is not the key enzyme responsible for turnover of extracellular PAF, a function most likely carried out by an unrelated, secreted PAFAH [104–107]. Second, PAFs possibly have other roles in cells because they are ubiquitously found in intracellular membranes, including those of Saccharomyces cerevisiae [108]. The relevant question for Golgi structure/function is how can PAFAHIb change membrane shape since PAF is structurally similar to LPLs? One possibility is that in vivo PAFAHIb hydrolyzes unidentified substrates. Another possibility is that PAF is nevertheless sufficiently different from LPLs to help generate outward membrane curvature when hydrolyzed on the cytoplasmic leaflet of Golgi membranes. Reconstitution experiments using defined phospholipid compositions will be required to definitively address these issues.
cPLA2α
Another PLA2 member, a cytoplasmic Group IV PLA2 named cPLA2α (gene name PLA2G4A) also exerts effects on membrane dynamics at the Golgi complex (Table). cPLA2α is one of the most studied PLA2 members for its roles in the generation of AA and its subsequent signaling pathways [109]. cPLA2α possess a C2 domain that binds to intracellular Ca2+, leading to its translocation from the cytosol to both ER and Golgi membranes [110, 111], where it regulates both Golgi structure and membrane trafficking.
Similar to the membrane tubulating activities of PAFAHIb, San Pietro et al. found that cPLA2α is capable of forming membrane tubules between Golgi cisternae [112], which provide direct continuities between cis, medial, and trans Golgi cisternae for anterograde intra-Golgi protein and membrane transport (Fig. 2, Step 3) [27, 28, 113]. These intra-cisternal membrane tubules are induced during times of rapidly increased secretory load [114], but it is unclear how stable they are and if they function under other conditions. Indeed, the same study goes on to demonstrate that cells from cPLA2α knockout mice are still capable of transporting VSV from the ER through the Golgi complex to the plasma membrane but the protein becomes sequestered within the Golgi when PAFAHIb α1 is knocked down. This clearly establishes redundancy of multiple PLA2 members in membrane trafficking. However, it remains possible that cPLA2α may serve to provide distinct trafficking and sorting services than that of PAFAHIb, and this is demonstrated through experiments showing that cPLA2α is specifically involved in transport of various transmembrane junction proteins such as VE-cadherin, occludin, and claudin-5 from the Golgi complex to cell-cell contacts [115]. Also, in Purkinje neurons, cPLA2α relies on Ca2+ influxes to translocate from the cytosol to the Golgi in order to regulate AMPA receptor levels at the plasma membrane [116]. Finally, other recent studies have provided evidence that cPLA2, in essence, negatively regulates COPI coated vesicle formation (Fig. 2, Step 4), likely by shifting the balance toward membrane tubule formation (discussed further below) [34].
Overexpression of cPLA2α in kidney epithelial cells leads to fragmentation of the Golgi ribbon and prevents anterograde transport as protein cargo sequesters within the ER, while leaving the cytoskeletal and microtubule networks intact [117]. It is possible that membrane tubules formed by cPLA2α may serve as precursors to membrane vesicles and this claim is buttressed by observations that cholesterol-induced vesiculation of the Golgi membranes requires cPLA2α recruitment to the Golgi and that this is inhibited by the cPLA2 inhibitor, methyl arachidonyl fluorophosphonate (MAFP) [118]. Interestingly, this same report goes on to show that this vesiculation is independent of clathrin coat complexes and requires dynamin 1 and 2, thus supporting the view that vesicles can possibly be pinched off of nascent membrane tubules.
The activation of cPLA2α and its targeting to membranes have been extensively examined. cPLA2α can be activated through three distinct phosphorylation sites by PKC, MAPK family member ERK1/2, and CaM kinase, but the phosphorylation of cPLA2α does not always lead to AA release [119–121]. This observation clearly implicates cPLA2α to be involved in other processes that do not involve liberating AA and may also explain a possible route in uncoupling the functions of releasing AA from Golgi phospholipids and of regulating Golgi structure. The relationship between phosphorylation and Ca2+-binding in regulating cPLA2α translocation to membranes remains unclear. However, one report notes that AA can possibly mimic Ca2+ ionophores in stimulating cPLA2α translocation to ER but not Golgi membranes in a Ca2+-independent manner [122]. The targeting of cPLA2α to Golgi membranes may be similar to PAFAHIb in that PIPs play a facilitative role. Indeed, reports show that PI(4,5)P2 enhances both cPLA2α activity and membrane-binding affinity in vitro through interactions with cationic residues within the membrane-binding surface of the protein [123, 124]. However, another study raises conflicting results by showing that PI(4,5)P2 only enhances cPLA2α activity and not its Ca2+-dependent membrane binding affinity [125]. Incidentally, Golgi membranes contain PI(4,5)P2 in addition to PI(4)P [126].
Both membrane tubulation and vesiculation may be facilitated through enhanced membrane fluidity, and PLA2 activity may be involved in increasing membrane fluidity, as the addition of palmitoyl trifluoromethylketone (PACOCF3), a PLA2 inhibitor, reduces membrane fluidity in rat hippocampal tissue [127, 128]. Enhanced membrane fluidity may facilitate lateral segregation of conically shaped LPLs generated by PLA2 enzymes into regions of high curvature such as membrane tubules [129]. Membrane fluidity also facilitates the formation of lipid rafts as exemplified by the observation that PLA2 activity is specifically responsible for the budding and fission of liquid ordered (Lo) phase rafts from liquid disordered (Ld) giant unilamellar vesicles (GUVs) [130]. This is potentially explained by the hypothesis that PLA2 enzymes produce conically-shaped LPLs within Lo rafts, which in turn lead to the budding of rafts into membrane vesicles and/or tubules and is overall energetically favorable because the protrusion of Lo rafts into vesicles/tubules away from GUVs decrease the free energy that is confined within Lo/Ld phase boundary [130]. In another twist, lipid phases can additionally lead to inhibition of cPLA2α. One study shows that although sphingomyelin (SPH) cannot be hydrolyzed by cPLA2α, Lo phases enriched in SPH can bind to and sequester cPLA2α from Ld phases that contain hydrolysable glycero-phospholipid substrates [131]. The same study showed that such inhibition is specifically due to sequestering cPLA2α within phases apart from substrate phases because the addition of cholesterol or ceramide leads to the abolishment of separated phases and the reestablishment of cPLA2α activity.
PLA2G6/iPLA2-β
A Group VIA-2 PLA2, PLA2G6/iPLA2-β (also known as iPLA2B, PNPLA9), is a third PLA2 member that regulates Golgi trafficking indirectly by regulating the ERGIC (Table) [132]. The ERGIC is an independent compartment located between the ER and the cis-face of the Golgi complex, facilitates bidirectional trafficking between the two organelles, and may serve as a precursor to the cis aspect of the Golgi stack [133, 134]. The ERGIC is comprised of tubulovesicular membrane structures and potentially serves roles in cargo protein quality, sorting, and concentration [133]. PLA2G6/iPLA2-β has multiple splice variants, of which the full length variant, called L-iPLA2, is catalytically active while its shorter splice variants, ankyrin-iPLA2-1 and -2 are inactive but thought to negatively regulate L-iPLA2 via forming oligomers through their ankyrin repeats [135, 136]. PLA2 involvement at the ERGIC is first implicated through the use of PLA2 drug inhibitors that prevent the recycling of ERGIC-localized protein ERGIC-53 in addition to the formation of ERGIC membrane tubules [137]. Recent work shows that Arf1 and Arf4 knockdown can generate ERGIC membrane tubules that are dependent on PLA2G6/iPLA2-β as knockdown of this protein also leads to inhibition of membrane tubule formation [132]. The same study noted that PLA2G6/iPLA2-β-induced membrane tubules may be viable alternatives to vesicles in quickly and efficiently directing cargo between ERGIC clusters and cargo containing COPI vesicles (Fig. 2, Step 5). ERGIC tubulation and mobility are also regulated by microtubules and their associated motor proteins and whether these tubule-inducing functions are independent or shared with PLA2G6/iPLA2-β remains unknown [138].
Aside from basic cell biological interest in understanding how phospholipid modifying enzymes and membrane tubules influence intracellular membrane trafficking, recent studies have revealed that the function of these enzymes and membrane structures might be linked to pathological conditions. For example, mutations in human PLA2G6A gene (and in mouse models) cause a spectrum of autosomal recessive neurodegenerative disorders, characterized by severe cognitive and motor regression, now referred to as PLA2G6-Associated Neurodegeneration (PLAN) [139–142]. The connection between PLA2G6’s function at the ERGIC and disease manifestations in PLAN is not understood but could involve specific defects in secretory trafficking.
iPLA1γ
The final cytoplasmic PLA protein that has effects on the Golgi complex is a PLA1 family member, iPLA1γ (gene name DDH2 or KIAA0725p) (Table) [143, 144]. The other two cytoplasmic PLA1 family members include iPLA1α (PA-PLA1) and iPLA1β (p125) [145]. While iPLA1γ shares a common characteristic with iPLA1β in that the overexpression of either results in structural disruption of both the ERGIC and the Golgi, only the overexpression of iPLA1γ lead to mislocalization of Golgi resident proteins such as p115 and GM130 [146]. One of the first iPLA1γ studies showed that the protein is localized to both Golgi and ERGIC membranes (Fig. 2) [143]. The same study indicated that siRNA knockdown of iPLA1γ disrupted COPI- and Rab6-independent, i.e., BFA stimulated, Golgi-to-ER retrograde trafficking, whereas VSVG-ts045-GFP anterograde transport was unaffected. However, a more recent study conflicts with these results, finding that iPLA1γ knockdown did not affect Golgi-to-ER retrograde trafficking but did inhibit VSVG-ts045-GFP anterograde transport [144]. These discrepancies can be explained by the observation that the original knockdown studies had an unintended off-target knockdown of Rab6 [144], which regulates Golgi retrograde trafficking [147]. In mammals, the physiological roles of the PLA1 family are unclear but it is known that iPLA1α is highly expressed in brain and testis and thus possibly linked to neural development and spermatogenesis, similar to PAFAHIb [106, 107, 148, 149]. In addition, the molecular mechanisms by which iPLA1γ influences trafficking through the Golgi complex are unknown.
Lysophospholipid Acyltransferases
The fruitful studies of PLA2 enzymes in Golgi membrane trafficking stimulated an interest in LPAT activity as a potential mediator of Golgi membrane trafficking, perhaps by counteracting the tubulating activity of PLA2 enzymes (Fig. 3). Given that in model membrane systems PLA2 and LPAT lipid products stabilize positive and negative curvature, respectively, and that PLA2 activity stimulates membrane tubulation in cells, it was hypothesized that LPAT activity might prevent tubule formation in cells [66]. It had also been hypothesized that the negative curvature at the neck of a budding vesicle could be stabilized by LPAT lipid products [150]. Finally, it was suggested that cytoplasmic PLA2 and LPAT enzymes could work in concert: cytoplasmic PLA2 enzymes could promote outward curvature or budding that would subsequently be acted on LPATs to produce negative curvature to facilitate coated vesicle production [66].
The first two reports of LPAT activity being involved in membrane trafficking were from studies of BARS and endophilin [151–153]. BARS is a mediator of Golgi membrane tubule fission [53], and endophilin is an N-BAR domain containing protein that was recently shown to be required for uncoating of coated vesicles at the plasma membrane but not fission per se [154]. Both of these proteins were reported to have low levels of acyltransferase activity; however, this activity was later determined to be associated with LPAAT enzyme contaminants [155].
The first solid indication that LPATs were involved in membrane trafficking came from studies that utilized a powerful new tool, the small molecule CI-976. CI-976 (2,2-methyl-N-(2,4,6,-trimethoxyphenyl)dodecanamide) was originally synthesized as a fatty acid anilide derivative designed to mimic fatty acyl-CoA for inhibition of acyl-CoA:cholesterol acyltransferase (ACAT) [156, 157]. It is a small hydrophobic, membrane permeant, and competitive ACAT inhibitor [158]. While CI-976 possesses only weak inhibitor activity against ACAT, a screen for inhibition of Golgi associated LPAT activity showed strong inhibition of LPC and lysophosphatidylethanolamine (LPE) acyltransferase activities [159–162]. With this potent inhibitor, the study of LPAT enzymes in mammalian membrane trafficking immediately progressed. Most intriguingly, treatment of mammalian cells with CI-976 stimulated a remarkable enhancement of Golgi membrane tubulation and retrograde trafficking [160, 161]. This tubulation activity was phenotypically similar to BFA induced tubulation, although comparatively delayed. Additionally, CI-976-induced Golgi tubulation was inhibited by concomitant PLA2 inhibition, indicating for the first time that both PLA2 and LPATs work in concert to regulate the dynamic structure of the Golgi complex [161]
Golgi-associated LPAT enzyme(s) could regulate membrane trafficking by one of, or a combination of, five mechanisms (Fig. 3 and 4): 1) production of conical phospholipids that resist the high positive curvature present in tubules; 2) conversion of inverted-cone shaped LPLs that stabilize positive curvature to phospholipids that are less stabilizing; 3) production of conical phospholipids that stabilize the high negative curvature at the neck of budding vesicles; 4) production of phospholipids that recruit vesicle-budding factors; and, 5) production of phospholipids that affect lipid and protein partitioning. There is evidence for all of these mechanisms and deciphering the relative contribution of each is a major challenge in the field.
Further studies identified a PC- and PE-forming LPAT tightly associated with the Golgi complex [159]. The connection between this enzyme and CI-976 stimulated tubulation of the Golgi complex and Golgi-to-ER retrograde trafficking is unclear [161], and the exact molecular identity of the LPC/LPEAT activity is still unknown. In fact, recent studies strongly indicate that the observed CI-976 effect on the Golgi could be due to inhibition of LPAAT mediated acylation of LPA to PA, which is catalyzed by AGPAT3/LPAAT3 (also called LPAATγ), a Golgi localized LPA specific acyltransferase (Table) [76].
AGPAT3/LPAAT3 was the first specific LPAT shown to be involved in the dynamic function of the Golgi complex. It is an integral membrane protein with two transmembrane domains and is found in both the Golgi complex and ER [163]. Overexpression of AGPAT3/LPAAT3 was shown to slow ERGIC-53 recycling from the cis-Golgi to the ER/ERGIC and to reduce BFA-stimulated membrane tubulation. Conversely, AGPAT3/LPAAT3 knockdown accelerated ERGIC-53 recycling. Importantly, overexpression of AGPAT3/LPAAT3 counteracted the tubule-inducing effects CI-976, suggesting that AGPAT3/LPAAT3 is a direct target of the drug. However, this result does not rule out the possibility that the CI-976-inhibitable Golgi LPC/LPEAT activity also contributes to Golgi membrane remodeling. For example, a Golgi LPC/LPEAT enzyme could come into play because AGPAT3/LPAAT3 generated PA can be hydrolyzed to DAG by PA phosphatase and then converted to PC or PE, followed by Lands Cycle remodeling via cPLA2α (Fig. 6). In addition, PC and PE can be generated via Golgi localized cholinephosphotransferase (CPT1) or a phosphoethanolamine transferase, respectively [164].
Figure 6.
Enzymatic pathways, several involved in Lands cycle reactions, which lead to the modification and interconversion of lipid species. Lysophosphatidic acid (LPA); phosphatidic acid (PA); diacylglycerol (DAG); phosphatidylcholine (PC); lysophosphatidylcholine (LPC); sphingomyelin (SM); ceramide (Cer); phosphatidylinositol 4-phosphate (PI4P); phosphatidylinositol 4, 5-phosphate (PI4,5P); 1-acylglycerol-3-phosphate acyltransferase 3 (AGPAT3); lysophosphatidic acid acyltransferase 3 (LPAAT3); phospholipase A2 (PLA2); diacylglycerol kinase (DAGK); phosphatidic acid phosphatase (PA P’tase); phospholipase D (PLD); PC specific phospholipase C (PC-PLC); phosphatidylinositol specific PLC (PI-PLC); sphingomyelin synthase (SMS).
Like PLA2 enzymes, LPATs are also involved in other secretory and endocytic trafficking steps. For example, CI-976 reversibly inhibited a very late step in COPII vesicle budding, resulting in the accumulation of secretory cargo at ER exit sites (ERESs) [165]. Interestingly, ERESs underwent Sar1p-dependent membrane tubulation in the presence of CI-976, suggesting the involvement of LPAT activity in the fission of COPII budding elements in vivo. In addition to secretory trafficking, CI-976 was also found to stimulate membrane tubules from endosomes and to inhibit recycling of transferrin and transferrin receptors from the endocytic recycling compartment, suggesting a role for LPATs in the budding of vesicles from endosome tubules [160]. Currently, the precise LPATs involved in COPII budding or endocytic recycling have yet to be identified.
Integration of cPLA2α and AGPAT3/LPAAT3 to Influence Golgi COPI Vesicle and Membrane Tubule Formation
The above studies on Golgi-associated PLA2 and LPAT enzymes suggest an intimate association between the formation of membrane tubules and coated vesicles. Support for this idea came from previous studies showing that CI-976 inhibited COPI vesicle formation [53]. This association was recently directly tested by Hsu and colleagues who discovered that cPLA2α and AGPAT3/LPAAT3 function to regulate the relative abundance of COPI vesicle and membrane tubule formation (Fig. 4) [34]. Using purified Golgi complex membranes in an in vitro COPI budding assay, they first found that COPI coatomer subunits and Arf1 are required for both vesicle and membrane tubule formation. They surmised that Arf1 and coatomer initiates the budding events that become vesicles and membrane tubules. When examining endogenous cPLA2α and AGPAT/LPAAT3 by immunofluorescence microscopy, they were shown to colocalize with endogenous coatomer proteins. The coatomer subunit α-COP was shown by immuno-EM to localize to the base and tip, but not the stem, of membrane tubules. In this assay, AGPAT3/LPAAT3 activity increased COPI vesicle formation, which was shown by the addition of CI-976 or AGPAT3/LPAAT3 antibody to budding reactions. The authors suspect that AGPAT3/LPAAT3-generated PA, a known recruiter of COPI coatomer subunits and BARS, a COPI vesicle fission protein [53], as well as a negative curve-inducing phospholipid [77, 166], are responsible for stimulating COPI coating of the nascent vesicle. In support of this idea, inhibition of AGPAT3/LPAAT3, by CI-976 or specific antibody, stimulated membrane tubulation at the expense of vesicle production. The final step of COPI vesicle fission required the conversion of PC to PA by PLD2, but not PLD1.
The addition of pharmacological PLA2 inhibitors or anti-cPLA2α antibody to cytosol in their reconstitution assay increased vesicle formation and concomitantly decreased tubule formation, which was reversed by the addition of purified cPLA2α. Their model posits that Arf1 and COPI coatomer recruitment initiates bud formation and that buds then mature into either vesicles or membrane tubules, depending on the balance of cPLA2α and AGPAT3/LPAAT3 activity (Fig. 2, Step 5: Fig. 4B–E). It is still a matter of debate as to whether generation of curve-inducing PA or recruitment of effector proteins, is most important in AGPAT3/LPAAT3-mediated vesicle biogenesis. They further suggest that AGPAT3/LPAAT3 generated PA initiates negative membrane curvature but is insufficient for complete vesicle budding, a process that is finalized by the additional generation of PA by PLD2 (Fig. 4F). The generation of PA by both enzymes could aid in the recruitment of the fission protein BARS [53]. Hsu and colleagues also assert that the biogenesis of Golgi membrane tubules, both intercisternal and intracisternal, which facilitate anterograde trafficking and Golgi ribbon formation respectively, are initiated as COPI buds (Fig. 4D). These results provide important insights into how Lands cycle phospholipid remodeling might influence the dynamic behavior of Golgi membranes. Interestingly, however, this model of membrane curvature initiation solely by COPI/Arfs cannot explain enhanced Golgi membrane tubule formation by BFA or knockdown of Arf GEFs, and underscores the notion that cytoplasmic PLA2 enzymes can induce membrane tubules on their own (Fig. 4G). In addition, because COPI vesicle budding and fission can be reconstituted using minimal components coatomer and Arf-GTP [50], these phospholipid modifying enzymes likely function to enhance the efficiency of the COPI budding machinery.
The central roles of DAG and PA in Golgi membrane tubule and vesicle formation have been extensively examined, and their participation raises important points for the analysis of how lipid/phospholipid changes affect membrane trafficking. Both DAG and PA have been shown to be required for membrane tubule and vesicle formation from the Golgi and TGN [59, 167–170], likely by generating both curvature-altering phospholipids and platforms for effector protein binding. How the Golgi complex controls the numerous pathways that lead to and from PA and DAG is still a mystery (Fig. 6). In addition, understanding how lipids/phospholipids can exert dramatic local effects in the face of rapid lateral diffusion and conversion to other products is a challenge. Nevertheless, it is becoming increasingly clear that remodeling of Golgi membrane phospholipids by PLA2 and LPAT enzymes adds a new layer of complexity to an already complex organelle. Moreover, it is likely that the full complement of PLA and LPAT enzymes involved in secretory (and endocytic) trafficking has not yet been revealed.
Highlights.
Membrane remodeling by PLA2 and LPAT enzymes influence mammalian Golgi complex structure and function
The phospholipases PAFAHIb, cPLA2α, PLA2G6/iPLA2-β, and iPLA1γ localize to the Golgi complex
PAFAHIb, cPLA2α, and PLA2G6/iPLA2-β induce Golgi membrane tubule formation
The transmembrane lysophospholipid acyltransferase AGPAT3/LPAAT3 influences Golgi structure and function
AGPAT3/LPAAT3 and cPLA2α regulate COPI vesicle and membrane tubule formation
Acknowledgments
This work was supported by NIH grant NIDDK R01 51596 to WJB.
Listing of abbreviations
- AA
Arachidonic acid
- ACAT
acyl-CoA cholesterol acyltransferase
- AGPAT
1-acylglycerol-3-phosphate acyltransferase
- Arf
ADP-ribosylating factor
- BARS
BFA-inhibited, ADP-ribosylated substrate
- BFA
Brefeldin A
- CPT1
Cholinephosphotransferase
- DAG
Diacylglycerol
- DAGK
Diacylglycerol kinase
- ER
Endoplasmic reticulum
- ERES
ER exit site
- ERGIC
ER-Golgi-intermediate complex
- GEF
Guanine nucleotide exchange factor
- GUV
Giant unilamellar vesicle
- Lo
Liquid ordered
- Ld
Liquid disordered
- LPA
Lysophosphatidic acid
- LPAAT
Lysophosphatidic acid acyltransferase
- LPAT
Lysophospholipid acyltransferase
- LPC
Lysophosphatidylcholine
- LPE
Lysophosphatidylethanolamine
- LPL
Lysophospholipid
- MAFP
Methyl arachidonyl fluorophosphonate
- MBOAT
Membrane bound O-acyltransferase
- MT
Microtubule
- MTOC
Microtubule organizing center
- PA
Phosphatidic acid
- PACOCF3
palmitoyl trifluoromethylketone
- PAFAH
Platelet activating factor acetylhydrolase
- PC
Phosphatidylcholine
- PIP
Phosphoinositide phosphate
- PLA
Phospholipase A
- PLAAp
PLA2 activating protein peptide
- PLD
Phospholipase D
- SM
Sphingomyelin
- SMS
Sphingomyelin synthase
- SPH
Sphingomyelin
- TGN
trans-Golgi network
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Farquhar MG, Palade GE. The Golgi apparatus (complex)-(1954–1981)-from artifact to center stage. J Cell Biol. 1981;91:77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Farquhar MG, Palade GE. The Golgi apparatus: 100 years of progress and controversy. Trends Cell Biol. 1998;8:2–10. doi: 10.1016/S0962-8924(97)01187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe M. Structural organization of the Golgi apparatus. Curr Opin Cell Biol. 2011;23:85–93. doi: 10.1016/j.ceb.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 4.Polishchuk RS, Capestrano M, Polishchuk EV. Shaping tubular carriers for intracellular membrane transport. FEBS Lett. 2009;583:3847–3856. doi: 10.1016/j.febslet.2009.10.031. [DOI] [PubMed] [Google Scholar]
- 5.Wilson C, Venditti R, Rega LR, Colanzi A, D'Angelo G, De Matteis MA. The Golgi apparatus: an organelle with multiple complex functions. Biochem J. 2011;433:1–9. doi: 10.1042/BJ20101058. [DOI] [PubMed] [Google Scholar]
- 6.Klumperman J. Architecture of the mammalian Golgi. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wei JH, Seemann J. Unraveling the Golgi ribbon. Traffic. 2010;11:1391–1400. doi: 10.1111/j.1600-0854.2010.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emr S, Glick BS, Linstedt AD, Lippincott-Schwartz J, Luini A, Malhotra V, Marsh BJ, Nakano A, Pfeffer SR, Rabouille C, Rothman JE, Warren G, Wieland FT. Journeys through the Golgi--taking stock in a new era. J Cell Biol. 2009;187:449–453. doi: 10.1083/jcb.200909011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bechler ME, de Figueiredo P, Brown WJ. A PLA1-2 punch regulates the Golgi complex. Trends Cell Biol. 2012;22:116–124. doi: 10.1016/j.tcb.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bankaitis VA. The Cirque du Soleil of Golgi membrane dynamics. J Cell Biol. 2009;186:169–171. doi: 10.1083/jcb.200907008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lands WE. Lipid Metabolism. Annu Rev Biochem. 1965;34:313–346. doi: 10.1146/annurev.bi.34.070165.001525. [DOI] [PubMed] [Google Scholar]
- 12.Lands WE. Stories about acyl chains. Biochim Biophys Acta. 2000;1483:1–14. doi: 10.1016/s1388-1981(99)00177-8. [DOI] [PubMed] [Google Scholar]
- 13.Burke JE, Dennis EA. Phospholipase A2 structure/function, mechanism, and signaling. Journal of lipid research. 2009;50(Suppl):S237–S242. doi: 10.1194/jlr.R800033-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harayama T, Shindou H, Ogasawara R, Suwabe A, Shimizu T. Identification of a novel noninflammatory biosynthetic pathway of platelet-activating factor. J Biol Chem. 2008;283:11097–11106. doi: 10.1074/jbc.M708909200. [DOI] [PubMed] [Google Scholar]
- 15.McIntyre TM, Prescott SM, Stafforini DM. The emerging roles of PAF acetylhydrolase. J Lipid Res. 2009;50(Suppl):S255–S259. doi: 10.1194/jlr.R800024-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shindou H, Hishikawa D, Nakanishi H, Harayama T, Ishii S, Taguchi R, Shimizu T. A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J Biol Chem. 2007;282:6532–6539. doi: 10.1074/jbc.M609641200. [DOI] [PubMed] [Google Scholar]
- 17.Das S, Castillo C, Stevens T. Phospholipid remodeling/generation in Giardia: the role of the Lands cycle. Trends Parasitol. 2001;17:316–319. doi: 10.1016/s1471-4922(01)01901-8. [DOI] [PubMed] [Google Scholar]
- 18.Bard F, Malhotra V. The formation of TGN-to-plasma-membrane transport carriers. Annu Rev Cell Dev Biol. 2006;22:439–455. doi: 10.1146/annurev.cellbio.21.012704.133126. [DOI] [PubMed] [Google Scholar]
- 19.Beck R, Rawet M, Wieland FT, Cassel D. The COPI system: molecular mechanisms and function. FEBS Lett. 2009;583:2701–2709. doi: 10.1016/j.febslet.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 20.Borgonovo B, Ouwendijk J, Solimena M. Biogenesis of secretory granules. Curr Opin Cell Biol. 2006;18:365–370. doi: 10.1016/j.ceb.2006.06.010. [DOI] [PubMed] [Google Scholar]
- 21.De Matteis MA, Luini A. Exiting the Golgi complex. Nat Rev Mol Cell Biol. 2008;9:273–284. doi: 10.1038/nrm2378. [DOI] [PubMed] [Google Scholar]
- 22.Hsu VW, Lee SY, Yang JS. The evolving understanding of COPI vesicle formation. Nat Rev Mol Cell Biol. 2009;10:360–364. doi: 10.1038/nrm2663. [DOI] [PubMed] [Google Scholar]
- 23.Braulke T, Bonifacino JS. Sorting of lysosomal proteins. Biochim Biophys Acta. 2009;1793:605–614. doi: 10.1016/j.bbamcr.2008.10.016. [DOI] [PubMed] [Google Scholar]
- 24.Luini A, Mironov AA, Polishchuk EV, Polishchuk RS. Morphogenesis of post-Golgi transport carriers. Histochem Cell Biol. 2008;129:153–161. doi: 10.1007/s00418-007-0365-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marsh BJ, Mastronarde DN, Buttle KF, Howell KE, McIntosh JR. Organellar relationships in the Golgi region of the pancreatic beta cell line, HIT-T15, visualized by high resolution electron tomography. Proc Natl Acad Sci U S A. 2001;98:2399–2406. doi: 10.1073/pnas.051631998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marsh BJ, Mastronarde DN, McIntosh JR, Howell KE. Structural evidence for multiple transport mechanisms through the Golgi in the pancreatic beta-cell line, HIT-T15. Biochem Soc Trans. 2001;29:461–467. doi: 10.1042/bst0290461. [DOI] [PubMed] [Google Scholar]
- 27.Marsh BJ, Volkmann N, McIntosh JR, Howell KE. Direct continuities between cisternae at different levels of the Golgi complex in glucose-stimulated mouse islet beta cells. Proc Natl Acad Sci U S A. 2004;101:5565–5570. doi: 10.1073/pnas.0401242101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Polishchuk RS, Polishchuk EV, Marra P, Alberti S, R B, Luini A, Mironov AA. Correlative light-electron microscopy reveals the tubular-saccular ultrastructure of carriers operating between Golgi apparatus and plasma membrane. J Cell Biol. 2000;148:45–58. doi: 10.1083/jcb.148.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lippincott-Schwartz J, Snapp E, Kenworthy A. Studying protein dynamics in living cells. Nat Rev Mol Cell Biol. 2001;2:444–456. doi: 10.1038/35073068. [DOI] [PubMed] [Google Scholar]
- 30.Lippincott-Schwartz J, Yuan L, Tipper C, Amherdt M, Orci L, Klausner RD. Brefeldin A's effects on endosomes, lysosomes, and the TGN suggest a general mechanism for regulating organelle structure and membrane traffic. Cell. 1991;67:601–616. doi: 10.1016/0092-8674(91)90534-6. [DOI] [PubMed] [Google Scholar]
- 31.Presley JF, Smith C, Hirschberg K, Miller C, Cole NB, Zaal KJ, Lippincott-Schwartz J. Golgi membrane dynamics. Mol Biol Cell. 1998;9:1617–1626. doi: 10.1091/mbc.9.7.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Waguri S, Dewitte F, Le Borgne R, Rouille Y, Uchiyama Y, Dubremetz JF, Hoflack B. Visualization of TGN to endosome trafficking through fluorescently labeled MPR and AP-1 in living cells. Mol Biol Cell. 2003;14:142–155. doi: 10.1091/mbc.E02-06-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wood SA, Park JE, Brown WJ. Brefeldin A causes a microtubule-mediated fusion of the trans-Golgi network and early endosomes. Cell. 1991;67:591–600. doi: 10.1016/0092-8674(91)90533-5. [DOI] [PubMed] [Google Scholar]
- 34.Yang JS, Valente C, Polishchuk RS, Turacchio G, Layre E, Moody DB, Leslie CC, Gelb MH, Brown WJ, Corda D, Luini A, Hsu VW. COPI acts in both vesicular and tubular transport. Nat Cell Biol. 2011;13:996–1003. doi: 10.1038/ncb2273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lippincott-Schwartz J, Donaldson JG, Schweizer A, Berger EG, Hauri HP, Yuan LC, Klausner RD. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990;60:821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- 37.Wood SA, Brown WJ. The morphology but not the function of endosomes and lysosomes is altered by brefeldin A. J Cell Biol. 1992;119:273–285. doi: 10.1083/jcb.119.2.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Donaldson JG, Finazzi D, Klausner RD. Brefeldin-A inhibits Golgi membrane-catalysed exchange of guanine nucleotide onto ARF protein. Nature. 1992;360:350–352. doi: 10.1038/360350a0. [DOI] [PubMed] [Google Scholar]
- 39.Peyroche A, Antonny B, Robineau S, Acker J, Cherfils J, Jackson CL. Brefeldin A acts to stabilize an abortive ARF-GDP-Sec7 domain protein complex: involvement of specific residues of the Sec7 domain. Mol Cell. 1999;3:275–285. doi: 10.1016/s1097-2765(00)80455-4. [DOI] [PubMed] [Google Scholar]
- 40.Peyroche A, Jackson CL. Functional analysis of ADP-ribosylation factor (ARF) guanine nucleotide exchange factors Gea1p and Gea2p in yeast. Methods Enzymol. 2001;329:290–300. doi: 10.1016/s0076-6879(01)29090-9. [DOI] [PubMed] [Google Scholar]
- 41.Robinson MS, Kreis TE. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992;69:129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- 42.Wong DH, Brodsky FM. 100-kD proteins of Golgi- and trans-Golgi network-associated coated vesicles have related but distinct membrane binding properties. J Cell Biol. 1992;117:1171–1179. doi: 10.1083/jcb.117.6.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hunziker W, Whitney JA, Mellman I. Selective inhibition of transcytosis by brefeldin A in MDCK cells. Cell. 1991;67:617–627. doi: 10.1016/0092-8674(91)90535-7. [DOI] [PubMed] [Google Scholar]
- 44.Volpicelli-Daley LA, Li Y, Zhang CJ, Kahn RA. Isoform-selective effects of the depletion of ADP-ribosylation factors 1–5 on membrane traffic. Mol Biol Cell. 2005;16:4495–4508. doi: 10.1091/mbc.E04-12-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Szul T, Grabski R, Lyons S, Morohashi Y, Shestopal S, Lowe M, Sztul E. Dissecting the role of the ARF guanine nucleotide exchange factor GBF1 in Golgi biogenesis and protein trafficking. J Cell Sci. 2007;120:3929–3940. doi: 10.1242/jcs.010769. [DOI] [PubMed] [Google Scholar]
- 46.Puertollano R, van der Wel NN, Greene LE, Eisenberg E, Peters PJ, Bonifacino JS. Morphology and dynamics of clathrin/GGA1-coated carriers budding from the trans-Golgi network. Mol Biol Cell. 2003;14:1545–1557. doi: 10.1091/mbc.02-07-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cluett EB, Wood SA, Banta M, Brown WJ. Tubulation of Golgi membranes in vivo and in vitro in the absence of Brefeldin A. J Cell Biol. 1993;120:15–24. doi: 10.1083/jcb.120.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Popoff V, Adolf F, Brugger B, Wieland F. COPI budding within the Golgi stack. Cold Spring Harb Perspect Biol. 2011;3:a005231. doi: 10.1101/cshperspect.a005231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Manneville JB, Casella JF, Ambroggio E, Gounon P, Bertherat J, Bassereau P, Cartaud J, Antonny B, Goud B. COPI coat assembly occurs on liquid-disordered domains and the associated membrane deformations are limited by membrane tension. Proc Natl Acad Sci U S A. 2008;105:16946–16951. doi: 10.1073/pnas.0807102105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beck R, Prinz S, Diestelkotter-Bachert P, Rohling S, Adolf F, Hoehner K, Welsch S, Ronchi P, Brugger B, Briggs JA, Wieland F. Coatomer and dimeric ADP ribosylation factor 1 promote distinct steps in membrane scission. J Cell Biol. 2011;194:765–777. doi: 10.1083/jcb.201011027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bonazzi M, Spano S, Turacchio G, Cericola C, Valente C, Colanzi A, Kweon HS, Hsu VW, Polishchuck EV, Polishchuck RS, Sallese M, Pulvirenti T, Corda D, Luini A. CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat Cell Biol. 2005;7:570–580. doi: 10.1038/ncb1260. [DOI] [PubMed] [Google Scholar]
- 52.Yang JS, Lee SY, Gao M, Bourgoin S, Randazzo PA, Premont RT, Hsu VW. ARFGAP1 promotes the formation of COPI vesicles, suggesting function as a component of the coat. J Cell Biol. 2002;159:69–78. doi: 10.1083/jcb.200206015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang JS, Lee SY, Spano S, Gad H, Zhang L, Nie Z, Bonazzi M, Corda D, Luini A, Hsu VW. A role for BARS at the fission step of COPI vesicle formation from Golgi membrane. EMBO J. 2005;24:4133–4143. doi: 10.1038/sj.emboj.7600873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yang JS, Zhang L, Lee SY, Gad H, Luini A, Hsu VW. Key components of the fission machinery are interchangeable. Nat Cell Biol. 2006;8:1376–1382. doi: 10.1038/ncb1503. [DOI] [PubMed] [Google Scholar]
- 55.Zhou X, Graham TR. Reconstitution of phospholipid translocase activity with purified Drs2p, a type-IV P-type ATPase from budding yeast. Proc Natl Acad Sci U S A. 2009;106:16586–16591. doi: 10.1073/pnas.0904293106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beck R, Adolf F, Weimer C, Bruegger B, Wieland FT. ArfGAP1 activity and COPI vesicle biogenesis. Traffic. 2009;10:307–315. doi: 10.1111/j.1600-0854.2008.00865.x. [DOI] [PubMed] [Google Scholar]
- 57.Brown HA, Gutowski S, Kahn RA, Sternweis PC. Partial purification and characterization of Arf-sensitive phospholipase D from porcine brain. J Biol Chem. 1995;270:14935–14943. doi: 10.1074/jbc.270.25.14935. [DOI] [PubMed] [Google Scholar]
- 58.Ktistakis NT, Brown HA, Waters MG, Sternweis PC, Roth MG. Evidence that phospholipase D mediates ADP ribosylation factor-dependent formation of Golgi coated vesicles. J Cell Biol. 1996;134:295–306. doi: 10.1083/jcb.134.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang JS, Gad H, Lee SY, Mironov A, Zhang L, Beznoussenko GV, Valente C, Turacchio G, Bonsra AN, Du G, Baldanzi G, Graziani A, Bourgoin S, Frohman MA, Luini A, Hsu VW. A role for phosphatidic acid in COPI vesicle fission yields insights into Golgi maintenance. Nat Cell Biol. 2008;10:1146–1153. doi: 10.1038/ncb1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murakami M, Taketomi Y, Miki Y, Sato H, Hirabayashi T, Yamamoto K. Recent progress in phospholipase A research: from cells to animals to humans. Prog Lipid Res. 2011;50:152–192. doi: 10.1016/j.plipres.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 61.Aoki J, Inoue A, Makide K, Saiki N, Arai H. Structure and function of extracellular phospholipase A1 belonging to the pancreatic lipase gene family. Biochimie. 2007;89:197–204. doi: 10.1016/j.biochi.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 62.Bonventre JV. Phospholipase A2 and signal transduction. J Am Soc Nephrol. 1992;3:128–150. doi: 10.1681/ASN.V32128. [DOI] [PubMed] [Google Scholar]
- 63.Scott KF, Sajinovic M, Hein J, Nixdorf S, Galettis P, Liauw W, de Souza P, Dong Q, Graham GG, Russell PJ. Emerging roles for phospholipase A2 enzymes in cancer. Biochimie. 2010;92:601–610. doi: 10.1016/j.biochi.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 64.Rosenson RS. Phospholipase A2 inhibition and atherosclerotic vascular disease: prospects for targeting secretory and lipoprotein-associated phospholipase A2 enzymes. Curr Opin Lipidol. 2010;21:473–480. doi: 10.1097/MOL.0b013e32833eb581. [DOI] [PubMed] [Google Scholar]
- 65.Cummings BS. Phospholipase A2 as targets for anti-cancer drugs. Biochem Pharmacol. 2007;74:949–959. doi: 10.1016/j.bcp.2007.04.021. [DOI] [PubMed] [Google Scholar]
- 66.Brown WJ, Chambers K, Doody A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: mediators of membrane shape and function. Traffic. 2003;4:214–221. doi: 10.1034/j.1600-0854.2003.00078.x. [DOI] [PubMed] [Google Scholar]
- 67.de Figueiredo P, Drecktrah D, Katzenellenbogen JA, Strang M, Brown WJ. Evidence that phospholipase A2 activity is required for Golgi complex and trans Golgi network membrane tubulation. Proc Natl Acad Sci U S A. 1998;95:8642–8647. doi: 10.1073/pnas.95.15.8642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tagaya M, Henomatsu N, Yoshimori T, Yamamoto A, Tashiro Y, Fukui T. Correlation between phospholipase-A2 activity and intra-Golgi protein transport reconstituted in a cell-free system. FEBS Lett. 1993;324:201–204. doi: 10.1016/0014-5793(93)81393-e. [DOI] [PubMed] [Google Scholar]
- 69.de Figueiredo P, Polizotto RS, Drecktrah D, Brown WJ. Membrane tubule-mediated reassembly and maintenance of the Golgi complex is disrupted by phospholipase A2 antagonists. Mol Biol Cell. 1999;10:1763–1782. doi: 10.1091/mbc.10.6.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Polizotto RS, de Figueiredo P, Brown WJ. Stimulation of Golgi membrane tubulation and retrograde trafficking to the ER by phospholipase A2 activating protein (PLAP) peptide. J Cell Biochem. 1999;74:670–683. [PubMed] [Google Scholar]
- 71.Baumgart T, Hess ST, Webb WW. Imaging coexisting fluid domains in biomembrane models coupling curvature and line tension. Nature. 2003;425:821–824. doi: 10.1038/nature02013. [DOI] [PubMed] [Google Scholar]
- 72.Shindou H, Eto M, Morimoto R, Shimizu T. Identification of membrane O-acyltransferase family motifs. Biochem Biophys Res Commun. 2009;383:320–325. doi: 10.1016/j.bbrc.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 73.Shindou H, Hishikawa D, Harayama T, Yuki K, Shimizu T. Recent progress on acyl CoA:lysophospholipid acyltransferase research. J Lipid Res. 2009;50(Suppl):S46–S51. doi: 10.1194/jlr.R800035-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shindou H, Shimizu T. Acyl-CoA:lysophospholipid acyltransferases. J Biol Chem. 2009;284:1–5. doi: 10.1074/jbc.R800046200. [DOI] [PubMed] [Google Scholar]
- 75.Lewin TM, Wang P, Coleman RA. Analysis of amino acid motifs diagnostic for the sn-glycerol-3-phosphate acyltransferase reaction. Biochemistry. 1999;38:5764–5771. doi: 10.1021/bi982805d. [DOI] [PubMed] [Google Scholar]
- 76.Schmidt JA, Brown WJ. Lysophosphatidic acid acyltransferase 3 regulates Golgi complex structure and function. J Cell Biol. 2009;186:211–218. doi: 10.1083/jcb.200904147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zimmerberg J, Kozlov MM. How proteins produce cellular membrane curvature. Nat Rev Mol Cell Biol. 2006;7:9–19. doi: 10.1038/nrm1784. [DOI] [PubMed] [Google Scholar]
- 78.De Matteis MA, D'Angelo G. The role of the phosphoinositides at the Golgi complex. Biochem Soc Symp. 2007:107–116. doi: 10.1042/BSS0740107. [DOI] [PubMed] [Google Scholar]
- 79.van Meer G, Voelker DR, Feigenson GW. Membrane lipids: where they are and how they behave. Nat Rev Mol Cell Biol. 2008;9:112–124. doi: 10.1038/nrm2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jackson CL. Mechanisms of transport through the Golgi complex. J Cell Sci. 2009;122:443–452. doi: 10.1242/jcs.032581. [DOI] [PubMed] [Google Scholar]
- 81.Baldridge RD, Graham TR. Identification of residues defining phospholipid flippase substrate specificity of type IV P-type ATPases. Proc Natl Acad Sci U S A. 2012;109:E290–E298. doi: 10.1073/pnas.1115725109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bankaitis VA, Grabon A. Phosphatidylinositol synthase and diacylglycerol platforms bust a move. Dev Cell. 2011;21:810–812. doi: 10.1016/j.devcel.2011.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ghosh R, Bankaitis VA. Phosphatidylinositol transfer proteins: negotiating the regulatory interface between lipid metabolism and lipid signaling in diverse cellular processes. Biofactors. 2011;37:290–308. doi: 10.1002/biof.180. [DOI] [PubMed] [Google Scholar]
- 84.Graham TR, Burd CG. Coordination of Golgi functions by phosphatidylinositol 4-kinases. Trends Cell Biol. 2011;21:113–121. doi: 10.1016/j.tcb.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Malhotra V, Campelo F. PKD regulates membrane fission to generate TGN to cell surface transport carriers. Cold Spring Harb Perspect Biol. 2011;3 doi: 10.1101/cshperspect.a005280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Natarajan P, Liu K, Patil DV, Sciorra VA, Jackson CL, Graham TR. Regulation of a Golgi flippase by phosphoinositides and an ArfGEF. Nat Cell Biol. 2009;11:1421–1426. doi: 10.1038/ncb1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Santiago-Tirado FH, Bretscher A. Membrane-trafficking sorting hubs: cooperation between PI4P and small GTPases at the trans-Golgi network. Trends Cell Biol. 2011;21:515–525. doi: 10.1016/j.tcb.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ho YS, Swenson L, Derewenda U, Serre L, Wei Y, Dauter Z, Hattori M, Adachi T, Aoki J, Arai H, Inoue K, Derewenda ZS. Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature. 1997;385:89–93. doi: 10.1038/385089a0. [DOI] [PubMed] [Google Scholar]
- 89.Hattori M, Adachi H, Aoki J, Tsujimoto M, Arai H, Inoue K. Cloning and expression of a cDNA encoding the beta-subunit (30-kDa subunit) of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem. 1995;270:31345–31352. doi: 10.1074/jbc.270.52.31345. [DOI] [PubMed] [Google Scholar]
- 90.Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase[corrected] Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- 91.Vallee RB, Tsai JW. The cellular roles of the lissencephaly gene LIS1, and what they tell us about brain development. Genes Dev. 2006;20:1384–1393. doi: 10.1101/gad.1417206. [DOI] [PubMed] [Google Scholar]
- 92.Vergnolle MA, Taylor SS. Cenp-F links kinetochores to Ndel1/Nde1/Lis1/dynein microtubule motor complexes. Curr Biol. 2007;17:1173–1179. doi: 10.1016/j.cub.2007.05.077. [DOI] [PubMed] [Google Scholar]
- 93.Yamada M, Toba S, Yoshida Y, Haratani K, Mori D, Yano Y, Mimori-Kiyosue Y, Nakamura T, Itoh K, Fushiki S, Setou M, Wynshaw-Boris A, Torisawa T, Toyoshima YY, Hirotsune S. LIS1 and NDEL1 coordinate the plus-end-directed transport of cytoplasmic dynein. The EMBO journal. 2008 doi: 10.1038/emboj.2008.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sasaki S, Mori D, Toyo-oka K, Chen A, Garrett-Beal L, Muramatsu M, Miyagawa S, Hiraiwa N, Yoshiki A, Wynshaw-Boris A, Hirotsune S. Complete loss of Ndel1 results in neuronal migration defects and early embryonic lethality. Mol Cell Biol. 2005;25:7812–7827. doi: 10.1128/MCB.25.17.7812-7827.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Manya H, Aoki J, Kato H, Ishii J, Hino S, Arai H, Inoue K. Biochemical characterization of various catalytic complexes of the brain platelet-activating factor acetylhydrolase. J Biol Chem. 1999;274:31827–31832. doi: 10.1074/jbc.274.45.31827. [DOI] [PubMed] [Google Scholar]
- 96.Manya H, Aoki J, Watanabe M, Adachi T, Asou H, Inoue Y, Arai H, Inoue K. Switching of platelet-activating factor acetylhydrolase catalytic subunits in developing rat brain. J Biol Chem. 1998;273:18567–18572. doi: 10.1074/jbc.273.29.18567. [DOI] [PubMed] [Google Scholar]
- 97.Bechler ME, Doody AM, Racoosin E, Lin L, Lee KH, Brown WJ. The phospholipase complex PAFAH Ib regulates the functional organization of the Golgi complex. J Cell Biol. 2010;190:45–53. doi: 10.1083/jcb.200908105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bechler ME, Doody AM, Ha KD, Judson BL, Chen I, Brown WJ. The phospholipase A2 enzyme complex PAFAH Ib mediates endosomal membrane tubule formation and trafficking. Mol Biol Cell. 2011;22:2348–2359. doi: 10.1091/mbc.E09-12-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Burkhardt JK, Echeverri CJ, Nilsson T, Vallee RB. Overexpression of the dynamitin (p50) subunit of the dynactin complex disrupts dynein-dependent maintenance of membrane organelle distribution. J Cell Biol. 1997;139:469–484. doi: 10.1083/jcb.139.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Corthesytheulaz I, Pauloin A, Pfeffer SR. Cytoplasmic dynein participates in the centrosomal localization of the Golgi complex. J Cell Biol. 1992;118:1333–1345. doi: 10.1083/jcb.118.6.1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Lam C, Vergnolle MA, Thorpe L, Woodman PG, Allan VJ. Functional interplay between LIS1, NDE1 and NDEL1 in dynein-dependent organelle positioning. J Cell Sci. 2010;123:202–212. doi: 10.1242/jcs.059337. [DOI] [PubMed] [Google Scholar]
- 102.Ding C, Liang X, Ma L, Yuan X, Zhu X. Opposing effects of Ndel1 and α1 or α2 on cytoplasmic dynein through competitive binding to Lis1. J Cell Sci. 2009 doi: 10.1242/jcs.048777. [DOI] [PubMed] [Google Scholar]
- 103.Tarricone C, Perrina F, Monzani S, Massimiliano L, Kim MH, Derewenda ZS, Knapp S, Tsai LH, Musacchio A. Coupling PAF signaling to dynein regulation: structure of LIS1 in complex with PAF-acetylhydrolase. Neuron. 2004;44:809–821. doi: 10.1016/j.neuron.2004.11.019. [DOI] [PubMed] [Google Scholar]
- 104.Arai H, Koizumi H, Aoki J, Inoue K. Platelet-activating factor acetylhydrolase (PAF-AH) J Biochem (Tokyo) 2002;131:635–640. doi: 10.1093/oxfordjournals.jbchem.a003145. [DOI] [PubMed] [Google Scholar]
- 105.Karasawa K, Harada A, Satoh N, Inoue K, Setaka M. Plasma platelet activating factor-acetylhydrolase (PAF-AH) Prog Lipid Res. 2003;42:93–114. doi: 10.1016/s0163-7827(02)00049-8. [DOI] [PubMed] [Google Scholar]
- 106.Koizumi H, Yamaguchi N, Hattori M, Ishikawa TO, Aoki J, Taketo MM, Inoue K, Arai H. Targeted disruption of intracellular type I platelet activating factor-acetylhydrolase catalytic subunits causes severe impairment in spermatogenesis. J Biol Chem. 2003;278:12489–12494. doi: 10.1074/jbc.M211836200. [DOI] [PubMed] [Google Scholar]
- 107.Yan W, Assadi AH, Wynshaw-Boris A, Eichele G, Matzuk MM, Clark GD. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nakayama R, Kumagai H, Saito K. Evidence for production of platelet-activating factor by yeast Saccharomyces cerevisiae cells. Biochim Biophys Acta. 1994;1199:137–142. doi: 10.1016/0304-4165(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 109.Leslie CC, Gangelhoff TA, Gelb MH. Localization and function of cytosolic phospholipase A2α at the Golgi. Biochimie. 2010;92:620–626. doi: 10.1016/j.biochi.2010.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Evans JH, Gerber SH, Murray D, Leslie CC. The calcium binding loops of the cytosolic phospholipase A2 C2 domain specify targeting to Golgi and ER in live cells. Mol Biol Cell. 2004;15:371–383. doi: 10.1091/mbc.E03-05-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Evans JH, Spencer DM, Zweifach A, Leslie CC. Intracellular calcium signals regulating cytosolic phospholipase A2 translocation to internal membranes. J Biol Chem. 2001;276:30150–30160. doi: 10.1074/jbc.M100943200. [DOI] [PubMed] [Google Scholar]
- 112.San Pietro E, Capestrano M, Polishchuk EV, DiPentima A, Trucco A, Zizza P, Mariggio S, Pulvirenti T, Sallese M, Tete S, Mironov AA, Leslie CC, Corda D, Luini A, Polishchuk RS. Group IV phospholipase A2α controls the formation of inter-cisternal continuities involved in intra-Golgi transport. PLoS Biol. 2009;7:e1000194. doi: 10.1371/journal.pbio.1000194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Patterson GH, Hirschberg K, Polishchuk RS, Gerlich D, Phair RD, Lippincott-Schwartz J. Transport through the Golgi apparatus by rapid partitioning within a two-phase membrane system. Cell. 2008;133:1055–1067. doi: 10.1016/j.cell.2008.04.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Trucco A, Polishchuk RS, Martella O, Di Pentima A, Fusella A, Di Giandomenico D, San Pietro E, Beznoussenko GV, Polishchuk EV, Baldassarre M, Buccione R, Geerts WJ, Koster AJ, Burger KN, Mironov AA, Luini A. Secretory traffic triggers the formation of tubular continuities across Golgi sub-compartments. Nat Cell Biol. 2004;6:1071–1081. doi: 10.1038/ncb1180. [DOI] [PubMed] [Google Scholar]
- 115.Regan-Klapisz E, Krouwer V, Langelaar-Makkinje M, Nallan L, Gelb M, Gerritsen H, Verkleij AJ, Post JA. Golgi-associated cPLA2α regulates endothelial cell-cell junction integrity by controlling the trafficking of transmembrane junction proteins. Mol Biol Cell. 2009;20:4225–4234. doi: 10.1091/mbc.E08-02-0210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mashimo M, Hirabayashi T, Murayama T, Shimizu T. Cytosolic PLA2α activation in Purkinje neurons and its role in AMPA-receptor trafficking. J Cell Sci. 2008;121:3015–3024. doi: 10.1242/jcs.032987. [DOI] [PubMed] [Google Scholar]
- 117.Choukroun GJ, Marshansky V, Gustafson CE, McKee M, Hajjar RJ, Rosenzweig A, Brown D, Bonventre JV. Cytosolic phospholipase A2 regulates golgi structure and modulates intracellular trafficking of membrane proteins. J. Clin Invest. 2000;106:983–993. doi: 10.1172/JCI8914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Grimmer S, Ying M, Walchli S, van Deurs B, Sandvig K. Golgi vesiculation induced by cholesterol occurs by a dynamin- and cPLA2-dependent mechanism. Traffic. 2005;6:144–156. doi: 10.1111/j.1600-0854.2005.00258.x. [DOI] [PubMed] [Google Scholar]
- 119.Geijsen N, Dijkers PF, Lammers JJ, Koenderman L, Coffer PJ. Cytokine-mediated cPLA2 phosphorylation is regulated by multiple MAPK family members. FEBS Lett. 2000;471:83–88. doi: 10.1016/s0014-5793(00)01373-9. [DOI] [PubMed] [Google Scholar]
- 120.Muthalif MM, Hefner Y, Canaan S, Harper J, Zhou H, Parmentier JH, Aebersold R, Gelb MH, Malik KU. Functional interaction of calcium-/calmodulin-dependent protein kinase II and cytosolic phospholipase A2. J Biol Chem. 2001;276:39653–39660. doi: 10.1074/jbc.M103136200. [DOI] [PubMed] [Google Scholar]
- 121.Xu J, Weng YI, Simonyi A, Krugh BW, Liao Z, Weisman GA, Sun GY. Role of PKC and MAPK in cytosolic PLA2 phosphorylation and arachadonic acid release in primary murine astrocytes. J Neurochem. 2002;83:259–270. doi: 10.1046/j.1471-4159.2002.01145.x. [DOI] [PubMed] [Google Scholar]
- 122.Wooten RE, Willingham MC, Daniel LW, Leslie CC, Rogers LC, Sergeant S, O'Flaherty JT. Novel translocation responses of cytosolic phospholipase A2α fluorescent proteins. Biochim Biophys Acta. 2008;1783:1544–1550. doi: 10.1016/j.bbamcr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Das S, Cho W. Roles of catalytic domain residues in interfacial binding and activation of group IV cytosolic phospholipase A2. J Biol Chem. 2002;277:23838–23846. doi: 10.1074/jbc.M202322200. [DOI] [PubMed] [Google Scholar]
- 124.Mosior M, Six DA, Dennis EA. Group IV cytosolic phospholipase A2 binds with high affinity and specificity to phosphatidylinositol 4,5-bisphosphate resulting in dramatic increases in activity. J Biol Chem. 1998;273:2184–2191. doi: 10.1074/jbc.273.4.2184. [DOI] [PubMed] [Google Scholar]
- 125.Six DA, Dennis EA. Essential Ca2+-independent role of the group IVA cytosolic phospholipase A2 C2 domain for interfacial activity. J Biol Chem. 2003;278:23842–23850. doi: 10.1074/jbc.M301386200. [DOI] [PubMed] [Google Scholar]
- 126.De Matteis M, Godi A, Corda D. Phosphoinositides and the Golgi complex. Curr Opin Cell Biol. 2002;14:434–447. doi: 10.1016/s0955-0674(02)00357-5. [DOI] [PubMed] [Google Scholar]
- 127.Roux A, Cuvelier D, Nassoy P, Prost J, Bassereau P, Goud B. Role of curvature and phase transition in lipid sorting and fission of membrane tubules. EMBO J. 2005;24:1537–1545. doi: 10.1038/sj.emboj.7600631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Schaeffer EL, Bassi F, Jr, Gattaz WF. Inhibition of phospholipase A2 activity reduces membrane fluidity in rat hippocampus. J Neural Transm. 2005;112:641–647. doi: 10.1007/s00702-005-0301-9. [DOI] [PubMed] [Google Scholar]
- 129.Derganc J. Curvature-driven lateral segregation of membrane constituents in Golgi cisternae. Phys Biol. 2007;4:317–324. doi: 10.1088/1478-3975/4/4/008. [DOI] [PubMed] [Google Scholar]
- 130.Staneva G, Angelova MI, Koumanov K. Phospholipase A2 promotes raft budding and fission from giant liposomes. Chemistry and physics of lipids. 2004;129:53–62. doi: 10.1016/j.chemphyslip.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 131.Klapisz E, Masliah J, Bereziat G, Wolf C, Koumanov KS. Sphingolipids and cholesterol modulate membrane susceptibility to cytosolic phospholipase A2. J Lipid Res. 2000;41:1680–1688. [PubMed] [Google Scholar]
- 132.Ben-Tekaya H, Kahn RA, Hauri HP. ADP ribosylation factors 1 and 4 and group VIA phospholipase A regulate morphology and intraorganellar traffic in the endoplasmic reticulum-Golgi intermediate compartment. Mol Biol Cell. 2010;21:4130–4140. doi: 10.1091/mbc.E10-01-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Appenzeller-Herzog C, Hauri HP. The ER-Golgi intermediate compartment (ERGIC): in search of its identity and function. J Cell Sci. 2006;119:2173–2183. doi: 10.1242/jcs.03019. [DOI] [PubMed] [Google Scholar]
- 134.Sannerud R, Saraste J, Goud B. Retrograde traffic in the biosynthetic-secretory route: pathways and machinery. Curr Opin Cell Biol. 2003;15:438–445. doi: 10.1016/s0955-0674(03)00077-2. [DOI] [PubMed] [Google Scholar]
- 135.Larsson PK, Claesson HE, Kennedy BP. Multiple splice variants of the human calcium-independent phospholipase A2 and their effect on enzyme activity. J Biol Chem. 1998;273:207–214. doi: 10.1074/jbc.273.1.207. [DOI] [PubMed] [Google Scholar]
- 136.Ackermann EJ, Kempner ES, Dennis EA. Ca2+-independent cytosolic phospholipase A2 from macrophage-like P388D1 cells. Isolation and characterization. J Biol Chem. 1994;269:9227–9233. [PubMed] [Google Scholar]
- 137.de Figueiredo P, Drecktrah D, Polizotto RS, Cole NB, Lippincott-Schwartz J, Brown WJ. Phospholipase A2 antagonists inhibit constitutive retrograde membrane traffic to the endoplasmic reticulum. Traffic. 2000;1:504–511. doi: 10.1034/j.1600-0854.2000.010608.x. [DOI] [PubMed] [Google Scholar]
- 138.Tomas M, Martinez-Alonso E, Ballesta J, Martinez-Menarguez JA. Regulation of ER-Golgi intermediate compartment tubulation and mobility by COPI coats, motor proteins and microtubules. Traffic. 2010;11:616–625. doi: 10.1111/j.1600-0854.2010.01047.x. [DOI] [PubMed] [Google Scholar]
- 139.Gregory A, Hayflick SJ. Genetics of neurodegeneration with brain iron accumulation. Curr Neurol Neurosci Rep. 2011;11:254–261. doi: 10.1007/s11910-011-0181-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kurian MA, Morgan NV, MacPherson L, Foster K, Peake D, Gupta R, Philip SG, Hendriksz C, Morton JE, Kingston HM, Rosser EM, Wassmer E, Gissen P, Maher ER. Phenotypic spectrum of neurodegeneration associated with mutations in the PLA2G6 gene (PLAN) Neurology. 2008;70:1623–1629. doi: 10.1212/01.wnl.0000310986.48286.8e. [DOI] [PubMed] [Google Scholar]
- 141.Malik I, Turk J, Mancuso DJ, Montier L, Wohltmann M, Wozniak DF, Schmidt RE, Gross RW, Kotzbauer PT. Disrupted membrane homeostasis and accumulation of ubiquitinated proteins in a mouse model of infantile neuroaxonal dystrophy caused by PLA2G6 mutations. Am J Pathol. 2008;172:406–416. doi: 10.2353/ajpath.2008.070823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shinzawa K, Sumi H, Ikawa M, Matsuoka Y, Okabe M, Sakoda S, Tsujimoto Y. Neuroaxonal dystrophy caused by group VIA phospholipase A2 deficiency in mice: a model of human neurodegenerative disease. J Neurosci. 2008;28:2212–2220. doi: 10.1523/JNEUROSCI.4354-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Morikawa RK, Aoki J, Kano F, Murata M, Yamamoto A, Tsujimoto M, Arai H. Intracellular phospholipase A1γ (iPLA1γ) is a novel factor involved in coat protein complex I- and Rab6-independent retrograde transport between the endoplasmic reticulum and the Golgi complex. J Biol Chem. 2009;284:26620–26630. doi: 10.1074/jbc.M109.038869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sato S, Inoue H, Kogure T, Tagaya M, Tani K. Golgi-localized KIAA0725p regulates membrane trafficking from the Golgi apparatus to the plasma membrane in mammalian cells. FEBS Lett. 2010;584:4389–4395. doi: 10.1016/j.febslet.2010.09.047. [DOI] [PubMed] [Google Scholar]
- 145.Inoue A, Aoki A. Phospholipase A1: structure, location and function. Future Lipidol. 2006;1:687–700. [Google Scholar]
- 146.Nakajima K, Sonoda H, Mizoguchi T, Aoki J, Arai H, Nagahama M, Tagaya M, Tani K. A novel phospholipase A1 with sequence homology to a mammalian Sec23p-interacting protein, p125. J Biol Chem. 2002;277:11329–11335. doi: 10.1074/jbc.M111092200. [DOI] [PubMed] [Google Scholar]
- 147.Darchen F, Goud B. Multiple aspects of Rab protein action in the secretory pathway: focus on Rab3 and Rab6. Biochimie. 2000;82:375–384. doi: 10.1016/s0300-9084(00)00219-4. [DOI] [PubMed] [Google Scholar]
- 148.Higgs HN, Glomset JA. Identification of a phosphatidic acid-preferring phospholipase A1 from bovine brain and testis. Proc Natl Acad Sci U S A. 1994;91:9574–9578. doi: 10.1073/pnas.91.20.9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.McMullen TW, Li J, Sheffield PJ, Aoki J, Martin TW, Arai H, Inoue K, Derewenda ZS. The functional implications of the dimerization of the catalytic subunits of the mammalian brain platelet-activating factor acetylhydrolase (Ib) Protein Eng. 2000;13:865–871. doi: 10.1093/protein/13.12.865. [DOI] [PubMed] [Google Scholar]
- 150.Scales SJ, Scheller RH. Lipid membranes shape up. Nature. 1999;401:123–124. doi: 10.1038/43582. [DOI] [PubMed] [Google Scholar]
- 151.Modregger J, Schmidt AA, Ritter B, Huttner WB, Plomann M. Characterization of Endophilin B1b, a brain-specific membrane-associated lysophosphatidic acid acyl transferase with properties distinct from endophilin A1. J Biol Chem. 2003;278:4160–4167. doi: 10.1074/jbc.M208568200. [DOI] [PubMed] [Google Scholar]
- 152.Schmidt A, Wolde M, Thiele C, Fest W, Kratzin H, Podtelejnikov AV, Witke W, Huttner WB, Soling HD. Endophilin I mediates synaptic vesicle formation by transfer of arachidonate to lysophosphatidic acid. Nature. 1999;401:133–141. doi: 10.1038/43613. [DOI] [PubMed] [Google Scholar]
- 153.Weigert R, Silletta MG, Spano S, Turacchio G, Cericola C, Colanzi A, Senatore S, Mancini R, Polishchuk EV, Salmona M, Facchiano F, Burger KN, Mironov A, Luini A, Corda D. CtBP/BARS induces fission of Golgi membranes by acylating lysophosphatidic acid. Nature. 1999;402:429–433. doi: 10.1038/46587. [DOI] [PubMed] [Google Scholar]
- 154.Milosevic I, Giovedi S, Lou X, Raimondi A, Collesi C, Shen H, Paradise S, O'Toole E, Ferguson S, Cremona O, De Camilli P. Recruitment of endophilin to clathrin-coated pit necks is required for efficient vesicle uncoating after fission. Neuron. 2011;72:587–601. doi: 10.1016/j.neuron.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gallop JL, Butler PJ, McMahon HT. Endophilin and CtBP/BARS are not acyl transferases in endocytosis or Golgi fission. Nature. 2005;438:675–678. doi: 10.1038/nature04136. [DOI] [PubMed] [Google Scholar]
- 156.Field FJ, Albright E, Mathur S. Inhibition of acylcoenzyme A: cholesterol acyltransferase activity by PD128O42: effect on cholesterol metabolism and secretion in CaCo-2 cells. Lipids. 1991;26:1–8. doi: 10.1007/BF02544016. [DOI] [PubMed] [Google Scholar]
- 157.Roth BD, Blankley CJ, Hoefle ML, Holmes A, Roark WH, Trivedi BK, Essenburg AD, Kieft KA, Krause BR, Stanfield RL. Inhibitors of acyl-CoA:cholesterol acyltransferase. 1. Identification and structure-activity relationships of a novel series of fatty acid anilide hypocholesterolemic agents. J Med Chem. 1992;35:1609–1617. doi: 10.1021/jm00087a016. [DOI] [PubMed] [Google Scholar]
- 158.Brown WJ, Schmidt JA. Use of acyltransferase inhibitors to block vesicular traffic between the ER and Golgi complex. Methods Enzymol. 2005;404:115–125. doi: 10.1016/S0076-6879(05)04012-7. [DOI] [PubMed] [Google Scholar]
- 159.Chambers K, Brown WJ. Characterization of a novel CI-976-sensitive lysophospholipid acyltransferase that is associated with the Golgi complex. Biochem Biophys Res Commun. 2004;313:681–686. doi: 10.1016/j.bbrc.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 160.Chambers K, Judson B, Brown WJ. A unique lysophospholipid acyltransferase (LPAT) antagonist, CI-976, affects secretory and endocytic membrane trafficking pathways. J Cell Sci. 2005;118:3061–3071. doi: 10.1242/jcs.02435. [DOI] [PubMed] [Google Scholar]
- 161.Drecktrah D, Chambers K, Racoosin EL, Cluett EB, Gucwa A, Jackson B, Brown WJ. Inhibition of a Golgi complex lysophospholipid acyltransferase induces membrane tubule formation and retrograde trafficking. Mol Biol Cell. 2003;14:3459–3469. doi: 10.1091/mbc.E02-11-0711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Harte RA, Yeaman SJ, Jackson B, Suckling KE. Effect of membrane environment on inhibition of acyl-CoA:cholesterol acyltransferase by a range of synthetic inhibitors. Biochim. Biophys. Acta. 1995;1258:241–250. doi: 10.1016/0005-2760(95)00113-q. [DOI] [PubMed] [Google Scholar]
- 163.Schmidt JA, Yvone GM, Brown WJ. Membrane topology of human AGPAT3 (LPAAT3) Biochem Biophys Res Commun. 2010;397:661–667. doi: 10.1016/j.bbrc.2010.05.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Henneberry AL, Wright MM, McMaster CR. The major sites of cellular phospholipid synthesis and molecular determinants of fatty acid and lipid head group specificity. Mol Biol Cell. 2002;13:3148–3161. doi: 10.1091/mbc.01-11-0540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Brown WJ, Plutner H, Drecktrah D, Judson BL, Balch WE. The lysophospholipid acyltransferase antagonist CI-976 inhibits a late step in COPII vesicle budding. Traffic. 2008;9:786–797. doi: 10.1111/j.1600-0854.2008.00711.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Kooijman EE, Chupin V, de Kruijff B, Burger KN. Modulation of membrane curvature by phosphatidic acid and lysophosphatidic acid. Traffic. 2003;4:162–174. doi: 10.1034/j.1600-0854.2003.00086.x. [DOI] [PubMed] [Google Scholar]
- 167.Asp L, Kartberg F, Fernandez-Rodriguez J, Smedh M, Elsner M, Laporte F, Barcena M, Jansen KA, Valentijn JA, Koster AJ, Bergeron JJ, Nilsson T. Early stages of Golgi vesicle and tubule formation require diacylglycerol. Mol Biol Cell. 2009;20:780–790. doi: 10.1091/mbc.E08-03-0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Baron CL, Malhotra V. Role of diacylglycerol in PKD recruitment to the TGN and protein transport to the plasma membrane. Science. 2002;295:325–328. doi: 10.1126/science.1066759. [DOI] [PubMed] [Google Scholar]
- 169.Sarri E, Sicart A, Lazaro-Dieguez F, Egea G. Phospholipid synthesis participates in the regulation of diacylglycerol required for membrane trafficking at the Golgi complex. J Biol Chem. 2011;286:28632–28643. doi: 10.1074/jbc.M111.267534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Yeaman C, Ayala MI, Wright JR, Bard F, Bossard C, Ang A, Maeda Y, Seufferlein T, Mellman I, Nelson WJ, Malhotra V. Protein kinase D regulates basolateral membrane protein exit from trans-Golgi network. Nat Cell Biol. 2004;6:106–112. doi: 10.1038/ncb1090. [DOI] [PMC free article] [PubMed] [Google Scholar]