Abstract
Objectives:
Injection drug use plays the most important role in transmission of hepatitis C. In Iran, surveys have been conducted on various high risk groups but this is the first announcement based study for hepatitis C virus HCV prevalence among cases with history of intravenous drug using (IVDU) in the country.
Methods:
The announcement-based detection and follow-up of patients with anti-HCV positive project in volunteers with history of intravenous drug using was conducted in Isfahan province. At the first step, six focus groups were conducted and 2 pilot studies were carried out in two cities to design the main study. Comprehensive community announcement was done in all of public places and for physicians. The volunteers were invited to Isfahan reference laboratories and the serum samples were sent to Infectious Diseases Research Center Laboratory in standard conditions and HCV-Ab was tested by ELISA method.
Results:
In this study, 1,747 individuals that are estimated 50% of all expected intravenous drug users in the community were presented themselves. The most important reasons of success in recruiting volunteers in this study were the perfect propaganda, appropriate cooperation of lab staffs, continuous evaluation and good cooperation in Isfahan province administrations. HCV-Ab was detected in 34% of them and the HCV-Ab positives were sent for further follow-up procedures including confirmatory test, education, and treatment.
Conclusions:
In spite of some limitations to select real cases, this study was considered as a successful experience. Compared to the surveys in Iran on HCV prevalence in intravenous drug users, the results of this study, which was based on volunteers by announcement seems to be noteworthy.
Keywords: Announcement, Community, Hepatitis C, Intravenous drug using
INTRODUCTION
Drug addiction is considered as a major health and social problem due to its several social and health consequences. Evidences suggest that, there are 200 million addict persons worldwide from which 13.2 million are IV drug users (IDUs) and more than 78% of these IDUs live in developing countries.[1] Prevalence of IDU is increasing in Central and South Asia. In the last 20 years, the prevalence of IDU in Iran has had a large increase due to various reasons including Afghan refugees. It is estimated that 200-300 thousand IDUs are living in Iran, currently.[2–4]
IDUs consider a high-risk group for parenterally transmitted infections including HCV.[5]
During past decades, HCV infection has become the cause of the second major epidemic of viral infection after human immunodeficiency virus (HIV). So, it is considered as a critical public health problem worldwide. HCV infection can progress to chronic hepatitis, cirrhosis, and hepatocellular carcinoma. Chronic hepatitis C is the second common cause of end-stage liver disease and the leading cause of liver transplants in Iran and many countries.[6–8]
Though, it is estimated that the incidence of new HCV infection would be decreased over the next decade, but its related mortality and costs will increase. The prevalence of HCV infection among high risk populations is reported 30-50%.[9,10] IDU considers the most important risk factor for HCV infection. The worldwide prevalence of HCV infection is reported 50-100% among IDUs and in Iran it is reported 38-47%, according to findings of different studies.[11–14]
Considering that most patients with acute hepatitis C are asymptomatic and risky parenteral and non-parenteral exposures would spread the infection among general population, it seems that screening of high risk population for HCV infection would prevent the increasing rate of the infection and its related complications. The potential for HCV infection in developing countries is diverse, so reduction in the risk of HCV infection among IDUs, the main risk factor for the infection, is an important issue for overall control of the disease.[15]
On the other hand, in order to assess an effective program for controlling HCV infection, determining the epidemiology of the infection in each region is needed.
Most of the studies in Iran for determining the prevalence of HCV infection have been conducted in particular groups such as prisoners, IDUs or people who were arrested by police, etc.[16] The prevalence of HCV infection among IDUs through a community based study has not been studied yet. It seems that, HCV infection screening among IDUs through public recall is a useful method due to the active involvement of the people. It would also improve the knowledge of general population about the disease.
Thus, the aim of this study was to determine the prevalence of HCV infection among IDUs or those people with background of IDU through a community announcement-based study, for the first time in Iran.
METHODS
In this cross-sectional study, volunteers with history of intravenous drug using in Isfahan province enrolled. The protocol was approved by the Institutional Review Board of Isfahan Infectious and Tropical Disease Research Center and Medical Ethics Committee of Isfahan University of Medical Sciences.
At the first step, six focus groups were conducted to determine the methods of identification of IDUs and measurements of HCV infection among them. The method of choice for this purpose was determined using content analysis and member check methods. After that, the protocol of the study was designed and 2 pilot studies were carried out in two cities (Tiran and Golpayegan) of Isfahan province to design the main study.
The protocol of the study was sent to all cities of Isfahan provinces. Comprehensive community announcement was done in all of public places and for physicians in all cities.
According to the protocol, all IDUs and those with background of IDU were invited to refer to the reference laboratory of each city for HCV infection test. Laboratory staffs in all cities were trained for appropriate contact with referees.
Written informed consent was obtained from each volunteer with the assurance that all obtained information would be just for research purposes and would remain confidential. Characteristics of studied population including demography, socioeconomic status and history of risky parenteral and non-parenteral exposures were recorded using a standard questionnaire.
Five ml venous blood sample obtained from each person and centrifuged and the sera were separated and transferred to the sterile tubes having the corresponding codes. The extracted sera were stored at -20 °C, until the specimen collection time (10 days) completed. After that period, all stored sera were transferred to the laboratory of Isfahan Infectious and Tropical Diseases Research Center for laboratory processing. HCV antibody (HCV-Ab) was detected using ELISA third generation (DIA. PRO, Italy) kit.
The progress of the project in each city was supervised by the hepatitis Committee of each city and reported to the Disease Control Unit of Isfahan Province Health Center.
Obtained data was analyzed using SPSS (ver 15, SPSS Inc., Chicago, IL) software. The experiments of persons which involved in the project was collected and analyzed by Content analysis.
RESULTS
In this study, 1,747 individuals that are estimated 50% of all expected intravenous drug users in the community were presented themselves. Mean age of volunteers was 35 ± 9.4 (range17-64) years old. The characteristics of studied population are presented in Table 1. HCV-Ab was detected in 595 (34%) of the volunteers. The prevalence of positive HCV-Ab in the cities of Isfahan province is presented in Table 2.
Table 1.
The characteristics of IDUs or those people with background history of IDU in Isfahan province
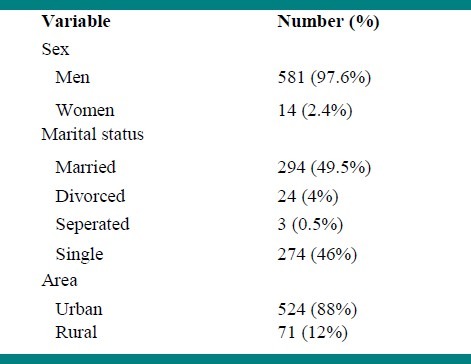
Table 2.
The prevalence of anti HCV antibody inIDUs or those people with background history of IDU in the cities of Isfahan province
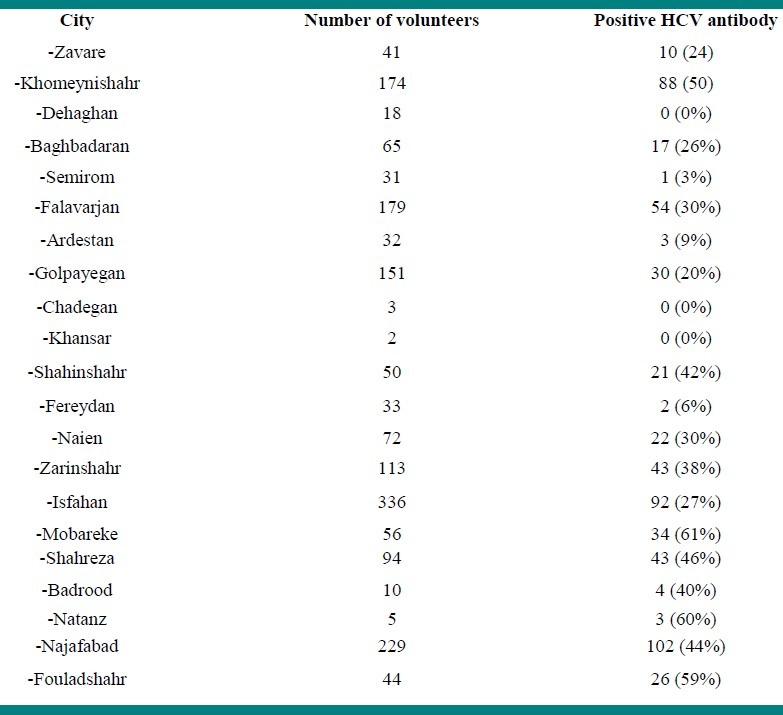
Volunteers with positive anti-HCV antibody were referred to Disease Control Unit of Isfahan Province Health Center for confirmatory test, education, and treatment.
The findings of content analysis indicated that, the most important reasons of success in recruiting volunteers in this study were the perfect propaganda, appropriate cooperation of lab staffs, continuous evaluation, and good cooperation in Isfahan province administrations.
DISCUSSION
In this community announcement-based study, the prevalence of HCV-Ab was investigated among IDUs or those people with background of IDU in Isfahan city-Iran. The results indicated that 34% of studied population had positive HCV-Ab.
It is estimated that 3,400 IDUs or those people with background of IDU are living in Isfahan province, except for those who identified in prisons, Drop in center (DIC) and other high risk centers.
Though, 50% of all expected intravenous drug users in the community were studied in this research, but it was considered as a successful experience in Iran, because in a short period (10 days) 50% of estimated IDUs referred and were studied.
Several studies have reported the prevalence of HCV-Ab among IDUs in different countries worldwide and different cities of Iran. As mentioned, current study was the first community announcement-based study in Iran and most previous studies in Iran were limited to special high risk groups of population, so the results of this study would be helpful for designing further nationwide studies.
Zamani et al. in Tehran investigated the prevalence of hepatitis C virus infection among injecting drug users in a community-based setting. In their study, IDUs from a drop-in center and neighbouring parks and streets in a drug-populated neighbourhood in Tehran enrolled. According to their results, 52% of IDUs were positive for HCV-Ab. There was positive correlation between HCV infection and duration of drug injection, lifetime incarcerations and history of being tattooed in prison.[17]
Mirahmadizadeh et al. in Shiraz indicated that HCV-Ab was positive in 80.1% of IDUs.[18]
The prevalence of HCV infection among IDU prisoners has reported to be 45%, 38%, 47%, 64.8% and 88.9% in Tehran, Hamadan, Zanjan, Bandar Abbas and Guilan, respectively.[12,19–22]
In another study in Lebanon, the prevalence of HCV-Ab among IDUs was 52.8% (56/106). Among IUDs with positive HCV-Ab there was a trend of increased risky behaviors.[23]
Xia et al. in China, in an epidemiological study showed that the prevalence of HCV infection among IDUs was 61.4%.[24]
It seems that reported prevalence of HCV-Ab among IDUs of Isfahan province is lower than those reported by others. However, considering that the studied population in this research was from general population, the obtained result could be explained.
The prevalence of HCV infection was higher in Mobareke (61%), Natanz (60%) and Fouladshahr (59%). It was lower in Semirom (3%), Fereydan (6%) and Ardestan (9%). In some cities such as Khansar and Chadegan, the number of volunteers was not enough for determining conclusive result. Further studies are needed for understanding the role of related risk factors in geographic variations of HCV prevalence in drug users.
Previous evidences suggest that variations in drug-related risk factors, sexual risk behavior and socioeconomic status are responsible for geographic variations of HCV infection in IDUs.[25] In this study, we did not study the relation between mentioned factors with HCV infection. It is recommended to determine mentioned relationship in future studies.
The limitations of this study were as follows: some limitations to select real cases, to determine the correlates of HCV infection in this high risk population. However, indication of how different variables influence on the infection of HCV would be helpful in this regard.
In addition, considering the importance of HCV genotypes as an epidemiological marker in providing the historical origin of the infection and their probable role in the clinical presentation, management and outcome of HCV infection,[26] it is recommended to study this variable in future studies.
In conclusion, the result of this study which was based on volunteers by announcement seems to be noteworthy. The findings of current study indicated that there are asymptomatic cases of HCV infection among general population. Moreover, there are large varieties of risk exposures, which are not limited to high risk population such as IDUs. So, it is recommended using this experience, we should implement proper pragmatic strategies for further evaluation of the infection with consideration of mentioned limitations in large scale. In addition, efforts should be made to improve the primary prevention of HCV infection by education, counselling for risk reduction and treatment of substance abuse, and finally HCV screening.
Footnotes
Source of Support: This paper is a part of research project which full article is published in Farsi.
Conflict of Interest: None declared
REFERENCES
- 1.Des Jarlais DC, Semaan S. HIV prevention for injecting drug users: The first 25 years and counting. Psychosom Med. 2008;70:606–11. doi: 10.1097/PSY.0b013e3181772157. [DOI] [PubMed] [Google Scholar]
- 2.Mojtahedzadeh V, Razani N, Malekinejad M, Vazirian M, Shoaee S, Saberi Zafarghandi MB, et al. Injection drug use in Rural Iran: Integrating HIV prevention into Iran's rural primary health care system. AIDS Behav. 2008;12(4 Suppl):S7–12. doi: 10.1007/s10461-008-9408-y. [DOI] [PubMed] [Google Scholar]
- 3.Razzaghi EM, Movaghar AR, Green TC, Khoshnood K. Profiles of risk: A qualitative study of injecting drug users in Tehran, Iran. Harm Reduct J. 2006;3:12. doi: 10.1186/1477-7517-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Drug Report. Vienna: UNODC; 2005. United Nations Office on Drugs and Crime. [Google Scholar]
- 5.Zeldis JB, Jain S, Kuramoto IK, Richards C, Sazama K, Samuels S, et al. Seroepidemiology of viral infections among intravenous drug users in northern California. West J Med. 1992;156:30–5. [PMC free article] [PubMed] [Google Scholar]
- 6.Sy T, Jamal MM. Epidemiology of hepatitis C virus (HCV) infection. Int J Med Sci. 2006;3:41–6. doi: 10.7150/ijms.3.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Saadany S, Coyle D, Giulivi A, Afzal M. Economic burden of hepatitis C in Canada and the potential impact of prevention. Results from a disease model. Eur J Health Econ. 2005;6:159–65. doi: 10.1007/s10198-004-0273-y. [DOI] [PubMed] [Google Scholar]
- 8.National Institutes of Health Consensus Development Conference Panel statement: Management of hepatitis C. Hepatology. 1997;26(3Suppl 1):2S–10S. doi: 10.1002/hep.510260701. [DOI] [PubMed] [Google Scholar]
- 9.Burattini M, Massad E, Rozman M, Azevedo R, Carvalho H. Correlation between HIV and HCV in Brazilian prisoners: Evidence for parenteral transmission inside prison. Rev Saude Publica. 2000;34:431–6. doi: 10.1590/s0034-89102000000500001. [DOI] [PubMed] [Google Scholar]
- 10.Weild AR, Gill ON, Bennett D, Livingstone SJ, Parry JV, Curran L. Prevalence of HIV, hepatitis B, and hepatitis C antibodies in prisonersin England and Wales: A national survey. Commun Dis Public Health. 2000;3:121–6. [PubMed] [Google Scholar]
- 11.Alavian SM, Fallahian F. Epidemiology of Hepatitis C in Iran and the World. Shiraz E Med J. 2009;10:162–72. [Google Scholar]
- 12.Mohammad-Alizadeh AH, Alavian SM, Jafari K, Yazdi N. Prevalence of hepatitis C virus infection andits related risk factors in drug abuser prisoners in Hamedan--Iran. World J Gastroenterol. 2005;11:4085–9. doi: 10.3748/wjg.v11.i26.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hosseini Asl K, Avijgan M, Mohamadnejad M. High prevalence of HBV, HCV, and HIV infections in Gypsy population residing in Shahr-E-Kord. Arch Iran Med. 2004;7:20–2. [Google Scholar]
- 14.Hajiani E, Masjedizadeh R, Hashemi J, Azmi M, Rajabi T. Hepatis C virus transmission and its risk factors within families of patients infected with hepatitis C virus in southern Iran: Khuzestan. World J Gastroenterol. 2006;12:7025–8. doi: 10.3748/wjg.v12.i43.7025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavanchy D. Hepatitis C: Public health strategies. J Hepatol. 1999;31(Suppl 1):146–51. doi: 10.1016/s0168-8278(99)80392-4. [DOI] [PubMed] [Google Scholar]
- 16.Zobeiri M, Adibi P, Alavian SM. Intravenous Drug Use and Hepatitis C Virus in Iran. Hepat Mon. 2011:11. doi: 10.5812/kowsar.1735143X.797. [In press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zamani S, Ichikawa S, Nassirimanesh B, Vazirian M, Ichikawa K, Gouya MM, et al. Prevalence and correlates of hepatitis C virus infection among injecting drug users in Tehran. Int J Drug Policy. 2007;18:359–63. doi: 10.1016/j.drugpo.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 18.Mirahmadizadeh AR, Kadivar MR, Hemmati AR, Javadi A. Infection with HIV and hepatitis C and B viruses among injecting drug users in Shiraz, Southern Iran. International Conference on AIDS. 2004;15:16–9. [Google Scholar]
- 19.Zali MR, Aghazadeh R, Nowroozi A, Amir-Rasouly H. Anti-HCV antibody among Iranian IV drug users: Is it a serious problem. Arch Iran Med. 2001;4:115–9. [Google Scholar]
- 20.Davoodian P, Dadvand H, Mahoori K, Amoozandeh A, Salavati A. Prevalence of selected sexually and blood-borne infections in Injecting drug abuser inmates of bandarabbas and roodan correction facilities, Iran, 2002. Braz J Infect Dis. 2009;13:356–8. doi: 10.1590/S1413-86702009000500008. [DOI] [PubMed] [Google Scholar]
- 21.Khani M, Vakili MM. Prevalence and risk factors of HIV, hepatitis B virus and hepatitis C virus infections in drug addicts among Zanjan prisoners. Arch Iran Med. 2003;6:1–4. [Google Scholar]
- 22.Mohtasham Amiri Z, Rezvani M, Jafari Shakib R, Jafari Shakib A. Prevalence of hepatitis C virus in fection and risk factors of drug using prisoners in Guilan province. East Mediterr Health J. 2007;13:250–6. [PubMed] [Google Scholar]
- 23.Mahfoud MZ, Kassak K, Kreidieh K, Shamra S, Ramia S. Distribution of hepatitis C virus genotypes among injecting drug users in Lebanon. Virol J. 2010;13:7–96. doi: 10.1186/1743-422X-7-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xia X, Luo J, Bai J, Yu R. Epidemiology of hepatitis C virus infection among injection drug users in China: Systematic review and meta-analysis. Public Health. 2008;122:990–1003. doi: 10.1016/j.puhe.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 25.Mathei C, Robaeys G, van Damme P, Buntinx F, Verrando R. Prevalence of hepatitis C in drug users in Flanders: Determinants and geographic differences. Epidemiol Infect. 2005;133:127–36. doi: 10.1017/s0950268804002973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mathei C, Wollants E, Verbeeck J, Van Ranst M, Robaeys G, Van Damme P, et al. Molecular epidemiology of hepatitis C among drug users in Flanders, Belgium: Association of genotypes with clinical parameters with sex and drug- related risk behaviors. Eur J Clin Microbiol Infect Dis. 2005;24:514–22. doi: 10.1007/s10096-005-1376-9. [DOI] [PubMed] [Google Scholar]


