Abstract
Objectives:
Iron chelators, such as maltol and kojic acid, have antioxidant and anti-inflammatory properties. They may have beneficial effects on inflammatory bowel disease (IBD) because iron can develop and aggravate inflammation in IBD. In the present study, the effect of selected iron chelators and anti-oxidants were evaluated on a model of trinitrobenzene sulfonic acid (TNBS)-induced colitis.
Methods:
Colitis was induced with instillation of 75 mg/kg TNBS in 0.25 ml ethanol 50% via the anus in fasted male Wistar rats. The animals were assigned randomly to 12 groups (n = 6) and treated once daily, started 2 hours before colitis induction, with normal saline (5 ml/kg), maltol (70, 140, 280 mg/kg), kojic acid (75, 150, 300 mg/kg), vitamin E (400 mg/kg), deferiprone (L1) (150 mg/kg) and prednisolone (4 mg/kg) orally and deferoxamine (50 mg/kg) intraperitoneally for 5 days. In the sixth day, rats were scarified and colon tissues were assessed macroscopically and pathologically.
Results:
Maltol (280 mg/kg) was able to reduce colon weight / length ratio, ulcer index and total colitis index similar to prednisolone, deferoxamine and deferiprone as positive controls. However, kojic acid and vitamin E could not significantly alleviate macroscopic and/or pathologic features of inflammation in comparison to normal saline.
Conclusions:
Maltol with the highest test dose was capable to protect against experimentally induced colitis. Kojic acid and vitamin E were not effective in this animal model of colon inflammation. More detailed studies are warranted to explore the mechanisms involved in anti-colitic property of maltol and to explain ineffectiveness of kojic acid and vitamin E.
Keywords: Anti-oxidant, Inflammatory bowel disease, Iron chelator, Kojic acid, Maltol
INTRODUCTION
Ulcerative colitis (UC) and Crohn's disease (CD) are two major categories of inflammatory bowel diseases (IBDs). Although the etiology and pathophysiology of IBD still remain unclear, immune dysfunction, reactive oxygen species (ROS), inflammatory mediators and cytokines play important roles in its development and recurrence.[1] It is supposed that homeostasis is disrupted in IBD patients because of over-expression of inflammatory cytokines (TNF-α, IL-1, IL-6) and/or lower expression of regulatory or anti-inflammatory cytokines (IL-2, IL-4, IL-10, TGF-β).[2] Common drugs that are administrated for the management of IBD include sulfasalazine, 5-ASA derivatives and glucocorticoids. Immunosuppressants, antibiotics and monoclonal antibodies (Infliximab) are also occasionally used for intractable disease conditions.[3] These therapeutic agents have side effects and they could not appropriately cure IBD patients.[4] Patients with IBD usually are also suffering from iron deficiency anemia because they lose blood especially in gastrointestinal (GI) tract. So patients have to use iron supplements usually by oral route to compensate this iron waste, but iron itself can develop inflammation because it can produce harmful oxygen radicals by Fenton reaction.[5] In the Fenton reaction, hydroxyl and hydrogen superoxide radicals are produced when iron interacts with hydrogen peroxide. Assembling of oxygen-free radicals lead to tissue damage and aggravation of inflammation because they increase permeability of mucus and vessels to water and inflammatory mediators and they stimulate migration of neutrophils and cytokines production.[6] So iron chelators can reduce inflammation by inhibiting oxygen radical production.[7] Maltol and kojic acid as iron chelators seem to be safe and their toxicity as food, drug or cosmetic agents with usual therapeutic doses have not been reported yet.[8,9] Maltol (3-hydroxy-2-methyl-4-pyron) is made from carbohydrate dehydration and found in coffee and soybean seeds. Maltol can protect tissues against free radicals because it is a strong iron chelator and free radical scavenger.[10] It can protect DNA against free radicals and oxidizing agents and result in anti-neoplasm and antioxidant effect.[11] Kojic acid (5-hydroxy-2-methyl-4-pyron) is an iron chelator and antioxidant agent too. It is an active metabolite of the fungus Aspergillus oryzae and extensively used in cosmetic preparations as de-pigmentary and skin whitening agent.[12] In the food industry, kojic acid is used as a flavor enhancer and food antioxidant for improving preservation. Kojic acid has antifungal, insecticidal, anticancer and bacteriostatic effects in experimental studies.[13] It can reduce ROS in the tissue and has antitumor and anti-neoplasm effects.[14] This study was designed to investigate the therapeutic effects of maltol and kojic acid on TNBS-induced colitis in rats in comparison to well-known iron chelators (deferiprone and deferoxamine), antioxidant (vitamin E) and reference drug (prednisolone).
METHODS
Animals
Male Wistar rats (180-220 g) bred and kept in animal house of Isfahan School of Pharmacy were allowed to adapt to laboratory environment for one week. They had free access to tap water and rat chow pellets and were housed singly in wire-bottomed cages under uniform and normal conditions of temperature, humidity and light/dark cycles. The experiments were carried out according to the ethical and research committee protocol of Isfahan University of Medical Sciences, Isfahan, Iran.
Chemicals
Prednisolone powder was procured from Iran Hormone Co. (Tehran, Iran). Vitamin E, kojic acid, maltol, deferoxamine and sulfasalazine procured from Sigma Co. (St. Louis, MO). Deferiprone (L1) powder was procured from Avesina Co. (Tehran, Iran). Maltol, deferiprone and prednisolone were reconstituted in tween 80 (1%) while kojic acid and deferoxamine were dissolved in normal saline (0.9%). Vitamin E was allowed to be liquefied in laboratory temperature (22 ± 2°C) and then was administered at specified dose.
Grouping
The animals were randomly divided into following groups of rats, six in each. All the treatments were made 2 hours before colitis induction and continued daily for 5 days.
Sham group: normal rats were treated with vehicle (1% tween 80 in normal saline) [5 ml/kg, orally (p.o.)].
Control group: rats with colitis were treated with vehicle (5 ml/kg, p.o.).
Test groups: rats with colitis were treated with low, middle and high doses of maltol (70, 140 and 280 mg/kg, p.o.) or kojic acid (75, 150 and 300 mg/kg, p.o.) according to the experimental protocol.
Reference groups: rats with colitis were treated with prednisolone (4 mg/kg, p.o.), iron chelators including deferiprone (150 mg/kg, p.o.) and deferoxamine (50 mg/kg, i.p.) and vitamin E (as an anti-oxidant) (400 mg/kg, p.o.) according to the experimental protocol.
Experimental Protocol
Rats were fasted for 36 hours with free access to water and observed to be healthful before induction of colitis. The rats were lightly anesthetized with ether. A flexible plastic rubber catheter with an outside diameter of 2 mm was inserted 8 cm into the colon via the anus. TNBS (75 mg/kg) dissolved in 0.25 ml ethanol 50% injected into the colon and the rats were maintained in a head-down position for 30 s.[15] Two hours before colitis induction, the test drugs, freshly prepared in solution or suspension forms, were administrated to animals and continued daily for 5 days. All treatments were made orally by using feeding tube with the exception of deferoxamine which administered parenterally (i.p.). At the sixth day, rats were sacrificed using ether overdose inhalation and colon biopsies were removed for macroscopic scoring and histopathological examination subsequently.[16]
Assessment of Colon Macroscopic Parameters:
The tissue of colon, 8 cm in length and 3 cm proximal to the anus was excised, opened longitudinally and washed in saline buffer. The specimens were weighed and weight/length ratio was measured for all the rats. Coworker pathologist unaware of treatments recorded macroscopic scoring parameters. The criteria of the macroscopic score used a previously validated scoring system from 0 to 4 according to Morris et al.[17] The scores were: 0 = no, 1 = mucosal erythema only, 2 = mild mucosal edema, slight bleeding or slight erosion, 3 = moderate edema, bleeding ulcers or erosions, 4 = severe ulceration, erosion, edema and tissue necrosis. Ulcer area was measured according to a method developed in our laboratory by using 3M® (USA) scaled surgical transpose tape, which was fixed on a light and transparent sheet. The sheet was then placed on the tissue and the cells (l mm2) were counted for determining the ulcer area for each colon.[18] Ulcer index was the later parameter, measured by summing the ulcer score and the ulcer area for each tissue specimen.[19]
Assessment of Colon Pathologic Parameters
Colon tissue was fixed in 10% formalin, dehydrated, and paraffin-embedded, processed and sectioned in 4-μm thick sections, and stained with hematoxylin and eosin (H and E).
Inflammation and crypt damage were assessed on H and E-stained coded sections using a modification of a validated scoring scheme described by Cooper et al,[20] and Dieleman et al.[21] Total colitis score was the sum of the three sub-scores (inflammation severity, inflammation extent, and crypt damage). Histological evaluation and scoring was performed using a Zeiss® microscope equipped with a Sony® color video camera for digital imaging.
Statistical Analysis
Results are expressed as the mean ± SEM. Statistical analysis was performed using SPSS 11 statistical software. Differences among groups were examined using parametric one-way ANOVA with Scheffe post-hoc test. Kruskal Wallis followed by Mann-Whitney U test analyzed non-parametric data. The minimal level of significance was identified at P < 0.05.
RESULTS
Macroscopic damage parameters in the colon of control group revealed colonic mucosal hyperemia, edema, ulceration and sometimes necrosis in reproducible manner [Figure 1a]. So weight/length (W/L) ratio was at the maximal level in this group. No changes were observed in sham group and W/L ratio was at lowest amount [Table 1].
Figure 1.
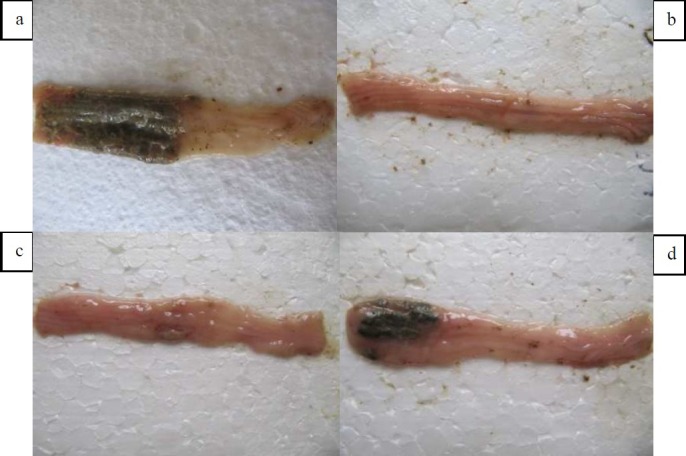
Macroscopic presentation of TNBS-induced colitis in rats. A: Negative control. B: Prednisolon (4 mg/Kg, p.o.) treated colitis. C: Kojic acid (300 mg/Kg, p.o.) treated colitis. D: Maltol (280mg/Kg) treated colitis. All treatments were made 2 h prior colitis induction and continued for 5 days later.
Table 1.
Effect of different iron chelators and anti-oxidants on macroscopic parameters of colitis induced by TNBS in rats
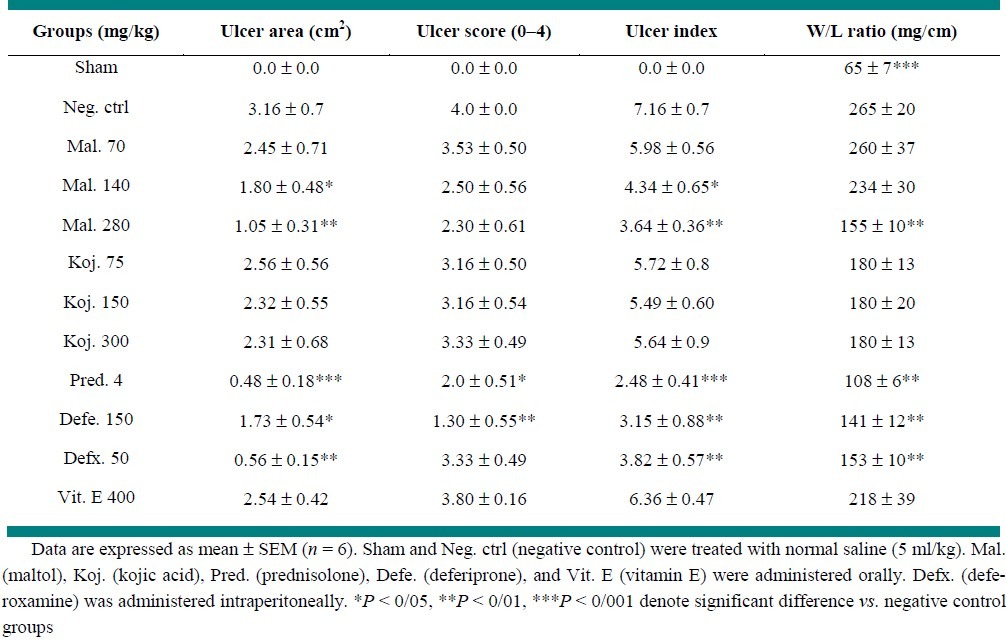
Prednisolone significantly reduced W/L ratio (P < 0.01), ulcer area (P < 0.001) and ulcer index (P < 0.001). Deferiprone and deferoxamine were both effective to alleviate macroscopic damage scores (P < 0.01) as well as W/L ratio significantly (P < 0.01) (Table 1). Treatment with maltol (280 mg/kg) reduced W/L ratio (P < 0.05) while at doses of 280, 140 mg/kg reduced ulcer index of colitis damage (P < 0.01, P < 0.05) [Figures 1b, 1c and 3, Table 1]. Vitamin E and kojic acid could not reduce the macroscopic parameters of colitis damage compared to control group [Figure 1d, Table 1].
Pathological Evaluation
Rats with TNBS-induced colitis and vehicle treatment (control groups) showed typical colitis features in pathological examination. These included destruction of epithelium, hemorrhage, edema, inflammatory cellular infiltration, crypt damage and ulceration at mucus and submucosal layers [Figure 2a, Table 2]. No histological damage and ulceration at mucus and submucosal layers was seen in sham groups [Figure 2b, Table 2]. Prednisolone was effective to reduce inflammatory severity and extent as well as crypt damage (P < 0.01). It was also effective to reduce total colitis index (P < 0.01) [Figure 2c, Table 2].
Figure 2.
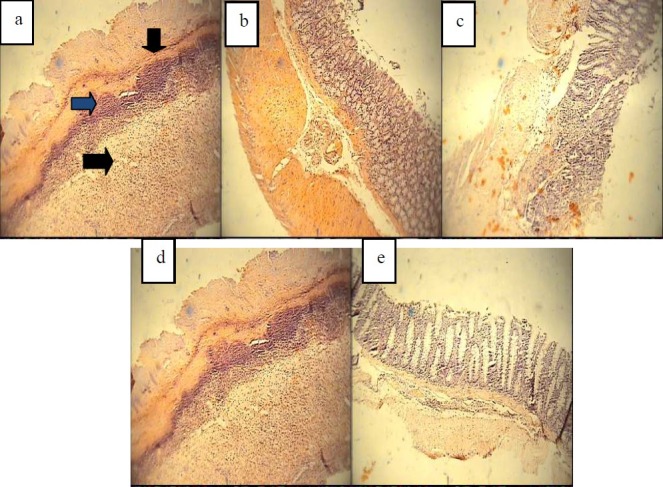
Microscopic presentation of TNBS-induced colitis in rats. a: Acute colitis induced by TNBS and treated by vehicle (negative control), as it is shown, necrotic destruction of mucosa, crypt damage and transmural acute inflammation are obvious. b: Normal rats treated by vehicle (Sham group) c: Acute colitis treated by prednisolone (4mg/kg). H&E staining and intermediate power (×40). d: Acute colitis treated by kojic acid (300mg/kg, p.o.) e: Acute colitis treated by maltol (280mg/kg)
Table 2.
Effect of different iron chelators and anti-oxidants on histopathologic parameters of colitis induced by TNBS in rats
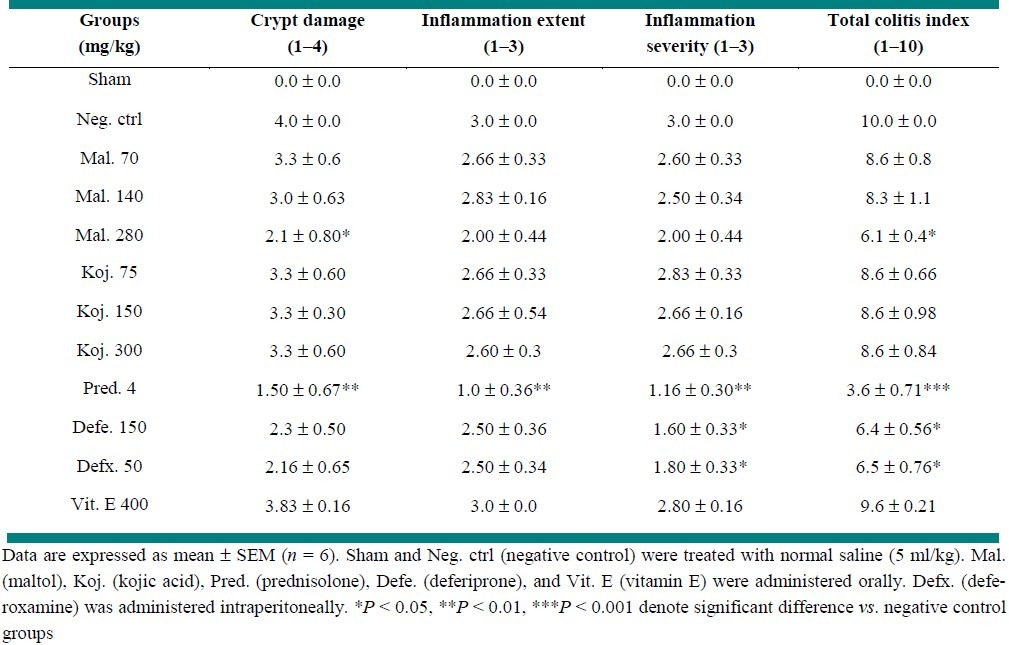
Deferiprone and deferoxamine were both effective to reduce total colitis index (P < 0.05) [Table 2].
Maltol at the greatest dose (280 mg/kg) was effective to reduced total colitis index (P < 0.01) as well as crypt damage score. Similar to macroscopic results, kojic acid vitamin E, and lowest dose of maltol (70 mg/kg) could not alleviate the histological features of colitis in test groups [Figures 2d, 2e and 3, Table 2].
DISCUSSION
The method of TNBS-induced colitis was used because its clinical features are similar to human ulcerative colitis. TNBS acts as a hapten and initiates a series of immune functions including pro-inflammatory cytokines and free radicals, which eventually result in an acute model of experimental IBD.[22] There are also evidences in which myenteric nervous system damage as well as pronounced colonic inflammation features take at least 72 h to occur in this model of induced colitis.[23] Therefore in current study, preventive therapy with test drugs were performed two hours before colitis induction and continued for five days later. Maltol but not kojic acid was effective to alleviate colitis both macroscopically and pathologically since maltol has probably a complex iron constant stability much higher than kojic acid.[24] Thus maltol is more selective than kojic acid for iron and it can eliminate iron faster than kojic acid within the gastrointestinal tract. On the other hand, maltol is more lipid soluble than kojic acid and this is indicative in its higher partition coefficient (K part of maltol = 0.5 versus k part of kojic acid = 0.077). Therefore, it is plausible that maltol could be absorbed with higher efficiency within the GI and this may explain its effectiveness in this study.[5] On the other hand, pharmacokinetic manipulation might provide a better condition for more pronounced effects of maltol by specific drug delivery to inflammatory regions of colon and increasing its access to the colonic mucosa. This can also prevent maltol-associated iron deficiency anemia as a troublesome adverse effect.[25] Results also demonstrated that the therapeutic effect of maltol was in part dependent to doses used, and at the highest test dose (280 mg/kg), it was comparable with prednisolone as standard anti-inflammatory drug and also deferoxamine and deferiprone as reference iron binding agents. Vitamin E as a common antioxidant was not able to protect against TNBS-induced colitis in our study. Bearing in mind the LD50 value of Vitamin E (5000 mg/kg in rats by oral intake), it is assumed that the applied dose of 400 mg/kg orally in current study would be rational. This is in contrary with one investigation, which revealed that vitamin E (2000 mg/kg) by unknown mechanism was orally effective against colitis induced by dextran sodium sulfate (DSS), while vitamin E could not reduce oxidative stress.[26] The much higher dose of vitamin E used in previous study and vast diversity of inflammatory mediators are involved in different experimental colitis, which may explain this controversy.[27] Deferiprone (L1) and deferoxamine as standard and potent iron chelators and antioxidants reduced inflammation in this study. Deferiprone (L1) and deferoxamine are using currently in iron overload disorders in clinic,[28] and there are reports indicating that deferiprone is capable to protect against experimental rat model of colitis and to reduce oxygen radicals result from iron.[29] Deferoxamine would be degraded within acidic medium of stomach and should be used by injection only while deferiprone is stable in gastric juice and would be effective after oral ingestion.[30] Therefore we applied these two drugs as reference iron chelators and found that they are both effective against TNBS-induced colitis in spite of difference in route of administration. Maltol at highest dose tested in current study was similarly effective to protect against colon inflammation induced by TNBS in comparison to deferoxamine and deferiprone. Bearing in mind the strength and affinity of deferoxamine (kf = 1031) and deferiprone (kf = 1037) for binding trivalent iron (ferric) ion versus maltol (kf = 1028) at pH~7, it is concluded that other factors like differences in anti-oxidant potencies of test drugs, better pharmacokinetic variables of maltol as well as dose-related properties might be involved.[31,32]
CONCLUSION
Taken together it is concluded that maltol is similar to well-known iron chelators such as deferoxamine and deferiprone and standard anti-inflammatory corticosteroid, prednisolone could protect against colitis-induced TNBS and alleviate all its features. This may be related to its oxidoradical scavengering and/or iron chelation; however, more experimental (based on biochemical and immunological variables) and clinical studies are needed to investigate the mechanisms involved. Bearing in mind the safety profile, availability and low cost of maltol as well as its current use as food or beverage additive suggest a suitable alternative agent for IBD therapy.
ACKNOWLEDGMENTS
This work was financially supported by Research Council of Isfahan University of Medical Sciences.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.Sellin JH, Pasricha PJ. Pharmacotherapy of inflammatory bowel diseases. In: Brunton LL, Lazo JS, Parker KL, editors. Goodman & Gilman's the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill companies; 2006. pp. 1009–11. [Google Scholar]
- 2.Lih-Brody L, Powell SR, Collier KP, Reddy GM, Cerchia R, Kahn E, et al. Increased oxidative stress and decreased antioxidant defenses in mucosa of inflammatory bowel disease. Dig Dis Sci. 1996;41:2078–86. doi: 10.1007/BF02093613. [DOI] [PubMed] [Google Scholar]
- 3.McQuaid KR. Drugs used in the treatment of gastrointestinal disease. In: Katzung BG, editor. Basic and clinical pharmacology. 10th ed. New York: McGraw Hill Companies; 2007. pp. 1029–35. [Google Scholar]
- 4.Cross RK, Lapshin O, Finkelstein J. Patient subjective assessment of drug side effect in inflammatory bowel disease. J Clin Gastroenterol. 2008;42:244–51. doi: 10.1097/MCG.0b013e31802f19af. [DOI] [PubMed] [Google Scholar]
- 5.Seril DN, Liao J, Ho KL, Warsi A, Yang CS, Yang GY. Dietary iron supplementation enhances DSS-induced colitis and associated colorectal carcinoma development in mice. Dig Dis Sci. 2002;47:1266–78. doi: 10.1023/a:1015362228659. [DOI] [PubMed] [Google Scholar]
- 6.Loguercio C, D’Argenio G, Delle Cave M, Cosenza V, Della Valle N, Mazzacca G, et al. Direct evidence of oxidative damage in acute and chronic phases of experimental colitis in rats. Dig Dis Sci. 1996;41:1204–11. doi: 10.1007/BF02088238. [DOI] [PubMed] [Google Scholar]
- 7.Hewitt SD, Hider RC, Sarpony P, Moriss CJ, Black DR. Investigation of the anti-inflammatory properties of hydroxypyridinones. Ann Rheum Dis. 1989;48:382–8. doi: 10.1136/ard.48.5.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anwar-Mohamed A, El-Kadi AO. Induction of cytochrome P450 1a1 by the food flavoring agent, maltol. Toxicol In Vitro. 2007;21:685–90. doi: 10.1016/j.tiv.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 9.Burnett CL, Bergfeld WF, Belsito DV, Hill RA, Klaassen CD, Liebler DC. Final report of the safety assessment of Kojic acid as used in cosmetics. Int J Toxicol. 2010;29(6 Suppl):244S–73. doi: 10.1177/1091581810385956. [DOI] [PubMed] [Google Scholar]
- 10.Kang KS, Yamabe N, Kim HY, Yokozawa T. Role of maltol in advanced glycation end products and free radicals: In-vitro and in-vivo studies. J Pharm Pharmacol. 2008;60:445–52. doi: 10.1211/jpp.60.4.0006. [DOI] [PubMed] [Google Scholar]
- 11.Kang KS, Kim HY, Pyo JS, Yokozawa T. Increase in the free radical scavenging activity of ginseng by heat processing. Biol Pharm Bull. 2006;29:750–4. doi: 10.1248/bpb.29.750. [DOI] [PubMed] [Google Scholar]
- 12.Brtko J, Rondahl L, Fickova M, Hudecova D, Eybl V, Uher M. Kojic acid and its derivatives: History and present state of ART. Cent Eur J Public Health. 2004;12(Suppl):S16–8. [PubMed] [Google Scholar]
- 13.Niwa Y, Akamatsu H. Kojic acid scavenges free radicals while potentiating leukocyte functions including free radical generation. Inflammation. 1991;15:303–15. doi: 10.1007/BF00917315. [DOI] [PubMed] [Google Scholar]
- 14.Yoo DS, Lee J, Choi SS, Rho HS, Cho DH, Shin WC, et al. A modulatory effect of novel kojic acid derivatives on cancer cell proliferation and macrophage activation. Pharmazie. 2010;65:261–6. [PubMed] [Google Scholar]
- 15.Minaiyan M, Ghannadi AR, Afsharipour M, Mahzouni P. Effect of hydroalcoholic and essential oil of Rosmarinus officinalis L. on TNBS-induced colitis in rats. Res Pharm Sci. 2011:6. [In press] [PMC free article] [PubMed] [Google Scholar]
- 16.Varshosaz J, Emami J, Tavakoli N, Minaiyan M, Rahmani N, Dorkoosh F, et al. Development of novel budesonide pellets based on CODESTM technology: In vitro/in vivo evaluation in induced colitis in rats. DARU. 2011;19:107–17. [PMC free article] [PubMed] [Google Scholar]
- 17.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. J Gastroenterol. 1989;96:795–803. [PubMed] [Google Scholar]
- 18.Minaiyan M, Ghassemi-Dehkordi, Mahzouni P, Ansari-Roknabadi M. Effect of Matricaria aurea (Loefl.) Shultz-Bip. hydroalcoholic extract on acetic acid-induced acute colitis in rats. Iran J Basic Med Sci. 2011;14:67–74. [Google Scholar]
- 19.Minaiyan M, Ghannadi AR, Nabi-Meibodi M, Mahzouni P. Anti-ulcerogenic effect of ginger (rhizome of Zingiber officinale Roscoe) hydroalcoholic extract on acetic acid-induced acute colitis in rats. Res Pharm Sci. 2008;3:15–22. [Google Scholar]
- 20.Cooper H, Murthy S, Shah R, Sedergran D. Clinicopathologic study of dextran sulfate sodium experimental murine colitis. Lab Invest. 1993;69:238–49. [PubMed] [Google Scholar]
- 21.Dieleman L, Palmen M, Akol H, Bloemena E, Pena A, Meuwissen S. Chronic experimental colitis induced by dextran sulfate sodium (DSS) is characterized by Th1 and Th2 cytokines. Clin Exp Immuno. 1998;114:385–91. doi: 10.1046/j.1365-2249.1998.00728.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Torres MI, Garcia-Martin M, Fernandez MI, Nietro N, Gil A, Rios A. Experimental colitis induced by trinitrobenzene sulfonic acid: an ultrastructural and histochemical study. Dig Dis Sci. 1999;44:2523–9. doi: 10.1023/a:1026651408998. [DOI] [PubMed] [Google Scholar]
- 23.Poli E, Lazzaretti M, Grandi D, Pozzoli C, Coruzzi G. Morphological and functional alterations of the myenteric plexus in rats with TNBS-induced colitis. Neurochem Res. 2001;26:1085–93. doi: 10.1023/a:1012313424144. [DOI] [PubMed] [Google Scholar]
- 24.Barrand MA, Callingham BA, Hider RC. Effect of the pyrones, maltol and ethyl maltol on iron absorption from the rat small intestine. J Pharm Pharmacol. 1987;39:203–11. doi: 10.1111/j.2042-7158.1987.tb06249.x. [DOI] [PubMed] [Google Scholar]
- 25.Ballester I, Daddaoua A, Lopez-Posadas R, Nieto A, Suarez MD, Zarzuelo A, et al. The bisphosphonate alendronate improves the damage associated with trinitrobenzen sulfonic acid-induced colitis in rats. Br J Pharmacol. 2007;151:206–15. doi: 10.1038/sj.bjp.0707227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carrier J, Aghdassi E, Cullen J, Allard JP. Iron supplementation increases disease activity and vitamin E ameliorates the effect in rats with dextran sulfate sodium-induced colitis. J Nutr. 2002;132:3146–50. doi: 10.1093/jn/131.10.3146. [DOI] [PubMed] [Google Scholar]
- 27.Yamada Y, Marshall S, Specian RD, Grisham MB. A comparative analysis of two models of colitis in rats. Gastroenterology. 1992;102:1524–34. doi: 10.1016/0016-5085(92)91710-l. [DOI] [PubMed] [Google Scholar]
- 28.Emara AM, El Kelany RS, Moustafa KA. Comparative study of the protective effect between deferoxamine and deferiprone on chronic iron overload induced cardiotoxicity in rats. Hum Exp Toxicol. 2006;25:375–85. doi: 10.1191/0960327106ht637oa. [DOI] [PubMed] [Google Scholar]
- 29.Ablin J, Shalev O, Okon E, Karmeli F, Rachmilewitz D. Deferiprone, an oral iron chelator, ameliorates experimental colitis and gastric ulceration in rats. Inflamm Bowel Dis. 1999;5:253–61. doi: 10.1097/00054725-199911000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Pepe A, Meloni A, Capra M, Cianciulli P, Prossomariti L, Malaventura C, et al. Deferasirox, deferiprone and desferrioxamine treatment in thalassemia major patients: Cardiac iron and function comparison determined by quantitative magnetic resonance imaging. Haematologica. 2011;96:41–7. doi: 10.3324/haematol.2009.019042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor PD, Morrison IEG, Hider RC. Microcomputer application of non-linear regression analysis to metal-ligand equilibria. Talanta. 1998;35:507–12. doi: 10.1016/0039-9140(88)80123-1. [DOI] [PubMed] [Google Scholar]
- 32.Dobbin PC, Hider RC. Iron chelation therapy. Chem Br. 1990;6:565–8. [Google Scholar]


