Abstract
Hepatitis C virus (HCV) infection is a serious public health concern throughout the world. Despite its public health prominence, however, how surveillance systems for hepatitis C should be designed is still a challenging issue especially in developing countries such as Iran. Establishing a surveillance system needs an ongoing process of case investigation, data collection, analysis of data and also dissemination of data to public health professionals and health care providers.
This review article tries to provide the best recommendations for planning and implantation a surveillance system for HCV infection.
Keywords: Epidemiology, Hepatitis C infection, Iran, surveillance system
INTRODUCTION
Hepatitis C virus (HCV) infection is a global public health problem.[1] At least five million people in the United States have hepatitis C virus (HCV) infection. That is about five times more than HIV infection.[2] The World Health Organization (WHO) estimated that 170 million individuals worldwide have been infected with hepatitis C virus (HCV).[3] Although, the incidence of this disease declined in recent years, but the prevalence is still high because of development of chronic hepatitis C (CHC) in approximately 75% of patients.[4] CHC is among the 15 leading causes of death in United States and half of them are attributable to HCV infection.[5] According to the last report of Center of Disease Control (CDC), the number of deaths related to CHC virus infection exceeded the one related to HIV.[6,7] This indicates the higher burden of disease and of course high medical expenditure for the patient and health system.[8,9] In the United States, medical care costs associated with the treatment of HCV infection are estimated to be more than $600 million a year[10] and projected to exceed $10.7 billion for the 10-year period from 2010 to 2019.[11] Unfortunately, there are insufficient data regarding to prevalence of HCV infection in Middle East as well as Iran.[12] Different serologic surveys characterized regional and local variations in prevalence of HCV infection, indicating the lack of a systematic surveillance system. A surveillance system is an ongoing process of case investigation, data collection, analysis of data and dissemination of data to public health professionals and health care providers.
The aim of this study is to present a practical approach for establishing a surveillance system for HCV infection in Iran and other developing countries.
EPIDEMIOLOGY OF HCV INFECTION
Hepatitis C virus (HCV) infection is the most common chronic blood borne infection in the United States with the highest incidence among persons between 20-39 years of age.[13] Also, chronic hepatitis C (CHC) is a major health burden throughout the world[14] and the epidemiological status of this disease is continuously evolving and may vary, significantly. This is due to several reasons such as variances in transmission routes or different public health policies and measures.
In the last report of European countries, this variation is indicated from the lowest prevalence (≤0.5%) in northern European countries to the highest rate (≥3%) in Romania and rural areas in Greece, as well as Italy and Russia.[15]
A recent systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt estimated that 49.3–64.0 million adults in these regions are anti-HCV positive with more HCV infections than all of Europe in China alone.[16] High prevalence rate of HCV reported in Egypt (15%), Pakistan (4.7%) and Taiwan (4.4%) in comparison to other parts of the world.
Studies from Iran reported heterogeneous results on prevalence of HCV. The prevalence of HCV infection reported differently from 0% in the Khuzestan[17] and Tehran province[18] to 0.31% in Golestan[19] and 1.3% in the Guilan province.[20] Moreover, a systematic review study showed that HCV infection prevalence rate in general population in Iran is 0.16%.[21]
However, the HCV prevalence is higher in high risk Iranian population.
Prevalence of HCV infection among IV drug users varies from 34%[22,23] to 43.4% in different surveys.[24] Another study on 106 IV drug users with HIV infection reported the HCV antibody positive in 75% of patients.[25]
Furthermore, the HCV prevalence among Iranian hemodialysis patients was reported as 4.5%[26] and 7.61%.[27] less than the prevalence showed by examining 298 hemodialysis patients in Guilan, 24.8%.[28]
Anti-HCV antibody prevalence in patients who receive multiple blood transfusions was also reported high[29] and a review study estimated the HCV seropositive prevalence up to18% among Iranian thalassemia patients.[30] The HCV antibody positive is estimated to be 11.5% in HIV positive patients.[31]
This high burden of HCV infection among high risk groups and also heterogeneity in results indicated an emergent need to design an efficient system for collection, analysis and interpretation of data.
SURVEILLANCE SYSTEM
Surveillance is defined as the “ongoing systematic collection, collation, analysis, interpretation of data; and the dissemination of information to those who need to know in order that action be taken”[32,33]
As noted by Donald Henderson (The leader of smallpox eradication in 1970s) surveillance is the neurologic system of public health that leads the public health in an effective and efficient way.[34]
According to World Health Organization (WHO), all surveillance systems consist of six key components: detection of health event, investigation and confirmation (epidemiological, clinical, laboratory), collection of data, analysis and interpretation of data, feedback and dissemination of results, response a link to public health programs, specifically actions for prevention and control.[35]
With considering these six elements, surveillance is useful to recognize cases or clusters of cases needed interventions, assess the public health impact of health events, demonstrate the need for public health intervention, monitor effectiveness of prevention and intervention strategies, identify high-risk population groups and develop hypotheses leading to analytic studies about risk factors for disease causation or progression.[36]
Additionally, it provides information for planning, implementation, monitoring, and evaluation of public health programs.[37] Furthermore, surveillance data are useful in resources allocation and evaluation of control and prevention strategies and programs at all levels.[38]
An effective system of public health surveillance must be simple, acceptable, sensitive, timely, flexible and also representative to be effective in public health actions.[39] The role of health authorities in maintaining appropriate supervision, training, and resources for the surveillance system is critical.
Furthermore, it is highly recommended that establishing a surveillance system in developing countries such as Iran should be less complex, more easily established, and sustainable.[36,40]
SURVEILLANCE SYSTEM FOR HCV
As noted above, hepatitis C has become an important public health issue. Despite its public health prominence, however, how surveillance systems for hepatitis C should be designed is still a challenging issue.[41] It should be emphasized that although cross sectional surveys are essential to estimate the burden of HCV infection, but they only reflect past transmission routes, providing no information on the current dynamic of HCV transmission. Moreover, the HCV infection is named as “long-time silent epidemic”;[42] so cross sectional studies are not able to detect people currently getting infected.
Surveillance system for acute HCV infection was first established in the United States in 1982.[43,44] There are 38 different surveillance systems in 27 European countries and 6 countries had more than one system.[45] But, there is still a gap in knowledge of prevalence of this disease in developing countries such as Iran.
Therefore, developing a national surveillance system for hepatitis C virus infection could provide a useful tool for estimating the burden of disease in Iran and reflecting changes in the trend of the disease. The knowledge gained from such programs can then be used to detect high risk population and develop effective public health policy and intervention.
ESTABLISHING A SURVEILLANCE SYSTEM FOR HCV INFECTION
Hepatitis C surveillance is a critical component to prevent and control HCV infection and HCV-related chronic liver disease. To achieve the goals of Hepatitis C surveillance, activities are needed to recognize persons with acute Hepatitis C, as well as persons with chronic HCV infection.[13]
For establishing a surveillance system for HCV infections, several steps should be undertaken.[35,46] [Table-1]
Table 1.
Steps in planning a surveillance system

1. Establish objectives:
The first step in planning a surveillance system is setting of specific, measurable, attainable, relevant and time based (SMART) objectives.[47] The goals of each surveillance system should be well specified. The key objective of surveillance is to offer information to guide interventions.[48] The surveillance system of HCV should set its objectives to: monitor secular trends and detect the high risk groups, detect epidemics, evaluate interventions and monitor preventive measures. Also, it should be able to monitor changes in HCV and also generate hypotheses for future research.[49]
2. Develop case definitions and case investigation
According to 2011 case definition of hepatitis C by CDC,[50] no symptom is required for diagnosis. The laboratory criteria for diagnosis of HCV infection are presented in Table 2.
Table 2.
Hepatitis C case definition (2011)
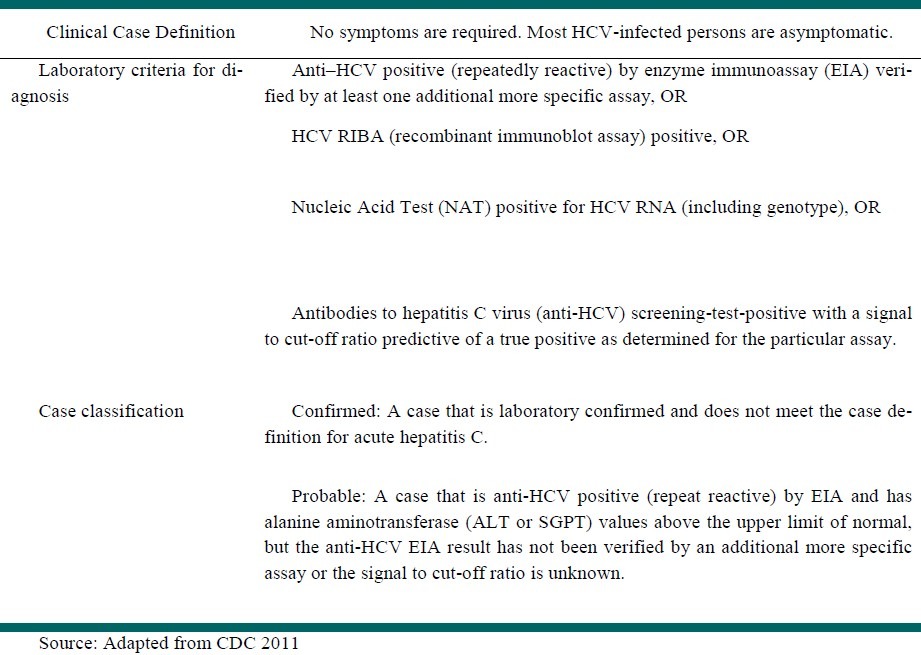
According to these criteria CDC classified cases into two groups:
Probable case: A case that is anti-HCV positive (repeat reactive) by EIA and has alanine aminotransferase (ALT or SGPT) values above the upper limit of normal, but the anti-HCV EIA result has not been verified by an additional more specific assay or the signal to cut-off ratio is unknown.
Confirmed: A case that is laboratory confirmed and does not meet the case definition for acute hepatitis C.[50]
Case Investigation and Follow-up:
After the diagnosis is made, all anti-HCV positive laboratory results should be reported to state and/or local health departments. Furthermore, a surveillance system should conduct the case investigation and follow up for all anti-HCV positive laboratory results.[13]
This investigation contains a confirmatory serologic test and also determines the risk factors. These risk factors include:
-
·
Blood transfusion prior to 1992
-
·
Organ transplant prior to 1992
-
·
Receipt of clotting factor concentrates made prior to 1987
-
·
Hemodialysis
-
·
Injection drug use
-
·
Number of sex partners
-
·
Contact with a person who had hepatitis
-
·
Employment involving contact with human blood[51]
Also, patients with HCV infection should receive counseling regarding how to reduce their risk of transmitting HCV to others and also prevention of further liver injury. In addition, there should be referral systems for medical evaluation and management.[52]
3. Determine data sources and data-collection mechanism
According to World Bank classification, the major surveillance methods for data collection are:[53]
1. Mandatory reports of certain diseases by clinicians or health care providers or facilities:
This is the traditional source of surveillance data and the most common method in Iran.[54] Despite its usefulness in collecting data, there are some limitations that should be considered: It needs the full collaboration of health care providers that depends on his/her perception and training; also as a rule, the more severe the illness the more likely it is to be reported.[55]
2. Reports by laboratories:
Laboratory reports are important means for collecting accurate data, especially in developed countries.[56] The utility of lab-based systems in developing countries is limited. It may be due to high costs of laboratory testing and also limited guidelines for accurate diagnosis.[57]
3. Sentinel surveillance:
In this method a sample of reporters (such as clinicians, hospitals, and local laboratories) are established as the reporting sources and they supervise the trend and the magnitude of disease in an accurate and high quality way.[58,59]
4. Periodic or ongoing prevalence surveys:
A periodic survey is usually based on a representative sample of the population. It can provide useful information on prevalence of risk factors, occurrence of exposures, service utilization and self-reported disease.[60]
5. Vital Records:
Vital records of births and deaths are the most important route of data collection, especially in developing countries who suffer from lack of other systematic methods.[61] This method is useful to estimate the magnitude, distribution and trend of certain diseases and injuries.[62]
4. Determine data-collection instruments
According to the method of data collection, instruments such as forms should be recognized,[63] and where suitable, computerized formats for each data element may be considered.[64] Different software packages are available and proposed by WHO and CDC for use in public health surveillance.[65] The most familiar and easy to use ones include:
Epi-Info and Epi-Map are designed by Centers for Disease Control and Prevention (CDC) to provide easy database construction, data entry, and analysis for the global community of public health practitioners and researchers.[66] Downloadable from: http://www.cdc.gov/epo/epi/software.htm, or http://www.who.int/whosis.
Prophet: This software offers advanced, easy-to-use software tools for data management, visualization, and statistical analysis.[36]
GIDEON: is an interactive computer program for diagnosis, especially in the field of microbiology, infectious diseases and epidemiology.[67]
5. Field-test methods:
The field testing of surveillance system is an important step in establishing the surveillance system.[68] These field-test projects can determine that, how the information can be obtained and analyzed. This step can demonstrate difficulties in data-collection and also problems in analysis of collected data.[35]
6. Develop and test analytic approach
A fundamental part of the planning of any surveillance system, is determining the analytical approach.[46] The surveillance system for HCV infection should contain basic analytical approaches to detect crude number of cases and rates and description of the population in which the condition occurs (person), where the condition occurs (place), and the period over which the condition occurs (time). More analysis is based on the objectives of surveillance system and for each objective the appropriate analytical plan should be developed.[58]
7. Develop dissemination mechanism
After analyzing the data, the data should be presented effectively to the audience of these data.[69,70] The characteristics of audiences and how they might use the data can determine the route of dissemination.[71] For HCV infection the primary users of surveillance information, are public health professionals and health-care providers. So, these data should contain the analyses and interpretation of surveillance results, as well as Graphs and maps for easy reading.
8. Assure use of analysis and interpretation.
Any surveillance system needs an evaluation system to assure the quality, completeness, and timeliness of data collection, analysis and dissemination.[72] This regular evaluation is helpful in finding the specific aspects of surveillance that need improvement.[4]
Every surveillance system needs a quality assurance program that should be assessed, routinely.[73] Some quality indicators include acceptability, timeliness, completeness, and representativeness of collected data.[74,75]
Timeliness reflects the time delay between any number of response steps in the public health surveillance system and can be measured by demonstrating the average length of time in days required for each step in the surveillance process.[76]
Furthermore, ethics should be considered in collecting, analysis and dissemination of data.[77]
Implantation of Surveillance system for HCV infection in developing countries:
By investing in public health surveillance for HCV infection, case detection and appropriate intervention will be in more effective and efficient way. But for establishing an effective surveillance system, some issues should be emphasized:
The first issue is cost. Establishing a foundation for data collection, analysis and dissemination is costly. So launching the surveillance system, based on existing public health systems, is effective to reduce cost, especially in developing countries. Successful implementation of disease surveillance requires comprehensive support within and beyond the health sector, and direct support from senior political levels and government.[78]
According to CDC guideline, case definitions vary from country to country depending on what resources (particularly laboratory resources) are available. Accordingly, case definition criteria for HCV infection must be standardized in each country. It needs a consensus between local experts and developing a national guideline for hepatitis C management.
As noted above, the case definition and diagnosis is mostly based on laboratory diagnosis. So, an effective surveillance system for HCV infection necessitates an investment in laboratory systems and providing reference laboratory and high quality laboratory reporting.[79] In low income countries, it can be through the cross-border collaboration to meet their needs.[36] A new approach suggested by WHO to strengthen the public health surveillance is the national public health laboratory networks that would connect the national laboratories with subregional, regional and international laboratories.[80] It means an assembly of laboratories with standard operative procedures and report information in a systematic approach.[81,82]
Finally, any surveillance system should consider training as an integral part.[83] All health care providers must be familiar with the basic concepts of surveillance of HCV case definitions, reporting, and appropriate case management in order to prevent avoidable death and disability related to hepatitis C infection.[84]
CONCLUSION
Surveillance system of hepatitis C infection is an important public health priority. This surveillance system can aid in determining the prevalence of this disease, identifying HCV-infected persons, prevention of secondary transmission and also establishing a chronic disease registry to support patients.
Every developing country such as Iran should characterize the national objective of HCV surveillance and definitive criteria for case identification and investigation. A comprehensive guideline for public health professionals and health care providers is needed.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Lavanchy D. Evolving epidemiology of hepatitis C virus. Clin Microbiol Infect. 2011;17:107–15. doi: 10.1111/j.1469-0691.2010.03432.x. [DOI] [PubMed] [Google Scholar]
- 2.Edlin BR. Perspective: Test and treat this silent killer. Nature. 2011;474:S18–9. doi: 10.1038/474S18a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lavanchy D. The global burden of hepatitis C. Liver Int. 2009;29:74–81. doi: 10.1111/j.1478-3231.2008.01934.x. [DOI] [PubMed] [Google Scholar]
- 4.Daniels D, Grytdal S, Wasley A. Surveillance for Acute Viral Hepatitis. United States, 2007: Department of Health and Human Services, Centers for Disease Control and Prevention; Centers for Disease Control and Prevention. 2009 [Google Scholar]
- 5.Jacobson IM, Davis GL, El-Serag H, Negro F, Trépo C. Prevalence and challenges of liver diseases in patients with chronic hepatitis C virus infection. Clin Gastroenterol Hepatol. 2010;8:924–33. doi: 10.1016/j.cgh.2010.06.032. [DOI] [PubMed] [Google Scholar]
- 6.Kathleen N, Ly JX, Klevens M, Jiles Ruth B, Ward John W, Holmberg Scott D. 49th Annual Meeting of the Infectious Diseases Society of America (IDSA) Boston, United States: 2011. The Growing Burden of Mortality from Viral Hepatitis in the United States, 1999-2007. [Google Scholar]
- 7.Canavan N. San Francisco, California: Nov 4-8, CDC: More Deaths From HCV Infection Than HIV. Paper presented at: The Liver Meeting 2011: American Association for the Study of Liver Diseases (AASLD) [Google Scholar]
- 8. Available from: http://www.medscape.com/viewarticle/753596 .
- 9.McCombs JS, Yuan Y, Shin J, Saab S. Economic Burden Associated With Patients Diagnosed With Hepatitis C. Clin Ther. 2011;33:1268–80. doi: 10.1016/j.clinthera.2011.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Davis KL, Mitra D, Medjedovic J, Beam C, Rustgi V. Direct economic burden of chronic hepatitis C virus in a United States managed care population. J Clin Gastroenterol. 2011;45:e17–24. doi: 10.1097/MCG.0b013e3181e12c09. [DOI] [PubMed] [Google Scholar]
- 11.Kim W, Gross JB, Jr, Poterucha JJ, Locke GR, 3rd, Dickson ER. Outcome of hospital care of liver disease associated with hepatitis C in the United States. Hepatology. 2001;33:201–6. doi: 10.1053/jhep.2001.20798. [DOI] [PubMed] [Google Scholar]
- 12.CDC. Evaluation of acute hepatitis C infection surveillance—United States, 2008. MMWR Morb Mortal Wkly Rep. 2010;59:1407–10. [PubMed] [Google Scholar]
- 13.Fallahian F, Najafi A. Epidemiology of hepatitis C in the middle east. Saudi J Kidney Dis Transpl. 2011;22:1–9. [PubMed] [Google Scholar]
- 14.Viral Hepatitis Surveillance Guidelines. New York State Department of Health Bureau of Communicable Disease Control. 2011 [Google Scholar]
- 15.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5:558–67. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 16.Cornberg M, Razavi HA, Alberti A, Bernasconi E, Buti M, Cooper C, et al. A systematic review of hepatitis C virus epidemiology in Europe, Canada and Israel. Liver Int. 2011;31:30–60. doi: 10.1111/j.1478-3231.2011.02539.x. [DOI] [PubMed] [Google Scholar]
- 17.Sievert W, Altraif I, Razavi HA, Abdo A, Ahmed EA, Alomair A, et al. A systematic review of hepatitis C virus epidemiology in Asia, Australia and Egypt. Liver Int. 2011;31(Suppl 2):61–80. doi: 10.1111/j.1478-3231.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- 18.Motlagh M, Makvandi M, Jalali M. Prevalence of anti-HCV among pregnant women. JQUMS. 2001;5:59–63. [Google Scholar]
- 19.Vahdani P, Hosseini-Moghaddam SM, Gachkar L, Sharafi K. Prevalence of hepatitis B, hepatitis C, human immunodeficiency virus, and syphilis among street children residing in southern Tehran, Iran. Arch Iran Med. 2006;9:153–5. [PubMed] [Google Scholar]
- 20.Poustchi H, Esmaili S, Mohamadkhani A, Nikmahzar A, Pourshams A, Sepanlou SG, et al. The Impact of Illicit Drug Use on Spontaneous Hepatitis C Clearance: Experience from a Large Cohort Population Study. PloS one. 2011;6:e23830. doi: 10.1371/journal.pone.0023830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mansour-Ghanaei F, Fallah M, Jafarshad R, Joukar F, Pourtahmasbi A, Bahari-Moghaddam A, et al. Seroprevalence of hepatitis B and C among residents of Guilan Nursing Home. Hepat Mon. 2007;7:139–141. [Google Scholar]
- 22.Alavian SM, Ahmadzad-Asl M, Lankarani KB, Shahbabaie MA, Bahrami Ahmadi A, Kabir A. Hepatitis C infection in the general population of Iran: A systematic review. Hepat Mon. 2009;9:211–23. [Google Scholar]
- 23.Fadaei Nobari R, Meshkati M, Ataei B, Heidari K, Kassaian N, Nokhodian Z, et al. Positive Hepatitis C Virus Antibody in Cases with History of Intravenous Drug Abuse via Community Announcement: A Useful Experience. J Isfahan Med Sch. 2011;1:1546–1552. [Google Scholar]
- 24.Rahimi-Movaghar A, Razaghi EM, Sahimi-Izadian E, Amin-Esmaeili M. HIV, hepatitis C virus, and hepatitis B virus co-infections among injecting drug users in Tehran, Iran. Int J Infect Dis. 2010;14:e28–33. doi: 10.1016/j.ijid.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Ataei B, Babak A, Yaran M, Kassaian N, Nokhodian Z, Meshkati M, et al. Hepatitis C in Intravenous Drug Users: Seroprevalence and Risk Factors. J Isfahan Med Sch. 2011;1:1537–1545. [Google Scholar]
- 26.Taeri K, Kasaeian N, Nobari RF, Ataei B. The prevalence of hepatitis B, hepatitis C and associated risk factors in intravenous drug addicts (IVDA) with HIV in Isfahan. J Isfahan Med Sch. 2008;26:273–278. [Google Scholar]
- 27.Alavian SM, Bagheri-Lankarani K, Mahdavi-Mazdeh M, Nourozi S. Hepatitis B and C in dialysis units in Iran: Changing the epidemiology. Hemodial Int. 2008;12:378–82. doi: 10.1111/j.1542-4758.2008.00284.x. [DOI] [PubMed] [Google Scholar]
- 28.Alavian SM, Kabir A, Ahmadi AB, Lankarani KB, Shahbabaie MA, Ahmadzad-Asl M. Hepatitis C infection in hemodialysis patients in Iran: A systematic review. Hemodial Int. 2010;14:253–62. doi: 10.1111/j.1542-4758.2010.00437.x. [DOI] [PubMed] [Google Scholar]
- 29.Amiri Z, Shakib A, Toorchi M. Seroprevalence of hepatitis C and risk factors in haemodialysis patients in Guilan, Islamic Republic of Iran. East Mediterr Health J. 2005;11:372–6. [PubMed] [Google Scholar]
- 30.Kassaian N, Babak A, Ataei B, Adibi P. Hepatitis C in Patients with Multi Blood Transfusion. J Isfahan Med Sch. 2011;1:1560–1564. [Google Scholar]
- 31.Alavian S, Tabatabaei S, Lankarani K. Epidemiology of HCV infection among thalassemia patients in eastern Mediterranean countries: A quantitative review of literature. Iran Red Crescent Med J. 2010;12:365–76. [Google Scholar]
- 32.Ataei B, Tayeri K, Kassaeian N, Farajzadegan Z, Babak A. Hepatitis B and C among patients infected with human immunodeficiency virus in Isfahan, Iran: Seroprevalence and associated factors. Hepat Mon. 2010;10:188–92. [PMC free article] [PubMed] [Google Scholar]
- 33.Sosin DM. Draft framework for evaluating syndromic surveillance systems. J Urban Health. 2003;80(Suppl 1):i8–i13. doi: 10.1007/PL00022309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.World Health Organization. Communicable disease surveillance and response systems. A guide to planning. 2008 [Google Scholar]
- 35.Halperin WE. More surveillance in child care, please! Public Health Rep. 1995;110:117–8. [PMC free article] [PubMed] [Google Scholar]
- 36.Teutsch SM, Thacker SB. Planning a public health surveillance system. Epidemiol Bull. 1995;16:1–6. [PubMed] [Google Scholar]
- 37.Berkelman RL, Sullivan P, Buehler JW, Detels R, Beaglehole R, Lansing M, et al. the methods of public health. Ed. 5. 2009. Public health surveillance. Oxford textbook of public health; pp. 699–715. [Google Scholar]
- 38.Horan TC, Andrus M, Dudeck MA. CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 2008;36:309. doi: 10.1016/j.ajic.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 39.Wilkins K, Nsubuga P, Mendlein J, Mercer D, Pappaioanou M. The data for decision making project: Assessment of surveillance systems in developing countries to improve access to public health information. Public Health. 2008;122:914–22. doi: 10.1016/j.puhe.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Last JM, Chin J, Fielding JE, Frank AL, Lashof JC, Wallace RB. New York: McGraw-Hill; 2010. Maxcy-Rosenau public health and preventive medicine. [Google Scholar]
- 41.Teutsch SM, Churchill RE. USA: Oxford University Press; 2000. Principles and practice of public health surveillance. [Google Scholar]
- 42.Qidwai W, Fahim A, Waheed S. Hepatitis C in Pakistan-A neglected challenge. Int J Hepatol. 2010;1:5–7. [Google Scholar]
- 43.Desenclos J. L. Infection with hepatitis C in the world: public health importance, modes of transmission and prospects. Virologie. 2003;7:177–91. [Google Scholar]
- 44.Klevens RM, Miller J, Vonderwahl C, Speers S, Alelis K, Sweet K, et al. Population-based surveillance for hepatitis C virus, United States, 2006–2007. Emerging infectious diseases. 2009;15(9):1499. doi: 10.3201/eid1509.081050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams IT, Bell BP, Kuhnert W, Alter MJ. Incidence and transmission patterns of acute hepatitis C in the United States, 1982-2006. Arch Intern Med. 2011;171:242–8. doi: 10.1001/archinternmed.2010.511. [DOI] [PubMed] [Google Scholar]
- 46.Rantala M, Van De Laar M. Surveillance and epidemiology of hepatitis B and C in Europea review. Euro Surveill. 2008;13:1–8. doi: 10.2807/ese.13.21.18880-en. [DOI] [PubMed] [Google Scholar]
- 47.Teutsch SM, Teutsch S. Principles and practice of public health surveillance. Oxford University Press; 1994. Considerations in planning a surveillance system; pp. 18–30. [Google Scholar]
- 48.Kleinman K, Abrams A, Mandl K, Platt R. Simulation and other evaluation approaches: Simulation for assessing statistical methods of biologic terrorism surveillance. Morb Mortal Wkly Rep. 2005;54:101–8. [PubMed] [Google Scholar]
- 49.Jamison DT. USA: Oxford University Press; 2006. Disease control priorities in developing countries. [PubMed] [Google Scholar]
- 50.Puoti C, Bellis L, Martellino F, Durola L, Galossi A, Guarisco R, et al. Surveillance of patients with chronic hepatitis C not on antiviral therapy: A practical approach. Rom J Gastroenterol. 2005;14:141–6. [PubMed] [Google Scholar]
- 51.Hepatitis C. Past or present [database on the Internet] [Last cited on 2011 Nov 25];2011 17(11) Available from: http://www.cdc.gov/osels/ph_surveillance/nndss/casedef/hepatitisc2011.htm . [Google Scholar]
- 52.Kamal SM. Acute hepatitis C: A systematic review. Am J Gastroenterol. 2008;103:1283–97. doi: 10.1111/j.1572-0241.2008.01825.x. [DOI] [PubMed] [Google Scholar]
- 53.Guidelines For Viral Hepatitis Surveillance And Case Management [database on the Internet] 2009. [Last cited on 2011 Nov 26]. Available from: http://www.cdc.gov/hepatitis/Statistics/SurveillanceGuidelines.htm .
- 54.Garcia-Abreu A, Danel I, Bank W, Division DC. Washington, DC: World Bank; 2002. Public Health Surveillance Toolkit: A Guide for Busy Task Managers. [Google Scholar]
- 55.Chorba TL, Berkelman RL, Safford SK, Gibbs NP, Hull HF. Mandatory reporting of infectious diseases by clinicians. JAMA. 1989;262:3018–26. [PubMed] [Google Scholar]
- 56.Kaldor JM, Dore GJ, Correll PK. Public health challenges in hepatitis C virus infection. J Gastroenterol Hepatol. 2000;15:E83–90. doi: 10.1046/j.1440-1746.2000.02134.x. [DOI] [PubMed] [Google Scholar]
- 57.Overhage JM, Grannis S, Mc Donald CJ. A comparison of the completeness and timeliness of automated electronic laboratory reporting and spontaneous reporting of notifiable conditions. Am J Public Health. 2008;98:344–50. doi: 10.2105/AJPH.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baker MG, Fidler DP. Global public health surveillance under new international health regulations. Emerg Infect Dis. 2006;12:1058–65. doi: 10.3201/eid1207.051497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Arita I, Nakane M, Nakano T. Surveillance of Disease: Overview. In: Editor-in-Chief. In: Kris H, editor. International Encyclopedia of Public Health. Oxford: Academic Press; 2008. pp. 275–89. [Google Scholar]
- 60.Talaat M, El-Sayed N, Kandeel A, Azab M, Afifi S, Youssef F, et al. Sentinel surveillance for patients with acute hepatitis in Egypt, 2001–2004. East Mediterr Health J. 2010;16:134–40. [PubMed] [Google Scholar]
- 61.Pottinger JM, Herwaldt LA, Perl TM. Basics of surveillance: An overview. Infect Control Hosp Epidemiol. 1997;18:513–27. doi: 10.1086/647659. [DOI] [PubMed] [Google Scholar]
- 62.Elder GH, Giele JZ. The Guilford Press; 2009. The craft of life course research; pp. 51–75. [Google Scholar]
- 63.Vander Stichele R, Elseviers MM, Ferech M, Blot S, Goossens H. European surveillance of antimicrobial consumption (ESAC): Data collection performance and methodological approach. Br J Clin Pharmacol. 2004;58:419–28. doi: 10.1111/j.1365-2125.2004.02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Blalock CL, Lichtenstein MJ, Owen S, Pruski L, Marshall C, Toepperwein MA. In pursuit of validity: A comprehensive review of science attitude instruments 1935–2005. Int J Sci Educ. 2008;30:961–77. [Google Scholar]
- 65.Yu P, de Courten M, Pan E, Galea G, Pryor J. The development and evaluation of a PDA-based method for public health surveillance data collection in developing countries. Int J Med Inform. 2009;78:532–42. doi: 10.1016/j.ijmedinf.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 66.Lautenbach E, Woeltje KF, Malani PN. Practical Healthcare Epidemiology: University of Chicago Press Journals. 2010;100 [Google Scholar]
- 67.Ma J, Otten M, Kamadjeu R, Mir R, Rosencrans L, Mc Laughlin S, et al. New frontiers for health information systems using Epi Info in developing countries: Structured application framework for Epi Info (SAFE) Int J Med Inform. 2008;77:219–25. doi: 10.1016/j.ijmedinf.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 68.Veenema TG, Toke J. Early detection and surveillance for biopreparedness and emerging infectious diseases. Online J Issues Nurs. 2006;11:3. [PubMed] [Google Scholar]
- 69.Gregg MB. USA: Oxford University Press; 2008. Field epidemiology. [Google Scholar]
- 70.German RR, Lee LM, Horan JM, Milstein RL, Pertowski CA, Waller MN, et al. Updated guidelines for evaluating public health surveillance systems: recommendations from the Guidelines Working Group. MMWR Recomm Rep. 2001;50:1–35. [PubMed] [Google Scholar]
- 71.Lee LM, Thacker SB, Teutsch SM. Principles and practice of public health surveillance: Oxford Univ Pr. 2010:418. [Google Scholar]
- 72.Green LW, Ottoson JM, García C, Hiatt RA. Diffusion theory and knowledge dissemination, utilization, and integration in public health. Annu Rev Public Health. 2009;30:151–74. doi: 10.1146/annurev.publhealth.031308.100049. [DOI] [PubMed] [Google Scholar]
- 73.Drewe J, Hoinville L, Cook A, Floyd T, Stärk K. Evaluation of animal and public health surveillance systems: A systematic review. Epidemiol Infect. 2011;1:1–16. doi: 10.1017/S0950268811002160. [DOI] [PubMed] [Google Scholar]
- 74.Ortiz JR, Sotomayor V, Uez OC, Oliva O, Bettels D, McCarron M, et al. Strategy to enhance influenza surveillance worldwide. Emerg Infect Dis. 2009;15:1271–8. doi: 10.3201/eid1508.081422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hadorn DC, Stärk KD. Evaluation and optimization of surveillance systems for rare and emerging infectious diseases. Vet Res. 2008;39:57. doi: 10.1051/vetres:2008033. [DOI] [PubMed] [Google Scholar]
- 76.Lin MY, Hota B, Khan YM, Woeltje KF, Borlawsky TB, Doherty JA, et al. Quality of traditional surveillance for public reporting of nosocomial blood-stream infection rates. JAMA. 2010;304:2035–41. doi: 10.1001/jama.2010.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jajosky RA, Groseclose SL. Evaluation of reporting timeliness of public health surveillance systems for infectious diseases. BMC Public Health. 2004;4:29. doi: 10.1186/1471-2458-4-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fairchild AL, Bayer R. Ethics and the conduct of public health surveillance. Science. 2004;303:631–2. doi: 10.1126/science.1094038. [DOI] [PubMed] [Google Scholar]
- 79.Katz R, Fernandez J, McNabb S. Disease surveillance, capacity building and implementation of the International Health Regulations (IHR [2005]) BMC Public Health. 2010;10(Suppl 1):S1. doi: 10.1186/1471-2458-10-S1-S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nkengasong JN. Strengthening laboratory services and systems in resource-poor countries. Am J Clin Pathol. 2009;131:774. doi: 10.1309/AJCP8GYX8KTKDATZ. [DOI] [PubMed] [Google Scholar]
- 81.Witt-Kushner J, Astles JR, Ridderhof JC, Martin RA, Wilcke B, Jr, Downes FP, et al. Core functions and capabilities of state public health laboratories. MMWR Recomm Rep. 2002;51:1–8. [PubMed] [Google Scholar]
- 82.Kebede S, Gatabazi JB, Rugimbanya P, Mukankwiro T, Perry HN, Alemu W, et al. Strengthening systems for communicable disease surveillance: Creating a laboratory network in Rwanda. Health Res Policy Syst. 2011;9:27. doi: 10.1186/1478-4505-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nsubuga P, Nwanyanwu O, Nkengasong J, Mukanga D, Trostle M. Strengthening public health surveillance and response using the health systems strengthening agenda in developing countries. BMC Public Health. 2010;10(Suppl 1):S5. doi: 10.1186/1471-2458-10-S1-S5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nsubuga P, White M, Fontaine R, Simone P. Training programmes for field epidemiology. Lancet. 2008;371:630–1. doi: 10.1016/S0140-6736(08)60281-0. [DOI] [PubMed] [Google Scholar]
- 85.Perry HN, Mc Donnell SM, Alemu W, Nsubuga P, Chungong S, Otten MW, Jr, et al. Planning an integrated disease surveillance and response system: A matrix of skills and activities. BMC Med. 2007;5:24. doi: 10.1186/1741-7015-5-24. [DOI] [PMC free article] [PubMed] [Google Scholar]


