Glioblastoma multiforme is the most aggressive of the gliomas, a collection of tumors arising from glia or their precursors within the central nervous system. Clinically, gliomas are divided into four grades; unfortunately, the most aggressive of these, grade 4 or glioblastoma multiforme (GBM), is also the most common in humans. Because most patients with GBMs die of their disease in less than a year and essentially none has long-term survival, these tumors have drawn significant attention; however, they have evaded increasingly cleaver and intricate attempts at therapy over the last half-century. The paper by Gromeier et al. (1) in this issue of PNAS is the newest chapter in this saga, describing a hybrid virus that infects and kills clonal human glioma cell lines, in culture and implanted in athymic mice, without affecting nonneoplastic cells within the brain. For those viewing this battle from a distance, the continued unsuccessful attempts at novel therapies for this disease may be difficult to understand. However, for those treating these patients, and certainly for the patients themselves, the importance and urgency of each attempt is clear.
One of the reasons for the resistance of GBM to therapeutic intervention is the complex character of the tumor itself. As the name implies, glioblastoma is multiforme. It is multiforme grossly, showing regions of necrosis and hemorrhage. It is multiforme microscopically, with regions of pseudopalisading necrosis, pleomorphic nuclei and cells, and microvascular proliferation. And it is multiforme genetically, with various deletions, amplifications, and point mutations leading to activation of signal transduction pathways downstream of tyrosine kinase receptors such as epidermal growth factor receptor (EGFR) and platelet-derived growth factor receptor (PDGFR), as well as to disruption of cell-cycle arrest pathways by INK4a-ARF loss or by p53 mutations associated with CDK4 amplification or Rb loss (2). These tumors also show intratumor genetic heterogeneity with subclones existing within the tumor cell population (3). It has been estimated that cultured neoplastic and p53-deficient cells may have mutations in any given gene at a rate as high as 1 in 1,000 cells (4). If this is approximately correct for GBMs in vivo, then one would expect a tumor of 109 cells to harbor as many as 106 cells with mutations in any given gene.
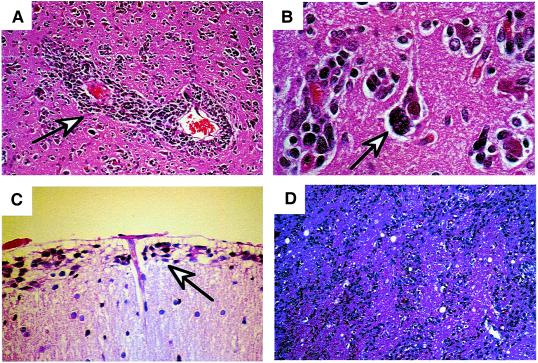
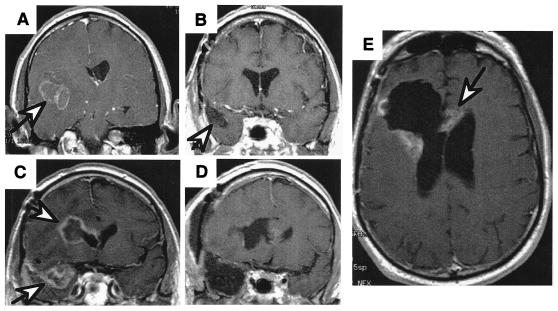
One of the main reasons that the gliomas are not cured by surgery is the topographically diffuse nature of the disease. In addition to the above-mentioned variability within the tumor proper, the location of the tumor cells within the brain also is variable, resulting in the inability to completely resect this tumor. In 1940, Scherer (5) described the appearance and behavior of glioma cells migrating away from the main tumor mass through the brain parenchyma. The patterns of glioma cell infiltration have since been referred to as the secondary structures of Scherer. These glioma cells migrate through the normal parenchyma, collect just below the pial margin (subpial spread), surround neurons and vessels (perineuronal and perivascular satellitosis), and migrate through the white matter tracks (intrafacicular spread) (Fig. 1). This invasive behavior of the individual cells may correspond to the neoplastic cell's reacquisition of primitive migratory behavior during central nervous system development. The ultimate result of this behavior is the spread of individual tumor cells diffusely over long distances and into regions of brain essential for survival of the patient. The extreme example of this behavior is a condition referred to as gliomatosis cerebri, in which the entire brain is diffusely infiltrated by neoplastic cells with minimal or no central focal area of tumor per se (6). Furthermore, ≈25% of patients with GBM have multiple or multicentric GBMs at autopsy (7). Although GBMs can be visualized on MRI scans as mass lesions that enhance with contrast, the neoplastic cells extend far beyond the area of enhancement. Fig. 2 illustrates a typical result of “gross total resection” of a temporal lobe GBM followed 6 months later by recurrence at the surgical margin and elsewhere. Even with repeat surgeries for tumor recurrences, the patients die from tumor spread into vital regions of the brain.
Figure 1.
Secondary structures of Scherer demonstrating migration of glioma cells through normal brain structures. (A) Glioma cells surrounding blood vessels (perivascular satellitosis) (arrow). (B) Perineuronal satellitosis (arrow). (C) Collection of cells below pial surface (subpial spread) (arrow). (D) Intrafascicular spread of tumor cells through the corona radiata.
Figure 2.
MRI scans of a patient with a right temporal GBM illustrating the spread of the disease. (A) Presurgical scan, GBM (arrow) is surrounded with edema. (B) Scan after surgery and radiation therapy showing “gross total resection” and clear resection cavity, and (C) six months later, showing recurrence not only at the resection margin (arrow) but a second focus of GBM across the Sylvian fissure in the frontal lobe (arrow). (D) Postresection scans of both recurrent tumors. (E) Scan 3 months later, showing the tumor recurring at the resection margin and crossing the corpus callosum to the other hemisphere (arrow).
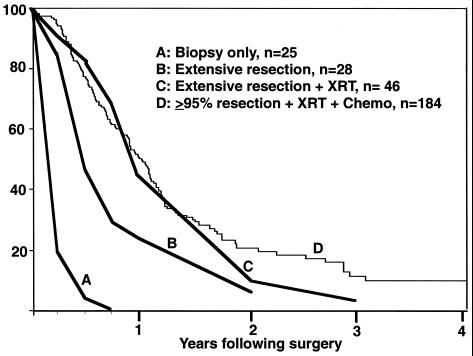
The standard of care for treatment of GBM has been essentially unchanged for many decades—surgical resection of as much of the tumor as is safe, followed by radiation therapy and chemotherapy (usually designed to damage DNA or to otherwise inhibit DNA replication). Even under the best of circumstances, in which essentially all of the enhancing tumor seen on MRI scan can be surgically removed and the patients are fully treated with radiation and chemotherapy, the mean survival of this disease is only extended from 2 to 3 months (8) to 1 year (Fig. 3).
Figure 3.
Kaplan–Meier survival plots for patients diagnosed with GBM. Curves A, B, and C are historical data from Jelsma and Bucy (8) published in 1967 before the availability of MRI scans: biopsy only (A), extensive resection (undefined) (B), and extensive resection followed by radiation therapy (C). Curve D is current data from the M. D. Anderson Cancer Center on patients with >95% resection (by volumetric MRI measurements) followed by both radiation therapy and chemotherapy. Although there are essentially no long-term survivors, removal of tumor mass clearly increases longevity.
Because of the poor outcome of the standard treatments for GBM and of the diffuse nature of the disease, a number of clever attempts at novel therapeutic approaches recently have been made with the aim of killing neoplastic cells far from the tumor proper. These approaches have been designed to entice the immune system to reject the tumor, to transfer lethal genes to the tumor cells with gene therapy, or, more recently, to infect with viruses that kill the tumor cells lytically.
The immunologic approach has been investigated extensively with many successes in laboratory animals. However, translation of success in rodents to humans has not occurred. Potential explanations for this apparent paradox center on the animal models used in the preclinical experiments. Until recently, animal models for gliomas have consisted of clonal glioma cell lines, maintained in culture, that are injected in the flanks or brains of rodents. These cells grow into mass lesions that eventually kill the animals (9, 10). To what extent either the genetic alterations selected for during passage of the cells in culture or the interactions between tumor cells and the host tissues in these experimental gliomas represents the biology of human gliomas is questionable, especially in the area of immune rejection. Early experiments scored treatment successes as rejection of the implanted allograph by the animals. Since then, syngenic grafts have been used to avoid the non-self-recognition by the host animal (11).
Another approach is the transfer of lethal genes to tumor cells by gene therapy. The classic example of this strategy was the retroviral transfer of the herpes simplex virus thymidine kinase (TK) gene to tumor cells followed by treatment with the antiviral compound gancyclovir to kill cells expressing TK. Early reports showed this strategy to eradicate experimentally implanted gliomas in rodents (12). Unfortunately, the application of this strategy to human gliomas has not seemed to have a therapeutic benefit in humans, presumably attributable, at least in part, to the low infection rate within the human tumors (13). In these rodent models, complete tumor regression was obtained with infection of substantially less than 100% of cells, caused by bystander effects in which infected cells are capable of killing adjacent, uninfected tumor cells. However, the rodent models used in these experiments, again, do not fully recapitulate the behavior of the human disease. Specifically, the invasive character of human gliomas rarely is recapitulated by grafting models. Although implanted rodent gliomas might appear to invade surrounding structures, they frequently do not do so on a cell-by-cell basis. Rather, implanted gliomas are comprised of cells in direct contact with each other and, therefore, a bystander effect probably is more likely to be seen in these animal models than in the diffuse portions of human gliomas, illustrated in Fig. 1.
A more recent approach to solving the low rate of infection and gene transfer is the use of viral vectors that replicate in and thereby lytically kill tumor cells. These approaches use viruses that normally infect the central nervous system that have been modified to become nonpathogenic to normal tissues but remain lytic to neoplastic cells. Attenuated, nonneurovirulent versions of herpes simplex virus have been used previously and have been shown to kill glioma cells in culture and implanted in rodents (14). These viruses are currently in clinical trials. The paper by Gromeier et al. (1) now reports the use of a polio virus-human rhinovirus that does not have the neurovirulence of polio virus but does infect and kill clonal human glioma cell lines both in culture and as xenographs in athymic mice (1).
All strategies for killing gliomas with viruses or viral vectors are hindered by the need for the tumor cells to undergo infection. Although it may seem obvious, it is worth pointing out that virus infection requires the target cell to express the viral receptor (specifically illustrated in the paper by Gromeier et al. (1) by the requirement for expression of the polio virus receptor CD155 for cells to be infected by the polio virus). Given the genomic instability and heterogeneity of gene expression within GBMs, it is likely that many cells within each tumor will be inherently resistant to viral infection because of lack of expression of the viral receptor. To this point, Gromeier et al. report that by immunohistochemical staining, 19 of 25 tumors showed expression of the polio virus receptor CD155, therefore 6 of 25 did not. The fact that these CD155 nonexpressing gliomas exist implies that CD155 expression is not required for survival or for the neoplastic glioma phenotype. Therefore, even within gliomas that predominately express CD155, infection-resistant subclones of CD155 nonexpressing cells may exist, giving rise to recurrence. This scenario is under the best of circumstances, where each cell has unrestricted access to viruses. In reality, however, cells diffusely spread throughout the brain and are not in contact with each other and are therefore unlikely to have access to viral particles.
Now that we have identified some of the theoretical difficulties of successfully treating this disease with viruses and other approaches, let us more clearly define success. Even though the current standard of care (surgery, radiation, and chemotherapy) ultimately fails, leading to the patient's death, refusing to treat GBM patients for this reason is more nihilistic than most of these patients, their families, and their physicians are comfortable with. It is clear that surgically resecting greater than 95% of a GBM in many cases results in an improvement of symptoms, even if only temporarily. Although surgeons realize they are ultimately not going to cure the patient, in many cases surgical resection is worth the effort because it frequently increases survival and quality of life. Fig. 3 demonstrates the improvement in survival of GBM patients fully treated to reduce tumor cell burden as compared with historical data of patients receiving biopsy only (8). If the goal of viral therapy is to similarly reduce the tumor cell burden significantly, such a strategy could be equally useful and potentially additive to current palliative treatments.
It should be with guarded optimism that we view each successive attempt at treating this devastating disease. It is equally important to clearly establish the useful effects each approach is expected to achieve, and to define success accordingly. Nonetheless, we should not lose sight of the final goal, actual cure. Cure for GBM will require testing treatments in better animal models that accurately recapitulate the histology and genetics of the human disease. Also, essential therapeutic targets for GBM are likely to be the pathways that represent the etiology of the disease, abnormalities of which lead to glioma formation. It is encouraging that experimental transgenic mouse models of melanoma and lymphoma, generated by inducible transgenes expressing Ras and Myc, respectively, are cured by removal of these initiating agents (15–17). These results are even more impressive given the genomic instability of the cells in these experimental tumors. In theory, these tumor cells could genetically evolve during tumor progression and no longer require the initiating agents for tumor maintenance. In reality, the tumors appear to evolve primarily so as to continue requiring elevated Myc and Ras activity; removal of these causative agents destroys them. These data imply that if the causative pathways for GBMs can be identified and pharmacologically blocked, then there is some hope of actual cure of this disease in humans. Then again, until we start curing patients of their GBMs by one of these new strategies, there remains the possibility that we are continuing to underestimate the complexity of this disease. These approaches may simply be added to the ever-growing list of attempts that work in mice but not humans.
Acknowledgments
I thank Greg Fuller and Raymond Sawaya for their thoughtful input on this manuscript and Dima Abi-Said for help with the M. D. Anderson GBM patient data set.
Footnotes
See companion article on page 6803.
References
- 1.Gromeier M, Lachmann S, Rosenfeld M R, Gutin P, Wimmer E. Proc Natl Acad Sci USA. 2000;97:6803–6808. doi: 10.1073/pnas.97.12.6803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.James C D, Olson J J. Curr Opin Oncol. 1996;8:188–195. doi: 10.1097/00001622-199605000-00004. [DOI] [PubMed] [Google Scholar]
- 3.Ishii N, Tada M, Hamou M F, Janzer R C, Meagher-Villemure K, Weistler O D, Tribolet N, Van Meier E G. Oncogene. 1999;18:5870–5878. doi: 10.1038/sj.onc.1203241. [DOI] [PubMed] [Google Scholar]
- 4.Livingstone L R, White A, Sprouse J, Livanos E, Jacks T, Tlsty T D. Cell. 1992;70:923–935. doi: 10.1016/0092-8674(92)90243-6. [DOI] [PubMed] [Google Scholar]
- 5.Scherer H J. Brain. 1940;40:631–635. [Google Scholar]
- 6.Russel D S, Rubenstein L J. Pathology of Tumors of the Nervous System. Philadelphia: Williams & Wilkins; 1989. pp. 146–147. [Google Scholar]
- 7.Batzdorf U, Malamud M D. J Neurosurg. 1963;20:122–136. doi: 10.3171/jns.1963.20.2.0122. [DOI] [PubMed] [Google Scholar]
- 8.Jelsma R, Bucy P C. J Neurosurg. 1967;27:388–400. doi: 10.3171/jns.1967.27.5.0388. [DOI] [PubMed] [Google Scholar]
- 9.Carson W E, III, Jakowatz J G, Yamamoto R, Fitzgerald T, Gupta S, Vayuvegula B, Lucci J A, III, Beckman M T, Dulkanchainun S, Granger G A, et al. J Immunother. 1991;10:131–140. [PubMed] [Google Scholar]
- 10.Holladay F P, Heitz T, Chen Y L, Chiga M, Wood G W. Neurosurgery. 1992;31:528–530. doi: 10.1227/00006123-199209000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Heimberger A B, Crotty L E, Archer G E, McLendon R E, Friedman A, Dranhoff G, Bigner D D, Sampson J H. J Neuroimmunol. 2000;103:16–25. doi: 10.1016/s0165-5728(99)00172-1. [DOI] [PubMed] [Google Scholar]
- 12.Culver K W, Ram Z, Wallbridge S, Ishii H, Oldfield E H, Blaese R M. Science. 1992;256:1550–1552. doi: 10.1126/science.1317968. [DOI] [PubMed] [Google Scholar]
- 13.Weyerbrock A, Oldfield E H. Curr Opin Oncol. 1999;11:168–173. doi: 10.1097/00001622-199905000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Mineta T, Rabkin S D, Yazaki T, Humter W D, Martuza R L. Nat Med. 1995;1:938–943. doi: 10.1038/nm0995-938. [DOI] [PubMed] [Google Scholar]
- 15.Felsher D W, Bishop J M. Mol Cell. 1999;4:199–207. doi: 10.1016/s1097-2765(00)80367-6. [DOI] [PubMed] [Google Scholar]
- 16.Pelengaris S, Littlewood T, Khan M, Elia G, Evan G. Mol Cell. 1999;3:565–577. doi: 10.1016/s1097-2765(00)80350-0. [DOI] [PubMed] [Google Scholar]
- 17.Chin L, Tam A, Pomerantz J, Wong M, Holash J, Bardeesy N, Shen Q, O'Hagan R, Pantginis J, Zhou, et al. Nature (London) 1999;400:468–472. doi: 10.1038/22788. [DOI] [PubMed] [Google Scholar]