Abstract
Objectives:
There is a little data about rectal administration of Ketamine as a postoperative analgesic, so we compared the efficacy of rectal ketamine with rectal acetaminophen, which is applied routinely for analgesia after painful surgeries like tonsillectomy.
Methods:
In this single-blinded comparative trial, we enrolled 70 children undergoing elective tonsillectomy, and divided them randomly in two groups. Patients received rectal ketamine (2 mg / kg) or rectal acetaminophen (20 mg / kg) at the end of surgery. The children's Hospital of Eastern Ontario Pain scale was used to estimate pain in children. Also the vital signs, Wilson sedation scale, and side effects in each group were noted and compared for 24 hours.
Results:
The ketamine group had a lower pain score at 15 minutes and 60 minutes after surgery in Recovery (6.4 ± 0.8, 7.4 ± 1 vs. 7.1 ± 1.2, 7.8 ± 1.2 in the acetaminophen group, P < 0.05) and one hour and two hours in the ward (7.2 ± 0.7, 7 ± 0.5 vs. 7.9 ± 1.2, 7.5 ± 1.2 in the acetaminophen group, P < 0.05), with no significant differences till 24 hours. Dreams and hallucinations were not reported in the ketamine group. Systolic blood pressure was seen to be higher in the ketamine group (104.4 ± 7.9 vs. 99.8 ± 7.7 in the acetaminophen group) and nystagmus was reported only in the ketamine group (14.2%). Other side effects were equivalent in both the groups.
Conclusions:
With low complications, rectal ketamine has analgesic effects, especially in the first hours after surgery in comparison with acetaminophen, and it can be an alternative analgesic with easy administration in children after tonsillectomy.
Keywords: Postoperative pain, Preventive analgesic, Rectal acetaminophen, Rectal ketamine, Tonsillectomy
INTRODUCTION
Pain relief after the surgeries with a suitable analgesic is an important issue for patients and anesthesiologists. The analgesic method has to be with high efficacy, low side effects, and easy administration.[1] The control of the pain during the surgery (intraoperative) and after it (postoperative) is called preventive analgesia, which results in accelerated recovery and improvement in the Health-Related Quality of Life (HRQL).[2]
Tonsillectomy is one of the painful operations, which is common in children and it is necessary to apply an analgesic drug to prevent this pain and the complications due to it.[3] Opioids including morphine are sometimes used and have high efficacy to suppress post-tonsillectomy pain, but the important side effects, especially respiratory depression, have limited use of them.[4]
Nonsteroidal Anti-Inflammatory Drugs (NSAIDs), including diclofenac, are also used as preventive analgesia, but they increase the risk of site-bleeding after tonsillectomy.[5] Acetaminophen is another analgesic drug that is used commonly in oral and rectal form, during and after tonsillectomy. Nausea, vomiting, and dysphagia after tonsillectomy have limited oral intake of acetaminophen, so rectal administration is the better route.[6]
Intravenous (IV) acetaminophen (15 mg / kg) was compared with the rectal form (40 mg / kg) in pediatric adenotonsillectomy and the rectal form showed better analgesic effect.[7]
Ketamine, a N-methyl-D-aspartate (NMDA) receptor antagonist, is a drug to induce anesthesia, and has also been used as a postoperative analgesic in surgeries like pediatric tonsillectomy, in several studies. IV and intra-muscular (i.m.) ketamine has compared with morphine and placebo.[8–11] Also the preventive analgesic effect of the peritonsillar administration of ketamine has been demonstrated in some studies.[12,13] Rectal ketamine also has analgesic efficacy in treating nociceptive and neuropathic pain.[14] Also some studies have been established to try rectal ketamine as a premedication before surgeries.[15]
However, there is not enough information to prove the hypothesized preventive effect of rectal ketamine for painful surgeries like tonsillectomy in children and there is no study to compare this effect with a routine analgesic like rectal acetaminophen. Thus, we designed this study to find out the preventive analgesic effect of rectal ketamine and compare it with rectal acetaminophen.
METHODS
After study approval by the Ethics Committee of the Isfahan University of Medical Sciences, 70 patients were selected to participate in this randomized, single-blinded comparative trial. On account of the difference in administration between rectal ketamine (needle removed syringe) and rectal acetaminophen (suppository form), the anesthesiologist was aware of the applied drug, but the person who recorded the data was unaware of it (blinded).
The inclusion criteria were children without neurological, renal, psychiatric or hepatic disease, 5 – 15 years old, American Society of Anesthesiologists (ASA) physical status I or II, and undergoing elective tonsillectomy. Patients with neurological, renal, psychiatric or hepatic disease, with history of allergy to any of the study drugs and usage of other analgesics were excluded from the study.
Informed consents were obtained from the parents before the study and they were randomly divided into two groups by selection of a sealed envelope with the drug code inside. One group received ketamine rectally (2 mg/kg) by syringe with the needle removed and lubricated, at the end of the surgery (even we were allowed to use up to 10 mg / kg ketamine rectally to obtain just analgesia without anesthesia).[16]
The other group received acetaminophen rectally (20 mg / kg), in suppository form, at the end of the surgery (the applied dose of rectal acetaminophen was safe and without side effects).[17]
The patients were fasted for eight hours before surgery; anesthesia was induced with sodium thiopental (5 mg / kg), atracurium (0.6 mg / kg), and fentanyl (1 μg / kg) and maintained with an equivalent of 1 MAC of isoflurane, a gas mixture of nitrous oxide (50%) – oxygen (50%), and morphine 0.1 mg / kg. The patients underwent basic monitoring including, echocardiogram (ECG), Pulse oximetry, and End tidal CO2 continuously during the surgery and blood pressure and pulse rate every five minutes for 15 minutes, and every 15 minutes till the end of operation.
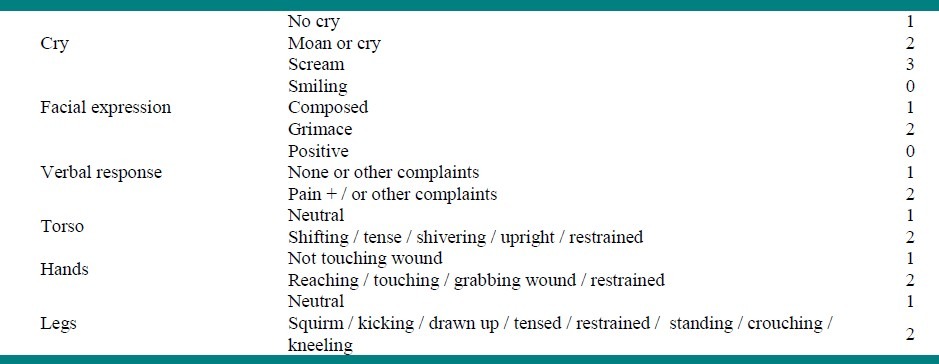
At the end of surgery, the anesthesiologist applied the drug for each patient and the children were transferred to Recovery Room and discharged from the Recovery Room to the ward, based on the Aldrete scale which scored the circulation, respiration, consciousness, and activity of the patient. A person, who was unaware about the used drug, recorded the information in a data form. The Children's Hospital of Eastern Ontario Pain Scale (CHEOPS) was the pain score assessed in the study[18] [Table 1], and was recorded 15, 30, and 60 minutes after the operation in the Recovery Room and one, six, 12, and 24 hours after transferring the patient to the ward. A patient with CHEOPS ≥ 11 was given morphine 0.1 mg / kg and excluded from study.
Table 1.
CHEOPS pain score (range 4 – 13)
The vital signs of the patients were recorded 15, 30, and 45 minutes after the operation in the Recovery Room and six, 12, and 18 hours after transferring them to the ward.
The Wilson sedation score was used to estimate and compare the level of consciousness between the two groups[19] [Table 2], and recorded 15, 30, and 60 minutes and six, 12, and 24 hours after the operation.
Table 2.
Wilson sedation scale

The time between discontinuation of nitrous oxide and extubation (extubation time), the time from tonsillectomy position until bleeding control (operation time), and the time the patient stayed in Recovery until discharge, based on the Aldrete score (time to recovery stay), were recorded.
Also side effects in the groups were evaluated, including, vomiting, hallucinations, dreams, disorientation, and nystagmus.
The sample size of 70 patients, was estimated based on 80% power, to detect a difference of at least 1 score between two groups with α-value = 0.05. The data were analyzed by SPSS software using an independent t-test for qualitative data and chi-square test for all the quantitative data. Also the Mann-Whitney test was used to compare the median of the Wilson sedation score between the groups, and the Fisher's exact test for comparing the side effects, where needed. Significant statistical data had P < 0.05 in the analysis.
RESULTS
All patients completed the study and no one was excluded.
There were no significant differences between the two groups in age and sex [Table 3]. The chi-square test showed that sexual distribution was similar in the groups (p = 0.46), and an independent t-test showed that the mean age was similar too (p = 0.26).
Table 3.
Demographic characteristic of patients, operation time, extubation time, time to first oral intake, and time to recovery stay

Also there was no significant difference between operation time, Extubation time, and the first time food was taken, between the two groups, based on the independent t-test. Moreover, all the patients got acetaminophen syrup, as they could take oral food. (p > 0.05), but the recovery time was significantly longer in the acetaminophen group (p = 0.02) [Table 3].
The CHEOPS scores were significantly lower in the ketamine group in 15 and 60 minutes in the Recovery Room and one and two hours after transferring to the ward (P < 0.05), but no significant difference after it, till 24 hours [Table 4].
Table 4.
CHEOPS scoring in the recovery room and ward after operation

The systolic blood pressure was seen to be higher in the ketamine group at all times (mean ± SD was 104.4 ± 7.9 in the ketamine group vs. 99.8 ± 7.7 in the acetaminophen group, P < 0.05) and also the pulse rate showed an increase 12 and 18 hours after surgery in the ketamine group (96.5 ± 3.8 and 94.5 ± 4.5 vs. 93.7 ± 5.3 and 89.6 ± 5.1 in the acetaminophen group, P < 0.05), but we found no special difference in the diastolic blood pressure and respiratory rate between the two groups (P > 0.05).
The patients in the two groups had an equivalent sedation score 15 minutes (P = 0.7) and 30 minutes (P = 0.9) after surgery. Both the groups were equivalent at 60 minutes and six hours in the level of conciseness (p = 1) and all the patients were completely awake and oriented after six hours.
Incidences of vomiting (5.7% in the ketamine group vs. 8.6% in the acetaminophen group), bleeding (8.6% in ketamine group vs. 11.4% in acetaminophen group) and disorientation (5.7% in both groups) were similar in the two groups, based on the chi-square test and Fisher's exact test. No hallucinations or dreams were reported at all. Some of the patients, only in the ketamine group, had nystagmus (14.2 %), which was absolutely significant.
DISCUSSION
The results showed that rectal ketamine reduced pain more effectively when compared with rectal acetaminophen in the first hours after surgery (one and two hours) and had an equivalent analgesic potential with acetaminophen at other times, for 24 hours (this might be because of the short half life of rectal ketamine).
Also the acetaminophen group had longer recovery station, which could be because of the higher pain scores in first hours after surgery.
Both groups had equivalent sedation scores for 24 hours and we could compare the CHEOPS based on it. Some parameters in the CHEOPS were related to the sedation score, so we needed to compare the CHEOPS in two groups with the equivalent level of consciousness.
Rectal administration of course was a suitable route of use, not invasive and was just done with a needle removed syringe. We used a sub-anesthetic dose of rectal ketamine (2 mg / kg). We were even allowed to use up to 10 mg / kg.[16] Some studies were done to detect this effect in tonsillectomy, which was common and painful in children, to evaluate if we could replace other analgesics with ketamine.
In one study that was done by R.J. Marcus et al., i.m. ketamine was compared with i.m. Morphine (0.1 – 015mg / kg) and the analgesic effect was comparable.[10] A similar study done by Spinal et al., and similar results were concluded.[11] Also i.m. ketamine (0.1 mg / kg) was compared with placebo in tonsillectomy, and the results demonstrated the pain relief effects of i.m. ketamine.[9] Heidari et al tried oral ketamine and compared it with placebo in one study and reported the analgesic effect of the oral form of this drug.[20] In another study i.v. ketamine had significant analgesic effects.[8]
The studies were usually performed based on oral, peritonsillar, i.v., and i.m. ketamine for postoperative analgesic effects, but there was no study that proved ketamine to be a postoperative analgesic when it was administered rectally, although rectal ketamine was useful in neuropathic pain.[15] Our study demonstrated the fact that rectal ketamine had an analgesic effect even for postoperative pain. On the other hand, in the studies with a control group, we avoided an analgesic in the patient that felt pain, so it was better to compare the analgesic with another one.
The analgesic effect of ketamine is due to some mechanisms. Ketamine is an NMDA receptor antagonist, and is known for analgesia in subanesthetic doses.[21] NMDA receptors are in the dorsal horn of the spinal cord and are involved in central sensitization to painful stimulation and ketamine reduces pain with the blockage of these receptors.[22] The blockage of sodium and potassium channels in the peripheral nerves is another mechanism that may also be effective.[23]
Serious side effects like Hallucinations and Dreams were not seen in the 2 mg / kg use of rectal ketamine, and other side effects like vomiting and bleeding were not significant and were equivalent in the two groups. Nystagmus was not severe and no other complications like vertigo were seen, so it was not an important point.
Ketamine has sympathetic activation potential, because of the inhibition of epinephrine reuptake, so the increasing of the blood pressure and pulse rate can be seen in ketamine usage. Acetaminophen has no significant effect on the patient's vital signs in the analgesic doses. The increase in systolic blood pressure, which has been reported in the ketamine group in comparison with the acetaminophen group, is not at a risk level to worry us, so we can forget about the hemodynamic effects of using rectal ketamine at 2 mg / kg.
Finally, we conclude by stating that rectal ketamine can be useful for pain relief in pediatric tonsillectomy, in comparison with acetaminophen, and it can be an alternative analgesic drug with acetaminophen in tonsillectomy, particularly in the first hours after the operation. In our study we have not recorded or compared anti-emetic and rescue analgesic drug doses or parent satisfaction. Higher doses of rectal ketamine can be tried in future studies.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.White PF. Ambulatory anesthesia advances into the new millennium. AnesthAnalg. 2000;90:1234–5. doi: 10.1097/00000539-200005000-00047. [DOI] [PubMed] [Google Scholar]
- 2.Miller RD, Eriksson LI, Fleisher LA. Miller's anesthesia. 7th ed. Philadelphia: Churchill Livingstone; 2010. pp. 2757–81. [Google Scholar]
- 3.Ravi R, Howell T. Anaesthesia for pediatric ear, nose and throat surgery. Contin Educ Anaesth Crit Care Pain. 2007;7:33–7. [Google Scholar]
- 4.Romsing J, Dstergaard D, Drozdziewicz D, Schultz P, Ravn G. Diclofenac or acetaminophen for analgesia in pediatric tonsillectomy in out-patients. ActaAnaesthesiolScand. 2000;44:291–5. doi: 10.1034/j.1399-6576.2000.440312.x. [DOI] [PubMed] [Google Scholar]
- 5.Moiniche S, Romsing J, Dahl JB, Tramer MR. Non-steroidal anti- inflammatory drugs and the risk of operative site-bleeding after tonsillectomy: A quantitative systemic review. AnesthAnalg. 2003;96:68–77. doi: 10.1097/00000539-200301000-00015. [DOI] [PubMed] [Google Scholar]
- 6.Anderson BJ, Woolard GA, Halford NH. Pharmaco-kinetics of rectal paracetamol after major surgery in children. PaediatrAnaesth. 1995;5:237–42. doi: 10.1111/j.1460-9592.1995.tb00291.x. [DOI] [PubMed] [Google Scholar]
- 7.Capici F, Ingelmo PM, Davidson A, Sacchi CA, Milan B, Sperti LR, et al. Randomized controlled trial of duration of analgesia following intravenous or rectal acetaminophen after adenotonsillectomy in children. Br J Anaesth. 2008;100:251–5. doi: 10.1093/bja/aem377. [DOI] [PubMed] [Google Scholar]
- 8.Murray WB, Yankelowitz SM, le Roux M, Bester HF. Prevention of post-tonsillectomy pain with analgesic doses of ketamine. S AfrMed J. 1987;72:839–42. [PubMed] [Google Scholar]
- 9.Elhakim M, Khalafallah Z, El-Fattah HA, Farouk S, Khattab A. Ketamine reduces swallowing-evoked pain after pediatric tonsillectomy. ActaAnaesthesiolScand. 2003;47:604–9. doi: 10.1034/j.1399-6576.2003.00111.x. [DOI] [PubMed] [Google Scholar]
- 10.Marcus RI, Victoria BA, Rushman SC, Thompson JP. Comparison of ketamine and morphine for analgesia after tonsillectomy in children. Br J Anaesth. 2000;84:739–42. doi: 10.1093/oxfordjournals.bja.a013585. [DOI] [PubMed] [Google Scholar]
- 11.Aspinall RL, Mayor A. A prospective randomized controlled study of the efficacy of ketamine for post operative pain relief in children after adenotonsillectomy. PaediatrAnaesth. 2001;11:333–6. doi: 10.1046/j.1460-9592.2001.00676.x. [DOI] [PubMed] [Google Scholar]
- 12.Honarmand A, Safavi MR, Jamshidi M. The preventative analgesic effect of preincisionalperitonsillar infiltration of two low dose of ketamine for postoperative pain relief in children following adenotonsillectomy. A randomized, double-blinded, placebo-controlled study. PediatrAnaesth. 2008;18:508–14. doi: 10.1111/j.1460-9592.2008.02461.x. [DOI] [PubMed] [Google Scholar]
- 13.Dal D, Celebi N, Elevan EG, Celiker V, Aypar U. The efficacy of intravenous or peritonsillar infiltration of ketamine for postoperative pain relief in children following adenotonsillectomy. PediatrAnaesth. 2007;17:263–9. doi: 10.1111/j.1460-9592.2006.02095.x. [DOI] [PubMed] [Google Scholar]
- 14.Kronenberg RH. Ketamine as an analgesic: Parentral, oral, rectal, subcutaneous, transdermal and intranasal administration. J Pain PalliatCare Pharmacother. 2002;16:27–35. doi: 10.1080/j354v16n03_03. [DOI] [PubMed] [Google Scholar]
- 15.Sayin MM, Marcan A, True H, Koner O, Sozubir S, Aykac B. The effect of two different concentration of rectal ketamine on its premedication features in children. Saudi Med J. 2008;29:683–7. [PubMed] [Google Scholar]
- 16.Pedraz JL, Calvo MB, Lanao JM, Muriel C, Satos-Lamas J, DominGuez-Gil A. Pharmacokinetics of rectal ketamine in children. Br J Anaesth. 1989;63:671–4. doi: 10.1093/bja/63.6.671. [DOI] [PubMed] [Google Scholar]
- 17.Sweetman SC. Martindale. 36th ed. London: Pharmaceutical Press; 2009. pp. 1–133. [Google Scholar]
- 18.McGrath PY, Johnson G, Goodman JT, Schillinger J, Dunn J, Chapman J. Advances in pain Research and Therapy. Vol. 9. New York: Raven Press; 1985. CHEOPS: A behavioral scale for rating operative pain in children; pp. 395–402. [Google Scholar]
- 19.Kakinohana M, Sugahara K. Level of consciousness affects the excitability of spinal motor neurons during propofol sedation in humans. Br J Anaesth. 2006;96:742–6. doi: 10.1093/bja/ael081. [DOI] [PubMed] [Google Scholar]
- 20.Heidari SM, Saghaei M, Hashemi SJ, Parvazinia P. Effect of oral ketamine on the postoperative pain and analgesic requirement following orthopedic surgery. ActaAnaesthesiolTaiwan. 2006;44:211–5. [PubMed] [Google Scholar]
- 21.White PF, Way WL, Trevor AJ. Ketamine: Its pharmacology and therapeutic uses. Anesthesiology. 1982;56:119–36. doi: 10.1097/00000542-198202000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Arendt-Nielsen L, Pertersen-Felix S, Fischer M, Bak P, Bjerring P, Zbinden AM. The effect of N-methyl-D-aspartate antagonist (ketamine) on single and repeated nociceptive stimuli: Aplacebo controlled experimental human study. AnesthAnalg. 1995;81:63–8. doi: 10.1097/00000539-199507000-00013. [DOI] [PubMed] [Google Scholar]
- 23.Pedersen JL, Galle TS, Kehlet H. Peripheral analgesic effects of ketamine in acuteinflammatory pain. Anesthesiology. 1998;89:58–66. doi: 10.1097/00000542-199807000-00011. [DOI] [PubMed] [Google Scholar]


