Abstract
After iron deficiency, zinc deficiency is the major micronutrient deficiency in developing countries, and staple food fortification is an effective strategy to prevent and improve it among at-risk-populations. No action has been taken to reduce zinc deficiency via flour fortification so far in Iran, and little is known about the influence of zinc fortification of flour on serum zinc and the iron status, and also about the optimum and effective amount of zinc compound that is used in food fortification. The objective of this study is to evaluate the influence of consuming zinc-fortified breads on the zinc and iron status in the blood serum. In this study, three types of bread were prepared from non-fortified and fortified flours, with 50 and 100 ppm elemental zinc in the form of sulfate. Eighty zinc-deficient women aged 19 to 49 years were randomly assigned to three groups; The volunteers received, daily, (1) a non-fortified bread, (2) a high-zinc bread, and (3) a low-zinc bread for one month. Serum zinc and iron were measured by Atomic Absorption before and after the study. Results showed a significant increase in serum zinc and iron levels in all groups (p < 0.001) except in the control (p > 0.05). Absorption of zinc and iron in the group that consumed high-zinc bread was significantly greater than that in the group that received low-zinc bread (p < 0.01). It was concluded that fortification of flour with 50–100 ppm zinc was an effective way to achieve adequate zinc intake and absorption in zinc-deficient people. It also appeared that consuming zinc-fortified bread improved iron absorption.
Keywords: Bread fortification, Serum iron, Serum zinc, Zinc deficiency, Zinc sulfate
INTRODUCTION
After iron deficiency, zinc deficiency is the most common micronutrient deficiency in the world especially in developing countries like Iran.[1].
Biochemical evidence suggests that the risk of zinc deficiency in Iran is high; approximately 20, 30, 40, and 30% of two-year-old children, six-year-old children, pregnant women, and the youth are zinc-deficient, respectively.[2]
Zinc deficiency is associated with poor growth,[3] depressed immunity,[4] adverse outcomes of pregnancy,[5] neurobehavioral abnormalities,[6] increased susceptibility to infections,[7,8] onset of night blindness, and decreased spermatogenesis.[9,10]
The higher incidence of zinc deficiency in many developing countries is due to the low consumption of animal source foods, which are rich in bioavailable zinc and also due to a high intake of plant-based diets including cereals and legumes.[11] Zinc bioavailability in foods based on plants is very low,[12] because such foods contain a large amount of phytate, which inhibits zinc absorption.[11]
One effective strategy to control zinc deficiency is the fortification of staple foods, to increase the zinc intake of the entire population. Wheat flour is the staple food in Iran, but it is a relatively poor source of zinc.
Studies over several years have shown that zinc supplementation in developing countries has led to a substantial reduction in the duration and severity of diarrhea and pneumonia,[7,8] improvement of neuropsychological performance and growth in children,[13,14] and decreased mortality and morbidity from respiratory diseases.[7]
At present, five zinc compounds are generally recognized as safe or generally recognized as safe (GRAS). Of the GRAS zinc salts, zinc sulfate is the best choice for fortification programs, because of its low cost, high solubility, bioavailability,[15] stability in fortified foods, and neutral effects on the organoleptic properties of fortified products.[16]
There are anxieties about the negative effect of zinc fortification on iron absorption. A few reviews considered the effect of zinc on iron absorption.[17] It appears that zinc may decrease iron absorption if it is given in an aqueous form,[18] but not from complex foods.[19]
According to the mentioned information, the objective of the present study was, therefore, to assess the effect of zinc-fortification of wheat flour on the iron and zinc status in the blood serum.
METHODS
The Study Population
One hundred and twenty women were recruited from among the staff and students of the Isfahan University of Technology. The subjects were considered eligible for the study if they were not currently pregnant or lactating; were free from any known chronic disease; were not participating in any similar program; and were not currently taking any vitamin or mineral supplements. Women meeting these criteria were screened for the zinc status, and 80 of them with low-zinc stores (serum zinc ≤ 70 μg / dl) were invited to participate in the study. The nature of the study, and its potential risks and requirements for participation were explained to the women and a written, informed consent was obtained from each volunteer. Human subjects’ approval for this study was obtained from the Research Department (Vice Chancellor) of the Isfahan University of Technology. The subjects were randomly divided into three groups. Group 1 (control) recipient of non-fortified breads; and Groups 2 and 3 were recipients of breads containing fortified flour with 50 and 100 mg Zn / Kg of zinc sulfate, respectively. Food record and sociodemographic questionnaires were filled out by the participants. The food record questionnaire was filled out for three days of each week during the study. At the baseline, the subjects’ height and weight were measured to calculate their body mass index (BMI).
Food Preparation
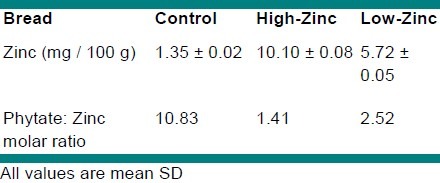
65.5Zn compounds were prepared in powdered form; zinc sulfate heptahydrate was purchased from Merck Corporation (Merck KGaA, Darmstadt, Germany). Ninety percent extraction wheat flour, salt, and active dried yeast were all purchased from the bakery of the University. As Taftoon bread was consumed the most among all the breads in Iran, it was used for the fortification. Zinc sulfate was weighed accurately and dissolved in water, to which wheat flour mixed with water, yeast, and salt was added to form a soft dough. After fermentation of the dough for 1.5 to 2 hours, its total mass was divided into equal portions, each about 200 g. Portions of the dough were fermented for 10 to 15 minutes, rolled into balls, flattened, and baked at 300°C for 90 seconds; following which the breads were put in individual labeled plastic containers until they were distributed among the volunteers. From the filled out questionnaires it was found that each volunteer ate one loaf of Taftoon bread daily on an average. Therefore, each subject received one bread every day for one month and was controlled to ensure that the whole bread was consumed. Table 1 shows the content of zinc and phytate: the zinc molar ratio of breads. The zinc and phytate content of the breads was determined using the American Association of Cereal Chemists (AACC) methods.[20]
Table 1.
The content of zinc and phytate: zinc molar ratio of breads
Sample Preparation and Biochemical Analyses
All the volunteers entered the study during the follicular phase of their menstrual cycle. In the morning hours, fasting participants had blood drawn for biochemical analyses. Blood serum was separated by centrifuging. Serum zinc was measured by flame atomic absorption spectrophotometry (Perkin-Elmer 2380) and serum iron was measured using the Zist Shimi kit (Zist Shimi Corporation, Tehran, Iran) and a spectrophotometer (Spectronic 20D+).
Data Management and Statistical Analyses
Statistical analyses were performed using SAS and SPSS 16.0. Differences among the three study groups for baseline characteristics and for daily intakes of nutrients were determined by the analysis of variance (ANOVA) and Duncan's test. The Paired t test was used to determine the changes in serum zinc and iron levels after one month and also the differences between Groups 2 and 3 with regard to the increase in the serum iron and zinc levels. Food Processor 2 was used to estimate the volunteers’ daily intakes of energy and nutrients. The results were considered statistically significant at the P value of < 0.05.
RESULTS
A total of 75 participants completed the study (25 subjects in each group).
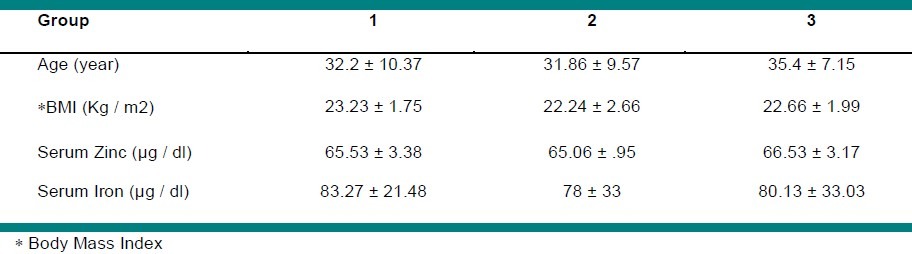
Mean values for serum iron level were in the normal range, but zinc deficiency was observed in all study groups. All subjects were healthy at the beginning of the study and remained so throughout the study. The baseline characteristics of the participants as well as their estimated daily intake of energy and nutrients did not differ among the study groups (P > 0.05). The same conditions for all groups were a positive point of the study. Table 2 reveals the baseline characteristics of the participants.
Table 2.
Baseline characteristics of the study subjects
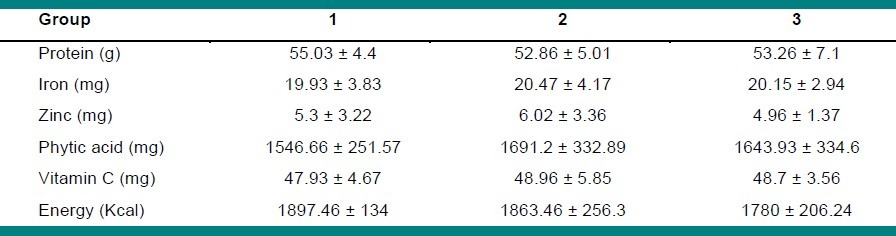
The estimated daily intake of protein, energy, zinc, iron, vitamin C, and phytic acid are shown in Table 3. The table indicates a low dietary zinc intake. The subjects received just 60 – 70% of the Recommended Dietary Allowance (RDA) from their diet (8 mg / day for adult women.[21]
Table 3.
The estimated daily intake of nutrients among the study groups
At the end of the study, the serum zinc level in Groups 2 and 3 increased significantly (P < 0.001) and rose up to more than 70 μg / dl. Also, the serum iron level showed a significant increase in Groups 2 and 3 (P<0.001). The serum iron and zinc concentration of control did not show significant changes (P > 0.05). Table 4 shows the effect of consuming fortified and non-fortified breads on serum zinc and iron concentrations at the end of the study.
Table 4.
Comparison of the serum zinc and iron level before and after the study
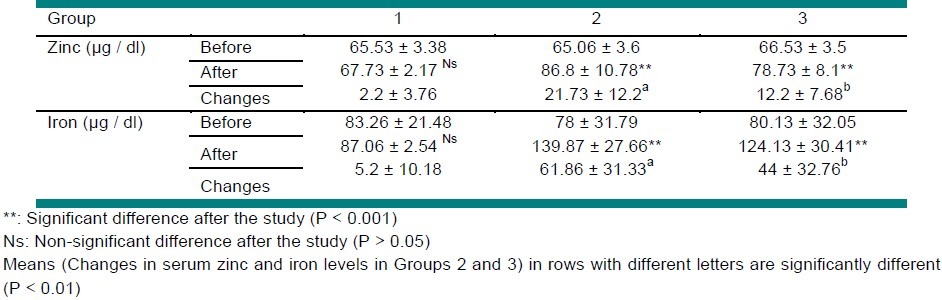
According to Table 4, the increase in serum zinc and iron levels in Group 2 was significantly greater than that in group 3 (P < 0.01).
DISCUSSION
The present study was carried out to evaluate the effect of consuming zinc-fortified bread on the serum iron and zinc status.
Breads were prepared with zinc-fortified wheat flour that contained relatively low or high amounts of zinc sulfate. Assuming there was 130 g of flour in a loaf of Taftoon bread containing 50 and 100 mg / Kg zinc ion (flour basis), there would be 9.15 and 16.16 mg of zinc per loaf, respectively. Thus, one loaf would contain 100% RDA of zinc for adult women. For men more than one loaf of low-zinc bread was required to meet their 100% RDA.
Improvement of zinc absorption was due to both increase of zinc intake and decrease of phytate : zinc molar ratio. According to the World Health Organization cut-offs, phytic acid: zinc molar ratio of ≥ 15, 5 – 15, and < 5 was equal to zinc bioavailability as low (10 – 15%), moderate (30 – 35%), and high (45 – 55%).[22] Indeed, fortification of breads decreased the inhibitory effect of phytic acid, changed the bioavailability of zinc from moderate to high, and improved the absorption of zinc from bread in this way.
The possible effects of zinc sulfate fortification of cereal products on the absorption of iron have been examined through both short-term tracer studies of iron absorption and larger term studies of the impact of zinc sulfate fortification on the biochemical indicators of iron status. One study in which iron absorption from a zinc-fortified product was compared with iron absorption from a similar non-fortified product, it showed a significant reduction in iron absorption.[17] Two studies were available in which the impact of zinc sulfate fortification on the indicators of iron status was measured.[23,24] In none of these studies were there any adverse effects of zinc fortification.
In the present study, zinc improved iron absorption significantly. According to the fact that the daily intake of nutrients in all groups was alike, it was obvious that zinc helped iron to be absorbed in more quatities. An alternative study found the positive effect of zinc on iron absorption in mice.[25]
Zinc is associated with more than 50 metalloenzymes, which have a diverse range of functions, including the synthesis of specific proteins such as transferrin, the iron key transporter in plasma.
One study showed that increasing zinc intake caused a higher production of transferrin, to absorb more iron in the plasma.[25]
It is reported that there is a strong positive association between plasma zinc and hemoglobin concentration.[26] There have been a few studies in which the addition of zinc has improved the hematological response of children who were thought to be zinc-deficient.[27] As is known, iron is one of the most necessary part of the heme molecule. As plasma zinc and hemoglobin synthesis is increased, more iron is needed to take part in hemoglobin production; thus, increase of serum iron concentration and iron absorption is expected. Several mechanisms may be involved whereby zinc affects the hemoglobin concentration: Zinc is implicated in hemoglobin synthesis through the activity of several zinc-dependent enzyme systems, including aminolevulinic acid dehydratase that mediates a step in the synthesis of heme and thymidine kinase and DNA polymerase, which are involved in DNA synthesis. The zinc-finger transcription factor, GATA-1, is also essential for erythropoiesis. Secretion of a plasma insulin-like growth factor-1 stimulated by plasma zinc is another possible mechanism.[26]
Previous studies showed that apical to basolateral iron transport across the Caco-2 TC7 cell monolayer was lower in cells exposed to high iron compared to those exposed to high zinc. Intriguingly, the mRNA expression of the basolateral iron transporter, IREG1, while not altered by exposure to high iron, is increased by zinc. IREG1 mRNA is increased in response to iron-deficiency anemia and hereditary hemochromatosis[11] A.T. McKie, P. Marciani, A. Rolfs, K. Brennan, K. Wehr, D. Barrow, S. Miret, A. Bomford, T.J. Peters, F. Farzaneh, M.A. Hediger, M.W. Hentze and R.J. Simpson, Mol. Cell 5 (2000), pp. 299-309. Article PDF (525 K) View Record in Scopus|Cited By in Scopus (643), but is unaffected by dietary iron loading. IREG1 expression can be influenced by dietary zinc. The mechanism involved in zinc stimulation is unclear, but it is possible that metal responsive elements may also be present in the IREG1 promoter regions.[28] This can be another mechanism for increasing the iron absorption by zinc.
The increase of serum iron and zinc in Group 2 (high-zinc bread) was significantly greater than that in Group 3 (low-zinc bread). This indicates that the more the zinc received from fortified breads, the more the zinc and iron absorbed through the intestine.
In our previous study (unpublished), the effect of the proposed levels of zinc fortification on the organoleptic characteristics of breads was evaluated. Although it was possible to detect a slight difference in taste between non-fortified and high-zinc bread, high-zinc breads were still acceptable. Therefore, addition of more than 100 mg Zn / Kg would change the organoleptic properties of breads negatively. Fortification with less than 50 mg / Kg seems not to have a significant effect on the serum zinc concentration, however, more research is needed to give a definite idea.
In conclusion, fortification of flour with 50 – 100 mg / Kg zinc is an effective way to achieve adequate zinc intake and absorption in zinc-deficient people. It also appears that consuming zinc-fortified breads does not affect iron absorption negatively, and also improves iron absorption from the diet.
ACKNOWLEDGMENTS
The authors would like to thank the Isfahan University of Technology for providing financial support for this study, as an MS thesis.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared
REFERENCES
- 1.West CE. Iron deficiency: The problem and approaches to its solution. Food Nutr Bull. 1996;17:37–41. [Google Scholar]
- 2.A study on the status of micronutrients in Iran. Iran: 2000. Iran ministry of health and medical education and health assistance. [Google Scholar]
- 3.Brown KH, Peerson JM, Rivera J, Allen LH. Effect of supplemental zinc on the growth and serum zinc concentrations of prepubertal children: A meta-analysis of randomized controlled trial. Am J Clin Nutr. 2002;75:1062–71. doi: 10.1093/ajcn/75.6.1062. [DOI] [PubMed] [Google Scholar]
- 4.Fraker PJ, Gershwin ME, Good RA, Prasad AS. Interrelationships between zinc and immune function. Fed Proc. 1986;45:1474–9. [PubMed] [Google Scholar]
- 5.Caulfield LE, Zavaleta N, Shankar AH, Meriald M. Potential contribution of maternal zinc supplementation during pregnancy to maternal and child survival. Am J Clin Nutr. 1998;68:499–508. doi: 10.1093/ajcn/68.2.499S. [DOI] [PubMed] [Google Scholar]
- 6.Black MM. Zinc deficiency and child development. Am J Clin Nutr. 1998;68:464S–95S. doi: 10.1093/ajcn/68.2.464S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bhutta ZA, Black RE, Brown KH Zinc Investigators Collaborative Group. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: A pooled analysis of randomized controlled trial. J Pediatr. 1999;135:689–97. doi: 10.1016/s0022-3476(99)70086-7. [DOI] [PubMed] [Google Scholar]
- 8.Bhutta ZA, Black RE, Brown KH Zinc Investigators Collaborative Group. Therapeutic effects of oral zinc in acute and persistent diarrhea in children in developing countries: a pooled analysis of randomized controlled trial. Am J Clin Nutr. 2000;72:1516–22. doi: 10.1093/ajcn/72.6.1516. [DOI] [PubMed] [Google Scholar]
- 9.Shankar AH, Prasad AS. Zinc and immune function: the biological basis of altered resistance to infection. Am J Clin Nutr. 1998;68:447S–63S. doi: 10.1093/ajcn/68.2.447S. [DOI] [PubMed] [Google Scholar]
- 10.Zlewski PD. Zinc and immunity: Implications for growth, survival and function of lymphoid cells. J Nutr Immunol. 1996;4:39–80. [Google Scholar]
- 11.Gibson R. Zinc nutrition in developing countries. Nutr Res Rev. 1994;7:151–73. doi: 10.1079/NRR19940010. [DOI] [PubMed] [Google Scholar]
- 12.Solomons NW. Dietary sources of zinc and factors affecting its bioavailability. Food Nutr Bull. 2001;22:138–51. [Google Scholar]
- 13.Sandstead HH, Penland JG, Alcock NW, Dayal HH, Chen XC, Li JS, et al. Effect of repletion with zinc and other micronutrients on neuropsycologic performance and growth of Chinese children. Am J Clin Nutr. 1998;67:470S–5S. doi: 10.1093/ajcn/68.2.470S. [DOI] [PubMed] [Google Scholar]
- 14.Umeta M, West CE, Haidar J, Deurenberg C, Haus-vast JG. Zinc supplementation and stunted infants in Ethiopia: A randomized controlled trial. Lancet. 2000;355:2021–6. doi: 10.1016/S0140-6736(00)02348-5. [DOI] [PubMed] [Google Scholar]
- 15.Ranum P. Zinc enrichment of cereal staples. Food Nutr Bull. 2001;22:169–72. [Google Scholar]
- 16.Kenneth H, Brown KH, Hambidge M, Ranum P. Zinc fortification of cereal flours: Current recommendations and research needs. Food Nutr Bull. 2010;31:62S–74S. doi: 10.1177/15648265100311S106. [DOI] [PubMed] [Google Scholar]
- 17.Herman S, Griffin IJ, Suwarti S, Ernawati F, Permaesih D, Abrams SA. Cofortification of iron-fortified flour with zinc sulfate, but not zinc oxide, decreases iron absorption in Indonesian children. Am J Clin Nutr. 2002;4:813–7. doi: 10.1093/ajcn/76.4.813. [DOI] [PubMed] [Google Scholar]
- 18.Crofton RW, Gvozdanovic S, Kin CC, Brunt PW, Mowat NA, Agget PJ. Inorganic zinc and the intestinal absorption of ferrous iron. Am J Clin Nutr. 1989;50:141–4. doi: 10.1093/ajcn/50.1.141. [DOI] [PubMed] [Google Scholar]
- 19.Rossander-Hulthen L, Brune M, Sandstrom B, Lonnerdal B, Hallberg L. Competetive inhibition of iron absorption by manganese and zinc in humans. Am J Clin Nutr. 1991;54:152–6. doi: 10.1093/ajcn/54.1.152. [DOI] [PubMed] [Google Scholar]
- 20.American Association of Cereal Chemists. The Approval Methods Committee, the Association: St. Paul, MN. 2003 [Google Scholar]
- 21.Dietary reference intakes for vitamin A, vitamin K, arsenic, boron, chromium, iodine, iron, manganese, molybdenum, nickel, silicon, vanadium, and zinc. National Washington, D.C: Academy Press; 2001. Institute of Medicine. [PubMed] [Google Scholar]
- 22.Brown KH, Wuehler SE, Peerson JM. The importance of zinc in human nutrition and estimation of the global prevalence of zinc deficiency. Food Nutr Bull. 2001;22:113–25. [Google Scholar]
- 23.Brown KH, Wessells KR, Hess SY. Zinc bioavailability from zinc-fortified foods. Int J Vitam Nutr Res. 2007;3:174–81. doi: 10.1024/0300-9831.77.3.174. [DOI] [PubMed] [Google Scholar]
- 24.de Romana D Lopez, Salazar M, Hambidge KM, Penny ME, Peerson JM, Krebs NF, et al. Longitudinal measurements of zinc absorption in Peruvian children consuming wheat products fortified with iron only or iron and 1 of 2 amounts of zinc [abstract] Am J Clin Nutr. 2005;81:637–47. doi: 10.1093/ajcn/81.3.637. [DOI] [PubMed] [Google Scholar]
- 25.Badie A. Faculty of pharmacy and pharmaceutical science. Isfahan, Iran: Isfahan University of Medicine; 1992. Study of relationships between metabolic ways of iron and zinc and their interactions [dissertation] [Google Scholar]
- 26.Gibson RS, Abebe Y, Stabler S, Allen RH, Westcott JE, Stoecker BJ, et al. Zinc, gravid, infection, and iron, but not vitamin B-12 or folate statuses, predict hemoglobin during pregnancy in southern Ethiopia. J Nutr. 2008;138:581–6. doi: 10.1093/jn/138.3.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Smith JC, Makdani D, Hegar A, Rao D, Douglass LW. Vitamin A and zinc supplementation of pre-school children. Am J Clin Nutr. 1999;18:213–22. doi: 10.1080/07315724.1999.10718854. [DOI] [PubMed] [Google Scholar]
- 28.Yamaji S, Tennant J, Tandy S, Williams M, Srai SK, Sharp P. Zinc regulates the function and expression of the iron transporters DMT1 and IREG1 in human intestinal Caco-2 cells. FEBS Lett. 2001;507:137–41. doi: 10.1016/s0014-5793(01)02953-2. [DOI] [PubMed] [Google Scholar]


