Abstract
Skin cancer is the most common form of cancer, and although its most common forms are rarely life threatening, the morbidity of this disease is substantial, especially in the southern regions of the United States. Mohs micrographic surgery (MMS) is a precise form of microscopically controlled excisional surgery created in the US but currently practiced worldwide and used to treat various cutaneous carcinomas. The history, technique, indications, and recurrence rates of MMS are discussed to allow the practicing physician to understand the specific instances when patients will best benefit from this procedure.
INTRODUCTION
The first Mohs micrographic surgeon was Fredric Mohs of Madison, Wisconsin, who earned his medical degree in 1934. At the time of his graduation he had already completed a large portion of the basic research on the technique that bears his name. A very humble man by nature, he did not refer to his technique as Mohs surgery but coined the term “chemosurgery” to denote that the process involved placement of a chemical on the skin that was involved with tumor (1). The chemical that he discovered fixed tissue in situ was zinc chloride paste. The paste was applied to the patient in the clinical setting, and the patient would go home to return the next day for removal of the fixed tissue. This process was repeated until the cutaneous neoplasm was extirpated when the tissue was examined histologically. This procedure was not without drawbacks: the process was time consuming and painful, and the wounds required second-intention healing because the zinc chloride paste devitalized the wound margins, making surgical closure imprudent. Dr. Mohs' landmark article on 440 patients treated with chemosurgery appeared in the Archives of Surgery in 1941 (2).
Fredric Mohs' initial paper was met with skepticism, but his confidence in the procedure never wavered. Through meticulous record keeping of literally thousands of successful cases, his surgical colleagues gained understanding and faith in the technique. One problem repeatedly presented itself when treating eyelid tumors: the zinc chloride paste would damage the eye if allowed to contact it. Dr. Mohs discovered a solution to this problem in 1953 by infiltrating the diseased tissue with local anesthesia and removing the carcinoma immediately by excising in a horizontal (tangential) manner. This approach became known as the “fresh-tissue technique.” The term chemosurgery was then replaced by “fixed-tissue technique” (1,3). The fresh-tissue technique revolutionized Mohs micrographic surgery; procedures could be conducted in a fraction of the time, with minimal discomfort. Cure rates remained unaffected and unparalleled as documented in 1974 by two of Dr. Mohs' prior fellows, Sam Stegeman and Ted Tromovitch, in the Archives of Dermatology (4).
Since the mid 1970s, 1- and 2-year fellowships in Mohs micrographic surgery (MMS) accredited by the Amercian College of Mohs Micrographic Surgery and Cutaneous Oncology have expanded in number to 64 and the number of Fellows of the Mohs College stands at 600. Dr. Mohs retired from practice in 1986 and passed away on July 1, 2002 at the age of 92.
TECHNIQUE
Mohs micrographic surgery is a unique process that is ideally suited for the treatment of processes that enlarge by contiguous extension. Nonmelanoma skin cancer (NMSC) exemplifies one such process. Basal cell carcinoma (BCC) and squamous cell carcinoma (SCC), in general, grow in the localized area of the primary tumor, only occasionally metastasize, and rarely demonstrate skip areas. However, they can be locally aggressive and frequently recur. These are the carcinomas most often treated with MMS to ensure that a malignant focus is not left at the site of excision, which could be responsible for local recurrence (1,3). It is generally accepted that residual foci of nonmelanoma skin cancer are areas from which the tumor can recur (5). Inflammation associated with the healing process cannot be expected to eradicate the remaining malignancy (6,7).
The medical literature is also replete with reports that standard histologic examination of the fusiform excisional specimen examines less than 1.0% of the surgical margin (8–10). An example of the portions of the tissue actually examined by standard histologic processing is shown in Figure 1A. Since certain histologic subtypes of BCC and aggressive SCC can grow in erratic patterns, analogous to tree roots growing in the earth, foci of tumor may be missed. Even more compulsive “breadloafing” or serial sectioning of the tissue, as shown in Figure 1B, still potentially misses the full extent of the tumor.
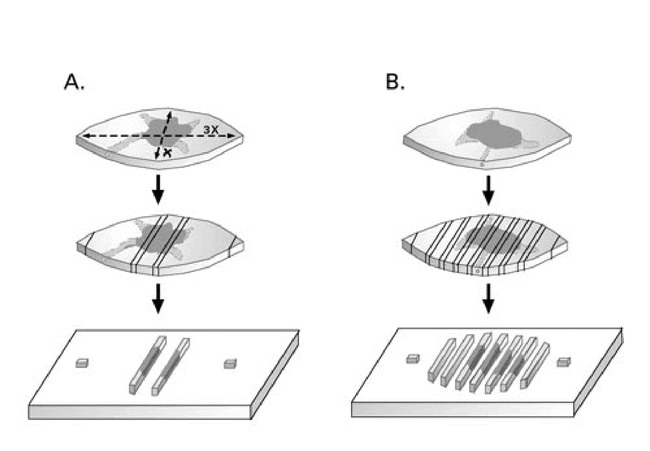
Figure 1. A. Standard histologic examination of 2 cross sections and the tips of an ellipse, a subclinical extension of the tumor represented by the stippled area is missed in this example.B. “Breadloafing” of the elliptical excision specimen for histologic examination could potentially miss tumor extending to the lateral margin.
If, in fact, an entire specimen is truly serially sectioned in an unbroken fashion and sections are 7 µ thick, the pathologist could expect 1500 sections per centimeter of tissue. This type of processing is not routinely conducted (8). Not uncommonly, intraoperative frozen sections on vertically excised tissue are done on the surgical specimen, and although this is a better estimate of the surgical margin, it is not examination of 100% of the surgical margin. Figure 2 illustrates how foci of infiltrating tumor could potentially escape detection with this type of processing. Mohs micrographic surgery is a precise technique that allows histologic examination of 100% of the surgical margins. An overview of the steps required to perform MMS are shown in Figures 3–7. The typical MMS patient has had a thorough preoperative evaluation to include an extensive history and physical examination prior to the procedure. The author requires a histologic diagnosis documented with paraffin-embedded permanent sections prior to initiation of MMS. An informed consent is obtained by the operating physician and appropriately witnessed (11).
Figure 2.
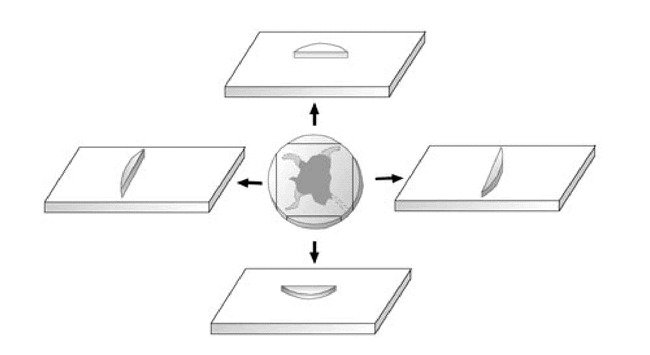
Random peripheral frozen sectioning of excisional specimen misses subclinical extension at the 4:30 clock face position in this representation.
Figure 3.
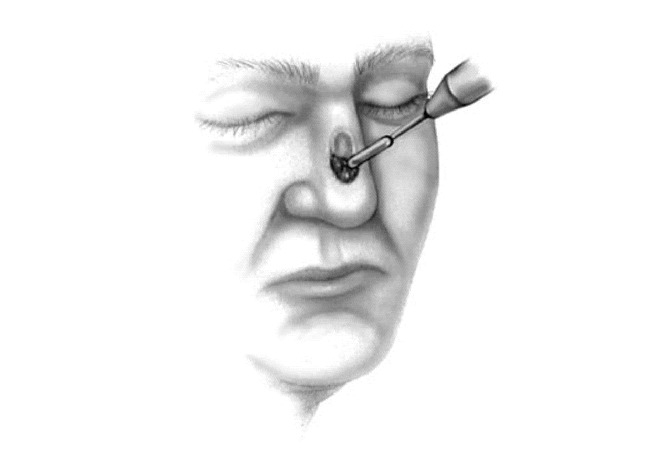
Debulking of surgical site prior to resection of first Mohs surgery layer.
Figure 7.
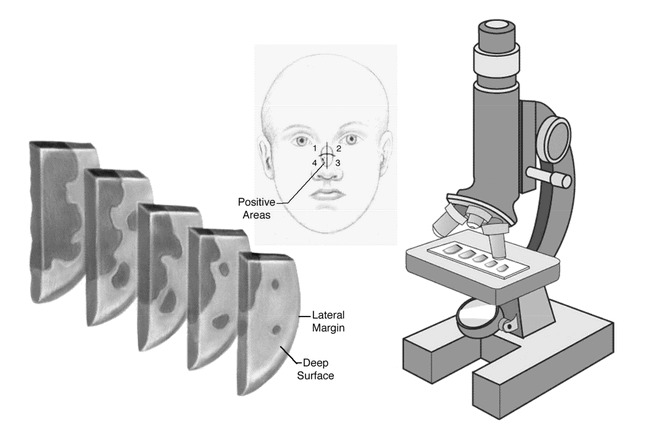
The processed material is examined under a microscope, any areas positive for tumor are mapped, and the process is repeated until a clear surgical margin is obtained.
The procedure itself usually takes place in the outpatient setting under local anesthesia. The patient is prepared, draped, and anesthetized in a sterile fashion. Lesional tissue is carefully identified by stretching the affected area and marking the area with a gentian violet skin-marking pen. Local anesthesia is achieved with 1.0% Xylocaine with epinephrine 1:200,000 infiltrated with a 1-inch, 30-gauge needle. The clinical extent of the tumor is then roughly identified by curettage of the tumor (Figure 8A–D). The tissue is incised with a #15 blade at a 45 degree angle from horizontal, beginning approximately 2 mm around the curettage defect. This tangential or “beveled” incision is continued until the deep margin is completely incised horizontally (Figure 9A–D).
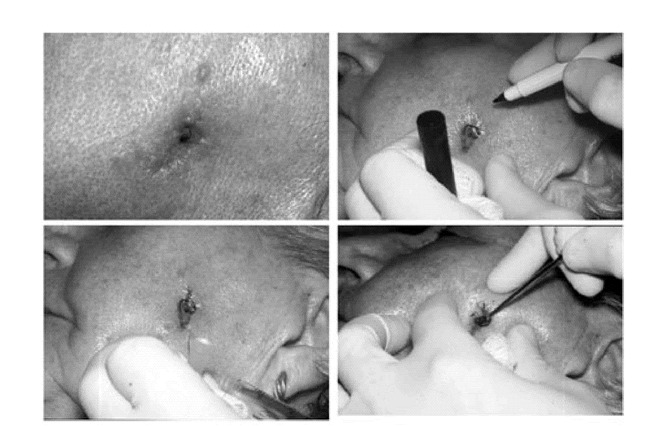
Figure 8. A. Basal cell carcinoma of the left cheek with histologic documentation of tumor at the deep and lateral margin of previous excision.B. Tumor carefully visualized and marked with skin marking pen.C. Area infiltrated with local anesthetic.D. Clinical extent of tumor approximated with dermal curette.
Figure 9.
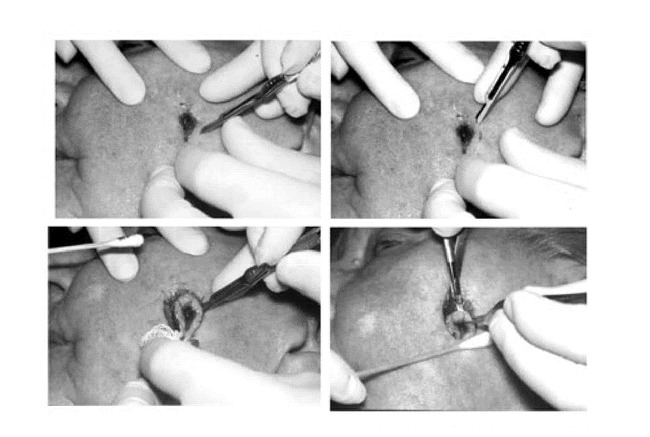
Sequential photographs A through D representing the horizontal nature of the incision angle.
Hemostasis is obtained with cautious electrocoagulation, the defect is measured, a detailed map is created, and a sterile dressing is applied to the wound while the tissue is processed (Figure 10). At all points during MMS, care is taken by the Mohs surgeon and the Mohs histotechnologist to always know the orientation of the specimen. Each specimen is grossed in by cutting the tissue along the orienting score marks that were made during the excision procedure. The tissue edges are dyed with tissue dye and mapped on the case map. The tissue is then delivered to the Mohs laboratory which is usually just a few feet from the operating suite (Figure 11).
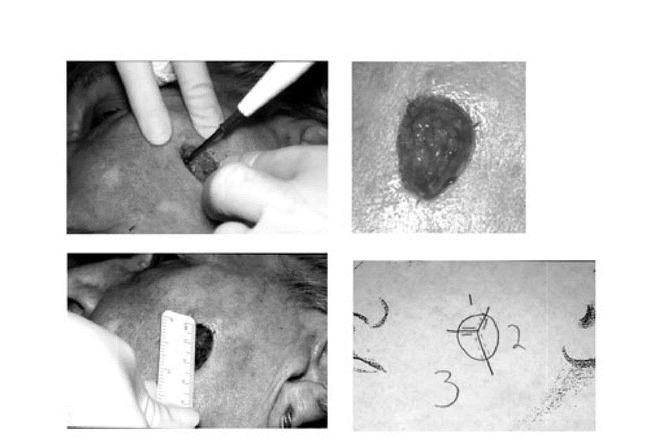
Figure 10. A. Electrocoagulation hemostasis.B. Mohs micrographic surgery wound after the first stage of surgery.C. Wound dimensions obtained.D. Mohs micrographic surgery map drawn.
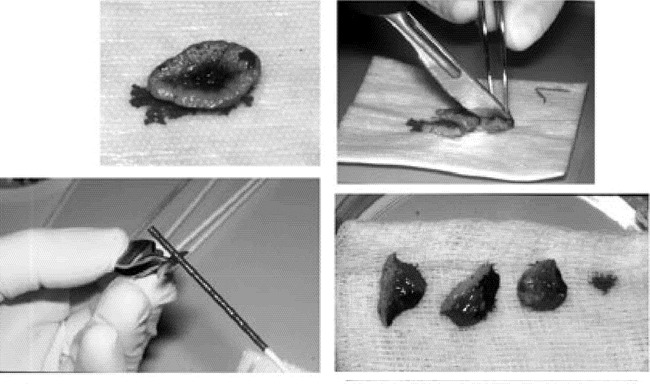
Figure 11. A. Specimen with proper orientation preserved, placed at grossing station.B. Tissue sectioned along score marks made previously.C. Tissue edges dyed in accordance with the case map.D. Tissue pieces placed in a uniform fashion in a petre dish with orientation preserved for transport to the Mohs laboratory.
Once the tissue arrives in the laboratory, frozen-section processing takes place. The tissue is placed on a heat extractor deep side down, a microtome chuck is prepared with optimum cutting temperature (OCT) medium, and the heat extractor, with the specimen in place, is inverted and positioned on the OCT. This embedded specimen is then sectioned on a microtome in the cryostat. The 4–6 µ sections are placed sequentially on glass slides (Figure 12). The glass slides are stained and placed in a slide holder prior to examination. This process is repeated for each piece of tissue submitted to the laboratory. The surgeon, also acting as the pathologist, examines the histologic material and reads it for the presence of carcinoma (12). The Mohs surgeon is constantly assessing the material to ensure the staining quality is high and that the entire specimen is visualized as he or she would expect. Residual carcinoma is exactly mapped (Figure 13). It is possible at this point that the tumor has been completely extirpated and the repair may now be done or second-intention healing begun. If a second stage of MMS is required, a second case map is made from the first map indicating the planned excision that will lead to a tumor-free plane. The patient is returned to the operating suite, the dressing removed, and the patient re-prepped and re-anesthetized. The second stage of MMS is conducted and the tissue processed the same as the first stage (Figure 14). The tissue sections from the second stage are examined under the microscope. In the example presented, a tumor-free plane was obtained at this point and primary closure of the defect accomplished (Figure 15). One-month follow-up is shown in Figure 16.

Figure 12. A. The first specimen is placed deep side down on a heat extractor and the epidermis is “teased” radially.B. The microtome chuck is prepared with OCT and the heat extractor is placed on to the chuck.C. The chuck is placed into the microtome and sectioned in the cryostat.D. The serial sections are placed sequentially on microscope slides.
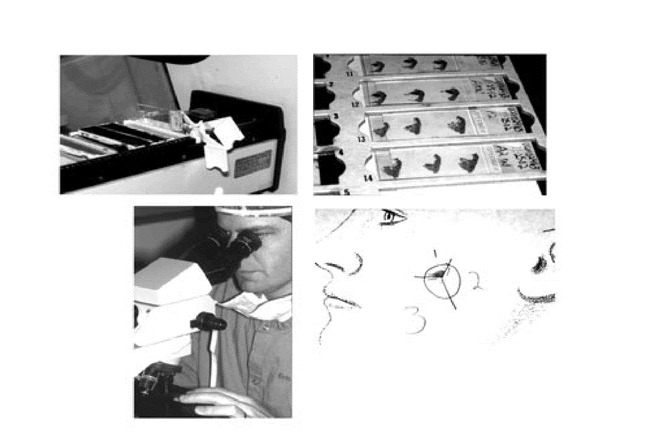
Figure 13. A. The slides are stained with hematoxylin and eosin.B. Two slides are made and accurately labeled for each piece of tissue submitted to the lab.C. Surgeon acting as pathologist for the case reads the histologic material.D. Area of residual tumor marked on case map.
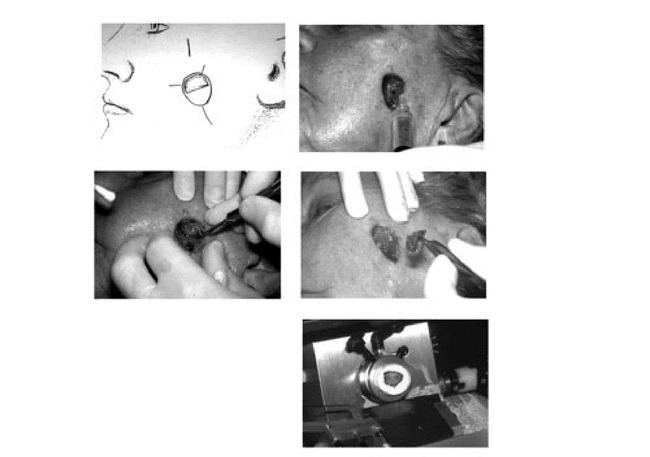
Figure 14. A. Case map drawn for second stage of MMS.B. Operative site infiltrated with local anesthesia again.C. Second stage of Mohs surgery performed.D. Tissue excised and grossed in as described above.E. Tissue processed by frozen section technique as previously outlined.
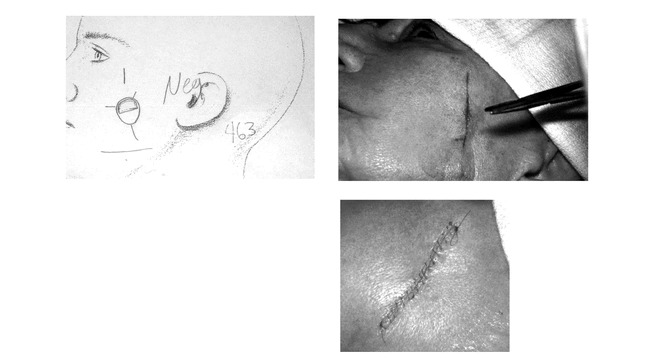
Figure 15. A. Histologic examination done on second stage and results documented on case map.B. Side to side closure done on tumor free wound.C. Closure completed on the morning of surgery.
Figure 16.
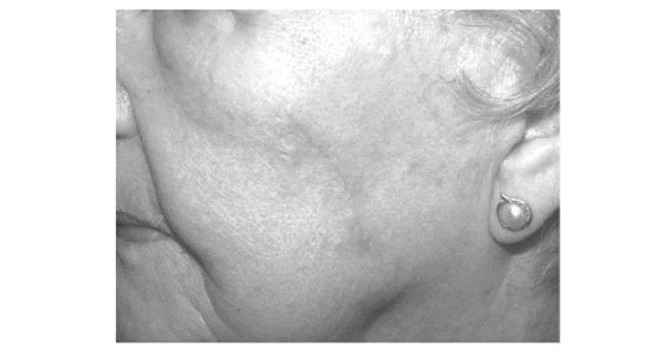
Surgical site at one month postoperatively.
INDICATIONS
The strongest indication for Mohs micrographic surgery is recurrent BCC and SCC. Over the last decade, MMS has become the gold standard for recurrent nonmelanoma skin cancer (11,13,14). Other situations in which Mohs surgery should be considered are primary BCC and SCC with clinically indistinct margins (1). Carcinomas on or around the eyes, nose, and ears are at greater risk for recurrence and are ideally treated with MMS. Tumors with aggressive growth patterns such as morpheaform (sclerosing), metatypical, keratinizing, adenoidal, multicentric, or those classified as “infiltrating” basal cell carcinoma should be treated with Mohs micrographic surgery (14,15). Tumors that exhibit perineural or perivascular invasion, are large in size (>2 cm), occur on previously irradiated skin, or have been incompletely excised, as in the example, are best treated with this technique. One should consider immediate MMS for tumors that display rapid growth or behave aggressively such as one might see in an immunosuppressed host. And finally, MMS is a consideration for any tumor occuring in an area where tissue conservation is of the utmost concern such as the genitalia or digits (12).
Other cutaneous carcinomas are treated with MMS less frequently than NMSC and are shown in Table 1 (3,11,12,14,16). The treatment of melanoma in situ and malignant melanoma with MMS remains controversial, with world-renown dermatologic surgeons differing in their opinions and recommendations (9,10,17). The author believes that single, atypical melanocytes that may accompany malignant pigmented lesions are very difficult to accurately and precisely identify on frozen sectioning. For this reason, MMS is not performed on melanocytic neoplasia at Ochsner Clinic Foundation (OCF).
According to a study that surveyed the Fellows of the American College of Mohs Micrographic Surgery and Cutaneous Oncology, a minority of Mohs surgeons treat melanoma in situ or malignant melanoma with this technique (18). In this same survey, only 30% of respondents treated Merkel cell carcinoma with MMS. The more rare cutaneous carcinomas are occasionally treated in a “combined” fashion in which the Mohs technique is used to clear the tumor by frozen sections: another layer is taken, and this is submitted for paraffin-embedded sections in the main pathology laboratory to verify resection. This is the approach taken at OCF for the infrequent dermatofibrosarcoma protuberans or sebaceous carcinoma.
RECURRENCE RATES
The pinnacle of evidence-based medicine is the double-blind, randomized, controlled, prospective study of a given topic. This type of analysis on the recurrence rate of a primary NMSC after MMS would be a randomized prospective controlled study of MMS versus surgical excision, or electrodesiccation and curettage, or cryosurgery. Unfortunately, these studies do not exist and probably will not be conducted because such a study would not be practical or ethical as documented by Thissen et al. and reiterated by McGovern and Leffell (13,15,19). Although an exhaustive systematic electronic review of the literature in six languages from 1970 to 1997 found 298 studies on this topic of the treatment of primary BCCs, only 18 met the study inclusion criteria and could be compared in the cross-study analysis. Findings from these studies were that primary BCCs treated with MMS showed the lowest recurrence rate after 5 years, followed by those treated with surgical excision, cryosurgery, and curettage and electrodesiccation. The overall conclusion was that the recurrence rates for different therapies could not be compared due to a lack of uniformity with the methods of reporting; “so evidence-based guidelines could not be developed”(15). In an extensive review of all studies that examined recurrence rates in the treatment of primary BCCs, Rowe et al. found that the 5-year recurrence rate for MMS was 1%; surgical excision, 10.1%; curettage and electrodesiccation, 7.7%; radiation therapy, 8.7%; and cryosurgery 7.5% (20). In a large study from the Cleveland Clinic, Roenigk et al. reported 4-year cure rates for primary BCC of 98.6% for MMS. Interestingly, the cure rate in this study for traditional methods when Mohs surgery was not indicated was 97.1% (21). Mohs micrographic surgery for recurrent BCC was also reported by Rowe et al. in a separate study by reviewing all existing literature on the subject from 1945 forward. The results were quite striking: the 5-year recurrence rate for recurrent BCC treated with MMS was 5.6% compared with 17.4% for surgical excision, 9.8% for radiation treatment, and 40% for curettage and electrodesiccation. The recurrence rate for cryosurgery was reported at 13% and was probably artificially low because the follow-up period on this modality was less than 5 years (22). The 4-year cure rate as reported by Roenigk et al. for recurrent tumors treated with MMS was 95.8%, whereas the cure rate for treatment of recurrent BCCs with non-Mohs modalities was 84.5% (21). Cure rates for primary well-differentiated SCC without metastases treated with MMS were reported at 97%–98% (11,23,24). Mohs himself reported a 5-year cure rate of 99% for SCC less than 2 cm in diameter treated with MMS. The 5-year recurrence rate for recurrent SCC treated with MMS is 10%. It is important for the clinician to realize that recurrent SCC, SCC with perineural involvement, SCC with diameters greater than 2 cm, and poorly differentiated SCC had significantly lower recurrence rates when Mohs surgery treatment was used versus non-Mohs modalities (24).
CLINICAL CONSIDERATIONS
Critics of MMS frequently cite the cost of the procedure as a major drawback in selecting this treatment option. However, in 1998 Cook and Zitelli assessed the cost of treating a series of skin cancers with MMS versus traditional methods of treatment. All costs associated with diagnosis and treatment of 400 consecutive tumors treated with MMS were calculated and compared with calculated estimates of traditional methods to treat an identical tumor. The cost of treating recurrences was factored into this analysis. MMS had an average calculated cost of $1243. This compared favorably with the alternatives of $1167 for excision with permanent-section margin control, $1400 for excision in the office with frozen-section margin control, and $1973 for excision with frozen-section margin control in an ambulatory surgery facility. Simple destruction (electrodesiccation and curettage) had a average cost of $642 and radiation therapy averaged $4558 based on a 1.5 cm facial tumor (25). Although cost should not be the most important consideration when treating patients, this study lets the reader put aside the meritless cost arguments of the MMS detractors. There are other advantages to MMS in addition to the lower recurrence rates. The inherent characteristic of maximal tissue conservation with this technique is to allow for greater preservation of normal tissue for the repair or reconstruction. This enables the surgeon to conduct more sophisticated and perhaps more cosmetically elegant reconstructions immediately after resection. Surgical margins are cleared without delay, making future re-excision for tumor at the excisional margin an unnecessary concern.
Scarring from MMS is the most significant sequelae of the procedure. Scarring is dependent upon the extent of the destruction caused by the tumor and the management of this wound upon completion of the procedure. Wounds that are smaller and repaired in a straightforward fashion have less conspicuous scarring than large mutilating cases that might require complex reconstructions. Mohs surgery wounds that heal by second intention can give rise to erratic scarring as well. Occasionally, a wound that has healed by second intention is more cosmetically elegant that one would expect, but usually such scars are conspicuous (26).
Patients that undergo MMS can expect some edema, ecchymosis, dysesthesia, discomfort, and possibly asymmetry, but no more than would be experienced with any other surgical procedure which would remove the tumor (12). Infrequently, edema and dysesthesia can be persistent, but this normally returns to baseline in a few to several months. Surgical complications that can occur include pigmentation disturbance, hematoma or seroma formation, postoperative bleeding, skin necrosis, wound dehiscence, infection, nerve damage, hypertrophic scarring, or keloidal scar formation. These complications are uncommon and are no more likely to occur in MMS than in any other type of cutaneous surgery (1).
Often in the treatment of difficult carcinomas, it is necessary to adopt a multidisciplinary approach. Frequently, optimum treatment is given when the Mohs surgeon removes the lesion while the appropriate surgical colleague reconstructs the defect. Excessively large defects, carcinomas on the eyelid or nose, or those on the hand or digits often generate a consultation to the occuloplastic, facial plastic, or plastic and reconstructive surgeon (1). Occasionally, high-risk SCCs, such as those with perineural involvement, are treated with adjuvant radiation therapy after the MMS extirpation, requiring consultation to radiation oncology (11,27).
All skin cancer patients should be followed postoperatively for evidence of recurrence in the operative site and generalized examination should be performed to look for new primary carcinomas. These patients should be reminded to compulsively apply sunscreens SPF 15 or greater, avoid the midday sun (10am – 4pm), stay in the shade when outdoors, and wear hats and clothing that block ultraviolet irradiation. These patients should wear sunglasses to avoid the deleterious effects of ultraviolet light on the eye as well.
CONCLUSION
Mohs micrographic surgery has existed for over 60 years as a way to treat cutaneous carcinomas that grow by local contiguous spread. The process has been refined over the years into the technique that is currently being utilized. Vital to the procedure is that the Mohs surgeon is the pathologist, which lowers the potential for human error in a situation where tissue orientation is of critical importance. MMS is the procedure of choice for recurrent NMSC but also has superior recurrence rates when used on primary carcinomas. MMS is an outpatient procedure performed most commonly under local anesthesia, and although scarring and the potential for complications exist, the complication rates are no higher than for any other similar cutaneous surgical procedure.
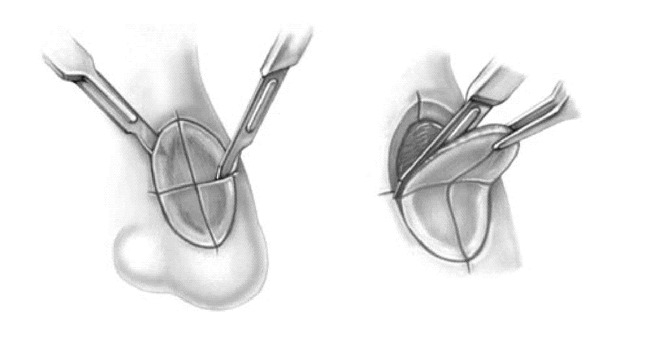
Figure 4. A. First stage of MMS conducted by excising in a tangential manner.B. The beveled excision is continued and the tissue scored for orientation purposes.
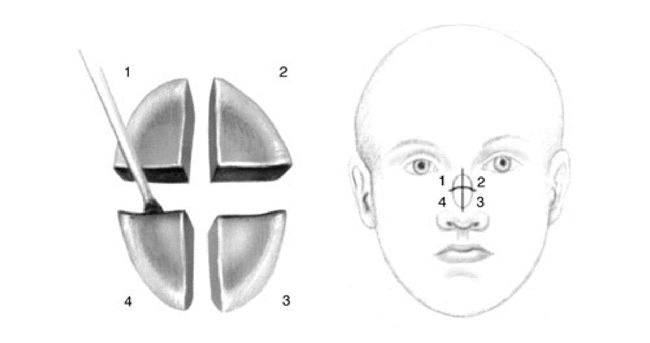
Figure 5. A. The tissue is sectioned along the score marks and dyed with tissue dye.B. A detailed map is drawn that helps preserve orientation and aids with histologic examination.
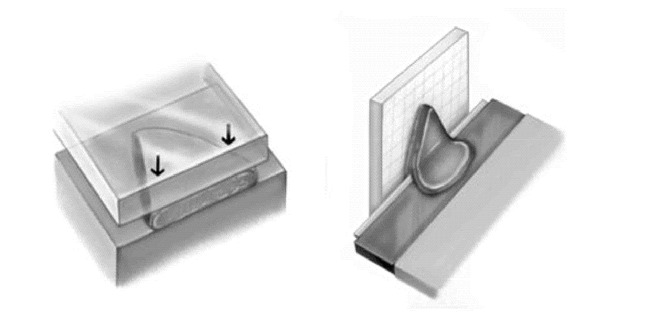
Figure 6. A. This tissue is inverted and processed by frozen section technique so that the entire deep and lateral margins fall into the same tissue plane.B. The embedded specimen is sectioned in a cryostat.

Dr. Finely is the Director of Dermatologic Surgery, at Ochsner Clinic Foundation.
Table 1.
Tumors other than BCC/SCC treated with Mohs Micrographic Surgery

REFERENCES
- Finley E. M., Ratz J. L. Mohs micrographic surgery. 1998:417–438. In: Ratz JL, editor. Textbook of Dermatologic Surgery. Philadelphia: Lippincott-Raven. [Google Scholar]
- Mohs F. E. Chemosurgery: a microscopically controlled method of cancer excision. Arch Surg. 1941;42:279–295. [Google Scholar]
- Dinehart S. M., Pollack S. V. Mohs micrographic surgery for skin cancer. Cancer Treat Rev. 1989;16:257–265. doi: 10.1016/0305-7372(89)90045-5. [DOI] [PubMed] [Google Scholar]
- Tromovitch T. A., Stegeman S. J. Microscopically controlled excision of skin tumors. hemotherapy (Mohs): fresh tissue technique. Arch Dermatol. 1974;110:231–232. [PubMed] [Google Scholar]
- Berlin J., Katz K. H., Helm K. F. The significance of tumor persistence after incomplete excision of basal cell carcinoma. J Am Acad Dermatol. 2002;46:549–553. doi: 10.1067/mjd.2002.117733. [DOI] [PubMed] [Google Scholar]
- Spencer J. M., Tannenbaum A., Sloan L. Does inflammation contribute to the eradication of basal cell carcinoma following curettage and electrodesiccation? Dermatol Surg. 1997;23:625–631. doi: 10.1111/j.1524-4725.1997.tb00379.x. [DOI] [PubMed] [Google Scholar]
- Holmkvist K. A., Rogers G. S., Dahl P. R. Incidence of residual basal cell carcinoma patients who appear tumor free after biopsy. J Am Acad Dermatol. 1999;41:600–605. [PubMed] [Google Scholar]
- Abide J. M., Nahai F., Bennett R. G. The meaning of surgical margins. Plast Reconstr Surg. 1984;73:492–497. doi: 10.1097/00006534-198403000-00030. [DOI] [PubMed] [Google Scholar]
- Zitelli J. A., Mohs F. E., Larson P. Mohs micrographic surgery for melanoma. Dermatol Clin. 1989;7:833–843. [PubMed] [Google Scholar]
- Zitelli J. A., Moy R. L., Abell E. The reliability of frozen section in the evaluation of surgical margins for melanoma. J Am Acad Dermatol. 1991;24:102–106. doi: 10.1016/0190-9622(91)70020-3. [DOI] [PubMed] [Google Scholar]
- Shriner D. L., McCoy D. K., Goldberg D. J. Mohs micrographic surgery. J Am Acad Dermatol. 1998;39:79–97. doi: 10.1016/s0190-9622(98)70405-0. [DOI] [PubMed] [Google Scholar]
- Drake L. A., Dinehart S. M., Goltz R. W. Guidelines of care for Mohs micrographic sugery. American Academy of Dermatology. J Am Acad Dermatol. 1995;33:271–278. doi: 10.1016/0190-9622(95)90261-9. [DOI] [PubMed] [Google Scholar]
- Nelson B. R., Railan D., Cohen S. Mohs' micrographic surgery for nonmelanoma skin cancers. Clin Plast Surg. 1997;24:705–718. [PubMed] [Google Scholar]
- Snow S. N., Madjar D. D., Jr Mohs surgery in the management of cutaneous malignancies. Clin Dermatol. 2001;19:339–347. doi: 10.1016/s0738-081x(01)00169-9. [DOI] [PubMed] [Google Scholar]
- Thissen M. R., Neumann M. H., Schouten L. J. A systematic review of treatment modalities for primary basal cell carcinomas. Arch Dermatol. 1999;135:1177–1183. doi: 10.1001/archderm.135.10.1177. [DOI] [PubMed] [Google Scholar]
- Hruza G. J. Mohs micrographic surgery. Otolaryngol Clin North Am. 1990;23:845–864. [PubMed] [Google Scholar]
- Barlow R. J., White C. R., Swanson N. A. Mohs' micrographic surgery using frozen sections alone may be unsuitable for detecting single atypical melanocytes at the margins of melanoma in situ. Br J Dermatol. 2002;146:290–294. doi: 10.1046/j.1365-2133.2002.04661.x. [DOI] [PubMed] [Google Scholar]
- McGillis S. T., Wheeland R. G., Sebben J. E. Current issues in the performance of Mohs micrographic surgery. J Dermatol Surg Oncol. 1991;17:681–684. doi: 10.1111/j.1524-4725.1991.tb01320.x. [DOI] [PubMed] [Google Scholar]
- McGovern T. W., Leffell D. J. Mohs surgery: the informed view. Arch Dermatol. 1999;135:1255–1259. doi: 10.1001/archderm.135.10.1255. [DOI] [PubMed] [Google Scholar]
- Rowe D. E., Carroll R. J., Day C. L., Jr Long-term recurrence rates in previously untreated (primary) basal cell carcinoma: implications for patient follow-up. J Dermatol Surg Oncol. 1989;15:315–328. doi: 10.1111/j.1524-4725.1989.tb03166.x. [DOI] [PubMed] [Google Scholar]
- Roenigk R. K., Ratz J. L., Bailin P. L. Trends in the presentation and treatment of basal cell carcinomas. J Dermatol Surg Oncol. 1986;12:860–865. doi: 10.1111/j.1524-4725.1986.tb01993.x. [DOI] [PubMed] [Google Scholar]
- Rowe D. E., Carroll R. J., Day C. L., Jr Mohs surgery is the treatment of choice for recurrent (previously treated) basal cell carcinoma. J Dermatol Surg Oncol. 1989;15:424–431. doi: 10.1111/j.1524-4725.1989.tb03249.x. [DOI] [PubMed] [Google Scholar]
- Roth J. J., Granick M. S. Squamous cell and adnexal carcinomas of the skin. Clin Plast Surg. 1997;24:687–703. [PubMed] [Google Scholar]
- Rowe D. E., Carroll R. J., Day C. L., Jr Prognostic factors for local recurrence, metastasis, and survival rates in squamous cell carcinoma of the skin, ear, and lip. Implications for treatment modality selection. J Am Acad Dermatol. 1992;26:976–990. doi: 10.1016/0190-9622(92)70144-5. [DOI] [PubMed] [Google Scholar]
- Cook J., Zitelli J. A. Mohs micrographic surgery: a cost analysis. J Am Acad Dermatol. 1998;39:698–703. doi: 10.1016/s0190-9622(98)70041-6. [DOI] [PubMed] [Google Scholar]
- Zitelli J. A. Wound healing by secondary intention, a cosmetic appraisal. J Am Acad Dermatol. 1983;9:407–415. doi: 10.1016/s0190-9622(83)70150-7. [DOI] [PubMed] [Google Scholar]
- Geohas J., Roholt N. S., Robinson J. K. Adjuvant radiotherapy after excision of cutaneous squamous cell carcinomas. J Am Acad Dermatol. 1994;30:633–636. doi: 10.1016/s0190-9622(94)70073-7. [DOI] [PubMed] [Google Scholar]


