Abstract
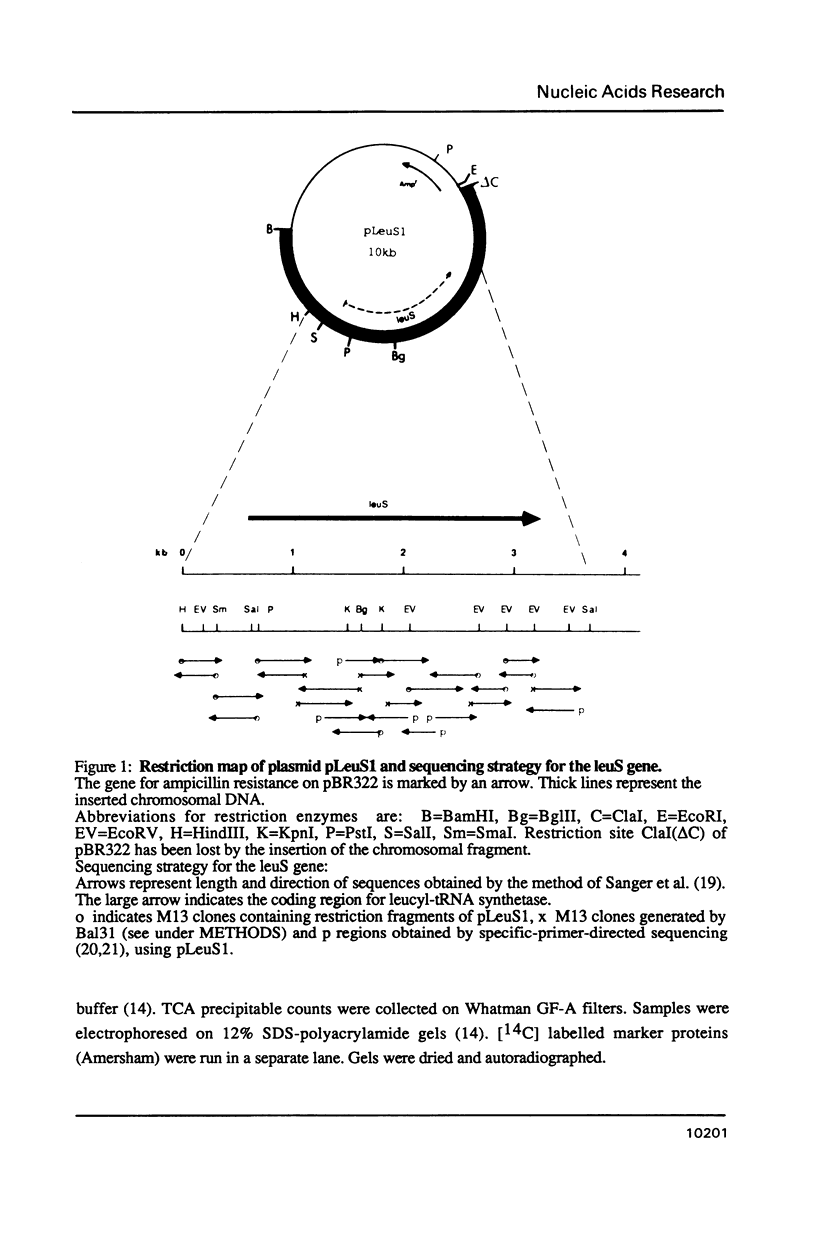
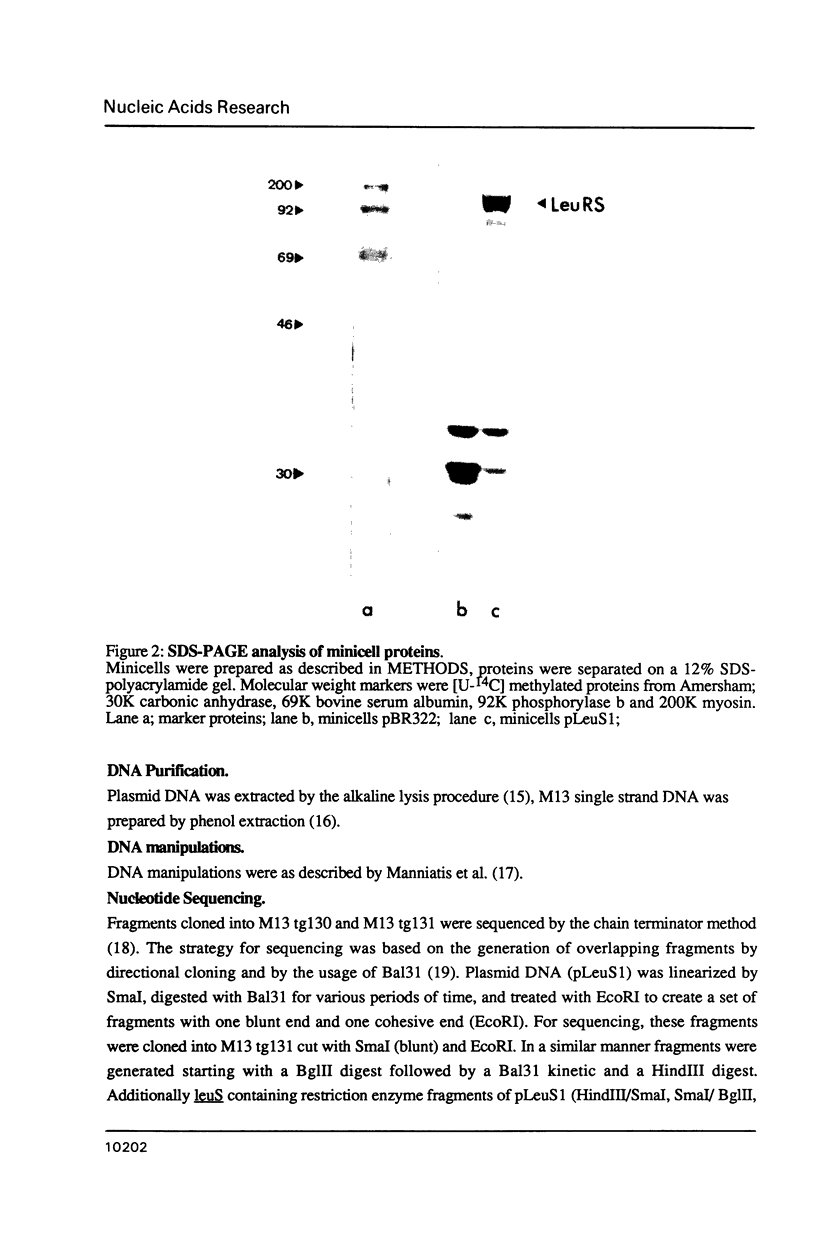
The gene for Escherichia coli leucyl-tRNA synthetase leuS has been cloned by complementation of a leuS temperature sensitive mutant KL231 with an E.coli gene bank DNA. The resulting clones overexpress leucyl-tRNA synthetase (LeuRS) by a factor greater than 50. The DNA sequence of the complete coding regions was determined. The derived N-terminal protein sequence of LeuRS was confirmed by independent protein sequencing of the first 8 aminoacids. Sequence comparison of the LeuRS sequence with all aminoacyl-tRNA synthetase sequences available reveal a significant homology with the valyl-, isoleucyl- and methionyl-enzyme indicating that the genes of these enzymes could have derived from a common ancestor. Sequence comparison with the gene product of the yeast nuclear NAM2-1 suppressor allele curing mitochondrial RNA maturation deficiency reveals about 30% homology.
Full text
PDF







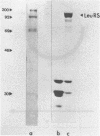
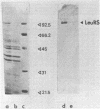
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brune M., Schumann R., Wittinghofer F. Cloning and sequencing of the adenylate kinase gene (adk) of Escherichia coli. Nucleic Acids Res. 1985 Oct 11;13(19):7139–7151. doi: 10.1093/nar/13.19.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crothers D. M., Seno T., Söll G. Is there a discriminator site in transfer RNA? Proc Natl Acad Sci U S A. 1972 Oct;69(10):3063–3067. doi: 10.1073/pnas.69.10.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dardel F., Fayat G., Blanquet S. Molecular cloning and primary structure of the Escherichia coli methionyl-tRNA synthetase gene. J Bacteriol. 1984 Dec;160(3):1115–1122. doi: 10.1128/jb.160.3.1115-1122.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furano A. V. Content of elongation factor Tu in Escherichia coli. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4780–4784. doi: 10.1073/pnas.72.12.4780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf F. M., Sträuli P. Use of the avidin-biotin-peroxidase complex (ABC) method for the localization of rabbit cathepsin B in cells and tissues. J Histochem Cytochem. 1983 Jun;31(6):803–810. doi: 10.1177/31.6.6341462. [DOI] [PubMed] [Google Scholar]
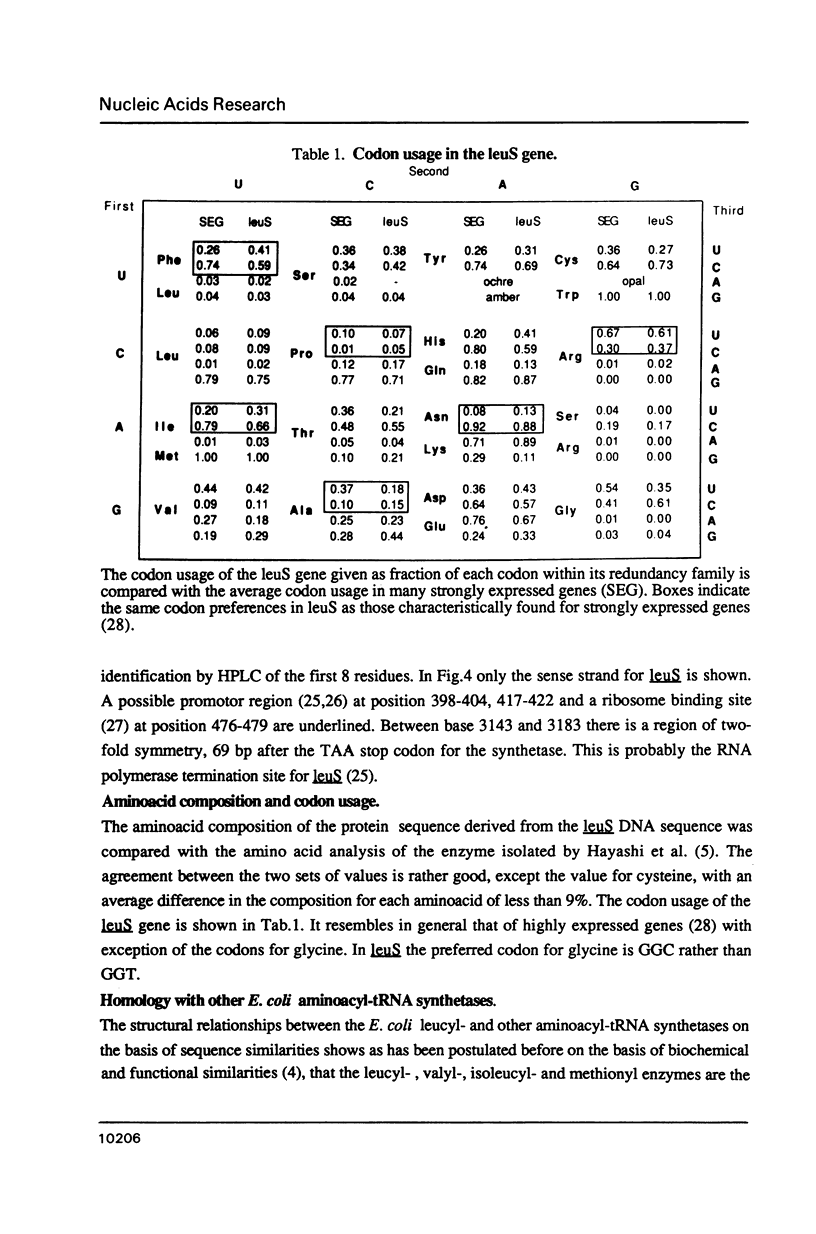
- Grosjean H., Fiers W. Preferential codon usage in prokaryotic genes: the optimal codon-anticodon interaction energy and the selective codon usage in efficiently expressed genes. Gene. 1982 Jun;18(3):199–209. doi: 10.1016/0378-1119(82)90157-3. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
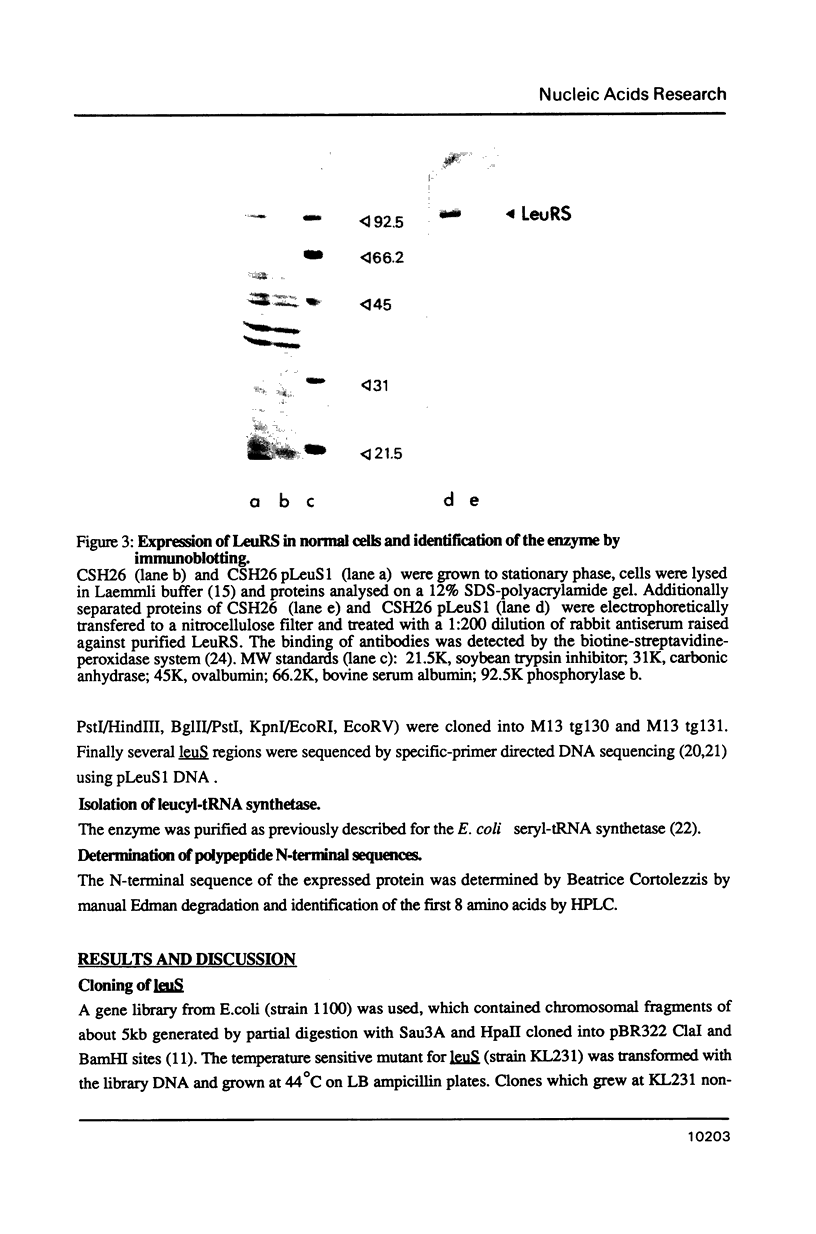
- Hayashi H., Knowles J. R., Katze J. R., Lapointe J., Söll D. Purification of leucyl transfer ribonucleic acid synthetase from Escherichia coli. J Biol Chem. 1970 Mar 25;245(6):1401–1406. [PubMed] [Google Scholar]
- Hountondji C., Dessen P., Blanquet S. Sequence similarities among the family of aminoacyl-tRNA synthetases. Biochimie. 1986 Sep;68(9):1071–1078. doi: 10.1016/s0300-9084(86)80181-x. [DOI] [PubMed] [Google Scholar]
- Härtlein M., Madern D., Leberman R. Cloning and characterization of the gene for Escherichia coli seryl-tRNA synthetase. Nucleic Acids Res. 1987 Feb 11;15(3):1005–1017. doi: 10.1093/nar/15.3.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieny M. P., Lathe R., Lecocq J. P. New versatile cloning and sequencing vectors based on bacteriophage M13. Gene. 1983 Dec;26(1):91–99. doi: 10.1016/0378-1119(83)90039-2. [DOI] [PubMed] [Google Scholar]
- Kunugi S., Uehara-Kunugi Y., von der Haar F., Schischkoff J., Freist W., Englisch U., Cramer F. Biochemical comparison of the Neurospora crassa wild type and the temperature-sensitive and leucine-auxotroph mutant leu-5. Purification of the cytoplasmic and mitochondrial leucyl-tRNA synthetases and comparison of the enzymatic activities and the degradation patterns. Eur J Biochem. 1986 Jul 1;158(1):43–49. doi: 10.1111/j.1432-1033.1986.tb09718.x. [DOI] [PubMed] [Google Scholar]
- Labouesse M., Herbert C. J., Dujardin G., Slonimski P. P. Three suppressor mutations which cure a mitochondrial RNA maturase deficiency occur at the same codon in the open reading frame of the nuclear NAM2 gene. EMBO J. 1987 Mar;6(3):713–721. doi: 10.1002/j.1460-2075.1987.tb04812.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levy S. B. R factor proteins synthesized in Escherichia coli minicells: incorporation studies with different R factors and detection of deoxyribonucleic acid-binding proteins. J Bacteriol. 1974 Dec;120(3):1451–1463. doi: 10.1128/jb.120.3.1451-1463.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. S., Irwin R., Chirikjian J. G. Isolation of a single polypeptide leucyl-tRNA synthetase from bakers' yeast. Nucleic Acids Res. 1979 Aug 10;6(11):3651–3660. doi: 10.1093/nar/6.11.3651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low B., Gates F., Goldstein T., Söll D. Isolation and partial characterization of temperature-sensitive Escherichia coli mutants with altered leucyl- and seryl-transfer ribonucleic acid synthetases. J Bacteriol. 1971 Nov;108(2):742–750. doi: 10.1128/jb.108.2.742-750.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poncz M., Solowiejczyk D., Ballantine M., Schwartz E., Surrey S. "Nonrandom" DNA sequence analysis in bacteriophage M13 by the dideoxy chain-termination method. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4298–4302. doi: 10.1073/pnas.79.14.4298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg M., Court D. Regulatory sequences involved in the promotion and termination of RNA transcription. Annu Rev Genet. 1979;13:319–353. doi: 10.1146/annurev.ge.13.120179.001535. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel P. R., Söll D. Aminoacyl-tRNA synthetases: general features and recognition of transfer RNAs. Annu Rev Biochem. 1979;48:601–648. doi: 10.1146/annurev.bi.48.070179.003125. [DOI] [PubMed] [Google Scholar]
- Schreier P. H., Cortese R. A fast and simple method for sequencing DNA cloned in the single-stranded bacteriophage M13. J Mol Biol. 1979 Mar 25;129(1):169–172. doi: 10.1016/0022-2836(79)90068-8. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. Determinant of cistron specificity in bacterial ribosomes. Nature. 1975 Mar 6;254(5495):34–38. doi: 10.1038/254034a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Smith T. F., Waterman M. S. Identification of common molecular subsequences. J Mol Biol. 1981 Mar 25;147(1):195–197. doi: 10.1016/0022-2836(81)90087-5. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. Site of aminoacylation of tRNAs from Escherichia coli with respect to the 2'- or 3'-hydroxyl group of the terminal adenosine. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3049–3053. doi: 10.1073/pnas.72.8.3049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss E. C., Kobori J. A., Siu G., Hood L. E. Specific-primer-directed DNA sequencing. Anal Biochem. 1986 Apr;154(1):353–360. doi: 10.1016/0003-2697(86)90536-1. [DOI] [PubMed] [Google Scholar]
- Theall G., Low K. B., Söll D. Regulation of the biosynthesis of aminoacyl-tRNA synthetases and of tRNA in Escherichia coli. IV. Mutants with increased levels of leucyl- or seryl-tRNA synthetase. Mol Gen Genet. 1979 Jan 31;169(2):205–211. doi: 10.1007/BF00271672. [DOI] [PubMed] [Google Scholar]
- Webster T., Tsai H., Kula M., Mackie G. A., Schimmel P. Specific sequence homology and three-dimensional structure of an aminoacyl transfer RNA synthetase. Science. 1984 Dec 14;226(4680):1315–1317. doi: 10.1126/science.6390679. [DOI] [PubMed] [Google Scholar]
- Wetzel R. Aminoacyl-tRNA synthetase families and their significance to the origin of the genetic code. Orig Life. 1978 Sep;9(1):39–50. doi: 10.1007/BF00929712. [DOI] [PubMed] [Google Scholar]