Since the classification by Dale of cholinergic responses as muscarinic or nicotinic and the subsequent identification of acetylcholine as the first neurotransmitter mediating the negative chronotropic response of cardiac muscarinic acetylcholine receptors (mAChR), studies on muscarinic receptors have provided a wealth of new insights that have had a wide impact on neurobiology and pharmacology. Muscarinic receptors are present in neurons in the central and peripheral nervous system, cardiac and smooth muscles, and a variety of exocrine glands. Mammals possess genes encoding five different subtypes of mAChR, termed M1–M5, which can be divided into two broad functional categories: the M1, M3, and M5 receptors preferentially couple to the Gq family of G-proteins whereas the M2 and M4 receptors preferentially couple to the Gi family of G-proteins (1–3).
Muscarinic receptors in the airways are important both in the normal physiology and the pathophysiology of pulmonary function. Acetylcholine released from parasympathetic nerve terminals causes contraction of airway smooth muscle. Animals with asthma or other chronic inflammation of the airways exhibit hypersensitivity of the airways to muscarinic agonists, and muscarinic antagonists are used therapeutically in patients with asthma and chronic obstructive pulmonary disease (4, 5). The paper by Hansen et al. (6) in this issue of PNAS provides surprising information on at least one of the signaling mechanisms for the regulation of airway function by mAChR. These authors produced mice with a targeted mutation in the gene encoding phosphodiesterase 4D (PDE4D), one of a group of PDEs that have high specificity for hydrolysis of cAMP and are selectively inhibited by the drug rolipram. Inhibitors of type 4 PDEs exhibit anti-inflammatory actions and cause bronchodilation, suggesting that they may be useful in alleviating the symptoms of airway disorders (7). Hansen et al. demonstrate that PDE4D accounts for approximately 50% of the total PDE activity in the lung. Surprisingly, both the basal contraction of the airways induced by mAChR agonist as well as the increased sensitivity of the airways to muscarinic agonist normally observed after antigen exposure (i.e., airway hyperreactivity) were lost in the PDE−/− mice, and treatment of wild-type mice with rolipram decreased mAChR-mediated bronchoconstriction. Even more surprising, the ability of muscarinic agonist to decrease intracellular cAMP levels was also lost in the PDE knockouts. The PDE4D−/− mice exhibit normal serotonin-mediated bronchoconstriction and normal numbers of airway mAChR. Thus, PDE4D appears to be a key component in the ability of mAChR to regulate both contractility and cAMP levels in the airways.
These results demonstrate a previously unsuspected level of complexity in mAChR-mediated signal transduction. The mechanisms responsible for signal transduction by mAChR, like other members of the G-protein-coupled receptor superfamily, grow increasingly complex virtually every year because of such factors as the identification of additional isoforms of signal transduction effectors, the recognition of new intersection points for crosstalk between signaling pathways, and the demonstration of cell-type specific differences in signaling. The functional specificity of mAChR is frequently summarized as the M1, M3, and M5 receptors mediating activation of phospholipase C (PLC) via pertussis toxin-insensitive G-proteins of the Gq family while not inhibiting adenylyl cyclase, and the M2 and M4 receptors mediating inhibition of adenylyl cyclase (using pertussis toxin-sensitive G-proteins of the Gi family) without stimulating PLC (2, 3). This specificity is not absolute, however, and M2 and M4 receptors can weakly couple to PLC (again by pertussis toxin-sensitive G-proteins) when expressed at high levels in certain cell types (8). The pertussis toxin-insensitive activation of phospholipase C is mediated by activation of PLCβ subtypes (i.e., β1–4) by α subunits of the Gαq family whereas pertussis toxin-sensitive activation is mediated by activation of PLCβ2 and β3 by Gβγ subunits (9).
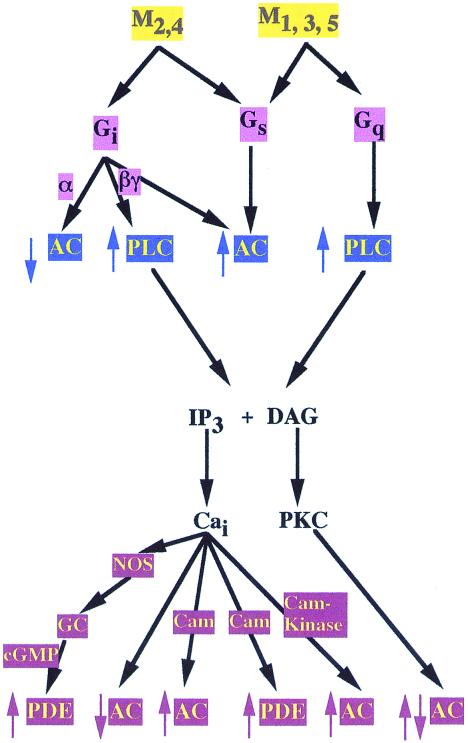
The mechanisms for the regulation of intracellular cAMP levels by mAChR activation are even more complex (Fig. 1). Thus, in addition to the “standard” mechanism for decreasing intracellular cAMP levels by Gi-mediated inhibition of adenylyl cyclase activity, mAChR can cause both increased and decreased levels of intracellular cAMP by multiple signaling pathways in a cell-type specific manner depending on the composition of other signaling proteins present. Thus, Gq-coupled mAChR in astrocytoma and thyroid cells activate PLC; the resulting increase in intracellular Ca2+ levels activates a calcium-calmodulin sensitive PDE that results in decreased levels of intracellular cAMP (10). In the heart, Gi-coupled mAChR activate nitric oxide synthetase; the NO activates guanylyl cyclase, leading to activation of a cGMP- stimulated PDE and decreased intracellular cAMP levels (11).
Figure 1.
Simplified outline of the potential mechanisms for regulation of intracellular cAMP levels by mAChR activation. The activation of a given subtype of mAChR can lead to either increases or decreases in intracellular cAMP levels, depending on the level of receptor expression, the cell type the receptor is expressed in, and the particular combination of effector molecules expressed in that cell.
There are also multiple mechanisms for mAChR-mediated increases in intracellular cAMP levels. When expressed at high levels, both Gi- and Gq-coupled mAChR can increase cAMP levels by “ectopically” coupling to Gs, leading to activation of adenylyl cyclase (12). Gq-coupled mAChR, by increasing intracellular calcium levels, can cause activation of calmodulin-sensitive adenylyl cyclases (13). In the olfactory bulb, Gi-coupled mAChR can cause Gβγ-mediated activation of (types II and IV) adenylyl cyclase, also leading to increased cAMP levels (14). Finally, various isoforms of adenylyl cyclase can also be regulated by phosphorylation by cAMP-dependent protein kinase A (PKA), protein kinase C (PKC), and calmodulin-dependent protein kinases (15). Because all of these kinases can be regulated by mAChR, it is likely that additional mechanisms for regulation of cAMP levels by mAChR will be found.
Since an initial report (16) that activation of hippocampal mAChR leads to increased protein tyrosine phosphorylation, the regulation of tyrosine kinase pathways by mAChR has received much attention in recent years. Like other members of the G-protein-coupled receptor superfamily, mAChR can activate tyrosine kinase and mitogen-activated protein kinase (MAPK) pathways by multiple mechanisms. Gq-coupled receptors can activate PYK2 via calmodulin-dependent protein kinases and PKC-mediated mechanisms; PYK2 can activate c-src to cause activation of the ERK pathway (17, 18). Transactivation of epidermal growth factor receptors is an important intermediary of G-protein-coupled receptor-mediated activation of MAPK pathways in certain cell types. The M1 receptor has been recently shown to cause PKC-mediated activation of a metalloprotease that releases an epidermal growth factor-like ligand that can activate the epidermal growth factor receptor to initiate signaling (19). Activation of the M1 receptor can also directly activate Bruton's tyrosine kinase through direct interaction with Gαq (20). Both M1 and M2 receptors have been shown to cause Ras activation by a Gβγ-mediated phosphorylation and activation of a guanine nucleotide exchange factor (21). The M2 receptor can activate MAPK through Gβγ- mediated activation of phosphatidylinositol-3-kinase and the subsequent involvement of a src-family kinase, ras, and raf (22). The M2 receptor can also activate a GTPase activator protein for Rap1 because of its interaction with Gαi; because Rap1 can act as an antagonist of Ras action, the resultant decrease in Rap1 activity increases MAPK activity (23). Finally, mAChR have been shown to directly or indirectly regulate many other signaling pathways, including c-Jun N-terminal and p38 kinases, phospholipases A2 and D, and rho and rac (24–28). Because of the potential crosstalk between signaling pathways and the differential expression of signal transduction components, there is an exceptionally large combination of potential signals that can be produced by a given mAChR in different cell types. Thus, although the M2 receptor activates the MAPK pathway and phosphatidylinositol-3-kinase when expressed in Rat 1a cells, the M1 receptor does not activate MAPK and inhibits MAPK activation in response to epidermal growth factor and platelet-derived growth factor. This inhibition of growth factor signaling results from M1-mediated increases in intracellular calcium, activation of a calcium-sensitive adenylyl cyclase, and PKA-mediated inhibition of activation of the MAPK kinase kinase Raf (29).
Not surprisingly, the regulation of ion channel function by mAChR can use virtually every mechanism discussed above. Thus, there are many examples of the regulation of ion channel function by mAChR-dependent changes in channel phosphorylation mediated by such kinases as PKA and PKC and by tyrosine kinases (1, 30, 31). G-proteins can also directly couple receptors to ion channels. Muscarinic receptor-mediated activation of inwardly rectifying potassium channels and inhibition of N and P/Q-type potassium channels are mediated by Gβγ subunits released from Gi-family proteins (32).
Despite this plethora of mAChR-regulated signaling pathways, there is a paucity of information on the mechanism(s) responsible for the interesting results of Hansen et al. (6). The airway smooth muscles contain M2 and M3 receptors, with M1 receptors having an excitatory role in the parasympathetic ganglia and M2 receptors inhibiting ACh release from parasympathetic nerve terminals. It is generally thought that increased intracellular calcium attributable to M3 receptor activation is the primary mechanism for mAChR-dependent contraction; the M2 receptor has generally been considered to act by blocking β-adrenergic receptor-mediated relaxation via Gi-dependent inhibition of adenylyl cyclase, although direct effects of M2 have also been suggested (4, 5, 33). Mice with a targeted mutation in the M2 receptor gene exhibit only a slight diminution in mAChR-mediated bronchoconstriction, consistent with M3 playing the predominant role (34). The work of Hansen et al. raises many questions. What are the effects of PDE4D mutation on mAChR-mediated activation of PLC, intracellular calcium, and inhibition of adenylyl cyclase activity? What is the relationship between the loss of mAChR-mediated decreases in intracellular cAMP and the loss of mAChR-mediated contractility? The decrease in mAChR-mediated lowering of intracellular cAMP by PDE4D mutation raises the possibility that the mAChR activates the enzyme. If this is the case, how does this occur, and which receptor subtype is responsible? As discussed above, other PDE isozymes can be stimulated by mAChR because of increased intracellular calcium or cGMP, but PDE4D does not appear to be regulated by these signaling molecules. PDE activation by growth factors and hormones that regulate signaling pathways, such as phosphatidylinositol-3-kinase and PKA, that are also regulated by mAChR have been reported (35, 36). Interestingly, the PDE4D gene gives rise to several splice variants, some of which can be regulated by phosphorylation by PKA and ERK (37, 38). It will thus be of great interest to determine whether mAChR activation does indeed stimulate PDE4D activity. Because of the importance of muscarinic and cyclic nucleotide signaling pathways in the functioning of the airways, the work of Hansen et al. (6) not only should lead to new insights into muscarinic receptor signaling pathways but also provides a further rationale for the continued development of PDE4D-selective inhibitors (7) for the pharmacological treatment of asthma and other inflammatory conditions of the airways.
Acknowledgments
I thank J. Bartoe, K. Belmonte, S. Hamilton, and L. Nadler for comments on the manuscript. Research in the author's laboratory is supported by grants from the National Institutes of Health and the Muscular Dystrophy Association.
Footnotes
See companion article on page 6751.
References
- 1.Nathanson N M. Annu Rev Neurosci. 1987;10:195–236. doi: 10.1146/annurev.ne.10.030187.001211. [DOI] [PubMed] [Google Scholar]
- 2.Wess J. Crit Rev Neurobiol. 1996;10:69–99. doi: 10.1615/critrevneurobiol.v10.i1.40. [DOI] [PubMed] [Google Scholar]
- 3.Caulfield M P, Birdsall N J. Pharmacol Rev. 1998;50:279–290. [PubMed] [Google Scholar]
- 4.Hirshman C A, Lande B, Croxton T L. Life Sci. 1999;64:443–448. doi: 10.1016/s0024-3205(98)00586-4. [DOI] [PubMed] [Google Scholar]
- 5.Watson N, Eglen R M. Pulm Pharmacol Ther. 1999;12:115–118. doi: 10.1006/pupt.1999.0185. [DOI] [PubMed] [Google Scholar]
- 6.Hansen G, Jin S-L C, Umetsu D T, Conti M. Proc Natl Acad Sci USA. 2000;97:6751–6756. doi: 10.1073/pnas.97.12.6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Torphy T J, Barnette M S, Underwood D C, Griswold D E, Christensen S B, Murdoch R D, Nieman R B, Compto C H. Pulm Pharmacol Ther. 1999;12:131–135. doi: 10.1006/pupt.1999.0181. [DOI] [PubMed] [Google Scholar]
- 8.Ashkenazi A, Winslow J W, Peralta E G, Peterson G L, Schimerlik M I, Capon D J, Ramachandran J. Science. 1987;238:672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- 9.Katz A, Wu D, Simon M I. Nature (London) 1992;360:686–689. doi: 10.1038/360686a0. [DOI] [PubMed] [Google Scholar]
- 10.Tanner L I, Harden T K, Wells J N, Martin M W. Mol Pharmacol. 1986;29:455–460. [PubMed] [Google Scholar]
- 11.Han X, Kobzik L, Severson D, Shimoni Y. J Physiol (London) 1998;509:741–754. doi: 10.1111/j.1469-7793.1998.741bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Migeon J C, Nathanson N M. J Biol Chem. 1994;269:9767–9773. [PubMed] [Google Scholar]
- 13.Choi E J, Wong S T, Hinds T R, Storm D R. J Biol Chem. 1992;267:12440–12442. [PubMed] [Google Scholar]
- 14.Olianas M C, Ingianni A, Onali P. J Neurochem. 1998;70:2620–2627. doi: 10.1046/j.1471-4159.1998.70062620.x. [DOI] [PubMed] [Google Scholar]
- 15.Hurley J H. J Biol Chem. 1999;274:7599–7602. doi: 10.1074/jbc.274.12.7599. [DOI] [PubMed] [Google Scholar]
- 16.Stratton K R, Worley P F, Huganir R L, Baraban J M. Proc Natl Acad Sci USA. 1989;86:2498–2501. doi: 10.1073/pnas.86.7.2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gutkind J S. J Biol Chem. 1998;273:1839–1842. doi: 10.1074/jbc.273.4.1839. [DOI] [PubMed] [Google Scholar]
- 18.Gudermann T, Grosse R, Schultz G. Naunyn-Schmieddeberg's Arch Pharmacol. 2000;361:345–362. doi: 10.1007/s002109900208. [DOI] [PubMed] [Google Scholar]
- 19.Prenzel N, Zwick E, Daub H, Leserer M, Abraham R, Wallasch C, Ullrich A. Nature (London) 1999;402:884–888. doi: 10.1038/47260. [DOI] [PubMed] [Google Scholar]
- 20.Bence K, Ma W, Kozasa T, Huang X Y. Nature (London) 1997;389:296–269. doi: 10.1038/38520. [DOI] [PubMed] [Google Scholar]
- 21.Mattingly R R, Macara I G. Nature (London) 1996;382:268–272. doi: 10.1038/382268a0. [DOI] [PubMed] [Google Scholar]
- 22.Lopez-Ilasaca M, Crespo P, Pellici P G, Gutkind J S, Wetzker R. Science. 1997;275:394–397. doi: 10.1126/science.275.5298.394. [DOI] [PubMed] [Google Scholar]
- 23.Mochizuki N, Ohba Y, Kiyokawa E, Kurata T, Murakami T, Ozaki T, Kitabatake A, Nagashima K, Matsuda M. Nature (London) 1999;400:891–894. doi: 10.1038/23738. [DOI] [PubMed] [Google Scholar]
- 24.Marinissen M J, Chiariello M, Pallante M, Gutkind J S. Mol Cell Biol. 1999;19:4289–4301. doi: 10.1128/mcb.19.6.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiyono M, Satoh T, Kaziro Y G. Proc Natl Acad Sci USA. 1999;96:4826–4831. doi: 10.1073/pnas.96.9.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmidt M, Voss M, Thiel M, Bauer B, Grannass A, Tapp E, Cool R H, de Gunzburg J, von Eichel-Streiber C, Jakobs K H. J Biol Chem. 1998;273:7413–7422. doi: 10.1074/jbc.273.13.7413. [DOI] [PubMed] [Google Scholar]
- 27.Coso O A, Teramoto H, Simonds W F, Gutkind J S. J Biol Chem. 1996;271:3963–3966. doi: 10.1074/jbc.271.8.3963. [DOI] [PubMed] [Google Scholar]
- 28.Bayon Y, Hernandez M, Alonso A, Nunez L, Garcia-Sancho J, Leslie C, Sanchez, Crespo M, Nieto M L. Biochem J. 1997;323:281–287. doi: 10.1042/bj3230281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Russell M, Winitz S, Johnson G L. Mol Cell Biol. 1994;14:2343–2351. doi: 10.1128/mcb.14.4.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huang X Y, Morielli A D, Peralta E G. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 31.Cantrell A R, Ma J Y, Scheuer T, Catterall W A. Neuron. 1996;16:1019–1026. doi: 10.1016/s0896-6273(00)80125-7. [DOI] [PubMed] [Google Scholar]
- 32.Clapham D E, Neer E J. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 33.Barnes P J, Haddad E-B, Rousell J. Life Sci. 1997;60:1015–1021. doi: 10.1016/s0024-3205(97)00042-8. [DOI] [PubMed] [Google Scholar]
- 34.Stengel P W, Gomeza J, Wess J, Cohen M L. J Pharmacol Exp Ther. 2000;292:877–885. [PubMed] [Google Scholar]
- 35.Burns F, Zhao A Z, Beavo J A. Adv Pharmacol. 1996;36:29–48. doi: 10.1016/s1054-3589(08)60575-x. [DOI] [PubMed] [Google Scholar]
- 36.Zhao A Z, Bornfeldt K E, Beavo J A. J Clin Invest. 1998;102:869–873. doi: 10.1172/JCI3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu H, Maurice D H. J Biol Chem. 1999;274:10557–10565. doi: 10.1074/jbc.274.15.10557. [DOI] [PubMed] [Google Scholar]
- 38.Oki N, Takahashi S I, Hidaka H, Conti M. J Biol Chem. 2000;275:10831–10837. doi: 10.1074/jbc.275.15.10831. [DOI] [PubMed] [Google Scholar]