Abstract
The kynurenine pathway (KP) is the principle route of catabolism of the essential amino acid tryptophan, leading to the production of several neuroactive and immunoregulatory metabolites. Alterations in the KP have been implicated in various neuropsychiatric and neurodegenerative diseases, immunological disorders, and many other diseased states. Although the role of the KP in the skin has been evaluated in small niche fields, limited studies are available regarding the effect of acute ultra violet exposure and the induction of the KP in human skin-derived fibroblasts and keratinocytes. Since UV exposure can illicit an inflammatory component in skin cells, it is highly likely that the KP may be induced in these cells in response to UV exposure. It is also possible that some KP metabolites may act as pro-inflammatory and anti-inflammatory mediators, since the KP is important in immunomodulation.
Keywords: fibroblasts, keratinocytes, kynurenine pathway, tryptophan, inflammation
Introduction
Representing a shield against harsh external environmental effects, the skin is the largest organ in the human body and serves an array of vital purposes.1,2 Apart from providing an integration of one’s physical aesthetics, the skin also represents the first line immune defence, temperature regulation, and provides a protective barrier from external insults such as mechanical stress and ultra violet (UV) radiation. Chronic exposure to UV rays has been implicated in skin aging and carcinogenesis.3,4 While most of the UV energy reaching the human skin is in the UVA range (>95% from 320 to 400 nm), most of the deleterious effects are due to UV radiation (290–320 nm). UV can induce numerous degenerative processes including inflammation, and oxidative stress leading to epidermal photodamage, and erythema.2,4 DNA-base dimerisation which can consequently occur as a consequence is considered a leading cause of skin cancers.3
The KP is the major route of catabolism of the essential amino acid tryptophan (TRYP), leading to the production of several neuroactive and immuno-regulatory metabolites.5–8 Alterations in the KP have been implicated in various neuropsychiatric and neurodegenerative diseases, immunological disorders, and many other diseased states.9–17 A number of recent studies have indicated that the KP may also be involved in acute and chronic UV damage.2,18 This review critically analyses our current understanding of KP in the regulation of skin physiology. It also discusses the importance of understanding KP metabolism in skin cells in response to UV damage, and highlights the lack of literature available in this increasingly growing field of research. Since UV exposure can generate an inflammatory response in skin cells, it is highly likely that the kynurenine pathway (KP) may also be subsequently induced in these cells.
Human Skin Physiology
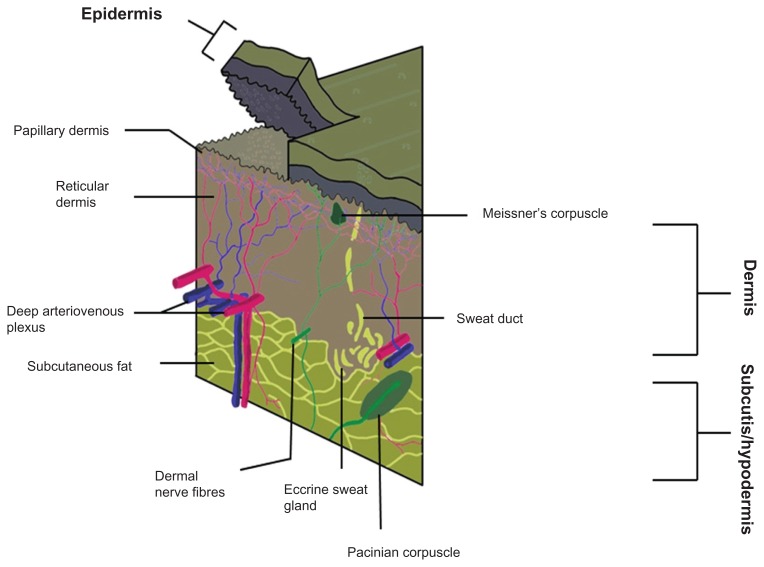
The human skin can be very generally divided into three sections: the epidermis, the outermost layer; dermis in the middle, and the hypodermis (or subcutaneous tissue)19 (Fig. 1 and Table 1). In humans UVA can penetrate deeper into the skin than UVB. Since UVA is the major component of UV energy, the basal layers of the epidermis are more susceptible to UV exposure where skin keratinocytic stem cells and melanocytes are present.
Figure 1.
Simplified three-dimensional diagram of the human skin.
Table 1.
Main layers of human skin including their composition and function.
| Skin layer | Composition | Function |
|---|---|---|
| Epidermis |
|
|
| Dermis |
|
|
| Hypodermis |
|
|
Epidermis
Containing no blood vessels, the epidermis is a stratified squamous epithelium. It is made up of many layers of closely packed cells, most of which are keratinocytes, but also contains melanocytes, Langerhan and Merkel cells.20 The epidermis can be divided into four sections, the horny layer, granular layer, prickle cell layer and the basal layer. These sections are characterised by their keratinocyte properties and their degree of differentiation as the cells move from the basal layer to the horny layer.20,21
In the time span of thirty to sixty days, dividing cells of the epidermis in the basal layer are mitotically migrated to the horny layer where they eventually die and shed, maintaining a constant thickness of skin through this cycle. The epidermis downwardly projects ridges to interlock with the upwardly projecting dermal papillae of the dermis, forming the dermal-epidermal junction which primarily acts to join these two layers and provide protection against external shearing forces.20
Dermis
The dermis makes up much of the bulk of the skin and gives it its pliability, tensile strength and elasticity. It is primarily composed of interwoven fibres, made up mainly of collagen. The main cells present in the dermis are fibroblasts, although phagocytes, lymphocytes, Langerhan, and mast cells are also present.20,21 Much of the skins nociceptors, mechanoreceptors, lymphatics, and smooth and striated muscle are present in the dermis. The dermis and the hypodermis are separated by a very abrupt transition from a fibrous connective tissue to an environment which is abundant in adipose tissue.20
Hypodermis
As well as working as an energy reserve, the hypodermis acts as an insulation barrier, protecting the skin and moulding the contours of the body. Loss of this subcutaneous fat would result in poorly balanced glucose, triglyceride and cholesterol regulation, as well as other cosmetic dysfunctions.20
The three layers of the skin work interdependently to serve as a barrier from external damage, to prevent loss of important body constituents, and ultimately to maintain normal physiological functions.20 Of the many cell types that are present in the skin, keratinocytes and fibroblasts are becoming increasingly important as we discover more about their implications in immune regulation and in various disease states21 (Fig. 2).
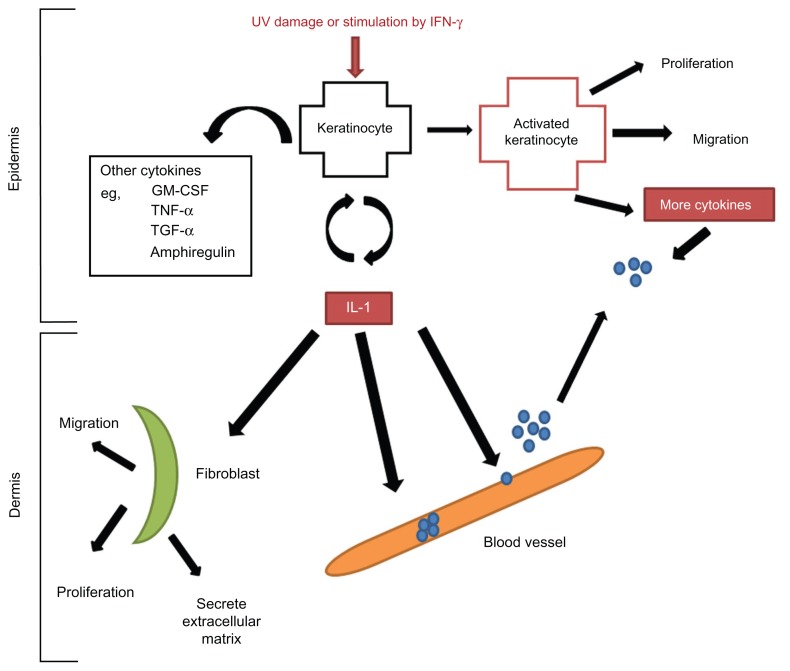
Figure 2.
Damaged keratinocytes (following exposure to UV or IFN-γ) release numerous cytokines including interleukin 1 (IL-1), which then in turn activate endothelial cells that express selectins, slowing down the migration of lymphocytes to the site of injury, IL-1 acts as a chemotactic factor to draw lymphocytes into the epidermis.
Notes: IL-1 simultaneously activates keratinocytes by binding to the IL-1 receptor, leading to increased production of other cytokines, including tumour necrosis factor α (TNF-α). TNF-α both activates keratinocytes, and keeps them in an activated state. Activated keratinocytes proliferate, migrate and release more cytokines.
Functional correlations
Keratinocytes primarily make up the outermost horny layer, although, they play a very important role in immunological function.20 They are composed of keratin filaments and have a predominantly structural role. When activated in an immune response caused by injury, or stimulated by exogenous factors such as UV radiation, they can secrete anti-microbial peptides.19 They also release inflammatory cytokines which are constitutively active in small amounts and are upregulated in injury. Keratinocytes also play a large role in the healing process after injury to the epidermis, since the cells self-regulate their differentiation and proliferation.20,21
On the other hand, fibroblasts are not fixed cells confined to a specific area, but migrate through the tissue, synthesising and degrading fibrous and non-fibrous material. They continuously secrete precursors to extracellular matrices, hence maintaining integrity of connective tissue. These cells play a crucial role in wound healing and scarring as they increase their proliferative activity in these times of stress.21 Keratinocytes and fibroblasts work in conjunction with other skin cells to maintain regular immune function (Table 2).
Table 2.
Main cells in the skin and their functional role in the maintenance of skin physiology.
| Skin cell | Function |
|---|---|
| Keratinocyte |
|
| Fibroblast |
|
| Langerhan |
|
| T-lymphocyte | Differentiate into subpopulations
|
| Mast |
|
There is a high degree of immune regulation within the papillary dermis, with macrophages, monocytes and dermal dendrocytes accounting for a phagocytic system within the skin. Macrophages, which ultimately differentiate into monocytes in the dermis, act through various mechanisms such as cytokine secretions to prevent infections.19,20 Unlike these cells, the dermal dendrocytes are fixed cells but they are antigen presenting phagocytic cells. These mechanisms cannot be overstated, as the integumentary system is the bodies’ first line of defense against disease.19
With immune regulation being such an important aspect of physiological maintenance, it seems important to examine the potential effect that the KP has in this physiological system, as it too has been implicated in immune function.22–26
The Role of Tryptophan Catabolism via the Kynurenine Pathway in Mammalian Cells
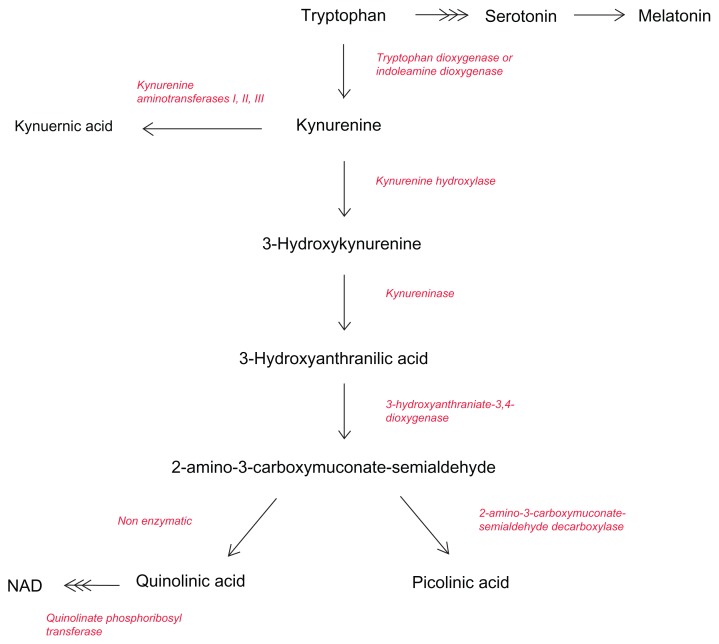
Although a lot of emphasis has been placed on its role as a precursor to the neurotransmitter 5- hydroxytryptophan (5-HT), most of the TRYP in the body is metabolized through the KP27–31 (Fig. 3). The KP represents the major route of TRYP catabolism, leading to the formation of the essential pyridine nucleotide, nicotinamide adenine dinucleotide (NAD+).32 This is achieved through an uncapped diversion in the KP to produce more NAD+ in the liver.33
Figure 3.
Simplified diagram of the kynurenine pathway.
As more than 95% of tryptophan in the CNS is metabolized through the KP,14 the role it plays in maintaining physiological homeostasis is very significant especially with regard to immune regulation. It has been shown very clearly that the KP and its metabolites are very important in immune modulation,32,33 and are associated with the pathogenesis of a variety of disease states.34–42 The activation of the KP pathway can be brought about by indoleamine-2,3-dioxygenase (IDO-1) which is the first rate limiting enzyme in this pathway.43 IDO is extremely important in immune suppression, particularly in the non-rejection of allogeneic foetuses.44 It has been found that cells which express IDO can suppress T-cell responses and hence induce immune tolerance.45 This theory has been supported by many studies and is the basis of Moffett and Namboodiri’s33 hypothesis that TRYP depletion suppresses T cell proliferation through a huge restriction in the availability of this amino acid. Conversely, TRYP loading has been shown to induce oxidative stress in healthy volunteers.46,47 Similarly, supplementation with TRYP in patients with depression in hopes of increasing serotonin availability has led to severe and debilitating immune disorders.48
Of the many metabolites expressed in the KP, the most important to consider are quinolinic acid (QUIN), a potent neurotoxin and agonist at glutamate receptors,49 and two generally neuroprotective metabolites, kynurenic acid (KYNA) and picolinic acid (PIC) (Guillemin et al14). These compounds are all able to affect oxidative stress, however in different capacities, as the various KP metabolites have either cytotoxic or protective effects. Interestingly, these metabolites can occasionally possess both of these properties depending on their concentration. In terms of their toxicity, several of the KP metabolites have been thoroughly investigated, using both in vitro and in vivo animal neurodegeneration models.28,29
Quinolinic acid
QUIN is a selective, but weak agonist of the N- methyl-D-aspartate (NMDA) receptor.50–52 QUIN is an extremely important metabolite. Recently, Braidy et al54 performed a study which supports the well accepted idea that the neurotoxic effects of QUIN is attributed to an increase in intracellular Ca2+ influx through NMDA glutamatergic receptor activation. 53,54 This calcium influx induces the activation of a cascade of downstream intracellular enzymes such as nitric oxide synthase and xanthine oxidase which then produce reactive oxygen species (ROS), leading to oxidative stress and macromolecular damage and consequently cell death. Under normal conditions, QUIN is a necessary precursor for the production of NAD+ at concentrations below 300 Nm.55 The neurotoxic capabilities of increased quinolinic acid levels (>300 nM) has been observed in many disease states, such as Alzheimer’s disease,56 Huntington’s disease,57 and Acquired Immunodeficiency Syndrome (AIDS).58
Kynurenic acid and picolinic acid
As opposed to QUIN, KYNA has been shown to be generally neuroprotective (Guillemin et al14). It is a non-selective antagonist at NMDA receptors, with high affinity for the glycine co-agonist site, and having the ability to antagonize the neurotoxic effects of QUIN.59,60 Despite its neuroprotective role in disease, KYNA is produced in too little amounts to attain any clinical significance.61
The effects of altered levels of KYNA have been studied in a variety of disease states, predominantly those of a neurodegenerative nature. In contrast to QUIN, KYNA appears to behave differently in different concentrations.34,35 Elevated levels have been associated with schizophrenia, and bipolar disorder40 and decreased levels have been associated with multiple sclerosis,62 and Amyotropic Lateral Sclerosis (ALS).63
Another endogenous neuroprotective metabolite in the KP, PIC is three times weaker than KYNA when blocking the effects of QUIN.64,65 Without affecting the excitatory effects of QUIN, it can block its neurotoxic effects through an unknown mechanism, as it does not seem to compete for the same agonist binding site on the NMDA receptor. Picolinic acid also increases chemokine release, and stimulates the releases of antimicrobial, anti-viral and anti-tumoral agents.66–71
Other kynurenines (3-HK, 3-HAA and 5-HAA)
Additional metabolites produced in the KP also show significant ability to cause toxic effects. 3-hydroxykynurenine (3-HK), 3- hydroxyanthranilic acid (3-HAA) and 5-hydroxyanthranilic acid (5-HAA) produce highly reactive free radicals that play an important role in the pathogenesis of neurodegenerative disorders.72 Okuda et al73 found that the toxicity induced by 3-HK was mainly related to the intracellular accumulation of peroxide, which was able to be attenuated greatly by the use of catalase.73,74 Similarly, Smith et al72 demonstrated the same principle for all three metabolites as well as showing p38 signalling activated cell death as a result of 3-HK and 5-HAA. 3-HK also represents a natural UV filter in the human lens, and other metabolites of tryptophan act as UV filters in some organisms.75,76 KP metabolites including 3-HK are of great interest when investigating the effects of UV in human skin.
Kynurenine pathway and human skin
A vast majority of the studies investigating the breakdown of tryptophan via the KP have focused on the pathogenesis of neurological disorders.5,6,28,29,31,52,77–82 Studies examining the KP in the skin are extremely limited, and so it is with the association of the NMDA receptor in the skin with which the implications of the KP pathway should be considered.
NMDA receptors have been recently found to be present in non-neuronal tissue, and have been demonstrated to have functional roles in both keratinocytes83 and fibroblasts.84 These studies suggest that Ca2+ influx through the NMDA receptors in keratinocytes inhibits the re-epithelialisation process, affecting cellular proliferation, differentiation and migration in the cell cycle. Albina et al85 found that glutamate is present in keratinocytes, and is upregulated during inflammation or wounding, implying that NMDA receptor activation is linked intrinsically to epidermal maintenance.85
Expression of Components of the Kynurenine Pathway in Fibroblasts and Macrophages
The KP is understandably differentially expressed in all cells, making the characterization of its expression in various important cell types very important, especially those which are known to be involved in immune responses. Having an understanding of how the KP is expressed in the cell types of interest is a crucial starting point for investigating the implications of alterations in this significant pathway.
Fibroblasts
The KP has recently been shown to be fully expressed in human dermal fibroblasts, with a considerable difference in the basal levels of expression across genes and individuals.18 These differences have been explained by variations across different cultural backgrounds. As with many other cells expressing the KP components, IDO1 expression is inducible with IFN-γ treatment leading to a significant increase in the production of KYN and its metabolite, KYNA.18
Macrophages
The KP has been shown to be fully expressed in macrophages. These inflammatory cells possess the ability to produce about 20 to 30 times more QUIN than microglial cells in the brain.86 This difference in excitotoxic producing ability is associated with lower expression of three key enzymes in microglia: IDO, KYNase and KYN-OHase.87
Effect of Pro-inflammatory Cytokines and Ultraviolet Exposure on Kynurenine Pathway Expression
As discussed earlier, IFN-γ is a pro-inflammatory cytokine that is released by activated T-cells during an immune response. The sustained catabolism of TRYP is a very well reproduced effect that occurs as a response to IFN- γ exposure. This is the crux of Moffett and Namboodiri’s33 hypothesis that TRYP depletion suppresses T-cell proliferation by drastically depleting the supply of this limited amino acid.
A recent study performed by Asp et al18 found that human skin fibroblasts showed a massive increase in transcripts encoding IDO1 when stimulated by IFN-γ. They also found that fibroblasts can release KYNA, which was greatly increased following the IFN-γ stimulation. This further supports the idea that IFN-γ treatment can stimulate cells to increase the rate of TRYP degradation, and that IDO1 is the major determinant of this response.
UV exposure can have similar effects on cellular functioning as IFN-γ exposure, as it can trigger the release of many endogenous cytokines. TNF-α expression has been shown to be increased in acute UV exposure, and this has potential to affect the KP in the skin as TNF-α is a potent inducer of IDO1. As IDO1 is the first and rate-limiting enzyme in the KP, this potential connection between UV exposure and the KP could potentially be the defining connection between these two pathways. Although, Asp et al18 showed that there was no significant change in levels of KYNA in response to TNF-α, there was a marked and very selective increase in the levels of transcripts encoding kynureninase, suggesting that different cytokines display selectivity for expression of certain genes in the KP in human skin cells.
Programmed cell death is also implicated in both acute UV irradiation and oxidative damage as p53 has an invaluable role in DNA repair, from the promotion of repair to the induction of apoptosis to prevent tumor colonization. Acute UV irradiation for example results in the immediate upregulation of p53 transcription.88 Cell-cycle arrest can be attributed to p21, also known as CIP1 and WAFI, which inhibits cyclin-dependent kinase (CDK) by binding to it to form a dysfunctional complex causing the cell cycle arrest.89 If DNA repair mechanisms are unable to rectify the damage, p53 as a transcriptional transactivator, then initiates apoptotic pathways to prevent the proliferation of damaged cells.88 It either upregulates pro-apoptotic factors such as Apo-1 and Bax, or down regulates apoptosis suppressing genes such as Bcl-2.90 p53 maintains physiological integrity through several other mechanisms including angiogenesis inhibition through BAII and TSP1 genes, and via promoting correct replication through the use of the GADD45 gene.91
Within this biochemical microcosm we can see another potential role for the KP. NAD+, the end metabolite of the KP, could have a paramount role in cellular repair.92–95 Sirtuins (in this case Sir2), a class of cellular conservationist proteins, are responsible for the NAD+ dependant deacetylation of a variety of protein substrates.96 In this case, p53 is deacetylated which attenuates its apoptotic cellular response and Sir2, along with other DNA repair genes are able to resume repair.97 As such, there is a potential role for the KP and its metabolites in maintaining anabolism of NAD+ within the skin.
Lack of Literature on the Kynurenine Pathway in Skin Cells
A clear majority of the work performed on the metabolic routes of the KP have focused on its implications in neurodegenerative disease. Much interest has been placed on determining the role of the KP in many different neuronal cells, and determining the intricate details of their expression pathways. There is however, very little work in the area of TRYP breakdown in human skin. Studies in the skin appear to have originated, like many other KP studies, in understanding TRYP metabolism and its connection to common dermatological pathologies.83,98–100 This lead to detailed studies into the presence and functionality of both TDO and IDO1 in skin cells, however these studies have all been relatively restricted to their subsequent effects on immune regulation. For example, the study recently performed by Asp et al18 provided the very first insight into the effect of inflammatory cytokines on KP transcripts and their downstream metabolites in human dermal fibroblasts. Moreover, Li et al99 extensively studied IDO1 in human keratinocytes and its role in MHC class I antigen suppression. This form of research has been developed further into studies regarding IDO1 as a potential pharmacological target for skin grafting.
This relatively uncharted area of study is becoming exponentially important as we aware of skin cancer incidences rising at an alarming rate, especially in Australia and New Zealand where the highest incidence rates of melanoma have been observed.19 A plethora of research has been undertaken in UV related immune suppression due to its frightening implications in carcinogenesis.2,4 KP metabolites have already been shown to play a major role in UV protection in the human eye and also in other organisms.101,102 Despite these widely accepted biochemical physiologies, there is little published research regarding any potential for an amplified synergistic immunosuppressive response between the kynurenine pathway and acute UV exposure.
Furthermore, it has been shown that proteomic analysis of skin fibroblasts from living patients displays significant differences in cellular proliferation and growth pathways between schizophrenic compared to control patients.103 Aberrant cell growth has been described in patients with bi-polar disorder,104 autism,105 and schizophrenia.106 Peripheral methods of diagnosing neurodegenerative disorders provide favourable non-invasive and un-biased judgements of their conditions. This can solve many issues for clinicians as diagnosis of neurodegenerative disorders can often be intimidating as a critically confirming biochemical tests are not available. With this understanding, further investigations into the role of TRYP degradation in the skin along with the implications of the KP in immune regulation means that metabolites of the KP in human fibroblasts and keratinocytes can be very useful in diagnosis of KP-related degenerative disorders.
Footnotes
Author Contributions
Wrote the first draft of the manuscript: DS, NB. Contributed to the writing of the manuscript: DS, NB. Jointly developed the structure and arguments for the paper: DS, NB, GJG. Made critical revisions and approved final version: NB, GJG. All authors reviewed and approved of the final manuscript.
Funding
Nady Braidy is the recipient of the Alzheimer’s Australia Viertel Foundation Postdoctoral Research Fellowship at the University of New South Wales.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Black H. The Defensive Role of Antioxidants in Skin Carcinogenesis. In: Fuchs J, editor. Oxidative Stress in Dermatology. Mercel Dekker; NY: 1993. pp. 243–69. [Google Scholar]
- 2.Sauvaigo S, et al. DNA repair capacities of cutaneous fibroblasts: effect of sun exposure, age and smoking on response to an acute oxidative stress. British Journal of Dermatology. 2007;157:26–32. doi: 10.1111/j.1365-2133.2007.07890.x. [DOI] [PubMed] [Google Scholar]
- 3.Brash D, et al. Sunlight and sunburn in human skin cancer: p53, apoptosis and tumor promotion. J Investig Dermatol Symp Proc. 1996;1:136–42. [PubMed] [Google Scholar]
- 4.Gasparro F. Sunscreens, skin photobiology and skin cancer: The need for UVA protection and evaluation of efficacy. Environ Health Perspect. 2000;108(Suppl 1):71–8. doi: 10.1289/ehp.00108s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stone TW. Endogenous neurotoxins from tryptophan. Toxicon. 2001;39(1):61–73. doi: 10.1016/s0041-0101(00)00156-2. [DOI] [PubMed] [Google Scholar]
- 6.Stone TW. Kynurenic acid antagonists and kynurenine pathway inhibitors. Expert Opin Investig Drugs. 2001;10(4):633–45. doi: 10.1517/13543784.10.4.633. [DOI] [PubMed] [Google Scholar]
- 7.Stone TW. Kynurenines in the CNS: from endogenous obscurity to therapeutic importance. Prog Neurobiol. 2001;64(2):185–218. doi: 10.1016/s0301-0082(00)00032-0. [DOI] [PubMed] [Google Scholar]
- 8.Stone TW. Purines and neuroprotection. Adv Exp Med Biol. 2002;513:249–80. doi: 10.1007/978-1-4615-0123-7_9. [DOI] [PubMed] [Google Scholar]
- 9.Beal MF, et al. Kynurenine pathway measurements in Huntington’s disease striatum: evidence for reduced formation of kynurenic acid. J Neurochem. 1990;55(4):1327–39. doi: 10.1111/j.1471-4159.1990.tb03143.x. [DOI] [PubMed] [Google Scholar]
- 10.Belladonna ML, et al. Immunosuppression via tryptophan catabolism: the role of kynurenine pathway enzymes. Transplantation. 2007;84(Suppl 1):S17–20. doi: 10.1097/01.tp.0000269199.16209.22. [DOI] [PubMed] [Google Scholar]
- 11.Guillemin G, Meininger V, Brew B. Implications for the kynurenine pathway and quinolinic acid in amyotrophic lateral sclerosis. Neurodegenerative diseases. 2006;2:166–76. doi: 10.1159/000089622. [DOI] [PubMed] [Google Scholar]
- 12.Guillemin GJ, Brew BJ. Implications of the kynurenine pathway and quinolinic acid in Alzheimer’s disease. Redox Rep. 2002;7(4):199–206. doi: 10.1179/135100002125000550. [DOI] [PubMed] [Google Scholar]
- 13.Guillemin GJ, Brew BJ. Chronic HIV Infection leads to an Alzheimer’s Disease Like Illness. In: Takai K, editor. Involvement of the Kynurenine Pathway, in International Congress Series. 2007. pp. 324–34. [Google Scholar]
- 14.Guillemin GJ, et al. Characterization of the kynurenine pathway in human neurons. J Neurosci. 2007;27(47):12884–92. doi: 10.1523/JNEUROSCI.4101-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guillemin GJ, Kerr SJ, Brew BJ. Involvement of quinolinic acid in AIDS dementia complex. Neurotox Res. 2005;7(1–2):103–23. doi: 10.1007/BF03033781. [DOI] [PubMed] [Google Scholar]
- 16.Guillemin GJ, et al. Quinolinic acid in the pathogenesis of Alzheimer’s disease. Adv Exp Med Biol. 2003;527:167–76. doi: 10.1007/978-1-4615-0135-0_19. [DOI] [PubMed] [Google Scholar]
- 17.Heyes MP. The kynurenine pathway and neurologic disease. Therapeutic strategies. Adv Exp Med Biol. 1996;398(125):125–9. doi: 10.1007/978-1-4613-0381-7_20. [DOI] [PubMed] [Google Scholar]
- 18.Asp L, et al. Effects of pro-inflammatory cytokines on expression of kynurenine pathway enzymes in human dermal fibroblasts. Journal of Inflammation. 2011;8:25. doi: 10.1186/1476-9255-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cotran RS, Kumar V, Collins T, editors. Pathological Basis of Disease. 6th ed. SaundersCompany; UK: 1999. [Google Scholar]
- 20.Eroschenko V, editor. DiFiore’s Atlas of Histology with Functional Correlations. 10th ed. Lippincott Williams and Wilkins; Baltimore, MD: 2005. [Google Scholar]
- 21.Chang H, et al. Diversity, topographic differentiation, and positional memory in human fibroblasts. Proc Natl Acad Sci U S A. 2002;99:12877–82. doi: 10.1073/pnas.162488599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee GK, et al. Tryptophan deprivation sensitizes activated T cells to apoptosis prior to cell division. Immunology. 2002;107(4):452–60. doi: 10.1046/j.1365-2567.2002.01526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mellor AL, et al. Tryptophan catabolism and T cell responses. Adv Exp Med Biol. 2003;527:27–35. doi: 10.1007/978-1-4615-0135-0_3. [DOI] [PubMed] [Google Scholar]
- 24.Mellor AL, Munn DH. Tryptophan catabolism and T-cell tolerance: immunosuppression by starvation? Immunol Today. 1999;20(10):469–73. doi: 10.1016/s0167-5699(99)01520-0. [DOI] [PubMed] [Google Scholar]
- 25.Mellor AL, Munn DH. Tryptophan catabolism and regulation of adaptive immunity. J Immunol. 2003;170(12):5809–13. doi: 10.4049/jimmunol.170.12.5809. [DOI] [PubMed] [Google Scholar]
- 26.Munn DH. Indoleamine 2,3-dioxygenase, tumor-induced tolerance and counter-regulation. Curr Opin Immunol. 2006;18(2):220–5. doi: 10.1016/j.coi.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 27.Stone TW, et al. Endogenous excitotoxic agents. Ciba Found Symp. 1987;126:204–20. doi: 10.1002/9780470513422.ch13. [DOI] [PubMed] [Google Scholar]
- 28.Stone TW, Darlington LG. Endogenous kynurenines as targets for drug discovery and development. Nat Rev Drug Discov. 2002;1(8):609–20. doi: 10.1038/nrd870. [DOI] [PubMed] [Google Scholar]
- 29.Stone TW, et al. Tryptophan, adenosine, neurodegeneration and neuroprotection. Metab Brain Dis. 2007 doi: 10.1007/s11011-007-9064-3. [DOI] [PubMed] [Google Scholar]
- 30.Stone TW, et al. Basic mechanisms of kynurenine actions in the central nervous system. Adv Exp Med Biol. 1996;398:195–201. doi: 10.1007/978-1-4613-0381-7_32. [DOI] [PubMed] [Google Scholar]
- 31.Stone TW, et al. Tryptophan metabolites and brain disorders. Clin Chem Lab Med. 2003;41(7):852–9. doi: 10.1515/CCLM.2003.129. [DOI] [PubMed] [Google Scholar]
- 32.Stone TW. Neuropharmacology of quinolinic and kynurenic acids. Pharmacol Rev. 1993;45(3):309–79. [PubMed] [Google Scholar]
- 33.Moffett JR, Namboodiri MA. Tryptophan and the immune response. Immunol Cell Biol. 2003;81(4):247–65. doi: 10.1046/j.1440-1711.2003.t01-1-01177.x. [DOI] [PubMed] [Google Scholar]
- 34.Erhardt S, Oberg H, Engberg G. Pharmacologically elevated levels of endogenous kynurenic acid prevent nicotine-induced activation of nigral dopamine neurons. Naunyn Schmiedebergs Arch Pharmacol. 2001;363(1):21–7. doi: 10.1007/s002100000325. [DOI] [PubMed] [Google Scholar]
- 35.Erhardt S, et al. Pharmacological elevation of endogenous kynurenic acid levels activates nigral dopamine neurons. Amino Acids. 2001;20(4):353–62. doi: 10.1007/s007260170032. [DOI] [PubMed] [Google Scholar]
- 36.Linderholm KR, et al. Activation of rat ventral tegmental area dopamine neurons by endogenous kynurenic acid: A pharmacological analysis. Neuropharmacology. 2007 doi: 10.1016/j.neuropharm.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Nilsson LK, Linderholm KR, Erhardt S. Subchronic treatment with kynurenine and probenecid: effects on prepulse inhibition and firing of midbrain dopamine neurons. J Neural Transm. 2005 doi: 10.1007/s00702-005-0343-z. [DOI] [PubMed] [Google Scholar]
- 38.Leonard BE, Myint A. Changes in the immune system in depression and dementia: causal or coincidental effects? Dialogues Clin Neurosci. 2006;8(2):163–74. doi: 10.31887/DCNS.2006.8.2/bleonard. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myint AM, Kim YK. Cytokine-serotonin interaction through IDO: a neurodegeneration hypothesis of depression. Med Hypotheses. 2003;61(5–6):519–25. doi: 10.1016/s0306-9877(03)00207-x. [DOI] [PubMed] [Google Scholar]
- 40.Myint AM, et al. Tryptophan breakdown pathway in bipolar mania. J Affect Disord. 2007 doi: 10.1016/j.jad.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 41.Myint AM, et al. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J Affect Disord. 2006 doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Myint AM, et al. Kynurenine pathway in major depression: Evidence of impaired neuroprotection. J Affect Disord. 2007;98(1–2):143–51. doi: 10.1016/j.jad.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 43.Fujigaki H, et al. Nitration and inactivation of IDO by peroxynitrite. J Immunol. 2006;176(1):372–9. doi: 10.4049/jimmunol.176.1.372. [DOI] [PubMed] [Google Scholar]
- 44.Baban B, et al. Indoleamine 2,3-dioxygenase expression is restricted to fetal trophoblast giant cells during murine gestation and is maternal genome specific. J Reprod Immunol. 2004;61(2):67–77. doi: 10.1016/j.jri.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Takikawa O. Biochemical and medical aspects of the indoleamine 2,3- dioxygenase-initiated l-tryptophan metabolism. Biochem Biophys Res Commun. 2005;338(1):12–9. doi: 10.1016/j.bbrc.2005.09.032. [DOI] [PubMed] [Google Scholar]
- 46.Christofides J, et al. Blood 5-hydroxytryptamine, 5-hydroxyindoleacetic acid and melatonin levels in patients with either Huntington’s disease or chronic brain injury. J Neurochem. 2006;97(4):1078–88. doi: 10.1111/j.1471-4159.2006.03807.x. [DOI] [PubMed] [Google Scholar]
- 47.Forrest CM, et al. Tryptophan loading induces oxidative stress. Free Radic Res. 2004;38(11):1167–71. doi: 10.1080/10715760400011437. [DOI] [PubMed] [Google Scholar]
- 48.Silver RM, et al. Alterations in tryptophan metabolism in the toxic oil syndrome and in the eosinophilia-myalgia syndrome. J Rheumatol. 1992;19(1):69–73. [PubMed] [Google Scholar]
- 49.Stone TW, Connick JH. Quinolinic acid and other kynurenines in the central nervous system. Neuroscience. 1985;15(3):597–617. doi: 10.1016/0306-4522(85)90063-6. [DOI] [PubMed] [Google Scholar]
- 50.Schurr A, Rigor BM. Quinolinate potentiates the neurotoxicity of excitatory amino acids in hypoxic neuronal tissue in vitro. Brain Res. 1993;617(1):76–80. doi: 10.1016/0006-8993(93)90615-t. [DOI] [PubMed] [Google Scholar]
- 51.Schwarcz R, Du F. Quinolinic acid and kynurenic acid in the mammalian brain. Adv Exp Med Biol. 1991;294:185–99. doi: 10.1007/978-1-4684-5952-4_17. [DOI] [PubMed] [Google Scholar]
- 52.Schwarcz R, Pellicciari R. Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. J Pharmacol Exp Ther. 2002;303(1):1–10. doi: 10.1124/jpet.102.034439. [DOI] [PubMed] [Google Scholar]
- 53.Braidy N, et al. Mechanism for quinolinic acid cytotoxicity in human astrocytes and neurons. Neurotox Res. 2009;16:77–86. doi: 10.1007/s12640-009-9051-z. [DOI] [PubMed] [Google Scholar]
- 54.Braidy N, et al. Neuroprotective effects of naturally occurring polyphenols on quinolinic-acid induced excitotoxicity in human neurons. FEBS J. 2010;277:368–82. doi: 10.1111/j.1742-4658.2009.07487.x. [DOI] [PubMed] [Google Scholar]
- 55.Braidy N, et al. Effects of Kynurenine pathway metabolites on intracellular NAD+ synthesis and cell death in human primary astrocytes and neurons. IJTR. 2009;2:61–9. doi: 10.4137/ijtr.s2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guillemin GJ, et al. Indoleamine 2,3 dioxygenase and quinolinic acid immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005;31(4):395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- 57.Guidetti P, et al. Neostriatal and cortical quinolinate levels are increased in early grade Huntington’s disease. Neurobiol Dis. 2004;17(3):455–61. doi: 10.1016/j.nbd.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 58.Fuchs D, et al. Cellular immune activation in the brain and human immunodeficiency virus infection. Ann Neurol. 1988;24:289. doi: 10.1002/ana.410240227. [DOI] [PubMed] [Google Scholar]
- 59.Kapoor V, Kapoor R, Chalmers J. Kynurenic acid, an endogenous glutamate antagonist, in SHR and WKY rats: possible role in central blood pressure regulation. Clin Exp Pharmacol Physiol. 1994;21(11):891–6. doi: 10.1111/j.1440-1681.1994.tb02460.x. [DOI] [PubMed] [Google Scholar]
- 60.Kloog Y, Lamdani-Itkin H, Sokolovsky M. The glycine site of the N- methyl-D-aspartate receptor channel: differences between the binding of HA-966 and 7-chlorokynurenic acid. J Neurochem. 1990;54:1576–83. doi: 10.1111/j.1471-4159.1990.tb01207.x. [DOI] [PubMed] [Google Scholar]
- 61.Boegman RJ, et al. Quinolinic acid neurotoxicity in the nucleus basalis antagonized by kynurenic acid. Neurobiol Aging. 1985;6(4):331–6. doi: 10.1016/0197-4580(85)90012-0. [DOI] [PubMed] [Google Scholar]
- 62.Rejdak K, et al. Decreased level of kynurenic acid in cerebrospinal fluid of relapsing-onset multiple sclerosis patients. Neurosci Lett. 2002;331(1):63–5. doi: 10.1016/s0304-3940(02)00710-3. [DOI] [PubMed] [Google Scholar]
- 63.Iwasaki Y, et al. Kynurenic acid in ALS patients. Acta Neurol Scand. 2004;109(3):236. doi: 10.1111/j.1600-0404.2004.0255a.x. author reply 437. [DOI] [PubMed] [Google Scholar]
- 64.Jhamandas K, et al. Quinolinate-induced cortical cholinergic damage: modulation by tryptophan metabolites. Brain Res. 1990;529(1–2):185–91. doi: 10.1016/0006-8993(90)90826-w. [DOI] [PubMed] [Google Scholar]
- 65.Jhamandas KH, et al. Excitotoxicity of quinolinic acid: modulation by endogenous antagonists. Neurotox Res. 2000;2(2–3):139–55. doi: 10.1007/BF03033790. [DOI] [PubMed] [Google Scholar]
- 66.Blasi E, et al. Differential host susceptibility to intracerebral infections with Candida albicans and Cryptococcus neoformans. Infect Immun. 1993;61(8):3476–81. doi: 10.1128/iai.61.8.3476-3481.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blasi E, et al. Protective effect of picolinic acid on mice intracerebrally infected with lethal doses of Candida albicans. Antimicrob Agents Chemother. 1993;37(11):2422–6. doi: 10.1128/aac.37.11.2422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bosco MC, et al. Macrophage activating properties of the tryptophan catabolite picolinic acid. Adv Exp Med Biol. 2003;527:55–65. doi: 10.1007/978-1-4615-0135-0_6. [DOI] [PubMed] [Google Scholar]
- 69.Cai S, et al. Antimicrobial activity of picolinic acid against extracellular and intracellular Mycobacterium avium complex and its combined activity with clarithromycin, rifampicin and fluoroquinolones. J Antimicrob Chemother. 2006;57(1):85–93. doi: 10.1093/jac/dki418. [DOI] [PubMed] [Google Scholar]
- 70.Evans GW, Johnson EC. Growth stimulating effect of picolinic acid added to rat diets. Proc Soc Exp Biol Med. 1980;165(3):457–61. doi: 10.3181/00379727-165-41004. [DOI] [PubMed] [Google Scholar]
- 71.Fernandez-Pol JA, Klos DJ, Hamilton PD. Antiviral, cytotoxic and apoptotic activities of picolinic acid on human immunodeficiency virus-1 and human herpes simplex virus-2 infected cells. Anticancer Res. 2001;21(6A):3773–6. [PubMed] [Google Scholar]
- 72.Smith AJ, Stone TW, Smith RA. Neurotoxicity of tryptophan metabolites. Biochem Soc Trans. 2007;35(Pt 5):1287–9. doi: 10.1042/BST0351287. [DOI] [PubMed] [Google Scholar]
- 73.Okuda S, et al. Hydrogen peroxide-mediated neuronal cell death induced by an endogenous neurotoxin, 3-hydroxykynurenine. Proc Natl Acad Sci U S A. 1996;93(22):12553–8. doi: 10.1073/pnas.93.22.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Okuda S, et al. 3-Hydroxykynurenine, an endogenous oxidative stress generator, causes neuronal cell death with apoptotic features and region selectivity. J Neurochem. 1998;70:299–307. doi: 10.1046/j.1471-4159.1998.70010299.x. [DOI] [PubMed] [Google Scholar]
- 75.Chiou SJ, et al. Purification of toxic compounds from larvae of the gray fleshfly: the identification of paralysins. Biochem Biophys Res Commun. 1998;246(2):457–62. doi: 10.1006/bbrc.1998.8644. [DOI] [PubMed] [Google Scholar]
- 76.Han Q, Beerntsen BT, Li J. The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid biosynthesis. J Insect Physiol. 2007;53(3):254–63. doi: 10.1016/j.jinsphys.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Colabroy KL, Begley TP. Tryptophan catabolism: Identification and characterization of a new degradative pathway. Journal of Bacteriology. 2005;187(22):7866–9. doi: 10.1128/JB.187.22.7866-7869.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reinhard JF., Jr Pharmacological manipulation of brain kynurenine metabolism. Ann N Y Acad Sci. 2004;1035:335–49. doi: 10.1196/annals.1332.020. [DOI] [PubMed] [Google Scholar]
- 79.Schwarcz R. The kynurenine pathway of tryptophan degradation as a drug target. Curr Opin Pharmacol. 2004;4(1):12–7. doi: 10.1016/j.coph.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 80.Stone TW. Inhibitors of the kynurenine pathway. Eur J Med Chem. 2000;35(2):179–86. doi: 10.1016/s0223-5234(00)00121-5. [DOI] [PubMed] [Google Scholar]
- 81.Stone TW. Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends Pharmacol Sci. 2000;21(4):149–54. doi: 10.1016/s0165-6147(00)01451-6. [DOI] [PubMed] [Google Scholar]
- 82.Stone TW, Addae JI. The pharmacological manipulation of glutamate receptors and neuroprotection. Eur J Pharmacol. 2002;447(2–3):285–96. doi: 10.1016/s0014-2999(02)01851-4. [DOI] [PubMed] [Google Scholar]
- 83.Nahm W, et al. Significance of N-Methyl-D-Aspartate (NMDA) Receptor-Mediated Signaling in Human Keratinocytes. J Cell Physiol. 2004;200:309–17. doi: 10.1002/jcp.20010. [DOI] [PubMed] [Google Scholar]
- 84.Cooper B, et al. Structural selectivity and molecular nature of L- glutamate transport in cultured human fibroblasts. Arch Biochem Biophys. 1998;353:356–64. doi: 10.1006/abbi.1998.0626. [DOI] [PubMed] [Google Scholar]
- 85.Albina JE, Abate JA, Mastrofrancesco B. Role of ornithine as a proline precursor in healing wounds. J Surg Res. 1993;55(1):97–102. doi: 10.1006/jsre.1993.1114. [DOI] [PubMed] [Google Scholar]
- 86.Guillemin GJ, Brew BJ. Microglia, macrophages, perivascular macrophages, and pericytes: a review of function and identification. J Leukoc Biol. 2004;75(3):388–97. doi: 10.1189/jlb.0303114. [DOI] [PubMed] [Google Scholar]
- 87.Guillemin GJ. Microglial activation. In: Microglia ANPP, editor. Functional Neuroanatomy. Vol. 4.1. ANPP; Paris: 2003. [Google Scholar]
- 88.Matsumura Y, Ananthaswamy H. Toxic effects of ultraviolet radiation on the skin. Toxicology and Applied Pharmacology. 2004;195:298–308. doi: 10.1016/j.taap.2003.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Harper J, et al. The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin dependant kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 90.Mullauer L, et al. Mutations in apoptosis genes: a pathogenic factor for human disease. Mutation Research. 2001;488:299–31. doi: 10.1016/s1383-5742(01)00057-6. [DOI] [PubMed] [Google Scholar]
- 91.Katsan M, et al. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–97. doi: 10.1016/0092-8674(92)90593-2. [DOI] [PubMed] [Google Scholar]
- 92.Braidy N, Guillemin G, Grant R. Promotion of cellular NAD+ anabolism: Therapeutic potential for oxidative stress in ageing and Alzheimer’s disease. Neurotox Res. 2008;13:173–84. doi: 10.1007/BF03033501. [DOI] [PubMed] [Google Scholar]
- 93.Burkle A. Poly(ADP-ribose). The most elaborate metabolite of NAD+ Febs J. 2005;272(18):4576–89. doi: 10.1111/j.1742-4658.2005.04864.x. [DOI] [PubMed] [Google Scholar]
- 94.Denu JM. Vitamins and aging: pathways to NAD+ synthesis. Cell. 2007;129(3):453–4. doi: 10.1016/j.cell.2007.04.023. [DOI] [PubMed] [Google Scholar]
- 95.di Lisa F, Ziegler M. Pathophysiological relevance of mitochondria in NAD+ metabolism. FEBS Lett. 2001;492:4–8. doi: 10.1016/s0014-5793(01)02198-6. [DOI] [PubMed] [Google Scholar]
- 96.Milne J, Denu JM. The Sirtuin family: Therapeutic targets to treat diseases of aging. Curr Pharm Des. 2008;12:11–7. doi: 10.1016/j.cbpa.2008.01.019. [DOI] [PubMed] [Google Scholar]
- 97.Vaziri H, et al. hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 2001;107:149–59. doi: 10.1016/s0092-8674(01)00527-x. [DOI] [PubMed] [Google Scholar]
- 98.Fujisawa H, et al. Effects of interferons on the production of interleukin- 6 and interleukin-8 in human keratinocytes. J Interferon Cytokine Res. 1997;17(6):347–53. doi: 10.1089/jir.1997.17.347. [DOI] [PubMed] [Google Scholar]
- 99.Li Y, Tredget EE, Ghahary A. Cell surface expression of MHC class I antigen is suppressed in indoleamine 2,3-dioxygenase genetically modified keratinocytes: implications in allogeneic skin substitute engraftment. Hum Immunol. 2004;65(2):114–23. doi: 10.1016/j.humimm.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 100.Maruyama K, et al. Regulatory effects of gamma-interferon on IL-6 and IL-8 secretion by cultured human keratinocytes and dermal fibroblasts. J Dermatol. 1995;22(12):901–6. doi: 10.1111/j.1346-8138.1995.tb03942.x. [DOI] [PubMed] [Google Scholar]
- 101.Chiarugi A, Rapizzi E, Moroni F. The kynurenine metabolic pathway in the eye: studies on 3-hydroxykynurenine, a putative cataractogenic compound. FEBS Lett. 1999;453(1–2):197–200. doi: 10.1016/s0014-5793(99)00724-3. [DOI] [PubMed] [Google Scholar]
- 102.Heyes MP, Quearry BJ. Increased L-tryptophan, 5-hydroxyindoleacetic acid, 3-hydroxykynurenine and quinolinic acid concentrations in cerebral cortex following systemic endotoxin administration. Adv Exp Med Biol. 1991;294:559–62. doi: 10.1007/978-1-4684-5952-4_66. [DOI] [PubMed] [Google Scholar]
- 103.Wang L, et al. Expression profiling of fibroblasts identifies cell cycle abnormalities in schizophrenia. J Proteome Res. 2010;9:521–7. doi: 10.1021/pr900867x. [DOI] [PubMed] [Google Scholar]
- 104.Persson M, et al. Aberrant amino acid transport in fibroblasts from patients with bipolar disorder. Neurosci Lett. 2009;457:49–52. doi: 10.1016/j.neulet.2009.03.095. [DOI] [PubMed] [Google Scholar]
- 105.Fernell E, et al. Aberrant amino acid transport in fibroblasts from children with autism. Neurosci Lett. 2007;418:82–6. doi: 10.1016/j.neulet.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 106.Flyckt L, et al. Aberrant tyrosine transport across the cell membrane in patients with schizophrenia. Arch Gen Psychiatry. 2007;58:953–8. doi: 10.1001/archpsyc.58.10.953. [DOI] [PubMed] [Google Scholar]