Abstract
Introduction
Typical spike-and-wave activity (TSWA) in the electroencephalogram (EEG) indicates idiopathic generalized epilepsy (IGE). IGE-related nonconvulsive status epilepticus (NCSE) is typically an absence status epilepticus (ASE). ASE and TSWA respond dramatically to benzodiazepines. Patients with no history of seizure/epilepsy may develop ASE “de novo” in the context of an acute brain disorder. However, we are aware of only one previous case of de novo ASE with TSWA in hypoxic-ischemic brain injury.
Case presentation
A 65-year-old man, with congestive heart failure and history of substance abuse, survived cardiorespiratory arrest after 18 minutes of cardiopulmonary resuscitation. Post-resuscitation, the patient was in coma with intact brainstem function. Toxicology was positive for cocaine and marijuana. Eyelid myoclonus suggested NCSE, which was initially treated with lorazepam and fosphenytoin. EEG monitoring showed sustained TSWA confirming NCSE and demonstrating de novo ASE (the patient and his family never had seizure/epilepsy). The TSWA was resistant to lorazepam, levetiracetam, and low-dose midazolam; it was eliminated only with midazolam at a dose that resulted in burst-suppression (≥1.2 mg/kg/hour).
Conclusion
This is an unusual case of TSWA and hypoxic-ischemic brain injury in a patient with no history of seizure/epilepsy. The TSWA was relatively resistant to benzodiazepines suggesting that cerebral hypoxia-ischemia spared the thalamocortical apparatus generating TSWA but impaired the cortical/thalamic inhibitory circuits where benzodiazepines act to suppress TSWA. Albeit rare, ‘post-hypoxic’ TSWA offers us some valuable insights for classifying and managing nonconvulsive status epilepticus.
Keywords: EEG, epileptiform, typical spike-and-wave activity, nonconvulsive status epilepticus, de novo absence status epilepticus, hypoxic-ischemic brain injury
Introduction
Patients with absence epilepsy or other idiopathic generalized epilepsy (IGE) syndromes manifest 2.5 Hz to 3.5 Hz (3 Hz on average) bisynchronous typical spike-and-wave activity (TSWA) in the interictal electroencephalogram (EEG) and during typical absence seizures.1 Nonconvulsive status epilepticus (NCSE) consists of sustained rhythmic/periodic epileptiforma activity in the EEG, along with alterations in mental status and/or behavior, in the absence of convulsive movements. There are other definitions of NCSE, but no definition (including ours) is universally accepted. A wide range of cognitive-behavioral changes occurs in NCSE, including mild inattention, confusion, stupor, and coma. An even wider range of EEG patterns is seen in NCSE, but many of these patterns are not conclusively epileptiform.2 NCSE is traditionally divided into complex partial status epilepticus (CPSE)3 and absence status epilepticus (ASE).4
Diagnosing NCSE can be difficult in the presence of coma.5,6 First, the EEG in coma may show a typical CPSE/ASE pattern even though the clinical course and response to treatment are not compatible with CPSE/ASE. Second, the EEG in coma may show epileptiform abnormalities that defy classification into CPSE or ASE. Third, the EEG in coma may show abnormalities that cannot be easily labeled as “epileptiform”. IGE-related NCSE usually takes the form of absence status epilepticus.4 Some adults with no history of seizure/epilepsy develop ASE “de novo” in the context of an acute brain disorder.4 The acute brain disorder in de novo ASE is often an encephalopathy from drug overdose/withdrawal, fluid/electrolyte imbalance, or systemic infections.7 However, de novo ASE is not routinely reported in hypoxic-ischemic encephalopathyb; we are aware of only one previous case of de novo ASE with TSWA in hypoxic-ischemic brain injury.8
We present an unusual case of hypoxic-ischemic brain injury in which the patient’s EEG showed benzodiazepine-resistant TSWA even though the patient had no past or family history of seizure or epilepsy. After presenting the case, we examine the pathophysiology of post-hypoxic TSWAc and its implications for classifying and managing NCSE.
Case Presentation
The patient, a 65-year-old man with congestive heart failure (left ventricular ejection fraction of 20%) suffered cardiorespiratory arrest resulting in hypoxic-ischemic brain injury. The history is also significant for hypertension, kidney disease, hepatitis C, and substance abuse with cocaine and marijuana. Home medications include digoxin, furosemide, spironolactone, carvedilol, lisinopril, aspirin, potassium chloride, and folic acid. Neither he nor his family had a history of seizure or epilepsy. The patient was found unconscious, apneic, asystolic, and pulseless on a street in his neighborhood. Emergency responders arrived within 15 minutes and performed cardiopulmonary resuscitation (CPR), including defibrillation, intubation, and artificial ventilation. Cardiorespiratory function was re-established after 18 minutes of CPR. Amiodarone was started and bradycardia was treated with pacing and norepinephrine. The patient arrived in the emergency room with a Glascow coma scale of 3; dopamine was started.
On admission to the intensive care unit (ICU), the patient was in coma with intact brainstem function and stable vital signs (RR = 14, HR = 73, BP = 100/70, T = 36 °C). He was maintained on mechanical ventilation without induction of hypothermia. Brain CT scan showed mild diffuse atrophy, chronic microvascular changes, and no acute lesions. Blood tests showed normal white cell count, low hemoglobin (11.5 g/dL), elevated serum glucose (255 mg/dL), normal blood urea, nitrogen and creatinine, and normal serum sodium, potassium, and calcium. Toxicology was positive for cocaine and marijuana, but not ethanol. Ventricular fibrillation occurred 21 hours after the first hypoxic-ischemic event; CPR was performed for 15 minutes. Post-resuscitation, the patient manifested eyelid myoclonus occurring spontaneously every 3 to 12 seconds without any clear relationship to external stimulation; this is most likely a form of acute posthypoxic myoclonus. Intravenous lorazepam was given: 7-mg over 7 hours (first 4-mg, then 2-mg, then 1-mg) resulting in cessation of eyelid myoclonus. More lorazepam (10-mg) was infused over the next 7 hours (0.01 mg/kg/hour over 4 hours, then 0.02 mg/kg/hour over 3 hours). Lorazepam was discontinued and fosphenytoin 1400-mg was loaded. Serum phenytoin was 18.6 μg/mL 10 hours after the loading dose and 18.0 μg/mL 38 hours later even though fosphenytoin was discontinued before the patient received any maintenance dose.
EEG monitoringd was initiated 23 hours after administering fosphenytoin and discontinuing lorazepam; at that time the patient was in deep coma with no involuntary movements. The EEG showed sustained typical spike-and-wave activity (TSWA) confirming NCSE and indicating typical absence status epilepticus (Fig. 1). Intravenous lorazepam was given: 2-mg initially and 2-mg more after 5 minutes. Intravenous levetiracetam 500-mg was also given. The EEG continued to show TSWA despite this treatment. Intravenous midazolam was started: 0.2 mg/kg bolus and 0.05 mg/kg/hour infusion. TSWA persisted so another 0.2 mg/kg bolus was given 2.5 hours after the first bolus. The EEG continued to show TSWA, albeit with somewhat lower discharge frequency (Fig. 2). The infusion rate was increased and at 0.5 mg/kg/hour the EEG showed intermittent suppression of spike-and-wave discharges (Fig. 3). The midazolam infusion rate was further increased to achieve EEG burst-suppression. At a rate of 1.2 mg/kg/hour, TSWA disappeared and was replaced by burst-suppression. Stable burst-suppression (6–12 bursts/minute) was achieved with an infusion rate of 1.4 to 1.6 mg/kg/hour. The patient’s family requested withdrawal of life support 72 hours after admission and the patient died the following day.
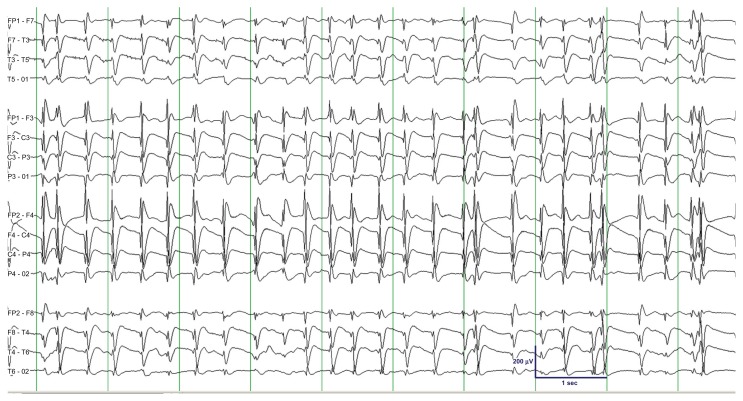
Figure 1.
Initial EEG showing sustained typical spike-and-wave activity (TSWA) with 3 Hz average frequency, uniform morphology, bilateral synchrony, bilateral symmetry, widespread distribution, and bifrontal voltage maximum.
Notes: Clinically, the patient was in coma with no voluntary movements. The epoch shown was recorded approximately 53 hours after the first hypoxic-ischemic event, 32 hours after the episode of ventricular fibrillation in the ICU, and 23 hours after loading fosphenytoin and discontinuing lorazepam. TSWA persisted after lorazepam 4-mg and levetiracetam 500-mg was given intravenously.
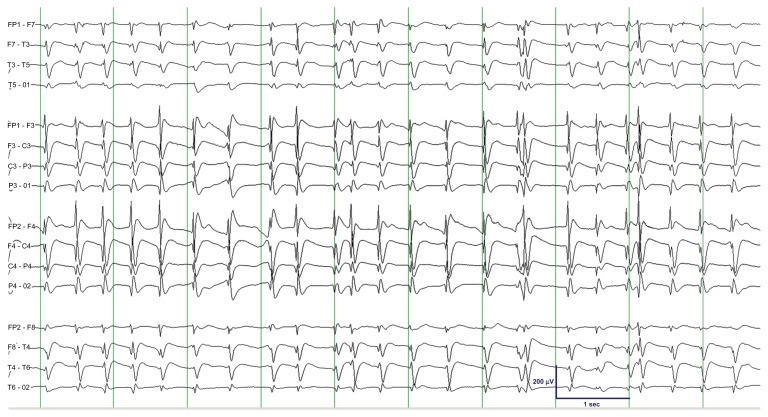
Figure 2.
EEG showing persistent TSWA with somewhat lower average frequency (compare with Fig. 1).
Note: The epoch shown was recorded approximately 10 minutes after the patient received two boluses of midazolam 0.2 mg/kg and initiation of midazolam drip at a rate of 0.05 mg/kg/hour.
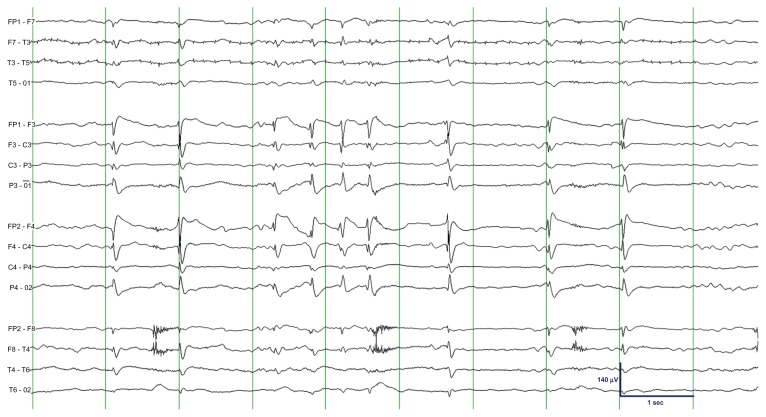
Figure 3.
EEG showing “fragmentation” of TSWA due to intermittent suppression of spike-and-wave activity.
Note: The epoch shown was recorded approximately 10 minutes after the midazolam infusion rate was increased to 0.5 mg/kg/hour. Midazolam was further titrated; an infusion rate of 1.2 mg/kg/hour resulted in disappearance of TSWA and appearance of burst-suppression in the EEG (not shown).
Discussion
Our patient suffered cardiorespiratory arrest resulting in hypoxic-ischemic brain injury. EEG showed typical spike-and-wave activity (TSWA) even though he had no history of seizure or epilepsy. Non-convulsive status epilepticus (NCSE) was diagnosed and intravenous lorazepam and midazolam was administered. The TSWA proved to be relatively resistant to benzodiazepines, raising some important issues regarding the pathophysiology of post-hypoxic TSWA and providing some insight into how NCSE should be classified and managed.
Pathophysiology of TSWA
Typical spike-and-wave activity (TSWA) is the EEG hallmark of absence seizure. It is also a routine interictal EEG finding in absence epilepsy and other IGE syndromes.1 For TWSA to occur, the thalamocortical-corticothalamic network must be functionally intact and in a state where intrinsically bursting cortical pyramidal and thalamic neurons are mildly to moderately hyperpolarized and susceptible to producing high-frequency bursts of action potentials.9
Meeren and colleagues published a review of the theories on the pathophysiology of TSWA.9 This can be summarized as follows: (1) The centrencephalic theory posits a diffusely projecting pacemaker in the midline thalamus as the origin of TSWA. (2) The thalamic clock theory is a refinement of the centrencephalic theory; it localizes the abnormal pacemaker to the reticular thalamic nucleus. (3) The corticoreticular theory claims that TSWA is generated by the abnormal interaction of the cortex and the reticular system of the thalamus and brainstem. (4) The cortical theory considers cortical hyperexcitability as the primary defect; a hyperexcitable cortex transforms the normal rhythms of the thalamus into TSWA. (5) The cortical focus theory is a synthesis of the cortical and corticoreticular theories; it asserts that a cortical focus (the perioral region of the somatosensory cortex is a prime suspect) initiates the epileptic activity, spreads to other cortical areas, and entrains the thalamus producing a resonant circuitry for expressing TSWA. To these five theories, we add Blumenfeld’s “comprehensive” theory which argues that TSWA can be provoked by different insults acting on different excitatory/inhibitory circuits.10
It is likely that absence status epilepticus (ASE) shares the basic mechanisms of absence seizure. Whether ictogenesis results in a self-limited absence seizure or in ASE depends on the brain’s seizure terminating mechanisms; such mechanisms are likely impaired in ASE.11 Patients with no history of IGE can develop ASE “de novo” in the context of an acute brain disorder.4 Pro and colleagues reported a patient with late-onset de novo ASE whose EEG consistently showed interictal TSWA over a 14-year period.12 Their case indicates that, in some individuals with “latent IGE”, exposure of the brain to toxic or metabolic factors can trigger a seizure later in life.
What does this case imply about post-hypoxic TSWA/ASE? The fact that post-hypoxic TSWA can look exactly like IGE-related TSWA implies that different insults acting on different brain circuits can “turn on” the basic mechanisms that lead to TSWA (see Blumenfeld’s theory above).10 This case also implies that extensive hypoxic-ischemic brain injury can spare the thalamocortical-corticothalamic network responsible for generating TSWA/absence seizure.9 As stated above, a genetic predisposition to develop TSWA/ASE may be the reason our patient manifested TSWA.12 It is also possible that all individuals have an inherent ability to produce TSWA and that, in our patient, the pattern of cerebral hypoxic-ischemic injury has “released” such ability.10 The patient’s eye-lid movements occurred repeatedly at irregular intervals within a 7-hour period and stopped after a total of 7-mg of lorazepam was given. These movements are consistent with post-hypoxic myoclonus and not with the more periodic eye-blink automatism seen in absence seizure. It should also be emphasized that eyelid movements were not present when TSWA was detected and that TSWA is not a known EEG correlate of post-hypoxic myoclonic status epilepticus; hence myoclonic status epilepticus would not be an appropriate diagnosis for our patient.
Did cocaine and/or marijuana interact with hypoxic-ischemic encephalopathy to trigger TSWA? The patient has a history of substance abuse and he tested positive for cocaine and marijuana. It can be hypothesized that these substances contributed to TSWA genesis. However, most drug-related de novo ASE cases in the literature were linked to withdrawal of benzodiazepine (BDZ) or to intoxication with alcohol or psychotropic drugs.4,7 Alcohol was not detected in the patient’s blood and we are not aware of any reports in which cocaine/marijuana was linked to de novo ASE. Still, this explanation cannot be ruled out.
Did fosphenytoin and/or lorazepam play a role in precipitating TSWA? The patient received a 1440-mg loading dose of fosphenytoin (a phenytoin prodrug) 23 hours prior to the onset of EEG monitoring. In the setting of IGE, phenytoin (and other antiepileptic drugs) can aggravate seizures13 and can even result in refractory ASE.14 However, we are not aware of any report of phenytoin/fosphenytoin causing ASE in patients with no IGE. The patient also received 17-mg of lorazepam (a BDZ and GABA-A receptor agonist) 23 to 37 hours before the onset of EEG recording. Lorazepam may have triggered TSWA directly or by means of a “withdrawal” mechanism. However, the absence of a history of chronic BDZ use makes it unlikely that the patient went through a BDZ withdrawal state. Enhanced GABA-A inhibition in the thalamus can trigger absence seizures in genetic and pharmacological models of absence epilepsy.15 However, we are not aware of any report of lorazepam causing ASE in patients with no IGE.
What is the significance of benzodiazepine-resistance in TSWA/ASE? As a rule, IGE-related ASE responds rapidly and completely to BDZ therapy.4,11 This is in line with the ability of GABA-A receptors to mediate the inhibition of the thalamic “pacemaker” of TSWA.15 A few cases of refractory ASE were described in the literature but all of these patients had IGE and were receiving phenytoin or carbamazepine.13 The rarity of post-hypoxic TSWA makes it much more difficult to find any report of BDZ-resistant post-hypoxic TSWA. We initially hypothesized that resistance indicates a difference in the mechanisms of post-hypoxic TSWA and IGE-related TSWA.16 This is not necessarily the case and the basic mechanism of TSWA genesis could still be the same, only that cerebral hypoxia-ischemia resulted in impairment of neural circuits where BDZ act to suppress TSWA. For example, impairment of GABA inhibitory circuits in the cerebral cortex can increase the chance of cortical ictogenesis, thalamic entrainment, and TSWA generation (see cortical focus theory).9
Classification of NCSE
In classifying seizures, the International League against Epilepsy (ILAE) made no formal reference to non-convulsive status epilepticus (NCSE).17–19 The 1981 ILAE seizure classification contains three diagnostic entities that qualify as NCSE – aura continua, complex partial status epilepticus, and absence status epilepticus (ASE).17 Subtle status epilepticus was added in the 2006 Report of the ILAE Classification Core Group bringing the number of NCSE seizures to four.18 In our patient, the finding of TSWA indicated ASE (more specifically typical ASE) but treatment response (BDZ-resistance) and clinical outcome (the patient died) are diametrically opposite to that of ASE (especially typical ASE). Such an “electroclinical paradox” is not uncommon in critically ill patients with NCSE. Unfortunately, the 1981 and 2006 seizure classifications ignore this issue by omitting the many variations of NCSE, especially NCSE in coma.5,6 The 2010 ILAE seizure classification also fails to address this issue.19 What’s more, the ILAE has completely excluded status epilepticus and focal seizures from the 2010 classification.20
The fact that NCSE is more common than previously thought underscores the urgency to create a better classification system for status epilepticus and NCSE. Recent studies have shown that NCSE constitutes 25%–50% of all cases of status epilepticus and the incidence of NCSE in the critically ill can be as high as 50%.6 Excluding some NCSE subtypes from the seizure classification system on “philosophical” grounds is disadvantageous to the patient, the physician, and the researcher.19 While a seizure classification system cannot be perfect at the outset, it should be heuristic and include all phenomena considered as NCSE, including the NCSE variations in comatose patients. Only a heuristic seizure classification system will result in better understanding and rational treatment of NCSE.
According to Bauer and Trinka, the confusion vis-à-vis NCSE in coma can be overcome by having a separate NCSE category—“comatose NCSE” or “coma-NCSE”.5 The authors classified CPSE and ASE as “NCSE proper”. NCSE proper has a good prognosis and, in most cases, the treatment of choice is “turning off” the seizure without aggressively anesthetizing the patient. Coma-NCSE is more ominous and its prognosis depends almost entirely on the underlying brain disorder.5 While there is no firm evidence that suppressing the seizure improves prognosis in coma-NCSE, anesthetizing the patient with midazolam or propofol may not be unreasonable if the patient is already on mechanical ventilation and is being monitored in the ICU. Maganti and colleagues proposed an etiology-based classification for NCSE, including NCSE in coma, NCSE in acute cerebral lesions, NCSE in metabolic disorders, and NCSE in those with pre-existing epilepsy.6 This more detailed approach may lend itself to better treatment recommendation, but most importantly better evaluation and testing of treatment recommendations to determine their value based on outcome.
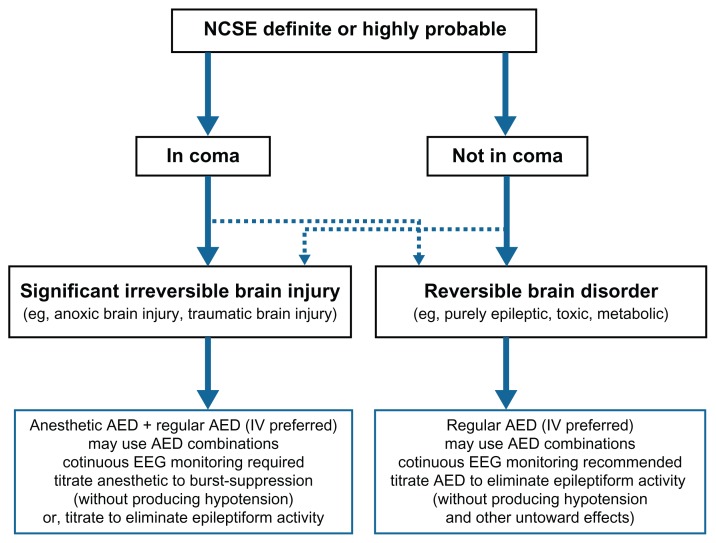
We agree with Bauer and Trinka that it is important to differentiate coma-NCSE from NCSE proper5 and with Maganti and colleagues that it is critical to account for etiology in classifying NCSE.6 By combining the Bauer/Trinka and Maganti proposals and adding some minor refinements, we were able to create a practical decision tree for NCSE (Fig. 4). As shown in this decision tree, as soon as NCSE is diagnosed (based on electroclinical data), the clinician should separate “coma NCSE” from “non-coma NCSE”. The next step is to differentiate between “NCSE with significant irreversible brain injury (SIBI)” and “NCSE with no evidence of SIBI”. Most “coma NCSE with SIBI” is due to hypoxic-ischemic, traumatic, ischemic, infectious, or inflammatory insults to the brain. Most “coma NCSE with no SIBI” is due to epileptic, toxic, or metabolic factors, often with the confounding effects of anesthetics or sedative drugs that are regularly employed in the ICU. Most “non-coma NCSE” is due to purely epileptic, toxic, or metabolic factors, the majority of which are reversible. Occasionally, SIBI of lesser severity may give rise to “non-coma NCSE”. Such a heuristic approach can facilitate our understanding of NCSE, especially NCSE in coma.
Figure 4.
Decision tree using a combination of Bauer and Trinka’s and Maganti’s suggested classification of NCSE.
Notes: A diagnosis of NCSE is made based on EEG, history, neurological exam and benzodiazepine-responsiveness (“Ativan Challenge”). The decision to treat aggressively and the end point of treatment are based primarily on two factors: (1) presence or absence of coma; and (2) the underlying brain disorder (this can be roughly categorized as irreversible and reversible). Dashed lines acknowledge that, on rare occasions, NCSE in coma may be due to a reversible condition (eg, severe metabolic encephalopathy) and NCSE without coma may be due to irreversible brain injury (eg, milder degrees or cerebral hypoxia-ischemia and traumatic brain injury).
While the incidence depends on how one defines NCSE, even more conservative estimates of the incidence of NCSE (eg, 25%) warrant a detailed analysis of NCSE cases with a goal toward increased understanding and better classification of NCSE. Further work is needed to better define NCSE, to determine which EEG patterns indicate NCSE, and to establish treatment paradigms for the different types of NCSE. A more collaborative approach across hospitals/universities is warranted given the sample size that would be needed to determine treatment efficacy in so many NCSE categories.
Conclusion
This is an unusual presentation of hypoxic-ischemic brain injury in that typical spike-and-wave activity (TSWA) appeared in the EEG even though the patient never had seizure/epilepsy. The TSWA was relatively resistant to benzodiazepines suggesting that cerebral hypoxia-ischemia spared the thalamocortical apparatus generating TSWA, but destroyed the inhibitory circuits where benzodiazepines act to suppress TSWA.
Post-hypoxic TSWA is rare; nevertheless, it highlights two important principles: First, the EEG allows us to diagnose NCSE but does not always tell us how to treat, or whether we should even treat, a particular NCSE pattern. Second, it is only by separating NCSE proper from comatose NCSE and taking into account the overall picture, including the presence of structural lesions, reversible causes, history of epilepsy, and prognosis that we can tailor NCSE treatment to the best interest of the patient.
Acknowledgment
We are grateful to the LSU Interim Public Hospital Critical Care, Pulmonary, Cardiology, and Internal Medicine staff and residents and to the Tulane University Neurology staff and residents for utilizing our EEG services and for implementing the recommendations we made in regards to the treatment of absence status epilepticus. We are also grateful to our EEG technologists for performing high-quality EEG recording in the ICU.
Footnotes
The term “epileptiform” describes a wave or a series of waves on the EEG tracing that corresponds directly to an epileptic event in the brain—either a seizure or an interictal epileptic event.
In this paper, “hypoxic-ischemic encephalopathy”, “hypoxic-ischemic brain injury”, and “anoxic brain injury” are considered synonymous.
The term “post-hypoxic TSWA” pertains to typical spike-and-wave activity that develops in the setting of hypoxic-ischemic brain injury.
The authors provided EEG services and recommendations for treating absence status epilepticus; other physicians were directly in charge of patient care.
Consent
The LSU Health Sciences Center Institutional Review Board (IRB) determined that this case presentation does not meet the definition of human subjects research and IRB approval is not required. The patient was in coma, did not recover, and died 72 hours after admission. Nothing in the presentation could lead to the patient’s identification. The article does not contain photographs or radiological images.
Authors Contributions
ECM, LSG, and SVK conceived the idea and prepared the manuscript. NRV and PWO provided a detailed review of the content and structure of the manuscript resulting in significant changes to the original document.
Funding
Author(s) disclose no funding sources.
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosure
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Mader EC, Jr, Olejniczak PW. Epilepsy Syndromes. In: Fisch BJ, editor. Epilepsy and Intensive Care Monitoring: Principles and Practice. Vol. 10. Demos Publishing; 2010. pp. 119–50. [Google Scholar]
- 2.Kaplan PW. The EEG of status epilepticus. J Clin Neurophysiol. 2006;23:221–9. doi: 10.1097/01.wnp.0000220837.99490.66. [DOI] [PubMed] [Google Scholar]
- 3.Heinz GW. Limbic Status Epilepticus. International League against Epilepsy. updated Jun 2003. http://www.ilae.org/Visitors/Centre/ctf/psychomotor_status.cfm.
- 4.Panayiotopoulos CP. Absence status epilepticus. International League against Epilepsy. updated Jan 18, 2005. http://www.ilae.org/Visitors/Centre/ctf/absence_status.cfm.
- 5.Bauer G, Trinka E. Nonconvulsive status epilepticus and coma. Epilepsia. 2009;1:1–14. doi: 10.1111/j.1528-1167.2009.02297.x. [DOI] [PubMed] [Google Scholar]; Epilepsia. 2010 Feb;51(2):177–90. doi: 10.1111/j.1528-1167.2009.02297.x. [DOI] [PubMed] [Google Scholar]
- 6.Maganti R, Gerber P, Drees C, Chung S. Nonconvulsive status epilepticus. Epilepsy Behav. 2008 May;12(4):572–86. doi: 10.1016/j.yebeh.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 7.Thomas P, Beaumanoir A, Genton P, Dolisi C, Chatel M. ‘De novo’ absence status of late onset: report of 11 cases. Neurology. 1992 Jan;42(1):104–10. doi: 10.1212/wnl.42.1.104. [DOI] [PubMed] [Google Scholar]
- 8.Ganji S, Palliyath S, Chemmale J, et al. Periodic complexes, absence-like status and suppression-burst pattern in coma following cardiorespiratory arrest. Clin Electroencephalography. 1997;28(2):76–86. doi: 10.1177/155005949702800205. [DOI] [PubMed] [Google Scholar]
- 9.Meeren H, van Luijtelaar G, Lopes da Silva F, Coenen A. Evolving concepts on the pathophysiology of absence seizures: the cortical focus theory. Arch Neurol. 2005 Mar;62(3):371–6. doi: 10.1001/archneur.62.3.371. [DOI] [PubMed] [Google Scholar]
- 10.Blumenfeld H. Cellular and network mechanisms of spike-wave seizures. Epilepsia. 2005;46(Suppl 9):21–33. doi: 10.1111/j.1528-1167.2005.00311.x. [DOI] [PubMed] [Google Scholar]
- 11.Shorvon S, Walker M. Status epilepticus in idiopathic generalized epilepsy. Epilepsia. 2005;46(S9):73–9. doi: 10.1111/j.1528-1167.2005.00316.x. [DOI] [PubMed] [Google Scholar]
- 12.Pro S, Vicenzini E, Randi F, Pulitano P, Mecarelli O. Idiopathic late-onset absence status epilepticus: a case report with an electroclinical 14 years follow-up. Seizure. 2011 Oct;20(8):655–8. doi: 10.1016/j.seizure.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Somerville ER. Some treatments cause seizure aggravation in idiopathic epilepsies (especially absence epilepsy) Epilepsia. 2009 Sep;50(Suppl 8):31–6. doi: 10.1111/j.1528-1167.2009.02233.x. [DOI] [PubMed] [Google Scholar]
- 14.Osorio I, Reed R, Peltzer J. Refractory idiopathic absence status epilepticus: a probable paradoxical effect of phenytoin and carbamazepine. Epilepsia. 2000;41:887–94. doi: 10.1111/j.1528-1157.2000.tb00258.x. [DOI] [PubMed] [Google Scholar]
- 15.Crunelli V, Cope DW, Terry JR. Transition to absence seizures and the role of GABA(A) receptors. Epilepsy Res. 2011 Dec;97(3):283–9. doi: 10.1016/j.eplepsyres.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kashirny S, Santana-Gould L, Mader EC, Olejniczak P. Benzodiazepine-resistant typical spike-and-waves in anoxic brain injury. Poster Presentation in the Annual Meeting of the American Clinical Neurophysiology Society; San Diego, CA. Feb 2010. [Google Scholar]
- 17.Commission on Classification and Terminology of the International League against Epilepsy. Proposal for revised clinical and electroencephalographic classification of epileptic seizures. Epilepsia. 1981;22:489–501. doi: 10.1111/j.1528-1157.1981.tb06159.x. [DOI] [PubMed] [Google Scholar]
- 18.Engel J. Report of the ILAE Classification Core Group. Epilepsia. 2006;47:1558–68. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- 19.Berg AT, Berkovic SF, Brodie MJ, et al. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005–2009. Epilepsia. 2010 Apr;51(4):676–85. doi: 10.1111/j.1528-1167.2010.02522.x. [DOI] [PubMed] [Google Scholar]
- 20.Panayiotopoulos CP. The new ILAE report on terminology and concepts for organization of epileptic seizures: a clinician’s critical view and contribution. Epilepsia. 2011 Dec;52(12):2155–60. doi: 10.1111/j.1528-1167.2011.03288.x. [DOI] [PubMed] [Google Scholar]