Abstract
Complement receptor one (CR1) is essential for removing circulating immune complexes (CIC), with malaria infection contributing to the formation of large amounts of CIC. We investigated CIC levels in children with malaria, of varying severity and seasonality. Two hundred age and sex-matched severe and mild malaria cases were studied during and after active disease. Pediatric controls had increased CIC levels (mean = 32 μg mEq/mL) compared to adult controls (mean = 26.9 μg mEq/mL). The highest levels of CIC were reported in severe malaria (mean = 39 μg mEq/mL). Higher levels of CIC were recorded in younger children and those with low E-CR1 copy numbers. Our data suggest that low levels of E-CR1 copy numbers, found in children with severe malaria, may adversely affect the ability to remove IC. Furthermore, the high background for circulating immune complex imply that Malian children are under constant assault by other pathogens that evoke a strong immune response.
Keywords: complement receptor, immune complex, malaria
Introduction
Complement receptor type one (CR1) is a membrane-bound glycoprotein expressed on most human peripheral blood cells as well as organs.1 Because erythrocytes are present in peripheral circulation at concentrations 103-fold higher than the peripheral blood mononuclear cells, they account for greater than 85% of CR1 in the blood. The CR1 gene resides at 1q32 and is comprised of 39 exons spread over ~133 kb of DNA.2 These exons encode regions called short consensus repeats (SCRs) of approximately 60 amino acids in the functional CR1 protein. Seven short consensus repeats are organized into larger units called long homologous repeats (LHR A, B, C and D). Four codominantly inherited alleles encode CR1 proteins of 190 kD, and these size differences suggest that concerted evolutionary events may have been responsible for duplications or deletions along the repeated sequence of this gene.3 We reported previously that several mutations of the CR1 gene have evolved that are either unique to Africans or appear with high frequency in Africa4–6 and these mutations may be contributing to the harmful consequences of malaria infection7 by impeding the ability of the innate immune system to remove potentially harmful ICs.
Primates clear immune complexes (IC) from their circulation by a unique pathway called immune adherence. In humans, IC clearance is mediated by CR1 that is expressed on a variety of blood cells. Marked reduction of either serum complement levels or the numbers of CR1 on erythrocytes is associated with impaired IC clearance and its deposition at inappropriate sites eg, the kidneys. We hypothesized that the previously identified CR1 polymorphisms adversely affect IC removal. This study was designed to determine the levels of circulating immune complex in the malaria disease and control state, determine the effect, if any, of erythrocyte CR1 expression and CR1 size variants on IC processing, alongside possible contribution to disease pathogenesis.
Materials and Methods
Subjects
The subjects recruited for this study include 100 children with severe malaria, 100 mild malaria cases, 100 pediatric healthy controls, and 100 random adults. The later were compared to 100 healthy Caucasians from the United States. The children diagnosed with malaria were tested during active disease and ~6 months later. The Malian adult and pediatric populations are similarly and equally exposed to malaria infectious assault. In addition, the protocols under which these samples were obtained received approvals from the respective institutional review boards.
Sample collection
Children aged between 3 months and 9 years presenting with severe malaria, according to World Health Organization criteria were recruited. Inclusion criteria included age, microscopic confirmation of asexual P. falciparum infection, and any of the following: cerebral malaria, Blantyre coma score of ≤2, repeated (3 or more per 24 hours) or 2 witnessed seizures in 24 hours, severe anemia hemoglobin < 7 gm/dL, respiratory distress, hypoglycemia (blood glucose < 40 mg/dL), renal failure and hyperparasitemia (>105 asexual forms/μL) and hyperpyrexia. Exclusion criteria include coexisting severe or chronic medical conditions unrelated to malarial infection. Following informed consent, 5–10 mL of venous blood was drawn into EDTA tubes and genomic DNA extracted, using Puregene DNA extraction kit, according to manufacturer’s instructions for other genomic studies (Gentra Systems, Minneapolis, MN). Serum was stored in −80 °C freezer until further analysis.
Quantification of erythrocyte CR1
Erythrocyte CR1 copy number was quantified using our previously described ELISA method.8 Briefly, erythrocyte ghosts are prepared using hypotonic lysis in the presence of protease inhibitors and the proteins solubilized in 1%NP40/PBS. CR1 copy number was determined using J3D3 for the capture antibody and E11 as the detection antibody. The binding site for E11 has previously been shown to reside in a non-duplicated region of CR1 and is not affected by the CR1 size or blood group polymorphisms. The plates were read spectrophotometrically at 450 nm and a standard curve was prepared for each assay from which test values were interpolated. A previously studied donor9 served as standard.
Determination of CR1 molecular weight
CR1 molecular weight was determined using a published Western blot method.10 A portion of the erythrocyte ghosts prepared for the ELISA is solubilized in SDS loading buffer and the proteins separated using non-reducing SDS-PAGE with a 3% stacking gel and a 5% resolving gel. The proteins are electrophoretically transferred to nylon membranes and blocked in 5% milk/PBS. The blots are washed and incubated with the anti-CR1 monoclonal antibody J3D3. The blot is washed again and incubated with sheep anti-mouse conjugated with horseradish-peroxidase. Following a final wash, the proteins are visualized using chemiluminescense (ECL, Amersham Pharmacia Biotech). Molecular weight determinations are made in comparison to known standards.
Measurement of circulating immune complex (IC) levels
Circulating IC levels were measured in serum using the commercial MicroVue CIC-Raji Cell Replacement EIA kit (Quidel, San Diego, CA), following manufacturer’s instruction. This technique measure a neo-epitope on activated C3 and, thus, detects IC produced by any of the three complement activation pathways. The measurements were carried out on blood collected during both time points, presentation with active disease during the high transmission period (September) and six months later during the low transmission period (April). CIC measurements were carried out in duplicates and mean total CIC levels were recorded for individual subjects. Serum CIC measurement was repeated twice. We used Student t test for comparison of two groups, with the Caucasian control group, serving as the base for comparison. P values presented here were unadjusted.
Results and Discussion
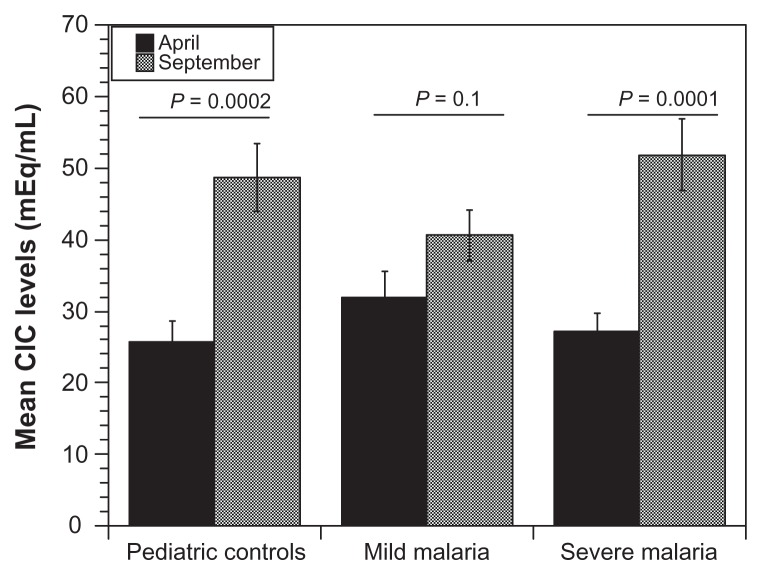
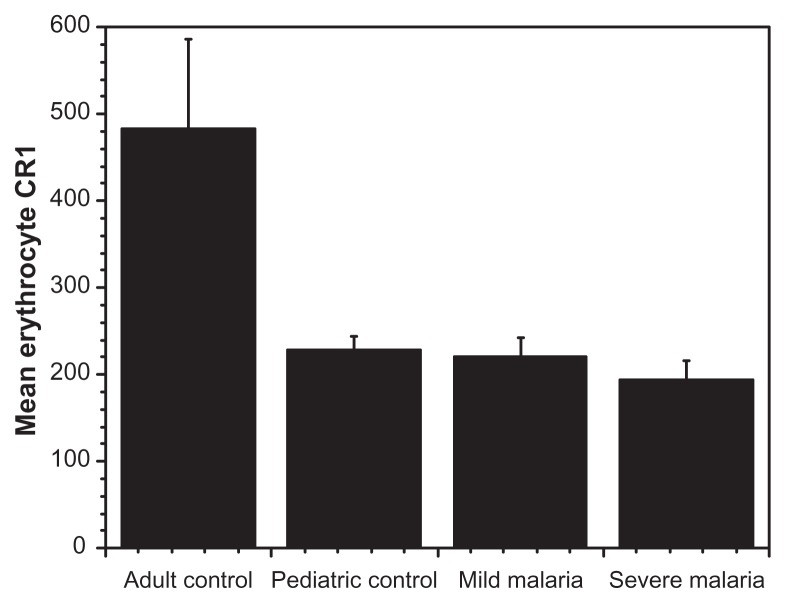
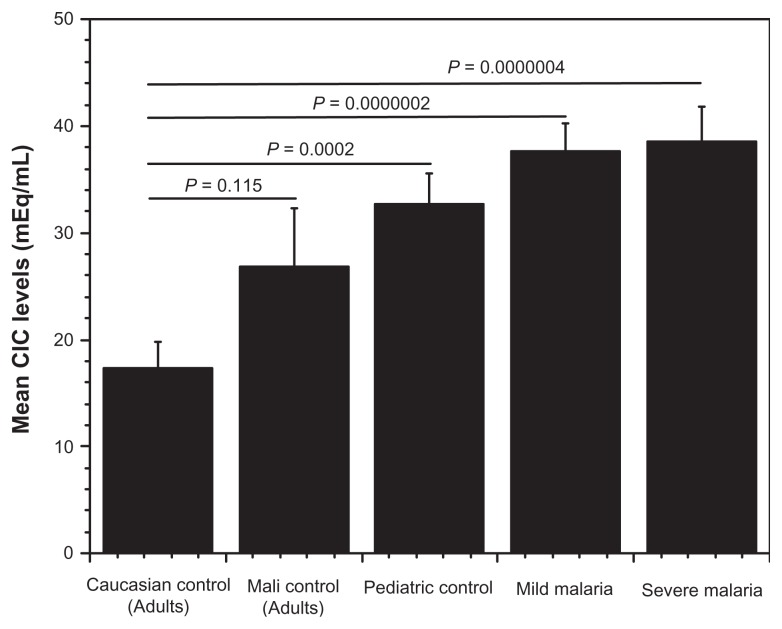
The levels of circulating immune complexes were measured among children presenting with varying degrees of malaria infection, healthy children as well as among healthy adult controls in Mali. Healthy Caucasian adults unexposed to malaria, served as baseline controls. The Caucasian adults had lower IC levels compared with healthy Malian adults. Pediatric controls had a higher CIC levels (mean 32 μg mEq/mL) when compared with healthy Malian adults (mean 26.9 μg mEq/mL). In the pediatric group, circulating IC levels correlated with disease severity. Children with severe malaria had the highest circulating IC levels (mean 39 μg mEq/mL) followed by those presenting with mild infection and the lowest in healthy pediatric controls (Fig. 1). Additionally, younger children had higher levels of circulating IC indicating a drop-off as age increases. Seasonally, we found higher CIC levels in all pediatric groups, during the high malaria transmission season (September recruitment) compared to the low transmission season (recruitment in April) (Fig. 2). In addition, we found no correlation between erythrocyte CR1 numbers and disease severity or sesonality. However, we did find a difference between adult controls and children, as expected (Fig. 3).
Figure 2.
Seasonal variation of circulating immune complex levels in malaria patients.
Notes: Figure reveals there is a significant difference in circulating immune complex levels between the rainy (September) and dry (April) seasons in all groups. The highest levels of CIC are during the dry season. The fact there are no clear disease differences in this figure speaks to the challenge of malaria transmission in the study area and the possibility of asymptomatic infection from numerous other pathogens.
Figure 3.
Mean erythrocyte CR1 levels and disease severity.
Notes: We found very high values for adult controls from Mali (as expected) and lower but comparable values for all disease groups. Erythrocyte CR1 levels are generally low for children and increases with age, confirmed by the age group of the recruited subjects.
During malaria infection, antibodies can bind to parasite antigens released from ruptured erythrocytes, activate complement and form immune complexes. Formation of large, potentially harmful IC requires multivalent binding of complement components that can block antigen-antibody interactions, solubilizing the immune complexes in the process. Additionally, C1q, C3b, C4b or MBL incorporated into the IC can act as ligands for CR1 on circulating erythrocytes. Following ligand binding, CR1 acts as a cofactor for the Factor I mediated cleavage of bound C3b to iC3b, which has a high affinity for CR3. As the erythrocytes bearing IC-iC3b circulate through the liver and spleen, they are proteolytically cleaved and transferred to resident macrophage by binding to CR3 and Fcγ receptors (FcγR). This process causes some loss of E-CR1 so that patients having high levels of IC lose E-CR1 more rapidly and, thus, begin a vicious downward cycle. Low levels of the early complement components such as C4 or C3 can adversely affect the transfer/removal process allowing IC to remain in the circulation, bind to polymorphonuclear cells (PMNs) and induce respiratory bursts.11 In addition, an increased loss of erythrocyte CR1 due to the processing of large quantities of IC may result in low levels of erythrocyte CR1 and similar binding of ICs to polymorphonuclear leukocytes. This loss of erythrocyte “buffering capacity” has been shown to be inversely proportional to the erythrocyte CR1 copy number and directly related to disease activity in systemic lupus erythematosus patients12 and those with IC-mediated diseases. A similar situation may exist in the malaria patient. In severe malaria anemia (Hgb < 5.0 gm/dL) the total available CR1 (CR1 copy number/erythrocyte x number erythrocytes) is significantly reduced because of the acute reduction in red cell mass and this may further hinder IC removal. In addition, the presence of ICs has been postulated to contribute to “reactive or innocent bystander lysis” which may be another mechanism resulting in the severe anemia observed in some malaria patients.13
Investigations into malaria pathogenesis have focused on the stimulation of neutrophils to produce cytokines, nitric oxide and reactive oxygen radicals.14 Although this stimulation may be directly due to the toxins produced by the malaria parasites, we propose an additional and equally plausible mechanism. As part of the natural immune response to Plasmodium infection, innate immunity is engaged, and immunoglobulins produced in response to malarial antigens can further activate the classical pathway and deposit complement components on circulating immune complexes targeting them for removal by CR1 and Fc receptors (FcRs). Although this is a protective mechanism, overloading of the system results in hypocomplementemia and decreased E-CR1 numbers. Subsequently, ICs are not processed and remain in the circulation where they become deposited in internal organs causing renal failure or coma. Furthermore, IC bearing iC3b can now bind to CR3 on netrophils causing their stimulation and increased production of cytokines such as TNF-α.
Healthy control children had comparable circulating IC levels to African adults. On the contrary, circulating IC levels were elevated but statistically insignificant in Mali adults compared to Caucasians (P = 0.115). Circulating immune complex levels increased with disease severity (pediatric controls < mild malaria < severe malaria), with the highest levels recorded in children with severe malaria (mean = 39 μg mEq/mL), indicating erythrocyte CR1 removal system is overburdened. Low levels of circulating IC were measured in April with higher concentrations recorded in September. These observations followed severity patterns as observed in the general population. Circulating IC levels in April were comparable for all disease status with increases in September recordings. The high background for circulating immune complex levels suggests analyses of results from vaccine or therapeutic trials should be based on local epidemiological values. This is also indicative that these children are under constant assault by other pathogens that evoke a strong immune response.
Figure 1.
Mean frequency of circulating immune complex in malaria patients.
Notes: The lowest level of circulating immune complex (CIC) was recorded among Caucasian control adults, followed by asymptomatic adults from Mali. Interestingly, the level of circulating immune complex increase as disease becomes severe, with the highest level recorded among children with severe malaria. Though there is no significant difference between mild and severe malaria, disease clearly affects CIC levels. Even though there is no difference between Caucasian and Malian adult controls, the fact there is higher CIC levels in Mali indicates the possibility of asymptomatic disease status in this group. There is statistical significant difference between Caucasian adults and pediatric controls, mild and severe malaria groups (P < 0.05).
Acknowledgement
We thank Sue Riester of Somerset Laboratories, New York for providing materials from United States Caucasians. We are grateful to Chris Plowe, University of Maryland and Ogobara Doumbo of the University of Bamako, who facilitated material collection from Mali.
Footnotes
Author Contributions
Conceived and designed the experiments: BNT, GTN, DAD, JMM. Analyzed the data: BNT, JMM. Wrote the first draft of the manuscript: BNT. Contributed to the writing of the manuscript: JMM. Agree with manuscript results and conclusions: BNT, GTN, DAD, JMM. Jointly developed the structure and arguments for the paper: BNT, JMM. Made critical revisions and approved final version: BNT, JMM. All authors reviewed and approved of the final manuscript. BNT, GTN, DAD, JMM.
Funding
This project was funded with Faculty Education and Development Grant, Rochester Institute of Technology (BNT) and NIAID/NIH grant R01-AI- 42367 (JMM).
Competing Interests
Author(s) disclose no potential conflicts of interest.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Gasque P, Chan P, Mauger C, Schouft M-T, Singhrao S, Dierich MP, et al. Identification and characterization of complement C3 receptors on human astrocytes. J Immunol. 1996;156:2247–55. [PubMed] [Google Scholar]
- 2.Vik DP, Wong WW. Structure of the gene for the F allele of complement receptor type 1 and sequence of the coding region unique to the S allele. J Immunol. 1993;151:6214–24. [PubMed] [Google Scholar]
- 3.Wong WW, Farrell SA. Proposed structure of the F′ allotype of human CR1. Loss of a C3b binding site may be associated with altered function. J Immunol. 1991;146:656–62. [PubMed] [Google Scholar]
- 4.Thomas BN, Donvito B, Cockburn I, et al. A complement receptor-1 polymorphism with high frequency in malaria endemic regions of Asia but not Africa. Genes Immun. 2005;6:31–6. doi: 10.1038/sj.gene.6364150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moulds JM, Thomas BN, Doubmo O, et al. Identification of the Kna/Knb polymorphism and a method for Knops gentotyping. Transfusion. 2004;44:164–9. doi: 10.1111/j.1537-2995.2004.00615.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moulds JM, Zimmerman PA, Doubmo OK, Kassambara L, Sagara I, Diallo DA, et al. Molecular identification of Knops blood group polymorphisms found in long homologous region D of complement receptor 1. Blood. 2001;97:2879–8. doi: 10.1182/blood.v97.9.2879. [DOI] [PubMed] [Google Scholar]
- 7.Krych-Goldberg M, Moulds JM, Atkinson JP. Immune adherence receptor CD35 binds a major malarial adhesion. Trends Mol Med. 2002;8:531–7. doi: 10.1016/s1471-4914(02)02419-x. [DOI] [PubMed] [Google Scholar]
- 8.Moulds JM, Moulds JJ, Brown MC, Atkinson JP. Antiglobulin testing for CR1-related (Knops/McCoy/Swain-Langley/York) blood group antigens: negative and weak reactions are caused by variable expression of CR1. Vox Sang. 1992;62:230–5. doi: 10.1111/j.1423-0410.1992.tb01204.x. [DOI] [PubMed] [Google Scholar]
- 9.Moulds JM, Brai M, Cohen J, Cortelazzo A, Cuccia M, Lin M, et al. Reference typing report for complement receptor 1 (CR1) Exp Clin Immunogenet. 1998;15:291–4. doi: 10.1159/000019084. [DOI] [PubMed] [Google Scholar]
- 10.Moulds JM, Reveille JD, Arnett FC. Structural polymorphisms of complement receptor 1 (CR1) in systemic lupus erythematosus (SLE) patients and normal controls of three ethnic groups. Clin Exp Immunol. 1996;105:302–5. doi: 10.1046/j.1365-2249.1996.d01-748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schifferli JA, Ng YC, Paccaud JP, Walport MJ. The role of hypocomplementaemia and low erythrocyte complement receptor type 1 numbers in determining abnormal immune complex clearance in humans. Clin Exp Immunol. 1989;75:329–35. [PMC free article] [PubMed] [Google Scholar]
- 12.Huw LC, Beynon HLC, Davies KA, Haskard DO, Walport MJ. Erythrocyte complement receptor type 1 and interactions between immune complexes, neutrophils, and endothelium. J Immunol. 1994;153:3160–7. [PubMed] [Google Scholar]
- 13.Thompson RA, Lachmann PJ. Reactive lysis: the complement-mediated lysis of unsensitized cells. I. The characterization of the indicator factor and its identification as C7. J Exp Med. 1970;131:629–41. doi: 10.1084/jem.131.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark IA, Cowden WB. The pathophysiology of falciparum malaria. Pharmacology Therapeutics. 2003;99:221–60. doi: 10.1016/s0163-7258(03)00060-3. [DOI] [PubMed] [Google Scholar]