Abstract
Hemodynamics following Fontan surgery are notable for right atrial (RA) hypertension and dilation. The effect of this stress on atrial cytoarchitecture has not been studied systematically, and may be relevant to arrhythmias and their treatment. Morphologic and histopathologic analysis was performed on RA and left atrial (LA) tissue from post-mortem specimens of Fontan hearts (n = 47) and compared to control samples from young patients with normal atrial hemodynamics (n = 15). Most Fontan specimens were from young patients who expired after a relatively short duration of Fontan physiology. Tissues were analyzed for wall thickness, fibrosis content, and fibrosis pattern. Mean wall thickness for both RA (3.0±1.0 mm) and LA (2.3±0.6 mm) in Fontan hearts was significantly greater than in control hearts (RA = 1.8±0.4 mm and LA = 1.8±0.5 mm, p<0.001 and p=0.024, respectively). Predictors for RA thickening included: a) older age at Fontan surgery, b) older age at death, and c) longer duration of Fontan circulation. Fontan hearts and controls exhibited nearly identical RA and LA fibrosis patterns. Neither wall thickness nor fibrosis varied with underlying heart defect or style of Fontan connection. In conclusion, atrial remodeling after Fontan surgery for univentricular heart physiology involves increased wall thickness in both the RA and LA. Interstitial fibrosis was also observed in Fontan atria, but because a similar pattern was present in control tissue, this likely represents normal fibroelastic atrial structure rather than a specific response to Fontan hemodynamics. The degree of wall thickening observed in Fontan atria was not so excessive as to preclude transmural lesions during catheter or surgical ablation of reentrant arrhythmias.
Keywords: Congenital heart disease, Fontan operation, Histology, Pathology, Pediatrics, Single ventricle
Introduction
The modern surgical approach to a univentricular heart is the Fontan operation [1-4]. Although the procedure offers effective hemodynamic palliation for most patients, problematic atrial tachycardias can develop as a late complication [5-10]. Macroreentrant circuits account for the majority of atrial tachycardias after Fontan surgery. These circuits typically involve one or more discrete conduction corridors related to suture lines and other fixed conduction obstacles [11], but may be further conditioned by diffuse remodeling of atrial cytoarchitecture in response to elevated pressure and volume loads [12]. Atrial tissue response to the hemodynamic stress of the Fontan circulation has not been investigated systematically. The current study was thus undertaken to examine histopathologic features of the right atrium (RA) and left atrium (LA) in post-mortem specimens from patients who underwent Fontan repairs, and compare findings to tissue from young patients with normal atrial hemodynamics. These data may add to the understanding of atrial tachycardias and their treatment in the Fontan population.
Methods
Morphologic and histopathologic analysis was performed on RA and LA tissue from all post-mortem specimens of Fontan hearts (n = 47) housed in the Cardiac Registry at Children′s Hospital Boston under a protocol approved by the hospital’s Committee on Clinical Investigation. Background clinical data were abstracted from pathology records. All hearts were fixed and stored in 10% formalin, all measurements taken in fixed state. Atrial wall thickness was measured at three different free-wall sites (superior, lateral, inferior) in both the RA and LA by two independent observers, and a value for mean thickness was calculated for each atrium. Three small tissue samples were harvested from each atrium near the site where wall thickness was determined. Care was taken to avoid sampling near suture lines, patches, visible scar sites and certain natural atrial structures (e.g. crista terminalis and large pectinate muscles) that could bias thickness measurements and fibrosis analysis. Formalin-fixed tissue samples were embedded in paraffin, cut to 5 micrometer sections, and stained with Masson Trichrome. Computerized microscopic morphometry with the MetaMorph® Imaging System (Molecular Devices, Sunnyvale CA) was used to quantitate the percentage fibrosis in the examined sections. Fibrosis was further characterized according to qualitative pattern: interstitial (diffuse fibrosis occurring as a fine network between myocytes), focal (broad areas of discrete fibrosis between myocytes), or mixed (combination of interstitial and focal). One observer who was blinded to the underlying cardiac diagnosis measured percentage fibrosis and determined fibrosis pattern in all samples.
Data from Fontan hearts were compared to 8 available post-mortem heart specimens in the Cardiac Registry from young patients who died of non-cardiac causes (age < 21 yrs). Similar measurements for wall thickness and fibrosis were performed in both the RA and LA for these control hearts.
Additional comparison was made between Fontan hearts and control RA samples taken intraoperatively from 7 patients (age <21 years) undergoing surgery for a diagnosis of isolated aortic stenosis. RA hemodynamics were normal according to pre-operative clinical assessment and studies have previously shown that RA myocardial cells of young patients with aortic stenosis have normal electrophysiological characteristics [13]. Approval for examination of surgical tissue was obtained prior to operation. RA thickness was measured, and single tissue samples were taken from atrial cannulation or atriotomy sites for microscopic analysis. No data were obtained for LA tissue in this surgical subgroup.
Data are expressed as frequency for nominal variables, and as mean ± SD for continuous variables. Differences between the two groups were assessed by unpaired Student’s t-test for variables with normal distribution. One-way ANOVA with Bonferroni post hoc tests was used to compare continuous variables in > 2 groups. Pearson’s correlation was used for determining possible association between continuous variables. A linear stepwise backward regression analysis was used to determine independent predictors, and the Pearson’s coefficient to determine correlation between two random variables. Data analysis was performed with SPSS for Windows (version 10).
Results
Age at the time of Fontan operation and the time of death, duration of Fontan physiology, anatomic diagnoses and Fontan technique for the 47 patients having undergone Fontan surgery are shown in Table 1. Patients with hypoplastic left heart syndrome and other forms of single right ventricle were the youngest to undergo Fontan surgery and the youngest at the time of death. Patients with tricuspid atresia were the oldest at the time of Fontan correction and had the longest survival.
Table 1.
Clinical features of Fontan hearts
| Male / female (number of patients) | 34 / 13 |
| Age at Fontan operation (years) | 5.9 ± 5.5 (median: 4, range: 0.4–23) |
| Age at Death (years) | 7.7 ± 9.2 (median: 4, range: 0.4–42) |
| Duration of Fontan physiology (years) | 1.73 ± 0.68 (median: 0.2, range: 0–20.2) |
|
| |
| Congenital Heart Disease | Number of patients (percent of total) |
| Tricuspid atresia | 10 (21 %) |
| Double outlet right ventricle | 12 (26 %) |
| Double inlet left ventricle | 5 (11 %) |
| Hypoplastic left heart syndrome | 7 (15 %) |
| Single right ventricle | 3 (6 %) |
| Single left ventricle | 7 (15 %) |
| Unbalanced AV canal defect | 3 (6 %) |
|
| |
| Fontan technique | |
| Right atrial – pulmonary artery connection | 25 (53 %) |
| Total cavopulmonary connection with intraatrial lateral tunnel | 12 (26 %) |
| Right atrial – right ventricular connection | 4 (8 %) |
| Superior vena cava – pulmonary artery connection (Kawashima operation) | 1 (2 %) |
| Other | 5 (11 %) |
| Intentional atrial fenestration | 2 (4 %) |
The diagnosis for the 8 post-mortem control hearts was diverse (leukemia, n = 3; neuroblastoma, n = 1; metabolic disease, n = 1; congenital agammaglobulinemia with sepsis, n =1; perforated ulcer, n = 1; and undetermined, n = 1), but none had a history of primary cardiac disease or cardiac surgery. Clinical evidence of right-sided heart failure had been present briefly before death in 2 cases, and bacteria were grown from 4 of the 8 hearts. The diagnosis for the 7 control RA specimens obtained intraoperatively from living patients included subaortic stenosis in 4, and valvar aortic stenosis in 3 patients. None of these 7 had undergone prior cardiac surgery. Mean age for the combined 15 controls at time of death or at the time of surgery was 8±7 yrs (median = 6.5 yrs, range 2 days - 21 yrs).
As shown in Table 2 and Figure 1, wall thickness for both RA and LA was significantly greater in Fontan hearts than in controls. In contrast, Fontan hearts and controls had nearly identical quantitative fibrosis scores for RA and LA muscle. Fibrosis occurred most commonly in a diffuse interstitial pattern for both controls and Fontan hearts (e.g. Figure 2A, 2B), with only a few examples of the focal (e.g. Figure 2C, 2D) or mixed patterns (e.g. Figure 2E, 2F). The percent fibrosis was not different between the RA and LA of Fontan hearts, while control hearts showed greater fibrosis in the LA compared to the RA. The percentage fibrosis did not correlate with measured atrial wall thickness. There were no differences between RA wall thickness and RA fibrosis quantification between the 8 post-mortem controls and the 7 intraoperative control specimens.
Table 2.
Atrial pathology of Fontan hearts versus control hearts
| RIGHT ATRIUM | p value | LEFT ATRIUM | p value | |||
|---|---|---|---|---|---|---|
|
Fontan (n=47) |
Controls* (n=15) |
Fontan (n=47) |
Controls* (n=8) |
|||
| Wall thickness (mm) | 3.0±1.0 (1.7-7.0) | 1.8±0.4 (1.0-2.5) | <0.001 | 2.3±0.6 (1.0-4.0) | 1.8±0.5 (1.0-2.3) | 0.024 |
| Fibrosis (%) | 21±10 (7.9-47.4) | 16±5 (9.3-23.9) | NS | 21±9 (6.8-43.2) | 28±8 (18.3-43.0) | NS |
|
|
||||||
| Fibrosis pattern§ | ||||||
| Interstitial | 36/40 | 8/13 | NS | 33/40 | 5/8 | NS |
| Focal | 1/40 | 0/13 | 4/40 | 1/8 | ||
| Mixed | 3/40 | 5/13 | 8/40 | 2/8 | ||
Control data available for 15 right atrial specimens, but only 8 left atrial specimens
Poor slide quality prevented accurate determination of fibrosis pattern for 7 Fontan and 2 control hearts
Figure 1. Examples of RA wall thickness.
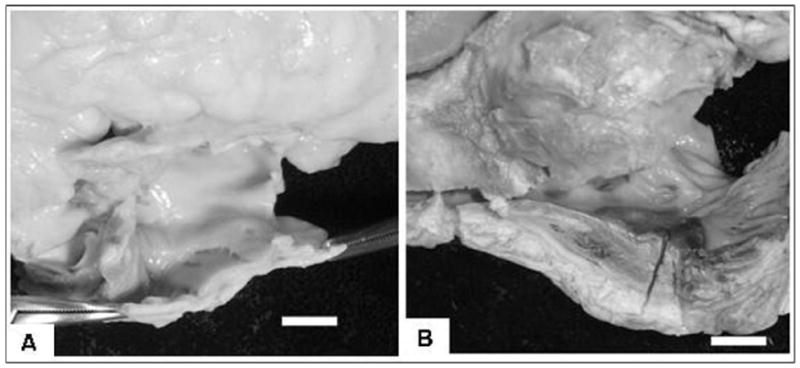
A) control patient: mean RA wall thickness = 1.6 mm; B) patient with tricuspid atresia: Fontan operation at age 9 years and death 3 months after surgery, mean RA thickness = 7.0 mm; (bar scale = 10 mm in both panels)
Figure 2. Examples of RA fibrosis.
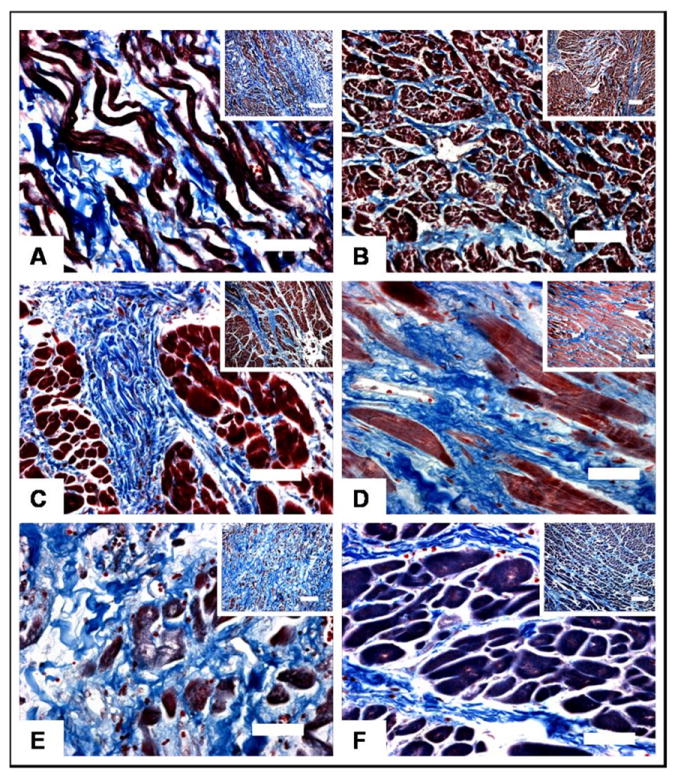
A) control patient with agammaglobulinemia who died of sepsis, RA fibrosis = 12.0%, interstitial pattern; B) patient with hypoplastic left heart syndrome who underwent RA-pulmonary artery Fontan at age 1 year and died in the early post-operative period, RA fibrosis = 23.7%, interstitial pattern; C) patient with double outlet right ventricle who underwent lateral tunnel Fontan at 3 years of age and died in the early post-operative period, RA fibrosis = 30.6%, focal pattern; D) patient with tricuspid atresia who underwent RA-right ventricle Fontan at 23 years of age and died after 19 years of Fontan physiology, RA fibrosis = 21.8%, focal pattern; E) patient with single left ventricle who underwent RA-pulmonary artery Fontan surgery at 18 years of age and died after 15 years of Fontan physiology, RA fibrosis = 43.9%, mixed pattern; F) patient with double outlet right ventricle who underwent lateral tunnel Fontan at 3 years of age and died 2 months after surgery, RA fibrosis = 45.5%, mixed pattern;
All panels shown at 400x magnification (bar scale = 50 micrometers) with broad-field inserts at 100x magnification; (bar scale = 200 micrometer)
Multivariate analysis was used to determine if severity of atrial tissue changes correlated with clinical features in Fontan hearts (Figure 3). With respect to wall thickness, independent predictors for RA thickening by linear regression analysis included: a) older age at time of Fontan surgery (p=0.024), b) older age at time of death (p=0.033), and c) longer duration of Fontan circulation (p=0.030). The only independent predictor for LA thickening in Fontan hearts was older age at time of death (p=0.008).
Figure 3. Independent predictors for RA wall thickening and the amount of RA fibrosis.
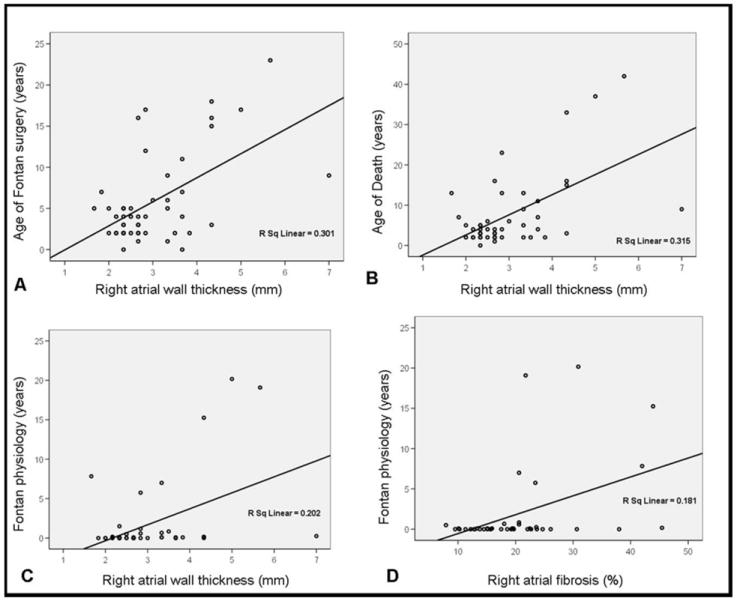
Scatter plots of RA wall thickness and age of Fontan surgery (A), age at the time of death (B) and Fontan duration (C), and of the amount of RA fibrosis and Fontan duration (D). R Sq Linear = R square (square of the correlation coefficient).
The only independent predictor for the extent of RA fibrosis was duration of Fontan physiology (p=0.007). The degree of RA fibrosis did not correlate with age at the time of Fontan surgery, as exemplified by specimens from patients who underwent Fontan correction at an age as young as age 3 years and exhibited the same amount of fibrosis as specimens from patients who were 15 years and older when Fontan surgery was performed (see Figures 2C vs. 2D). Likewise, age at the time of death did not directly influence RA fibrosis content, as exemplified by high levels of fibrosis in specimens from patients who expired as young as age 3 years (see Figure 2F). There were no independent predictors for the extent of fibrosis in the LA.
No difference was noted between the underlying anatomic diagnosis and either the degree of wall thickening or percent fibrosis in Fontan hearts. Likewise, there were no differences in wall thickening or fibrosis according to the subtype of surgical Fontan connection.
Discussion
As a consequence of the Fontan operation, atrial muscle becomes modified at both the macroscopic [14-16] and microscopic [17] levels. Suture lines and other conduction obstacles are involved in the macroreentrant circuits causing reentrant tachycardias seen in Fontan hearts [11, 18, 19], but the contribution of global atrial thickening and fibrosis is less clear. The magnitude of atrial remodeling in univentricular hearts after Fontan surgery as quantified by atrial wall thickness and fibrosis in post-mortem specimens was examined in the current study.
The data show that RA and LA wall thickening, and not fibrosis, was the dominant remodelling response of atrial tissue in univentricular hearts after the Fontan surgery. One heart had a mean RA wall thickness up to 7.0 mm (see Figure 1B), but overall the magnitude of atrial wall thickening was relatively modest being approximately one millimeter thicker than the atrial walls of control hearts. This finding might have practical implications for rhythm treatment in Fontan patients. A high recurrence rate after catheter ablation has been attributed to exaggerated tissue thickness [20], but the current data suggest that in the majority of cases especially after a relative short duration of Fontan physiology the average degree of wall thickening did not exceed the usual lesion dimensions achieved with radiofrequency energy or cryoablation.
Widespread interstitial fibrosis observed in Fontan hearts appeared to be a nonspecific finding, and likely reflects normal atrial structure since atrial muscle contains a prominent network of elastic fibers running from the epicardial to endocardial surfaces [21]. Control hearts exhibited a similar pattern for atrial fibrosis. Although Fontan hearts must endure the large discrete scars from an atriotomy and surgical patches, extensive fibrosis does not seem to be characteristic of tissue purposely sampled away from these regions of surgical injury.
There are obvious limitations to this study due to its retrospective nature and the restricted heart specimens available for analysis. Most critically the duration of Fontan physiology was < 1 year in the majority of Fontan hearts, so that the data are biased towards shorter-term consequences of Fontan surgery. In addition, the study was not designed to determine the precise cause of atrial tissue changes. Measurements were done in post-operative Fontan specimens only without comparison to pre-operative specimens, so that tissue changes could be intrinsic to the univentricular heart rather than a specific response to a Fontan circulation. Also, wall thickness measurements and tissues samples taken from 3 atrial sites might miss right atrial inhomogeneity. Additionally, there is shrinkage with tissue processing. No data are available about the exact amount in cardiac muscle but given that all tissues were handled the same way comparison can still be made. However tissue fixation and processing might bias our extrapolation to real wall thickness in hearts after Fontan surgery.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fontan F, Baudet E. Surgical repair of tricuspid atresia. Thorax. 1971;26:240–248. doi: 10.1136/thx.26.3.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Leval MR, Kilner P, Gewillig M, Bull C. Total cavopulmonary connection: a logical alternative to atriopulmonary connection for complex Fontan operations. Experimental studies and early clinical experience. J Thorac Cardiovasc Surg. 1988;96:682–695. [PubMed] [Google Scholar]
- 3.Doty DB, Marvin WJ, Jr, Lauer RM. Modified Fontan procedure. Methods to achieve direct anastomosis of right atrium to pulmonary artery. J Thorac Cardiovasc Surg. 1981;81:470–475. [PubMed] [Google Scholar]
- 4.Corno A, Becker AE, Bulterijs AH, Lam J, Nijveld A, Schuller JL, Marcelletti C. Univentricular heart: can we alter the natural history? Ann Thorac Surg. 1982;34:716–727. doi: 10.1016/s0003-4975(10)60917-4. [DOI] [PubMed] [Google Scholar]
- 5.Balaji S, Gewillig M, Bull C, de Leval MR, Deanfield JE. Arrhythmias after the Fontan procedure. Comparison of total cavopulmonary connection and atriopulmonary connection. Circulation. 1991;84(Suppl):III162–167. [PubMed] [Google Scholar]
- 6.Engelfriet P, Boersma E, Oechslin E, Tijssen J, Gatzoulis MA, Thilen U, Kaemmerer H, Moons P, Meijboom F, Popelova J, Laforest V, Hirsch R, Daliento L, Thaulow E, Mulder B. The spectrum of adult congenital heart disease in Europe: morbidity and mortality in a 5 year follow-up period. The Euro Heart Survey on adult congenital heart disease. Eur Heart J. 2005;26:2325–2333. doi: 10.1093/eurheartj/ehi396. [DOI] [PubMed] [Google Scholar]
- 7.Gates RN, Laks H, Drinkwater DC, Jr, Lam L, Blitz A, Child JS, Perloff JK. The Fontan procedure in adults. Ann Thorac Surg. 1997;63:1085–1090. doi: 10.1016/s0003-4975(96)01256-8. [DOI] [PubMed] [Google Scholar]
- 8.Gelatt M, Hamilton RM, McCrindle BW, Gow RM, Williams WG, Trusler GA, Freedom RM. Risk factors for atrial tachyarrhythmias after the Fontan operation. J Am Coll Cardiol. 1994;24:1735–1741. doi: 10.1016/0735-1097(94)90181-3. [DOI] [PubMed] [Google Scholar]
- 9.Mott AR, Feltes TF, McKenzie ED, Andropoulos DB, Bezold LI, Fenrich AL, Bedford SL, El-Said H, Stayer SA, Fraser CD., Jr Improved early results with the Fontan operation in adults with functional single ventricle. Ann Thorac Surg. 2004;77:1334–1340. doi: 10.1016/j.athoracsur.2003.10.011. [DOI] [PubMed] [Google Scholar]
- 10.Fishberger SB, Wernovsky G, Gentles TL, Gauvreau K, Burnett J, Mayer JE, Jr, Walsh EP. Factors that influence the development of atrial flutter after the Fontan operation. J Thorac Cadiovas Surg. 1997;113:80–86. doi: 10.1016/s0022-5223(97)70402-1. [DOI] [PubMed] [Google Scholar]
- 11.Bromberg BI, Schuessler RB, Gandhi SK, Rodefeld MD, Boineau JP, Huddleston CB. A canine model of atrial flutter following the intra-atrial lateral tunnel Fontan operation. J Electrocardiol. 1998;30:85–93. doi: 10.1016/s0022-0736(98)80038-1. [DOI] [PubMed] [Google Scholar]
- 12.Wong T, Davlouros PA, Li W, Millington-Sanders C, Francis DP, Gatzoulis MA. Mechano-electrical interaction late after Fontan operation: relation between P-wave duration and dispersion, right atrial size, and atrial arrhythmias. Circulation. 2004;109:2319–2325. doi: 10.1161/01.CIR.0000129766.18065.DC. [DOI] [PubMed] [Google Scholar]
- 13.Bush HL, Gelband H, Hoffman BF, Malm JR. Electrophysiological Basis for Supraventricular Arrhythmias. Arch Surg. 1971;103:620–625. doi: 10.1001/archsurg.1971.01350110122020. [DOI] [PubMed] [Google Scholar]
- 14.Shirakura R, Kawashima Y, Hirose H, Matsuda H, Shimazaki Y, Sano T. Autopsy findings 14 years after septation for single ventricle. Ann Thorac Surg. 1989;48:124–125. doi: 10.1016/0003-4975(89)90197-5. [DOI] [PubMed] [Google Scholar]
- 15.Thoele DG, Ursell PC, Ho SY, Smith A, Bowman FO, Gersony WM, Anderson RH. Atrial morphologic features in tricuspid atresia. J Thorac Cardiovasc Surg. 1991;102:606–610. [PubMed] [Google Scholar]
- 16.Yoshikawa Y, Ishibashi-Ueda H, Uemura H, Kawahira Y, Yagihara T. Pathologic findings in atrial musculature seven years after the intraatrial tunnel Fontan. Ann Thorac Surg. 2002;73:663–664. doi: 10.1016/s0003-4975(01)03109-5. [DOI] [PubMed] [Google Scholar]
- 17.McMahon CJ, Vatta M, Fraser CD, Jr, Towbin JA, Chang AC. Altered dystrophin expression in the right atrium of a patient after Fontan procedure with atrial flutter (abst) Heart. 2004;90:e65. doi: 10.1136/hrt.2004.044370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakagawa H, Shah N, Matsudaira K, Overholt E, Chandrasekaran K, Beckman KJ, Spector P, Calame JD, Rao A, Hasdemir C, Otomo K, Wang Z, Lazzara R, Jackman WM. Characterization of reentrant circuit in macroreentrant right atrial tachycardia after surgical repair of congenital heart disease: isolated channels between scars allow “focal” ablation. Circulation. 2001;103:699–709. doi: 10.1161/01.cir.103.5.699. [DOI] [PubMed] [Google Scholar]
- 19.Collins KK, Love BA, Walsh EP, Saul JP, Epstein MR, Triedman JK. Location of acutely successful radiofrequency catheter ablation of intraatrial reentrant tachycardia in patients with congenital heart disease. Am J Cardiol. 2000;86:969–974. doi: 10.1016/s0002-9149(00)01132-2. [DOI] [PubMed] [Google Scholar]
- 20.Triedman JK, Alexander MA, Love BA, Collins KK, Berul CI, Bevilacqua LM, Walsh EP. Influence of patient factors and ablative technologies on outcomes of radiofrequency ablation of intra-atrial tachycardia in patients with congenital heart disease. J Am Coll Cardiol. 2002;39:1827–1835. doi: 10.1016/s0735-1097(02)01858-2. [DOI] [PubMed] [Google Scholar]
- 21.Bloom W. Blood and lymph vascular systems. In: Bloom W, Fawcett DW, editors. A Textbook of Histology. Philadelphia: WB Saunders; 1975. pp. 417–418. [Google Scholar]


