Abstract
Objective
MicroRNAs (miRs) regulate angiogenesis by posttranscriptional silencing of target genes. The significance of angiostatic miR-200b in switching on skin wound angiogenesis was tested.
Methods and Results
Wounding caused imminent and transient downregulation of miR-200b in dermal wound-edge endothelial cells. Derailing this injury response by lentiviral delivery of miR-200b in vivo impaired wound angiogenesis. Computational prediction, target reporter luciferase assay, and Western blot analysis provided first evidence that miR-200b targets globin transcription factor binding protein 2 (GATA2) and vascular endothelial growth factor receptor 2 (VEGFR2). Overexpression of GATA2 or VEGFR2 in endothelial cells rescued the angiostatic effect of miR-200b in vitro. Downregulation of miR-200b derepressed GATA2 and VEGFR2 expression to switch on wound angiogenesis, which was disrupted in diabetic wounds. Treatment of endothelial cells with tumor necrosis factor-α, a proinflammatory cytokine abundant in diabetic wounds, induced miR-200b expression, silenced GATA2 and VEGFR2, and suppressed angiogenesis. These outcomes were attenuated using anti-miR-200b strategy. Neutralization of tumor necrosis factor-α in the diabetic wounds improved wound angiogenesis and closure, which was accompanied by downregulation of miR-200b expression and desilencing of GATA2 and VEGFR2.
Conclusion
Injury-induced repression of miR-200b turned on wound angiogenesis. In mice with diabetes mellitus, excessive tumor necrosis factor-α induced miR-200b blunting proangiogenic functions of GATA2 and VEGFR2.
Keywords: angiogenesis, GATA binding protein 2, microRNAs, vascular endothelial growth factor receptor 2, wound healing
Chronic nonhealing cutaneous wounds lead to loss of limbs and represent a major public health threat affecting over 6.5 million Americans annually.1 Patients with peripheral vasculopathies such as those associated with diabetes mellitus are more susceptible to develop chronic nonhealing wounds, indicating that reestablishment of blood flow, mainly driven by angiogenesis, is essential in the wound healing process.2 Understanding the molecular mechanisms regulating wound angiogenesis would help develop therapeutic strategies to prevent amputation.
MicroRNAs (miRs), small noncoding RNAs inducing posttranscriptional gene regulation, are emerging as master switches that control the angiogenic response of endothelial cells.3 Through sequence-specific interaction with transcript, miRs negatively modulate gene expression via inhibition of protein translation or transcript degradation.3 Our group and others have reported that angiogenesis is tightly regulated by these small noncoding RNAs.4,5 Several miRs have been identified to control angiogenesis under physiological and diseased conditions.3 miR-200b is an miR-200 family member which is clustered with miR-200a and miR-429 in chromosome 1p36.6 This miR is expressed in a wide variety of cells including ovarian cancer cells, mammary stem cells, renal mesangial cells, and endothelial cells,6–9 regulating multiple cellular functions such as proliferation, motility, apoptosis, and stemness.7 Our laboratory has recently reported that hypoxia inhibits the expression of miR-200b in human dermal microvascular endothelial cells (HDMECs), promoting angiogenesis.6 Endothelial miR-200b targets a cluster of proteins that intercept vascular endothelial growth factor (VEGF) signaling.8 Thus, miR-200b serves as a potent rheostat to restrain angiogenesis. In this work, we sought to elucidate the significance of miR-200b in the regulation of cutaneous wound angiogenesis. This work recognizes that wound itself represents a signal that transiently represses gene silencers (miR) to unleash tissue development in an adult setting.
Methods
Secondary-Intention Excisional Mouse Dermal Wound Models
Male C57BL/6 mice were obtained from Harlan Laboratory. Mice homozygous (BKS.Cg-m +/+ Leprdb/J or db/db) for spontaneous mutation of the leptin receptor (Leprdb) or their respective nondiabetic lean control littermates m+/db (aged 10–12 weeks) were obtained from Jackson Laboratory. For wound-edge collection study, two 8 × 16 mm full-thickness excisional wounds were placed on the dorsal skin, equidistant from the midline and adjacent to the 4 limbs.10 For lentivirus delivery and soluble tumor necrosis factor (TNF) receptor 1 (sTNFR1) treatment study, 2 or four 6-mm diameter full-thickness excisional wounds were developed on the dorsal skin of mice with a 6-mm disposable biopsy punch. All animal studies were performed in accordance with protocols approved by the Laboratory Animal Care and Use Committee of The Ohio State University. During the wounding procedure, mice were anesthetized by low-dose isoflurane inhalation for 5 to 10 minutes per standard recommendation. Each wound was digitally photographed at the time point indicated. Wound size was calculated by the software ImageJ. The animals were euthanized at the indicated postwounding time point, and wound-edge tissues (1 mm away from the wound, snap frozen) or the wound tissues (in optimal cutting temperature compound) were harvested.
In Vivo Dermal Delivery of Lentivirus or sTNFR1
In vivo dermal delivery of lentivirus was achieved by intradermal injection of miR overexpressing lentivirus.11 Briefly, miR-200b or control miR overexpression lentivirus (Applied Biological Materials) at titer 2 × 107 cfu/mL (50 μL per wound) was intradermally injected into the skin 1 mm away from the wound edge 3 days before the induction of 6-mm diameter full-thickness cutaneous wound on C57BL/6 mice under anesthetization as described above. sTNFR1 treatment was based on a previous study with slight modification.12 Briefly, a 6-mm diameter wound was created as described above. On days 2 and 5 postwounding, sTNFR1 (Sigma) at dose 5 μg/wounds (total volume 50 μL) or vehicle was intradermally injected into the wounds in m+/db nondiabetic mice and db/db diabetic mice.
Cell culture, transfection, lentiviral overexpression, miR target reporter luciferase assay, RNA extraction, real-time polymerase chain reaction, immunohistochemistry, immunocytochemistry, Matrigel tube formation assay, laser capture microdissection, laser Doppler, and statistical analyses are described in detail in the online-only Data Supplement.
Results
Downregulation of Endothelial miR-200b Supports Wound Angiogenesis
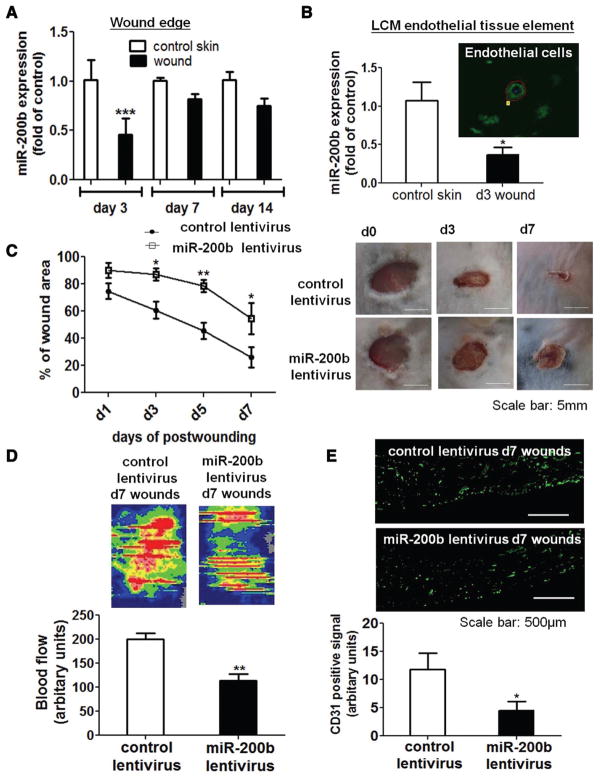
To validate whether miR-200b is implicated in the skin wound healing process, 8 × 16 mm full-thickness cutaneous wounds were induced on the dorsal skin of C57B6 mice, and wound edges (1-mm skin wound margin) on days 3, 7, and 14 post-wounding were collected to study the expression of miR-200b. miR-200b was transiently downregulated on day 3 postwounding (Figure 1A). Previously, our group and others reported that miR-200b exerts antiangiogenic effects in endothelial cells.6,8,9 Thus, we sought to investigate the response of endothelial miR-200b in the wound-edge tissue. Real-time polymerase chain reaction performed on laser microdissected wound-edge endothelial cells recognized that wound-dependent transient lowering of miR-200b was prominent in the endothelial cells (Figure 1B). The expression pattern of miR-200a, an miR that is encoded in the same cluster as for miR-200b, was comparable to that of miR-200b (Figure I in the online-only Data Supplement). Wounding did not cause significant changes in miR-200b expression of the nonendothelial compartment of wound-edge tissue (Figure II in the online-only Data Supplement). To test the significance of transiently downregulated endothelial miR-200b in wound angiogenesis, miR-200b overexpressing lentivirus was delivered intradermally into skin 3 days before wounding. Real-time polymerase chain reaction from laser capture microdissected dermal tissue element indicated that miR-200b was significantly upregulated after delivery of the miR-200b overexpressing lentivirus, compared with that of the control lentivirus–delivered dermal fraction (Figure III in the online-only Data Supplement). Overexpression of miR-200b significantly impaired wound angiogenesis, as evident by compromised wound blood flow measured by laser Doppler (Figure 1D). This observation was in agreement with lower CD-31 positive cells at the wound edge after miR-200b overexpression (Figure 1E). Consistent with these findings, miR-200b overexpression significantly impeded wound closure (Figure 1C and Figure IV in the online-only Data Supplement), suggesting that wound-induced downregulation of endothelial miR-200b serves as a turn-on signal for cutaneous wound angiogenesis supporting closure.
Figure 1.
Acute transient downregulation of endothelial microRNA-200b (miR-200b) was essential in supporting cutaneous wound angiogenesis. Quantitative polymerase chain reaction (PCR) analysis of miR-200b expression of (A) days 3, 7, and 14 wound-edge tissue (8 × 16 mm full-thickness excisional wounds, n = 6) and (B) laser microdissected endothelial tissue element (n = 4) from day 3 wounds, compared with their respective control skin sample. Control or miR-200b overexpressing lentiviral particle (2 × 107 cfu/mL, 50 μL per site) was intradermally delivered to the skin. Three days after delivery of lentivirus, excisional wounds were created using 6-mm diameter punches as described in the Methods section. C, Wound closure was monitored on days 1, 3, 5, and 7 postwounding after treatment with miR-200b or control miR overexpressing lentiviral particle by digital planimetry and was presented as percentage of wound closure (n = 8). Wound angiogenesis, as depicted by (D) laser Doppler (n = 6) and (E) quantification of CD31 positive staining (n = 7), of the cutaneous wound was analyzed on day 7 postwounding. Results are mean ± SEM. ***P < 0.001, **P < 0.01, *P < 0.05 compared with respective control. LCM indicates laser capture microdissection.
miR-200b Targets Globin Transcription Factor Binding Protein 2 and Vascular Endothelial Growth Factor Receptor 2 in Endothelial Cells
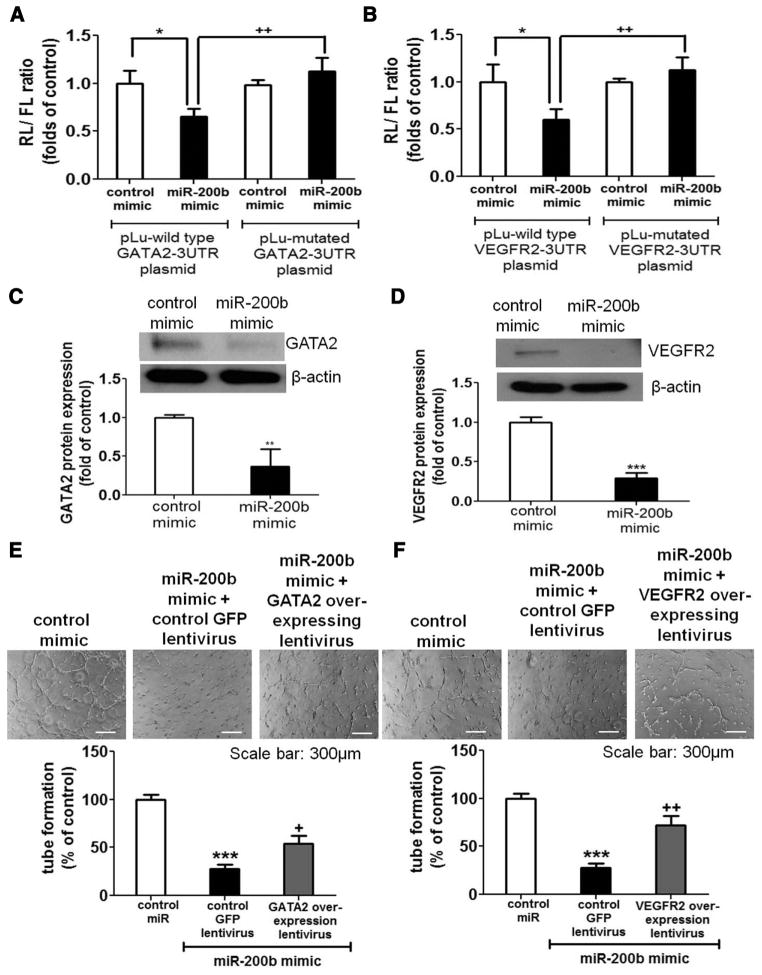
Next, we performed in silico study to search for potential targets that could regulate angiogenic response using TargetScan, PicTar, miRDB, miRanda, and Diana-MicroT algorithms. Such exercise revealed that 3′-untranslated regions (3′UTRs) of globin transcription factor binding protein 2 (GATA2) and VEGF receptor 2 (VEGFR2) contain a single and 2 binding site(s) for miR-200b (Figure V in the online-only Data Supplement), respectively. To validate whether miR-200b- dependent binding exerts translational repression, GATA2 3′UTR and VEGFR2 3′UTR reporter luciferase assay were performed using human embryonic kidney 293 cells. Delivery of miR-200b mimic significantly suppressed both GATA2 3′UTR and VEGFR2 3′UTR reporter luciferase activity (Figure 2A and 2B). Mutation of the predicted binding sites in the 3′UTR of construct significantly abolished miR-200b-dependent translational repression on 3′UTR of both GATA2 and VEGFR2 (Figure 2A and 2B), indicating that miR-200b specifically binds to the predicted sites to induce translational repression.
Figure 2.
MicroRNA-200b (miR-200b) modulated antiangiogenic effects by targeting globin transcription factor binding protein 2 (GATA2) and vascular endothelial growth factor receptor 2 (VEGFR2). miR target reporter luciferase assay using construct with either (A) GATA2 3′ untranslated region (3′UTR) or (B) VEGFR2 3′UTR in miR-200b mimic delivered human embryonic kidney 293 cells (HEK-293 cells). Results were normalized with renilla luciferase activity (n = 4). *P < 0.05 compared with control mimic transfected cells, ++P < 0.01 compared with respective wild-type plasmid-transfected cells. Western blot analysis of (C) GATA2 and (D) VEGFR2 protein expression in miR-200b mimic delivered human dermal microvascular endothelial cells (HDMECs; n = 4). β-actin serves as loading control. Matrigel tube formation at 8 hours in HDMEC treated with control mimic, miR-200b mimic, and miR-200b with either (E) GATA2 or (F) VEGFR2 overexpressing lentivirus (n = 4). ***P < 0.001, **P < 0.01, *P < 0.05 compared with respective control; +P < 0.05, ++P < 0.01 compared with miR-200b mimic alone. GFP indicates green fluorescent protein.
To determine whether miR-200b modulates the expression of GATA2 and VEGFR2 in endothelial cells, Western blot and immunocytochemistry analyses were performed. Delivery of miR-200b mimic lowered GATA2 and VEGFR2 protein expression in HDMECs (Figure 2C and 2D) and primary HDMEC (Figure VIA and VIB in the online-only Data Supplement). These data were in agreement with immunocytochemistry observations (Figure VIIA in the online-only Data Supplement), indicating that miR-200b negatively regulates the expression of GATA2 and VEGFR2. Delivery of miR-200b mimic inhibited the angiogenic tube-forming ability of endothelial cells (Figure VIIB in the online-only Data Supplement). Silencing GATA2 or VEGFR2 alone displayed comparable effects on angiogenesis in vitro (Figure VIII in the online-only Data Supplement). To examine whether the antiangiogenic effects of miR-200b are mediated by GATA2 or VEGFR2, endothelial cells were infected with lentiviral vector overexpressing miR-200b-resistant forms of GATA2 or VEGFR2 (without the 3′UTR of transgene in the clone). This was followed by miR-200b mimic delivery to cells and Matrigel culture. Lentiviral overexpression of GATA2 significantly induced protein expression by ≈2-fold at multiplicity of infection 1 (Figure IXA in the online-only Data Supplement). Similar results were obtained with lentiviral overexpression of VEGFR2 at multiplicity of infection 0.5 (Figure IXB in the online-only Data Supplement). Overexpression of GATA2 or VEGFR2 partially reversed miR-200b-dependent inhibition of tube formation (Figure 2E and 2F). Simultaneous overexpression of both GATA2 and VEGFR2 rescued the angiostatic effect of miR-200b in a seemingly additive but not synergistic manner (Figure X in the online-only Data Supplement). GATA2 controls the expression of VEGFR2.13,14 Silencing GATA2 alone using specific small interfering RNA downregulated the expression of VEGFR2 (Figure XIA in the online-only Data Supplement). Overexpression of GATA2 significantly induced VEGFR2 expression in the presence of miR-200b (Figure XIB in the online-only Data Supplement). Overexpression of VEGFR2 partially rescued the antiangiogenic effect caused by GATA2 knockdown (Figure XIC in the online-only Data Supplement), establishing that the VEGFR2-GATA2 signaling axis helps sustain wound angiogenesis.
Endothelial Expression of GATA2 and VEGFR2 Is Negatively Regulated by miR-200b in Cutaneous Wound-Edge Tissue
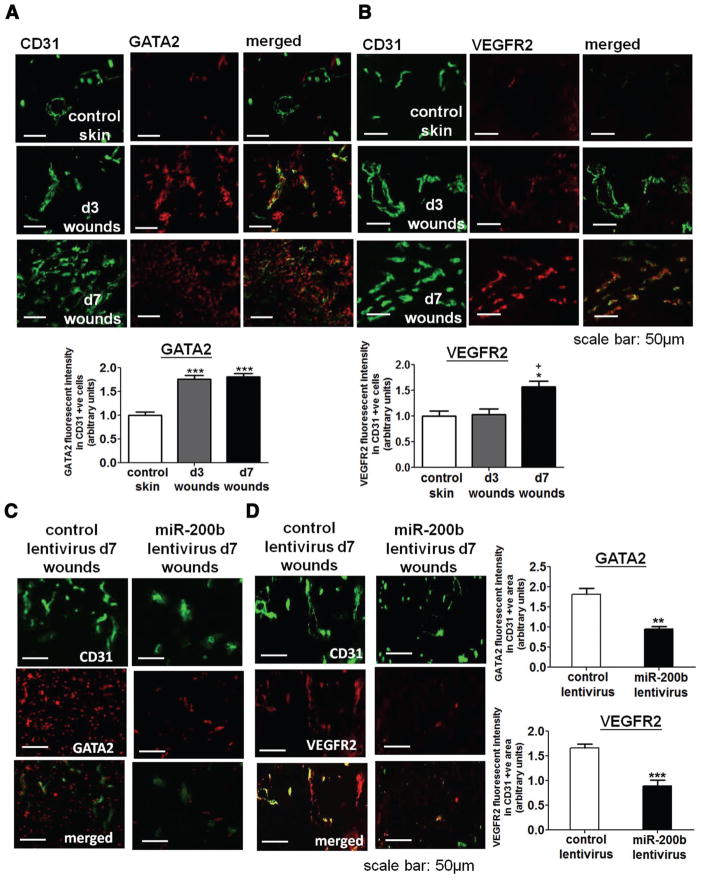
Given that the expression of endothelial miR-200b was down-regulated on day 3 postwounding (Figure 1A and 1B), it was anticipated that the expression of its target proteins GATA2 and VEGFR2 would be upregulated. Immunohistochemical studies demonstrated that endothelial GATA2 expression was significantly upregulated on both days 3 and 7 postwounding (Figure 3A). In contrast, endothelial VEGFR2 expression was upregulated on day 7 but not on day 3 postwounding (Figure 3B). To establish the significance of miR-200b in modulating GATA2 and VEGFR2 expression in the cutaneous wounds, the expression levels of GATA2 and VEGFR2 in day 7 wounds treated with either miR-200b overexpressing or control lentivirus were investigated. Lentiviral overexpression of miR-200b blunted GATA2 and VEGFR2 upregulation on day 7 postwounding (Figure 3C and 3D), establishing that wound-inducible endothelial GATA2 and VEGFR2 expression is, at least partially, mediated via downregulation of endothelial miR-200b.
Figure 3.
Expression of endothelial globin transcription factor binding protein 2 (GATA2) and vascular endothelial growth factor receptor 2 (VEGFR2) was induced during wound healing process and was modulated by microRNA-200b (miR-200b). Representative diagram shows (A) GATA2 and (B) VEGFR2 immunohistochemistry (red), in the intact skin, wound sample from days 3 and 7 postwounding. Representative diagram shows (C) GATA2 and (D) VEGFR2 immunohistochemistry (red) in day 7 wound sample treated with control or miR-200b overexpressing lentivirus. Colocalization of the GATA2 or VEGFR2 signaling with endothelial marker CD31 (green) was achieved by coincubation of anti-CD31 and anti-GATA2 or anti-VEGFR2 antibodies. Quantification of GATA2 or VEGFR2 intensity in CD31-positive cells. Results are mean ± SEM (n = 4). ***P < 0.001, **P < 0.01 compared with respective control; +P < 0.05 compared with day 3 wounds.
miR-200b-GATA2-VEGFR2 Signaling Axis Is Disrupted in Diabetic Wounds
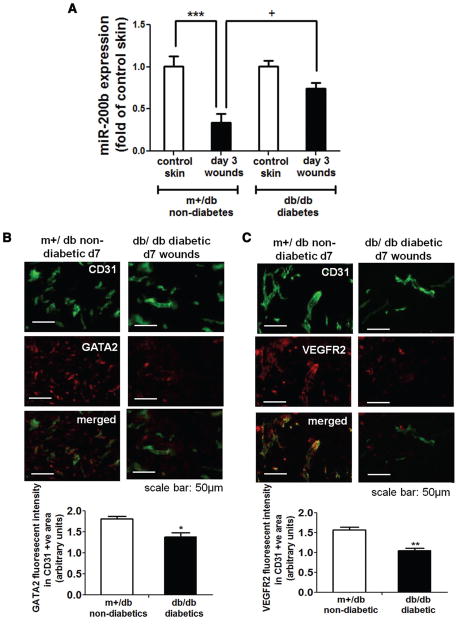
We investigated whether the intrinsic miR-200b signaling is disrupted in diabetic wounds. Mice homozygous for spontaneous mutation of the leptin receptor (db/db mice) display obesity and hyperglycemic phenotype and serve as an established model for type 2 diabetes mellitus. These mice exhibited impaired wound closure and significant impairment in wound angiogenesis (Figure XII in the online-only Data Supplement). Investigation of miR-200b expression level in cutaneous wound-edge tissue from db/db diabetic mice revealed that acute transient downregulation of miR-200b in the early phase of wound healing was disrupted (Figure 4A). This observation was consistent with GATA2 and VEGFR2 immunohistochemistry outcomes. Expressions of GATA2 and VEGFR2 proteins in wound-edge endothelial cells of db/db mice were significantly lower compared with that from m+/db nondiabetic lean control littermates (Figure 4B and 4C).
Figure 4.
Diabetic wounds exhibited disrupted microRNA-200b (miR-200b)-globin transcription factor binding protein 2 (GATA2)-vascular endothelial growth factor receptor 2 (VEGFR2) signaling. A, Quantitative polymerase chain reaction (PCR) analysis of miR-200b expression of day 3 wound-edge tissues isolated from m+/db nondiabetic mice and db/db diabetic mice. Fold changes of the wound samples were compared with their corresponding intact skin control. Results are mean ± SEM (n = 5). ***P < 0.001, +P < 0.05 compared with m+/db d3 wounds. Representative diagram shows (B) GATA2 and (C) VEGFR2 immunohistochemistry (red) in the day 7 wound sample from m+/db nondiabetic mice and db/db diabetic mice. Colocalization of the GATA2 or VEGFR2 signaling with endothelial marker CD31 (green) was achieved by coincubation of anti-CD31 and anti-GATA2 or anti-VEGFR2 antibodies. Quantification of GATA2 or VEGFR2 intensity in CD31-positive cells. Results are mean ± SEM (n = 4). **P < 0.01, *P < 0.05 compared with day 7 m+/db nondiabetic control mice.
TNF-α-Inducible miR-200b Inhibits Angiogenic Responses by a GATA2/VEGFR2-Dependent Mechanism
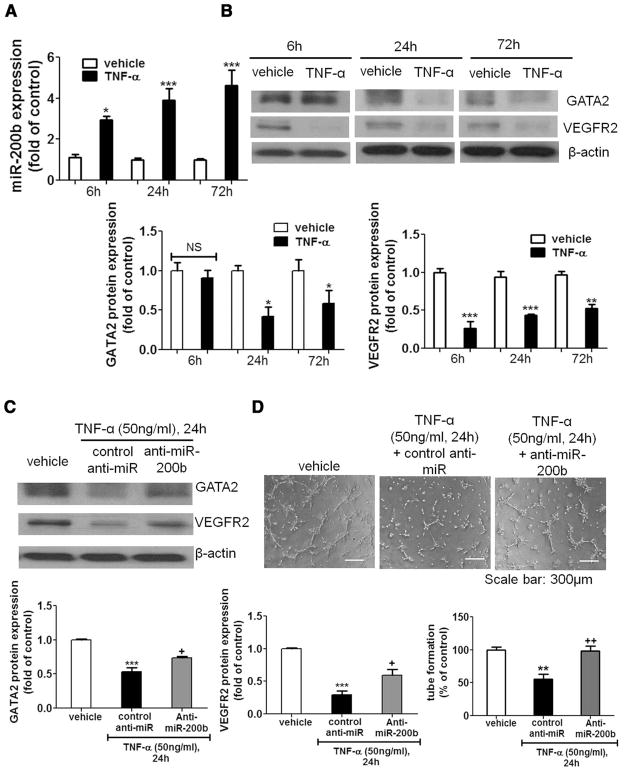
To dissect the molecular mechanism underlying aberrant expression of miR-200b in diabetic wound-edge tissue, endothelial cells were challenged with wound-relevant stimuli. Diabetic wounds are refractory to resolution of inflammation and display a prolonged abundance of proinflammatory mediators. Our group and others reported prolonged overproduction of proinflammatory TNF-α in diabetic wounds.12,15 Treatment of HDMECs with recombinant human TNF-α, known to inhibit angiogenic responses,16 significantly induced miR-200b expression at 6 hours, 24 hours, and 72 hours (Figure 5A). Interestingly, Western blot analyses revealed that the expressions of both GATA2 and VEGFR2 were concurrently downregulated in response to TNF-α at 24 hours and 72 hours (Figure 5B). Treatment of primary HDMEC with TNF-α also induced miR-200b expression (Figure XIIIA in the online-only Data Supplement), resulting in lower GATA2 and VEGFR2 protein expression (Figure XIIIB and XIIIC in the online-only Data Supplement). To characterize the significance of miR-200b in TNF-α-associated downregulation of GATA2 and VEGFR2, we delivered the short hairpin loop against miR-200b (anti-miR-200b) in HDMECs and studied the expression of GATA2 and VEGFR2. Delivery of anti-miR-200b blunted TNF-α-induced miR-200b upregulation (Figure XIV in the online-only Data Supplement). Inhibition of miR-200b by anti-miR-200b attenuated TNF-α-dependent downmodulation of GATA2 and VEGFR2 (Figure 5C). To further investigate the functional significance of the TNF-α-miR-200b signaling axis in endothelial cells, HDMECs were pretreated with TNF-α and were allowed to display angiogenic outcomes on Matrigel in the presence of TNF-α at the same dose. TNF-α significantly inhibited tube formation, which was rescued by pretreatment of anti-miR-200b (Figure 5D). These data demonstrate that TNF-α-dependent induction of endothelial miR-200b restrains angiogenic response via a GATA2/VEGFR2-dependent mechanism.
Figure 5.
MicroRNA-200b (miR-200b) served as a mediator of tumor necrosis factor-α (TNF-α)–induced downregulation of globin transcription factor binding protein 2 (GATA2) and vascular endothelial growth factor receptor 2 (VEGFR2) expression, and antiangiogenic response of endothelial cells. A, Quantitative polymerase chain reaction (PCR) analysis of miR-200b expression after treatment of TNF-α as indicated time in human dermal microvascular endothelial cells (HDMECs). B, Western blot analysis of GATA2 and VEGFR2 protein expression in TNF-α-treated HDMECs at different time points. C, Western blot analysis of GATA2 and VEGFR2 protein expression in TNF-α-treated HDMECs in the presence or absence of anti-miR-200b. β-actin serves as loading control. D, Matrigel tube formation visualized by phase contrast microscopy at 8 hours in TNF-α-treated HDMECs in the presence or absence of anti-miR-200b. Results are mean ± SEM (n = 4). ***P < 0.001, **P < 0.01, *P < 0.05 compared with respective control; ++P < 0.01, +P < 0.05 compared with cells treated with TNF alone. NS indicates not statistically significant.
Neutralization of TNF-a Rectifies Aberrant miR-200b-GATA2-VEGFR2 Signaling and Improves Diabetic Wound Angiogenesis
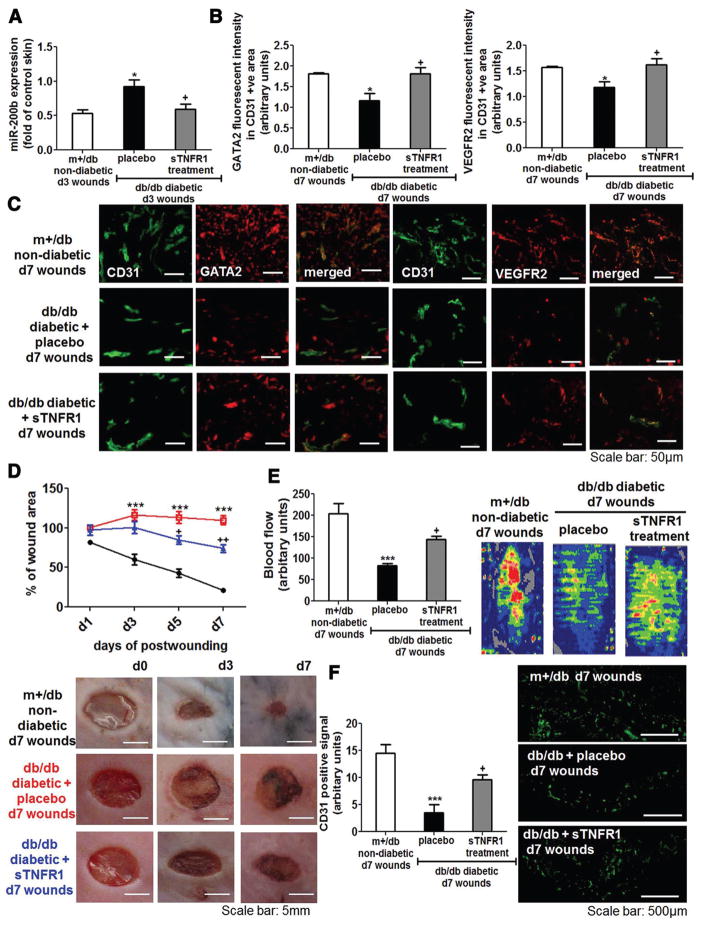
To test the significance of TNF-α in deregulated miR- 200b-dependent pathway in diabetic wounds in vivo, sTNFR1 was intradermally injected into the wounds on days 2 and 5 postwounding to specifically neutralize the effect of overproduced TNF-α. Antagonizing the effect of TNF-α significantly reduced the overproduction of miR-200b in the diabetic wounds (Figure 6A). Interestingly, results from immunohistochemistry revealed that loss of endothelial GATA2 and VEGFR2 in diabetic wounds was significantly reversed by the treatment of sTNFR1 (Figure 6B and 6C). Neutralization of overproduced TNF-α significantly improved wound closure (Figure 6D and Figure XV in the online-only Data Supplement). This observation was in agreement with the improvement of blood flow and endothelial cell abundance (Figure 6E and 6F). These results demonstrate that diabetic wound–associated exaggerated TNF-α production induces miR-200b expression, leading to loss of both GATA2 and VEGFR2, subsequently resulting in poor wound vascularization and closure.
Figure 6.
Neutralization of tumor necrosis factor-α (TNF-α) reversed the aberrant microRNA-200b (miR-200b) induction, rescued the loss of globin transcription factor binding protein 2 (GATA2) and vascular endothelial growth factor receptor 2 (VEGFR2) expression, and improved wound closure and angiogenesis. A, Quantitative polymerase chain reaction (PCR) analysis of miR-200b expression of d3 wound-edge tissue isolated from m+/db nondiabetic mice, db/db diabetic mice treated with placebo, and db/db diabetic mice treated with soluble TNF receptor 1 (sTNFR1; n = 5). Fold changes of the wound samples were compared with their corresponding intact skin control. B, Quantification of GATA2 or VEGFR2 intensity in CD31 positive cells (n = 4). C, Representative diagram shows GATA2 and VEGFR2 immunohistochemistry (red) in the day 7 wound sample from m+/db nondiabetic mice, db/db diabetic mice treated with placebo, and db/db diabetic mice treated with sTNFR1. Colocalization of the GATA2 or VEGFR2 signaling with endothelial marker CD31 (green) was achieved by coincubation of anti-CD31 and anti-GATA2 or anti-VEGFR2 antibodies. D, Wound closure was monitored on days 1, 3, 5, and 7 postwounding and was presented as percentage of wound closure (n = 6): black, m+/db nondiabetic mice; red, db/db diabetic mice treated with placebo; and blue, db/db diabetic mice treated with sTNFR1. Wound angiogenesis, as depicted by (E) cutaneous blood flow measured by laser Doppler (n = 6) and (F) quantification of CD31 positive staining (n = 4) on day 7 postwounding. Results are mean ± SEM. ***P < 0.001, *P < 0.05 compared with m+/db nondiabetic wounds; ++P < 0.01, +P < 0.05 compared with db/db diabetic wounds.
Discussion
miR-200b belongs to the miR-200 family member which controls epithelial-mesenchymal transition.7 Loss of miR-200b induces a mesenchymal phenotype, enhanced motility, and invasiveness of wide variety of transformed cells.7 Our group and others have reported that miR-200b not only modulates cell motility but also controls several proangiogenic proteins regulating endothelial cell function. The current work establishes that acute transient down-regulation of endothelial miR-200b is critical for successful angiogenic outcome during the cutaneous wound healing process. It also provides first evidence demonstrating that GATA2 serves as a novel direct target of miR-200b- regulating angiogenic response. GATA2, a zinc finger transcription factor involved in hematopoietic and endothelial development, is the most abundantly expressed GATA factor in endothelial cells.17 Besides its role in regulating the promoter of many endothelial specific genes including endothelin-1, von Willebrand factor, and VE-cadherin,17 GATA2 modulates angiogenesis in response to mechano-sensitive stimuli in a p190RhoGAP-dependent fashion.14 It also controls several genes including matrix metallo-proteinase-218 and endomucin19 to support endothelial cell migration. This is consistent with the findings from the current investigation indicating that endogenous GATA2 critically supports angiogenesis. Although the transcriptional control of GATA2 has been well studied,17 little is known about its posttranscriptional regulation. So far, only 2 studies addressing miR-dependent posttranscriptional modification of GATA2 have been reported.20,21 The current investigation provides first evidence establishing that miR-200b directly binds to GATA2 3′UTR and induces translational repression. During the preparation of this article, Choi et al8 reported that miR-200b directly targets VEGFR2, which is in agreement with our observation in the reporter assay and Western blot analysis. Observations from rescue experiments reported herein validate that the miR-200b-dependent antiangiogenic response is mediated by posttranscriptional silencing of GATA2 and VEGFR2. Given that GATA2 directly transactivates the promoter of VEGFR2,13 which is consistent with our observation that GATA2 supports VEGFR2 expression in endothelial cells, this work elucidates a novel regulatory mechanism wherein VEGFR2 expression is regulated by miR-200b at 2 levels: GATA2-dependent transcriptional and posttranscriptional silencing (direct interaction with the 3′UTR). In fact, the miR-200 family not only targets key players in the VEGF-signaling cascade but also modulates many mediators that potentially block angiogenesis. miR-200a, for example, can target β-catenin,22 a membrane-bound protein enabling adherens junction formation and thus stabilizing blood vessel.23 miR-200 family, via silencing notch ligand Jagged-1,24 can potentially suppress endothelial proliferation and sprouting.25 Taken together, observations of the present study and the current literature consolidate the concept that miR-200b and its associated family members silence clusters of molecules in different angiogenic circuits to dictate endothelial cell phenotype and functional outcomes.
Several studies have reported the involvement of miR dysregulation in the diabetes mellitus–associated pathogenic angiogenesis. In type 2 diabetic Goto-Kakizaki rats, upregulation of miR-320 inhibited angiogenic response of myocardial microvascular endothelial cells by targeting insulin-like growth factor 1.26 Elevation of miR-503 silenced cyclin E1 and cdc25A, subsequently inhibiting angiogenesis in diabetic ischemic muscle.27 High glucose-induced loss of miR-93, an miR targeting renal endothelial VEGF, resulted in aberrant angiogenesis.28 In the current work, we demonstrate that diabetic wound-edge endothelial cells suffer from silencing of GATA2 and VEGFR2, which is consistent with previous findings,29,30 via an miR-200b-dependent mechanism.
Excessive TNF-α is known to complicate closure of diabetic wounds.31 Strategies to antagonize TNF-α improve perfusion32 and closure of nonhealing wounds.12,31 In diabetic wounds, prolonged overproduction of TNF-α induces fibroblast apoptosis12 and inhibits fibroblast filopodia formation,33 resulting in compromised granulation tissue formation. TNF-α overload also desensitizes insulin signal and downregulates insulin receptor in diabetic keratinocytes leading to impairments in keratinocyte proliferation and differentiation.34 Intriguingly, TNF-α plays a dual role in regulating the angiogenic behavior of endothelial cells. Physiological low dose of TNF-α induces angiogenesis whereas supraphysiological high dose of TNF-α suppresses angiogenic response.16,35 Persistent stimulation of TNF-α inhibits angiogenic response whereas pulse of TNF-α primes endothelial cells for sprouting.36 Because of prolonged and unresolved inflammatory response in diabetic wounds, it is expected that dermal microvascular endothelial cells will shift to an antiangiogenic phenotype because of high TNF-α. This work establishes that persistent elevated TNF-α exerts substantial antiangiogenic effects via an miR-200b-GATA2-VEGFR2 mechanism. It also unveils the prospect of using local neutralization of TNF-α as a productive therapeutic strategy.
TNF-α may induce miR-200b via activation of p53. Promoter analysis and chromatin immunoprecipitation studies revealed that p53 can bind and transactivate the promoter of miR-200b-200a-429 cluster.37 Knockdown of p53 resulted in suppression of primary miR-200b expression, suggesting that p53 can induce the de novo synthesis of miR-200b.37 Interestingly, TNF-α may induce p53.33 This is further supported by the finding from the Kane et al38 study showing that p53 transcript was aberrantly upregulated in the diabetic wounds after day 3 postwounding. TNF-α-induced expression of miR-200b-200a-429 cluster was attenuated by p53 knockdown (Figure XVI in the online-only Data Supplement), suggesting a p53-dependent transcriptional control of endothelial miR-200b in response to TNF-α stimulation. Thus, it is tempting to speculate that in diabetic wounds exaggerated TNF-α upregulates miR-200b via activation of p53. In addition, it is possible that miR-200b upregulation could be sustained in a GATA2-dependent self–feed-forward mechanism. Putative GATA binding site has been identified in the miR-200b promoter.39 Binding of this site by GATA3, a GATA2 homolog, inhibited the expression of miR-200b cluster,39 suggesting that GATA2 might serve as a negative regulator of miR-200b expression. In this regard, TNF-α-associated upregulation of miR-200b silences GATA2, which might relieve the transrepression of miR-200b promoter, leading to sustained miR-200b expression under conditions of prolonged inflammation. It is noteworthy that this positive feed-forward loop could be further intensified by the self-regulatory mechanism of GATA2 owing to the positive regulation of GATA2 on its own promoter.17
In summary, this work reports that downregulation of endothelial miR-200b is crucial in turning on cutaneous wound angiogenesis by derepression of GATA2 and VEGFR2 expression. These findings support the concept that injury-responsive acute transient repression of miR serves as a potent signal, which unleashes reparative tissue development in an adult setting. GATA2 is recognized as a validated target of miR-200b. Diabetic wounds exhibit silencing of GATA2 and VEGFR2, which is a consequence of miR-200b being refractory to injury. Elevated TNF-α, as expected in dysregulated diabetic wounds, induces miR-200b which in turn silences angiogenic GATA2 and VEGFR2 (Figure XVII in the online-only Data Supplement). Taken together, this work sheds new light on the regulation of wound angiogenesis by miRs and develops the significance of such regulation in the context of diabetic complications.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health awards GM077185 and GM069589 to C.K. Sen and DK076566 to S. Roy.
Footnotes
Disclosures
None.
References
- 1.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, Gottrup F, Gurtner GC, Longaker MT. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17:763–771. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clemens MW, Attinger CE. Functional reconstruction of the diabetic foot. Semin Plast Surg. 2010;24:43–56. doi: 10.1055/s-0030-1253239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sen CK, Gordillo GM, Khanna S, Roy S. Micromanaging vascular biology: tiny microRNAs play big band. J Vasc Res. 2009;46:527–540. doi: 10.1159/000226221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shilo S, Roy S, Khanna S, Sen CK. Evidence for the involvement of miRNA in redox regulated angiogenic response of human microvascular endothelial cells. Arterioscler Thromb Vasc Biol. 2008;28:471–477. doi: 10.1161/ATVBAHA.107.160655. [DOI] [PubMed] [Google Scholar]
- 5.Suárez Y, Fernández-Hernando C, Pober JS, Sessa WC. Dicer dependent microRNAs regulate gene expression and functions in human endothelial cells. Circ Res. 2007;100:1164–1173. doi: 10.1161/01.RES.0000265065.26744.17. [DOI] [PubMed] [Google Scholar]
- 6.Chan YC, Khanna S, Roy S, Sen CK. miR-200b targets Ets-1 and is down-regulated by hypoxia to induce angiogenic response of endothelial cells. J Biol Chem. 2011;286:2047–2056. doi: 10.1074/jbc.M110.158790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brabletz S, Brabletz T. The ZEB/miR-200 feedback loop–a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi YC, Yoon S, Jeong Y, Yoon J, Baek K. Regulation of vascular endothelial growth factor signaling by miR-200b. Mol Cells. 2011;32:77–82. doi: 10.1007/s10059-011-1042-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McArthur K, Feng B, Wu Y, Chen S, Chakrabarti S. MicroRNA-200b regulates vascular endothelial growth factor-mediated alterations in diabetic retinopathy. Diabetes. 2011;60:1314–1323. doi: 10.2337/db10-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roy S, Khanna S, Rink C, Biswas S, Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008;34:162–184. doi: 10.1152/physiolgenomics.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Woodley DT, Keene DR, Atha T, Huang Y, Ram R, Kasahara N, Chen M. Intradermal injection of lentiviral vectors corrects regenerated human dystrophic epidermolysis bullosa skin tissue in vivo. Mol Ther. 2004;10:318–326. doi: 10.1016/j.ymthe.2004.05.016. [DOI] [PubMed] [Google Scholar]
- 12.Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, Behl Y, Alikhani M, Yang J, Braasch C, Graves DT. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1) Diabetologia. 2010;53:378–388. doi: 10.1007/s00125-009-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minami T, Murakami T, Horiuchi K, Miura M, Noguchi T, Miyazaki J, Hamakubo T, Aird WC, Kodama T. Interaction between hex and GATA transcription factors in vascular endothelial cells inhibits flk-1/KDR-mediated vascular endothelial growth factor signaling. J Biol Chem. 2004;279:20626–20635. doi: 10.1074/jbc.M308730200. [DOI] [PubMed] [Google Scholar]
- 14.Mammoto A, Connor KM, Mammoto T, Yung CW, Huh D, Aderman CM, Mostoslavsky G, Smith LE, Ingber DE. A mechanosensitive transcriptional mechanism that controls angiogenesis. Nature. 2009;457:1103–1108. doi: 10.1038/nature07765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, Bhasker V, Gordillo GM, Sen CK, Roy S. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PLoS ONE. 2010;5:e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen JX, Chen Y, DeBusk L, Lin W, Lin PC. Dual functional roles of Tie-2/angiopoietin in TNF-alpha-mediated angiogenesis. Am J Physiol Heart Circ Physiol. 2004;287:H187–H195. doi: 10.1152/ajpheart.01058.2003. [DOI] [PubMed] [Google Scholar]
- 17.De Val S, Black BL. Transcriptional control of endothelial cell development. Dev Cell. 2009;16:180–195. doi: 10.1016/j.devcel.2009.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han X, Boyd PJ, Colgan S, Madri JA, Haas TL. Transcriptional up-regulation of endothelial cell matrix metalloproteinase-2 in response to extracellular cues involves GATA-2. J Biol Chem. 2003;278:47785–47791. doi: 10.1074/jbc.M309482200. [DOI] [PubMed] [Google Scholar]
- 19.Kanki Y, Kohro T, Jiang S, Tsutsumi S, Mimura I, Suehiro J, Wada Y, Ohta Y, Ihara S, Iwanari H, Naito M, Hamakubo T, Aburatani H, Kodama T, Minami T. Epigenetically coordinated GATA2 binding is necessary for endothelium-specific endomucin expression. EMBO J. 2011;30:2582–2595. doi: 10.1038/emboj.2011.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pase L, Layton JE, Kloosterman WP, Carradice D, Waterhouse PM, Lieschke GJ. miR-451 regulates zebrafish erythroid maturation in vivo via its target gata2. Blood. 2009;113:1794–1804. doi: 10.1182/blood-2008-05-155812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fiedler J, Jazbutyte V, Kirchmaier BC, Gupta SK, Lorenzen J, Hartmann D, Galuppo P, Kneitz S, Pena JT, Sohn-Lee C, Loyer X, Soutschek J, Brand T, Tuschl T, Heineke J, Martin U, Schulte-Merker S, Ertl G, Engelhardt S, Bauersachs J, Thum T. MicroRNA-24 regulates vascularity after myocardial infarction. Circulation. 2011;124:720–730. doi: 10.1161/CIRCULATIONAHA.111.039008. [DOI] [PubMed] [Google Scholar]
- 22.Saydam O, Shen Y, Würdinger T, Senol O, Boke E, James MF, Tannous BA, Stemmer-Rachamimov AO, Yi M, Stephens RM, Fraefel C, Gusella JF, Krichevsky AM, Breakefield XO. Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol. 2009;29:5923–5940. doi: 10.1128/MCB.00332-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cavallaro U, Liebner S, Dejana E. Endothelial cadherins and tumor angiogenesis. Exp Cell Res. 2006;312:659–667. doi: 10.1016/j.yexcr.2005.09.019. [DOI] [PubMed] [Google Scholar]
- 24.Brabletz S, Bajdak K, Meidhof S, Burk U, Niedermann G, Firat E, Wellner U, Dimmler A, Faller G, Schubert J, Brabletz T. The ZEB1/miR-200 feedback loop controls Notch signalling in cancer cells. EMBO J. 2011;30:770–782. doi: 10.1038/emboj.2010.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Benedito R, Roca C, Sörensen I, Adams S, Gossler A, Fruttiger M, Adams RH. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137:1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Wang XH, Qian RZ, Zhang W, Chen SF, Jin HM, Hu RM. MicroRNA-320 expression in myocardial microvascular endothelial cells and its relationship with insulin-like growth factor-1 in type 2 diabetic rats. Clin Exp Pharmacol Physiol. 2009;36:181–188. doi: 10.1111/j.1440-1681.2008.05057.x. [DOI] [PubMed] [Google Scholar]
- 27.Caporali A, Meloni M, Völlenkle C, Bonci D, Sala-Newby GB, Addis R, Spinetti G, Losa S, Masson R, Baker AH, Agami R, le Sage C, Condorelli G, Madeddu P, Martelli F, Emanueli C. Deregulation of microRNA-503 contributes to diabetes mellitus-induced impairment of endothelial function and reparative angiogenesis after limb ischemia. Circulation. 2011;123:282–291. doi: 10.1161/CIRCULATIONAHA.110.952325. [DOI] [PubMed] [Google Scholar]
- 28.Long J, Wang Y, Wang W, Chang BH, Danesh FR. Identification of microRNA-93 as a novel regulator of vascular endothelial growth factor in hyperglycemic conditions. J Biol Chem. 2010;285:23457–23465. doi: 10.1074/jbc.M110.136168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keswani SG, Katz AB, Lim FY, Zoltick P, Radu A, Alaee D, Herlyn M, Crombleholme TM. Adenoviral mediated gene transfer of PDGF-B enhances wound healing in type I and type II diabetic wounds. Wound Repair Regen. 2004;12:497–504. doi: 10.1111/j.1067-1927.2004.12501.x. [DOI] [PubMed] [Google Scholar]
- 30.Krishnan ST, Quattrini C, Jeziorska M, Malik RA, Rayman G. Neurovascular factors in wound healing in the foot skin of type 2 diabetic subjects. Diabetes Care. 2007;30:3058–3062. doi: 10.2337/dc07-1421. [DOI] [PubMed] [Google Scholar]
- 31.Lai JJ, Lai KP, Chuang KH, Chang P, Yu IC, Lin WJ, Chang C. Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-alpha expression. J Clin Invest. 2009;119:3739–3751. doi: 10.1172/JCI39335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sugano M, Tsuchida K, Makino N. Intramuscular gene transfer of soluble tumor necrosis factor-alpha receptor 1 activates vascular endothelial growth factor receptor and accelerates angiogenesis in a rat model of hindlimb ischemia. Circulation. 2004;109:797–802. doi: 10.1161/01.CIR.0000112579.61522.67. [DOI] [PubMed] [Google Scholar]
- 33.Gadea G, Roger L, Anguille C, de Toledo M, Gire V, Roux P. TNFalpha induces sequential activation of Cdc42- and p38/p53-dependent pathways that antagonistically regulate filopodia formation. J Cell Sci. 2004;117(Pt 26):6355–6364. doi: 10.1242/jcs.01566. [DOI] [PubMed] [Google Scholar]
- 34.Spravchikov N, Sizyakov G, Gartsbein M, Accili D, Tennenbaum T, Wertheimer E. Glucose effects on skin keratinocytes: implications for diabetes skin complications. Diabetes. 2001;50:1627–1635. doi: 10.2337/diabetes.50.7.1627. [DOI] [PubMed] [Google Scholar]
- 35.Ligresti G, Aplin AC, Zorzi P, Morishita A, Nicosia RF. Macrophage-derived tumor necrosis factor-alpha is an early component of the molecular cascade leading to angiogenesis in response to aortic injury. Arterioscler Thromb Vasc Biol. 2011;31:1151–1159. doi: 10.1161/ATVBAHA.111.223917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sainson RC, Johnston DA, Chu HC, Holderfield MT, Nakatsu MN, Crampton SP, Davis J, Conn E, Hughes CC. TNF primes endothelial cells for angiogenic sprouting by inducing a tip cell phenotype. Blood. 2008;111:4997–5007. doi: 10.1182/blood-2007-08-108597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim T, Veronese A, Pichiorri F, Lee TJ, Jeon YJ, Volinia S, Pineau P, Marchio A, Palatini J, Suh SS, Alder H, Liu CG, Dejean A, Croce CM. p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med. 2011;208:875–883. doi: 10.1084/jem.20110235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kane CD, Greenhalgh DG. Expression and localization of p53 and bcl-2 in healing wounds in diabetic and nondiabetic mice. Wound Repair Regen. 2000;8:45–58. doi: 10.1046/j.1524-475x.2000.00045.x. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y, Ahn YH, Gibbons DL, Zang Y, Lin W, Thilaganathan N, Alvarez CA, Moreira DC, Creighton CJ, Gregory PA, Goodall GJ, Kurie JM. The Notch ligand Jagged2 promotes lung adenocarcinoma metastasis through a miR-200-dependent pathway in mice. J Clin Invest. 2011;121:1373–1385. doi: 10.1172/JCI42579. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.