Abstract
The elicitation of broadly cross-reactive HIV-1 neutralizing antibodies in humans remains a major challenge in developing a viable AIDS vaccine. We hypothesized that prolonged exposure to candidate vaccine immunogens could enhance the elicitation of such antibodies. In an attempt to develop HIV-1 vaccine immunogens with prolonged half-lives and increased stability, we constructed a fusion protein, gp41Fc, in which a truncated HIV-1 gp4189.6 was fused to a human IgG1 Fc. Gp41Fc is stable in solution, retains its antigenic structure and is highly immunogenic in rabbits. The serum titers reached 1:102,400 for the gp41Fc and 1:5,120 for gp14089.6. Rabbit IgG neutralized diverse HIV-1 isolates and HIV-2, and the neutralization activity was attributed to gp41-specific IgG. The concentration of the gp41Fc in the serum correlated with the neutralization activity of rabbit IgG which recognized mostly conformation-independent epitopes on gp41 and predominantly bound to peptides derived from the gp41 immunodominant loop region. These results suggest that the prolonged half-life of gp41Fc in the serum may enhance the generation of cross-reactive neutralizing antibodies. Further research is needed to confirm and extend these results which may have implications for the development of vaccine immunogens with enhanced capability to elicit cross-reactive HIV-1-neutralizing antibodies.
Keywords: HIV/AIDS, Vaccines, Gp41
1. Introduction
Human immunodeficiency virus type 1 (HIV-1) has become a growing pandemic since its discovery in the early 1980s. After more than two decades of an intensive search for a viable AIDS vaccine, the goal remains elusive. The elicitation of broadly cross-reactive HIV-1 neutralizing antibodies in humans remains a major challenge in this effort largely due to HIV-1 genetic diversity.
The HIV-1 envelope glycoprotein (Env) is known to exist as trimers of heterodimer on native virions that is composed of a non-covalently associated extracellular subunit gp120 and a transmembrane subunit gp41. In natural infections, Env induces a robust antibody response, but these antibodies usually have a narrow spectrum of neutralization activity. Occasionally some HIV-1-positive individuals induce broadly cross-reactive neutralizing antibodies that are believed to account for the containment of the virus [1,2]. Env-based vaccines have been extensively studied. Monomeric gp120 s can elicit neutralizing antibodies in vivo, but the neutralization activity is restricted to autologous viruses or the spectrum of neutralization activity is narrow [3]. Oligomeric forms of Env ectodomain from HIV-1, strain R2 (gp140R2), which contain both gp120 and a truncated gp41 that lacks both transmembrane domains and cytoplasmic tails, induced better antibody response than gp120R2 alone. Rabbit sera immunized with gp140R2 neutralized not only the autologous virus, but also heterologous viruses from diverse HIV-1 subtypes, suggesting that induction of broad spectrum neutralizing antibodies is an achievable goal in HIV-1 vaccine development [4].
This study utilizes gp41 derived from a dual tropic HIV-1 primary isolate of 89.6. Gp41 is relatively conserved compared to gp120, but unstable in the absence of gp120. To stabilize gp41 in the absence of gp120, we constructed a fusion protein, designated as gp41Fc, in which a truncated gp4189.6 that lacked a fusion peptide, transmembrane domain and cytoplasmic tail was fused to human Fc by a long flexible linker. The goal of this fusion protein was to increase its half-life in vivo and enhance its binding to immune cells like macrophages, dendritic cells, and B cells that express receptors specific for Fc [5]. We hypothesize that prolonged exposure to candidate vaccine immunogens could enhance the elicitation of broadly cross-reactive antibodies based on the observation that broadly cross-reactive HIV-1 neutralizing antibodies are typically elicited later than isolate-specific antibodies after infection. Initially gp41Fc was used to localize the epitopes of a panel of gp41-specific neutralizing antibodies by alanine scanning mutagenesis. We found that gp41Fc not only bound to our recently identified monoclonal antibodies (mAbs) recognizing conformational epitopes, m44 [6], m46 [7] and m48 [8], but also to known broadly neutralizing human mAbs that include 2F5, 4E10, Z13 that recognize linear epitopes in the membrane proximal region (MPER), suggesting that gp41Fc retains critical conformation required for presenting neutralization epitopes and may induce neutralizing antibodies against HIV-1 in vivo. This prompted us to test the possibility of developing this fusion protein as HIV-1 vaccine immunogen by immunizing rabbits with purified recombinant gp41Fc. Here we report the results from the characterization of immune rabbit sera and purified IgGs from two rabbits that had different immune response to gp41Fc.
2. Materials and methods
2.1. Cells, viruses, plasmids, gp120, gp140 and antibodies
293T cells were purchased from ATCC. Other cell lines and HIV-1 isolates were obtained from the NIH AIDS Research and Reference Reagent Program (ARRRP). Recombinant gp120 and gp140 Envs from primary isolates were produced as described previously [9]. Human mAbs 2F5 and 4E10 were obtained from the ARRRP. Mouse mAbs NC-1 was generously provided by Dr. Shibo Jiang (New York Blood Center). Mouse mAbs T3 and D61 were obtained from Dr. Christopher Broder (Uniformed Services University of the Health Sciences). Fab Z13 and other gp41-specific mAbs m44, m46, m48 were produced in our laboratory. Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of healthy donors. HRP-conjugated streptavidin was purchased from Zymed Laboratories Inc., San Francisco, CA. HIV-1 MN Env (15-mer) peptides (complete set, cat# 6451, Lot# 12) were obtained from ARRRP.
2.2. Preparation of gp41Fc fusion protein
To prepare gp41Fc fusion protein, a gp4189.6 ectodomain gene lacking the fusion peptide region was cloned in a pEAK10 plasmid upstream of a human Fc. The recombinant plasmid DNA for transient transfection of free-style 293 cells (Invitrogen) was produced in E. coli. The culture supernatant was collected four days post transfection and gp41Fc fusion protein purified from the culture supernatant using a protein A affinity purification.
2.3. Rabbit immunization
Two female New Zealand white (NZW) rabbits were immunized using a standard 87 day protocol utilizing Freund’s Complete Adjuvant (FCA)/Freund’s Incomplete Adjuvant (FIA) as outlined below:
| Day: | Procedure: |
|---|---|
| 0 | NZW or Elite NZW female rabbit |
| Pre-bleed | |
| Primary intradermal inoculation: 250μg with FCA | |
| 14 | Boost subcutaneous: 125μg with FIA |
| 35 | Boost subcutaneous: 125μg with FIA |
| 45 | Test bleed |
| 49 | Boost subcutaneous: 125μg with FIA |
| 59 | Production bleed (PB1) |
| 70 | Boost subcutaneous: 125μg with FIA |
| 80 | Production bleed (PB2) |
| 81–87 | ELISA Titer assay of bleed |
| 87 | Terminal bleed (EX) |
2.4. Binding assays
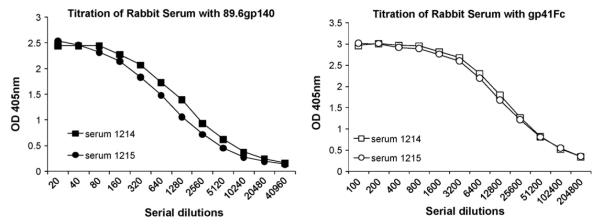
Binding of gp41-specific mAbs to recombinant gp41Fc (Fig. 1) was performed by coating 5 μg/mL gp41Fc on 96-well plates followed by the addition of 3-fold serially diluted biotinylated mAbs. The bound mAbs was detected using horse radish peroxidase (HRP) conjugated to streptavidin (1:10,000) as a second antibody and ABTS as a substrate. Titration of immune rabbit serum (Fig. 2) was done by coating 1 μg/mL gp41Fc or gp14089.6 on maxisorp 96-well plates, then washing and blocking with 3% BSA in PBS followed by the addition of two-fold serially diluted rabbit immune sera. Bound antibodies were detected using HRP conjugated to goat anti-rabbit IgG (H + L) (1:10,000) as a second antibody and ABTS as a substrate. The optical density at 405 nm was measured after a 20 min color development.
Fig. 1.
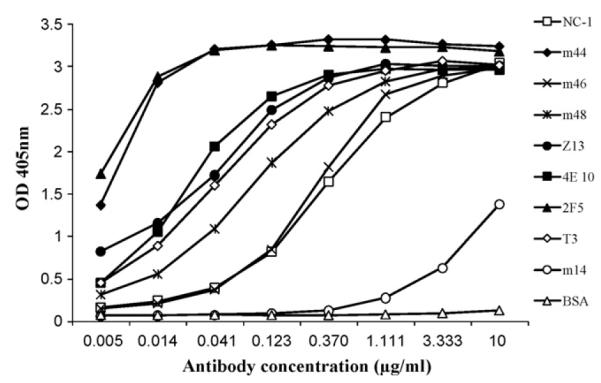
Binding of anti-HIV-1 Env mAbs to gp41Fc fusion protein. Three-fold serially diluted anti-gp41 mAbs were added to 96-well plates coated with 5 μg/mL of purified gp41Fc. Bound Abs were detected using HRP conjugated to anti-human IgG, F(ab’)2 (for human Abs) or anti-mouse IgG (H + L) (for mAbs NC-1 and T3) and ABTS as a substrate.
Fig. 2.
Titration of rabbit serum. One μg/mL 89.6gp140 linker protein or 1 μg/mL gp41Fc was coated on microwell plates. After blocking the wells, 2-fold serially diluted rabbit serum was added to the plates. Bound Abs were detected using HRP-anti-rabbit IgG, F(ab’)2 and ABTS as a substrate.
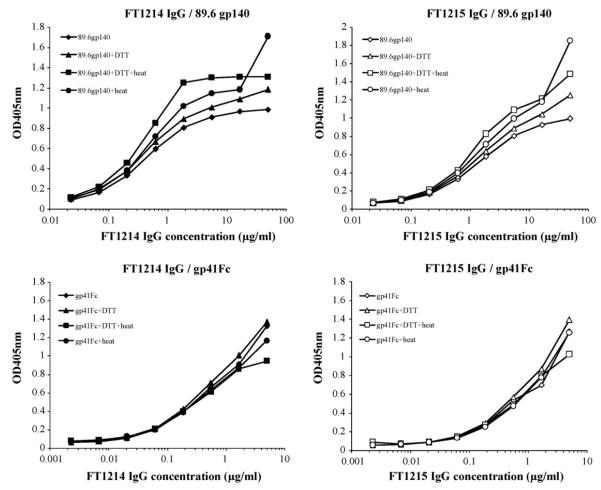
Binding of immune rabbit IgG to denatured gp140 and gp41Fc (Fig. 4) was carried out by diluting purified gp140 and gp41Fc to 20 μg/mL in PBS containing 50 mM DTT, then boiling the mixture for 5 min and diluting 1:10 in bicarbonate coating buffer (100 mM NaHCO3 and 500 mM NaCl, pH 8.3) followed by coating on 96-well plates. The bound antibodies were detected using HRP conjugated to either anti-rabbit IgG or anti-human IgG, F(ab’)2 as secondary antibodies and ABTS as a substrate.
Fig. 4.
Binding of immune rabbit IgG to non-denatured and denatured gp14089.6 and gp41Fc. Two μg/mL non-denatured and denatured gp14089.6 and gp41Fc were coated on microwell plates. Three-fold serially diluted rabbit IgG were added to the wells. Bound antibodies were detected using HRP conjugated to anti-rabbit IgG (H + L) and ABTS as substrate.
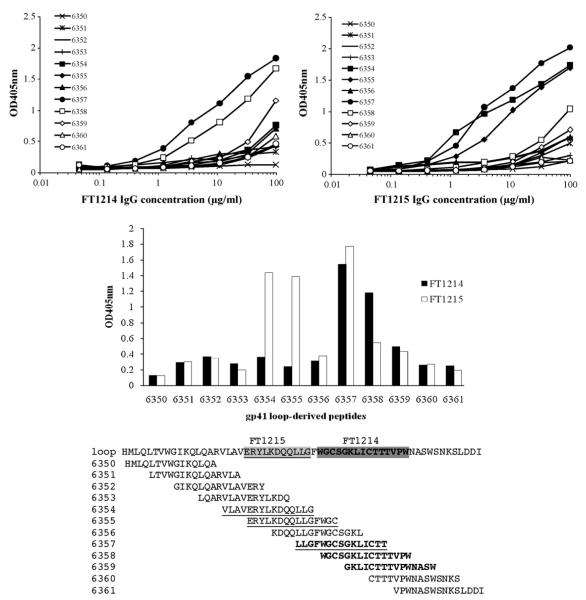
ELISA assay with HIV-1 MN gp41-derived peptides (6341–6377) (Fig. 5) was carried out by coating 5 μg/mL of each peptide on Easy-wash microplates followed by the addition of 3-fold serially diluted immune rabbit IgG. The bound rabbit IgG was detected as described above.
Fig. 5.
Binding of rabbit IgGs to peptides derived from gp41 immunodominant loop region. Five μg/mL of each peptide was coated on high-binding microwell plates. Three-fold serially diluted rabbit IgGs FT1214 (top left) and FT1215 (top right) added to the wells and bound antibodies detected using HRP-conjugated anti-rabbit IgG and ABTS as a substrate. Binding of IgGs FT1214 and FT1215 at 33 μg/mL to each peptide was plotted for comparison (middle panel). The amino acid sequences of the peptides tested were shown and the regions IgGs FT1214 (underlined) and FT1215 (in bold) bound to were indicated.
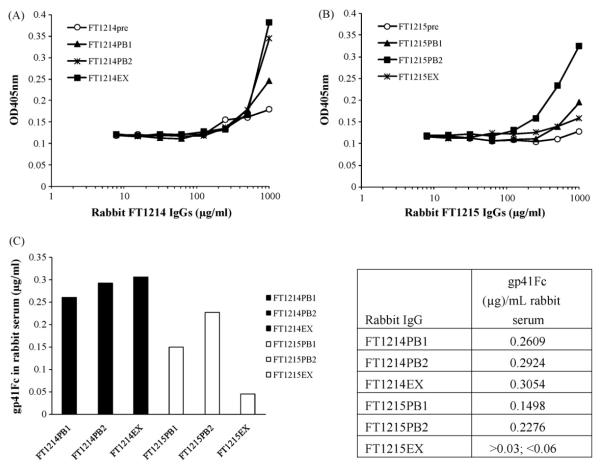
Capture ELISA was used to measure gp41Fc concentration in immune rabbit IgG sera utilizing the following procedure: gp41Fc was captured by conformation-dependent mouse mAb T3 coated on 96-well plates. Bound gp41Fc was detected by using biotinylated mouse mAb D61 and HRP conjugated to streptavidin. The optical density was measured 30 min after addition of ABTS. The concentration of gp41Fc in purified rabbit IgG was calculated using purified recombinant gp41Fc for making a standard curve and then converted to the concentration of gp41Fc in rabbit serum.
2.5. Absorption of immune rabbit IgG with human Fc to remove Fc-specific antibodies
Recombinant human Fc was coupled to a CNBr-activated Sepharose™ 4B column. The immune rabbit IgG was loaded onto the column. The flow-through was collected for further characterization. Fc-specific rabbit IgG bound to the column was eluted using a low pH buffer and neutralized immediately after elution.
2.6. PBMC-based HIV-1 neutralization assay
The PBMC-based assay was carried out as previously described [10]. Briefly, Fresh human PBMCs, seronegative for HIV and HBV, were isolated from blood of screened donors (Biological Specialty Corporation; Colmar, PA) using Lymphocyte Separation Medium (LSM; Cellgro® by Mediatech, Inc.; density 1.078 ± 0.002 g/mL) following the manufacturer’s instructions. Cells were stimulated by incubation in 4 μg/mL Phytohemag-glutinin (PHA; Sigma) for 48-72 h. Mitogenic stimulation was maintained by the addition of 20 U/mL recombinant human IL-2 (R&D Systems, Inc) to the culture medium. PHA-stimulated PBMCs from at least two donors were pooled, diluted in fresh medium and added to 96-well plates at 5 × 104 cells/well. Cells were infected (final MOI ≅ 0.1) in the presence of 9 different concentrations of antibody (triplicate wells/concentration; 50 μg/mL high-test concentration) and incubated for 7 days. To determine the level of virus inhibition, cell-free supernatant samples were collected for analysis of reverse transcriptase activity [11]. Following removal of supernatant samples, cytotoxicity was measured by the addition of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS; CellTiter 96 Reagent, Promega) following the manufacturer’s instructions.
2.7. Cell line-based HIV-1 neutralization assay
The cell line-based assay was carried out in triplicate using an HIV-1 Env pseudotyping system and HOS CD4+CCR5+ or HOS CD4+CXCR4+ target cells containing a tat-inducible luciferase reporter that express CD4, CCR5 or CXCR4. The degree of virus neutralization by antibody was achieved by measuring luciferase activity as described previously [9].
3. Results
3.1. Recombinant gp41Fc retains its antigenic structure
Purified gp41Fc separated on SDS-PAGE gels appear as a single band on denatured gels and several higher order bands on non-denatured gels. These results indicate the existence of oligomers, which could contribute to its avidity (data not shown).
Recombinant gp41Fc retains its antigenic structure as measured by ELISA (Fig. 1). Recombinant gp41Fc binds to all gp41-specific mAbs tested including human mAbs m44, m46 and m48, and mouse NC-1 and T3 that recognize the conformational epitopes of gp41, and the best characterized human mAbs, 2F5, 4E10 and Z13 that recognize linear epitopes of the MPER (Fig. 1). BSA and anti-gp120 human mAb m14 were used as controls. BSA did not bind to gp41Fc, but m14 showed weak binding at high concentrations likely due to non-specific interactions.
3.2. Recombinant gp41Fc is highly immunogenic
Two female rabbits were immunized with Purified gp41Fc. Antisera taken at exsanguination of both rabbits was titered for binding to the immunogen gp41Fc and the recombinant Env ectodomain gp14089.6. Both rabbit FT1214 and FT1215 generated high titer sera against gp41Fc that reached 1:102,400, indicating that the gp41Fc is highly immunogenic. Both rabbit antisera bound to gp14089.6 with a similar titre of 1:5,120 (Fig. 2).
3.3. Gp41-specific rabbit IgG accounts for neutralization activity of the immune serum IgG
We purified rabbit IgG from the antisera using a protein A affinity column and tested the rabbit IgG with a PBMC assay against a panel of HIV-1 primary isolates from group M and O, and HIV-2 (Table 1). Interestingly, rabbit FT1214EX IgG neutralized half of the HIV-1 primary isolates tested from group M (clades A, B, C, D, B/F and G), one out of three isolates from group O and two HIV-2 isolates with IC50 values lower than 50 μg/mL, the highest concentration tested. Rabbit FT1215EX IgG did not neutralize the HIV-1 isolates tested, but did neutralize one of the two HIV-2 isolates, CSC310342. We further confirmed that the neutralization activity of FT1214EX IgG was attributed to the gp41-specific IgG of the immune rabbit IgG (Fig. 3). Neutralization activity of immune rabbit IgG against Bal, a pseudo-typed HIV-1 virus, was decreased in the presence of an increased concentration of gp41Fc. Gp41Fc alone did not significantly affect the neutralization even at high concentrations (Fig. 3).
Table 1.
Neutralization activity (IC50) of rabbit IgGs FT1214 and FT1215 against a panel of primary isolates of HIV-1 and HIV-2 in a PBMC assay. AZT was used as a positive control with 100% inhibition at a concentration equal to 0.1μM, where the measured cell toxicity was undetectable (IC50 = 0.002μM, TC50 >1μM). TC50s of both FT1214 and FT1215 IgGs were over 50μg/mL for all isolates tested. IC50s were converted to the serum dilutions based on the amount of FT1214, 1215 IgG isolated from the corresponding serum, 6.7mg and 6.0mg per mL serum, respectively.
| Virus | Subtype | Coreceptor | FT1214 IgG IC50 (μg/mL) | FT1215 IgG IC50 (μg/mL) | AZT IC50 (nM) | FT1214 serum dilution | FT1215 serum dilution |
|---|---|---|---|---|---|---|---|
| 92UG037 | A | R5 | 43.8 | >50.0 | 0.55 | 1:152 | <1:120 |
| 92UG029 | A | X4 | >50.0 | >50.0 | 8.5 | <1:133 | <1:120 |
| 92BR014 | B | R5X4 | >50.0 | >50.0 | <0.10 | <1:133 | <1:120 |
| 92TH026 | B | R5 | 3.31 | >50.0 | 0.86 | 1:2,015 | <1:120 |
| Ba-L | B | R5 | 22.2 | >50.0 | 3.67 | 1:300 | <1:120 |
| 92BR025 | C | R5 | 8.91 | >50.0 | 1.12 | 1:749 | <1:120 |
| 93IN101 | C | R5 | 22.7 | >50.0 | 3.92 | 1:294 | <1:120 |
| 92UG001 | D | R5X4 | >50.0 | >50.0 | 1.67 | <1:133 | <1:120 |
| UG92024 | D | X4 | 35.3 | >50.0 | 5.63 | 1:189 | <1:120 |
| 92UG046 | D | X4 | >50.0 | >50.0 | 2.35 | <1:133 | <1:120 |
| CMU08 | E | X4 | >50.0 | >50.0 | 0.79 | <1:133 | <1:120 |
| 93BR019 | B/F | X4 | >50.0 | >50.0 | 2 | <1:133 | <1:120 |
| 93BR020 | F | R5X4 | >50.0 | >50.0 | 1.42 | <1:133 | <1:120 |
| 93BR029 | B/F | R5 | 9.93 | >50.0 | 1.12 | 1:672 | <1:120 |
| RU132 | G | R5 | >50.0 | >50.0 | 3.31 | <1:133 | <1:120 |
| G3 | G | R5 | 1.75 | >50.0 | 0.54 | 1:3,811 | <1:120 |
| BCF01 | Group O | R5 | >50.0 | >50.0 | 1.59 | <1:133 | <1:120 |
| BCF02 | Group O | R5 | 12.4 | >50.0 | 1.98 | 1:538 | <1:120 |
| BCF03 | Group O | R5 | >50.0 | >50.0 | 2.92 | <1:133 | <1:120 |
| CDC310319 | HIV-2 | 45 | >50.0 | 1.54 | 1:148 | <1:120 | |
| CDC310342 | HIV-2 | 1.8 | 2.84 | 0.21 | 1:3,706 | 1:2,113 | |
| Percentage of virus neutralized | 52 | 5 | 100 | ||||
Fig. 3.
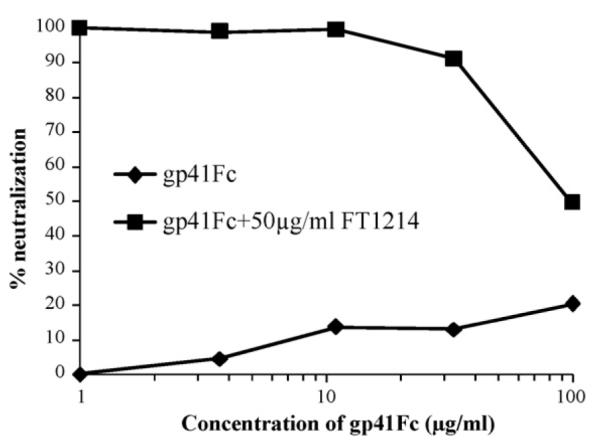
Neutralization activity of immune rabbit IgG was decreased in the presence of increased concentration of gp41Fc in neutralizing pseudo-typed HIV-1 (Bal). Fifty μg/mL FT1214 IgG was tested in cell line-based pseudovirus assay against HIV-1 isolate Bal in the presence of serially diluted gp41Fc. Gp41Fc alone was also tested in the assay.
3.4. Immune rabbit IgG recognizes conformation-independent epitopes on gp41 and preferentially binds to peptides derived from the immunodominant loop region
Because each rabbit IgG immune serum had similar titres to gp41Fc and gp14089.6, but showed very different neutralization activity against HIV-1 isolates, we investigated the composition of the rabbit IgG by measuring their binding to denatured gp41Fc and gp14089.6 (Fig. 4). Binding profiles were strikingly similar, suggesting that rabbit IgG recognizes mainly conformation-independent epitopes on gp41 (Fig. 4). Based on this observation, we measured the binding of the rabbit IgG to a panel of peptides derived from gp41MN (peptides 6341–6377). We found that rabbit IgG bound predominantly to some of the peptides derived from the immunodominant-loop region of gp41, but each rabbit IgG showed a different binding profile (Fig. 5). The neutralizing FT1214EX IgG bound to peptides 6357 (LLGFWGCSGKLICTT), 6358 (WGCSGKLICTTTVPW) and 6359 (GKLICTTTVPWNASW) that are located in the C-terminal immunodominant loop, while the weak-neutralizing FT1215EX IgG bound to peptides 6354 (VLAVERYLKDQQLLG) and 6355 (ERYLKDQQLLGFWGC), as well as 6357, 6358 and 6359. FT1214EX IgG bound more strongly to peptides 6358 and 6359 than FT1215EX IgG. Both bound well to peptide 6357 (Fig. 5). No binding was observed to peptides derived from MPER and other regions of gp41 (data not shown).
3.5. Prolonged existence of the immunogen was detected in the neutralizing rabbit IgG
In an effort to explain why two rabbit IgG sera with similar titers had different neutralization activity, we measured gp41Fc level in rabbit IgG (Fig. 6). The FT1214EX IgG at 87 days post immunization contained a much higher gp41Fc level (about 46 ng/mg rabbit IgG, equivalent to 305 ng/mL rabbit serum) than the FT1215EX IgG (< 10 ng/mg rabbit IgG, or < 60 ng/mL rabbit serum). The gp41Fc level in the FT1214PB1 and FT1214PB2 rabbit IgG purified from the production bleed after the 3rd and 4th booster was comparable to that in FT1215PB1 and FT1215PB2. These data indicate that gp41Fc in rabbit FT1214 show no decline at 17 days after the 4th booster while the level of gp41Fc in rabbit FT1215 dropped rapidly from the 2nd production bleed to the terminal bleed. The prolonged existence of the immunogen in rabbit sera seems to correlate with the neutralization activity of the antisera although further experiments with more animals are needed to confirm this observation (Fig. 6).
Fig. 6.
Capture ELISA of gp41Fc in immune rabbit IgG. Gp41Fc in the rabbit IgGs purified from immune rabbit sera collected from different time of bleed (see the immunization schedule in Materials and Methods) was captured by mouse mAb T3 coated on 96-well microplates. Bound gp41Fc was detected by using biotinylated mouse mAb D61 and HRP-conjugate to streptavidin. The optical density was measured 30 min after addition of ABTS (6A and 6B). The concentration of gp41Fc in purified rabbit IgG was calculated using purified recombinant gp41Fc as a standard and then converted to the concentration of gp41Fc in rabbit serum (6C).
4. Discussion
This study describes the results from two rabbits immunized with a gp41Fc fusion protein. The major observation of this study is that prolonged existence of the immunogen in rabbit serum correlates with the neutralization activity of rabbit IgG. Although data from more animals is required to derive statistically significant data, these results suggest that prolonging the existence of the immunogen in vivo may enhance elicitation of broadly cross-reactive HIV-1 neutralizing Abs. This hypothesis may have important implications for the design of an HIV-1 vaccine immunogen. It has been reported that the neonatal Fc receptor (FcRn) plays an important role in modulating IgG serum half-life, IgG homeostasis and maternofetal IgG transport [12,13]. Studies on antibody-FcRn interaction across species showed that human FcRn binds to human, rabbit and guinea pig IgG, but not to rat, bovine, sheep or mouse IgG (with the exception of weak binding to mouse IgG2b). In contrast, mouse FcRn binds to all IgG from these species [14]. Further study showed that bovine FcRn binds stronger to human IgG than to bovine IgG, and human IgG has two times longer serum half-life in normal and transchromosomic calves than its bovine counterpart [15]. So far there is no study reported on the interaction of human IgG with rabbit FcRn, but the results from the current study suggests that rabbit FcRn may bind to human Fc and mediates elongated human Fc serum half-life in rabbits. Based on this observation, it might be worth exploring the immunogenicity of gp140-Fc fusion in rabbits. In the current study we used human Fc fused to gp41 ectodomain as immunogen to immunize rabbits. The immunogen level of the two rabbits during the course of immunization was comparable, but dropped quickly in rabbit FT1215 after the last booster, indicating a difference in the half-life of the immunogen in different host individuals. Further studies will be required to elucidate the mechanism for what leads to a fast drop of the immunogen level in vivo and how to extend the in vivo half-life of the immunogen. We determined the percentage of Fc-specific antibodies in the rabbit IgG by absorption of the rabbit IgG with human Fc-conjugated sepharose 4B column (data not shown). Rabbit anti-Fc antibodies accounts for 30% of total rabbit IgG. Generation of human Fc-specific antibodies in rabbits may shorten the half-life of gp41Fc in rabbits. Administration of gp41Fc in humans may result in a better immune response than in rabbits because human Fc is non-immunogenic in human and it can bind to human FcRn on immune cells including macrophages and dendritic cells thus contributing to a potentially longer half-life. The prolonged serum half-life of gp41Fc in rabbit FT1214 may contribute to the high level of cross-reactive HIV-neutralizing antibodies, but we do not exclude the other mechanisms that may explain the difference in the level of cross-reactive HIV-neutralizing antibodies in the two rabbits used in this study.
The expected positive reaction of rabbit IgG to the gp41 immunodominant loop region was in contrast to the unexpected difference of the two rabbit IgG binding profiles to the loop-derived peptides. We do not know the reason for this anomaly or whether it may be related to the difference in neutralization activity. We also found that peptides 6358 and 6359, which bind preferentially to FT1214EX IgG rather than FT1215EX IgG, are conserved among HIV-1 clade A, B, C and D (data not shown). We did not observe binding of rabbit IgG to peptides derived from the MPER although the MPER-specific human mAbs 2F5, 4E10 and Z13 bound well to the recombinant gp41Fc. This observation suggests a difference in antigenicity and immunogenicity. Lack of MPER peptide-specific rabbit Abs may be due to lack of a lipid membrane that helps present the MPER epitopes to the immune system and/or the limit of the antibody gene repertoire in rabbits capable of expressing and maturing to 2F5, 4E10 and Z13-like antibodies [16]. Likewise, antibodies against the MPER of gp41 were not found in naturally infected humans [2], suggesting the poor immunogenicity of the MPER.
Acknowledgements
We thank Dr. Shibo Jiang for providing mouse mAb NC-1 and Dr. Christopher Broder for mouse mAbs T3 and D61. We also thank Drs. Shibo Jiang and Xiaodong Xiao for helpful discussions, Vidita Choudhry for help with pseudovirus assay and John Owens for help with manuscript preparation. We thank one of the reviewers for suggesting to explore gp140Fc fusion proteins as vaccine immunogens. This research was supported by the NIH Intramural AIDS Targeted Antiviral Program (IATAP), the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research, and the Gates Foundation to DSD, and by internal funds from Southern Research Institute. This project has been funded in part with federal funds from the National Cancer Institute, National Institutes of Health, under contract N01-CO-12400. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government.
References
- [1].Karlsson Hedestam GB, Fouchier RA, Phogat S, Burton DR, Sodroski J, Wyatt RT. The challenges of eliciting neutralizing antibodies to HIV-1 and to influenza virus. Nat Rev Microbiol. 2008 Feb 2;6:143–55. doi: 10.1038/nrmicro1819. [DOI] [PubMed] [Google Scholar]
- [2].Li Y, Migueles SA, Welcher B, Svehla K, Phogat A, Louder MK, et al. Broad HIV-1 neutralization mediated by CD4-binding site antibodies. Nat Med. 2007 Sep 9;13:1032–4. doi: 10.1038/nm1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Li Y, Svehla K, Mathy NL, Voss G, Mascola JR, Wyatt R. Characterization of antibody responses elicited by human immunodeficiency virus type 1 primary isolate trimeric and monomeric envelope glycoproteins in selected adjuvants. J Virol. 2006 Feb 3;80:1414–26. doi: 10.1128/JVI.80.3.1414-1426.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Zhang PF, Cham F, Dong M, Choudhary A, Bouma P, Zhang Z, et al. Extensively cross-reactive anti-HIV-1 neutralizing antibodies induced by gp140 immunization. Proc Natl Acad Sci U S A. 2007 Jun 24;104:10193–8. doi: 10.1073/pnas.0608635104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Zhu X, Meng G, Dickinson BL, Li X, Mizoguchi E, Miao L, et al. MHC class I-related neonatal Fc receptor for IgG is functionally expressed in monocytes, intestinal macrophages, and dendritic cells. J Immunol. 2001 Mar 5;166:3266–76. doi: 10.4049/jimmunol.166.5.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Zhang MY, Vu BK, Choudhary A, Lu H, Humbert M, Ong H, et al. Cross-reactive human immunodeficiency virus type 1-neutralizing human monoclonal antibody that recognizes a novel conformational epitope on gp41 and lacks reactivity against self-antigens. J Virol. 2008 Jul 14;82:6869–79. doi: 10.1128/JVI.00033-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Choudhry V, Zhang MY, Sidorov IA, Louis JM, Harris I, Dimitrov AS, et al. Cross-reactive HIV-1 neutralizing monoclonal antibodies selected by screening of an immune human phage library against an envelope glycoprotein (gp140) isolated from a patient (R2) with broadly HIV-1 neutralizing antibodies. Virology. 2007 Jun 1;363:79–90. doi: 10.1016/j.virol.2007.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zhang MY, Choudhry V, Sidorov IA, Tenev V, Vu BK, Choudhary A, et al. Selection of a novel gp41-specific HIV-1 neutralizing human antibody by competitive antigen panning. J Immunol Methods. 2006 Dec 1–2;317:21–30. doi: 10.1016/j.jim.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang MY, Shu Y, Phogat S, Xiao X, Cham F, Bouma P, et al. Broadly cross-reactive HIV neutralizing human monoclonal antibody Fab selected by sequential antigen panning of a phage display library. J Immunol Methods. 2003 Dec 1–2;283:17–25. doi: 10.1016/j.jim.2003.07.003. [DOI] [PubMed] [Google Scholar]
- [10].Ptak RG, Gallay PA, Jochmans D, Halestrap AP, Ruegg UT, Pallansch LA, et al. Inhibition of human immunodeficiency virus type 1 replication in human cells by Debio-025, a novel cyclophilin binding agent. Antimicrob Agents Chemother. 2008 Apr 4;52:1302–17. doi: 10.1128/AAC.01324-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Buckheit RW, Jr, Swanstrom R. Characterization of an HIV-1 isolate displaying an apparent absence of virion-associated reverse transcriptase activity. AIDS Res Hum Retroviruses. 1991 Mar 3;7:295–302. doi: 10.1089/aid.1991.7.295. [DOI] [PubMed] [Google Scholar]
- [12].Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol. 2000;18:739–66. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- [13].Roopenian DC, Akilesh S. FcRn: the neonatal Fc receptor comes of age. Nat Rev Immunol. 2007 Sep 9;7:715–25. doi: 10.1038/nri2155. [DOI] [PubMed] [Google Scholar]
- [14].Ober RJ, Radu CG, Ghetie V, Ward ES. Differences in promiscuity for antibody-FcRn interactions across species: implications for therapeutic antibodies. Int Immunol. 2001 Dec 12;13:1551–9. doi: 10.1093/intimm/13.12.1551. [DOI] [PubMed] [Google Scholar]
- [15].Kacskovics I, Kis Z, Mayer B, West AP, Jr, Tiangco NE, Tilahun M, et al. FcRn mediates elongated serum half-life of human IgG in cattle. Int Immunol. 2006 Apr 4;18:525–36. doi: 10.1093/intimm/dxh393. [DOI] [PubMed] [Google Scholar]
- [16].Lin G, Nara PL. Designing immunogens to elicit broadly neutralizing antibodies to the HIV-1 envelope glycoprotein. Curr HIV Res. 2007 Nov 6;5:514–41. doi: 10.2174/157016207782418489. [DOI] [PubMed] [Google Scholar]