Abstract
Glucans are known to promote wound repair. Non-cellulosic β-glucans are recognized as potent immunological activators. β-Glucans are generally safe and are known to attenuate the rate of postoperative infection. Glyc101 is a particulate β-glucan isolated from Saccharomyces cerevisiae. In this study, the hypothesis that Glyc101 regulates wound macrophage function was tested. Glyc101 induced TNFα transcription in macrophages isolated from murine wound site. Multiplex assay identified IL-10 and TNFα as two cytokines that are induced by Glyc101 in human blood monocyte derived macrophages. Glyc101-induced TNFα production was observed to be mediated via the TLR-2 and dectin-1 receptors, receptor tyrosine kinases and NFκB activation. In murine wound macrophages, Glyc101 potentiated PMA-induced respiratory burst. In vivo, implantation of Glyc101 enriched PVA-sponges at the wound-site induced TNFα expression in macrophages. Consistently, Glyc101 induced TNFα expression in wound-site macrophages isolated from two patients with chronic wounds. These observations establish the translational significance of the net findings of this study. Activation of wound macrophages by Glyc101 represents one of the potential mechanisms by which this beta-glucan may benefit chronic wounds where inefficient inflammatory response is one of the underlying causes of impaired healing.
Keywords: β-glucan, inflammation, macrophage, NFκB, TNFα, wound healing
INTRODUCTION
Chronic wounds fail to proceed through an orderly and timely reparative process to restore structural and functional integrity of the injured site (1). Often disguised as a comorbid condition, chronic wounds represent a silent epidemic that affects a large fraction of the world population and poses a major and gathering threat to public health and the economy (2). In the United States alone, chronic wounds affect 6.5 million patients (3). The immense economic and social impact of wounds in our society calls for enhancing our understanding of the biological mechanisms underlying cutaneous wound complications (2). Such understanding will help design improved therapeutic strategies for non-healing chronic wounds.
Dysregulated inflammatory response is a key characteristic feature of most chronic ulcers (4). During the early inflammatory period, macrophages in granulation tissue act as a source of growth and inflammation-mediating cytokines promoting the proliferation and differentiation of various wound-related cell types (5). Non-cellulosic β-glucans are generally recognized as potent immunological activators (6). The β-glucans consist of a backbone of glucose residues linked by beta-(1 →3)-glycosidic bonds, often with attached side-chain glucose residues joined by beta-(1 →6) linkages. β-Glucans are not synthesized by humans. The human immune system therefore recognizes this class of compounds as being exogenous and triggers both innate and adaptive immune responses (6). Structurally different β-glucans appear to have different affinities toward receptors of our immune systems and thus generate markedly different host responses. Traditionally, β-glucans have been used as vehicles for fibroblast formulations or as bandage-like coverings for burns or autograft sites. In a rat wound model, β-glucan markedly increased leukocyte recruitment to the wound site (7). Interventions promoting early migration of immune cells into a wound area may be helpful to mount a more effective healing response (8). Clinically, β–glucans have been reported to be generally safe and well tolerated as well as effective to attenuate the rate of postoperative infection (9). The response of wound-site macrophages to β–glucans remains unknown.
Glucans can be short or long, branched or unbranched, α or β isomers, and soluble or particulate (10). Current studies addressing the immune responses to β–glucans fail to recognize the unique properties of each variety of β–glucan resulting in a somewhat confusing literature (10). In this work we therefore sought to characterize the mechanism of action of a specific β-glucan, Glyc101. Glyc101 is a particulate β–glucan isolated from Saccharomyces cerevisiae (Figure 1). Macrophage function is known to be compromised under specific conditions such as diabetes (11, 12). Loss of macrophage function impairs healing responses (13). We were therefore led to test whether Glyc 101 may promote wound macrophage activity, a critical factor for prompt and healthy healing. The primary objective of this study was to investigate the mode of action of Glyc101 in modifying the function of wound-derived macrophages from mice and humans.
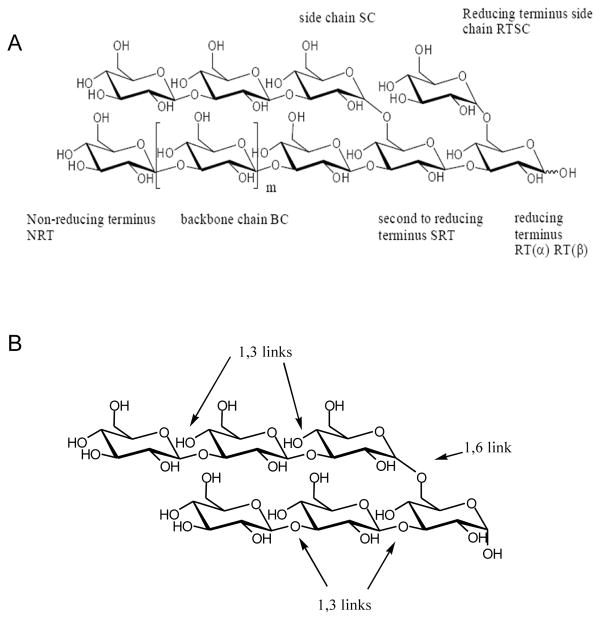
Figure 1. GLYC101 structure and nomenclature.
GLYC101 is a poly-(1,3)- β-D-glucopyranosyl-(1,6)- β-D-glucopyranose or (1→3;1→6)- β-D-glucan. A. The illustration of the structural features and nomenclature system of GLYC101; B. Structure showing repeating 1,3 and 1,6 glucopyranosyl units.
MATERIALS & METHODS
Implantation of polyvinyl alcohol (PVA) sponges
Circular (8 mm) sterile PVA sponges were implanted subcutaneously on the back of 8–10 wks old C57BL6 mice (11). In brief, following induction of anesthesia by isofluorane inhalation, the dorsal area was shaved and cleaned with betadine. A midline 1cm incision was created with a scalpel. Small subcutaneous pockets were created by blunt dissection, two pockets per animal. Two PVA sponges (8 mm) were inserted to each pocket. Incisions were closed with skin staples (9 mm) or suture (5-0 Surgiline™). Animals were then returned to clean cages for the monitoring of recovery. For final harvest of the PVA sponges, the animals were euthanized by CO2 inhalation (11). All animal studies have been approved by Ohio State University’s Institutional Animal Care and Use Committee.
Isolation of wound macrophages from PVA sponges
Subcutaneously implanted PVA sponges were harvested on a designated day and a single wound cell suspension was generated from sponges by repeated compression. The cell suspension was filtered through a 70 μm nylon cell strainer (Falcon) to remove all the sponge debris. For macrophage isolations, magnetic cell sorting was carried out using mouse anti-CD11b tagged microbeads (Miltenyi Biotec, Auburn, CA). This procedure yields a purified (>95%) population of wound macrophages as determined by F4/80 staining (11). Subcutaneously implanted polyvinyl alcohol (PVA) sponges are extensively used as model for wound-healing studies especially those addressing inflammation (14).
Human subjects and sample collection
All human studies have been approved by Ohio State University’s Institutional Review Board (IRB). Subjects participating in the study were chronic wound patients seen at the Ohio State University (OSU) Comprehensive Wound Center (CWC) clinics and have been undergoing NPWT (negative pressure wound therapy) therapy for their wounds. The demographic characteristics of patients and wound related information have been presented in Figure 9B. Protocols were approved by the Ohio State University’s Institutional Review Board. Declaration of Helsinki protocols was followed and patients gave their written, informed consent. The NPWT dressing (sponges) were collected. Wound fluids and cells were derived from the NPWT dressing by lavaging the wound dressing with saline solution.
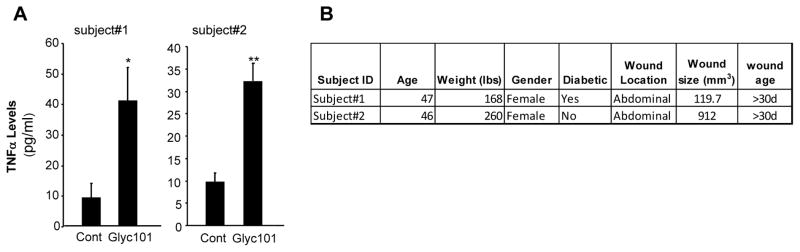
Figure 9. Ex vivo treatment of human chronic wound-derived macrophages with Glyc101 induced TNFα production.
Macrophages were isolated from chronic wounds of human subjects undergoing NPWT therapy. The isolated wound macrophages were plated and then treated with Glyc101 (20 μg/ml) for 24 h. TNFα levels were measured from cells using ELISA. Data from wound macrophages of two individuals are shown. Data are mean ± SD (n=4) for each subject. *, p<0.05; **, p<0.001 compared to untreated cells.
Human chronic wound macrophage isolation
The lavaged fluid was centrifuged to obtain wound cells. Wound macrophages were isolated from NPWT sponge derived wound cells using Ficoll density centrifugation followed by plating in culture plate for 3 h. The non-adherent cells were washed and removed.
Macrophage treatments
β-Glucan
Glyc101 (Glycotex Inc.) suspensions were prepared by adding appropriate amounts of dry Glyc101 to the macrophage culture media. The suspension was mixed thoroughly and then vortexed for an additional 30 sec. The suspension was then added to macrophage cultures directly at desired concentrations. Solutions/suspensions were prepared fresh for each treatment.
Blocking antibodies
To determine significance of TLR-2, TLR-4 or dectin-1 in Glyc101 mediated signaling, macrophages were treated with anti-TLR-2 (Biolegend San Diego, CA), TLR-4 (abcam, Cambridge, MA) and dectin-1 (R&D systems Minneapolis, MN) antibodies or corresponding IgG antibody prior to Glyc101 treatment. For treatment, the antibodies were directly added to the macrophage culture medium.
Cell viability
Macrophage viability was assessed by measuring lactate dehydrogenase (LDH) leakage from cells to media using in vitro toxicology assay kit from Sigma Chemical Co. (St. Louis, MO, USA) as previously described (15). Cell viability was determined using the following equation: viability = LDH activity of cells in monolayer/total LDH activity (i.e., LDH activity of cells in monolayer + LDH activity of detached cells + LDH activity in the cell culture media).
Wound macrophage cytokine analysis
Macrophages were seeded in 6-well or 12-well plates and cultured in RPMI 1640 medium containing 10% heat inactive bovine serum for 24 h under standard culture conditions. After 24 h, the media was collected and the cytokine levels in culture media were measured using commercially available ELISA kits (R & D Systems).
Isolation of RNA, reverse transcription and quantitative RT-PCR (qRT-PCR)
Total RNA was extracted using the mirVana RNA isolation kit (Ambion), according to the manufacturer’s instructions. The mRNA was quantified by real-time or quantitative (Q) PCR assay using the double-stranded DNA binding dye SYBR Green-I, as described previously (16, 17).
Phospho-RTK array
RTK antibody arrays were purchased from R&D Systems (#ARY-001, Minneapolis, MN). Cell harvest, sample preparation and RTK array assay were performed as recommended by the manufacturer.
Western Blot
Western blot was performed as described previously (11). Primary antibody against the phospho-MCSF-R (Tyr723) antibody and MCSF-R were purchased from Cell Signaling Technology, Inc (Danvers, MA).
Nitric oxide measurement
Nitric oxide production by wound macrophages was assessed using the Griess reaction that measures nitrite, a stable end product of the reaction of nitric oxide and oxygen. Wound macrophages were plated and activated with lipopolysaccharide (LPS; 1 μg/ml). After 12 h treatment, supernatants from wound macrophages were collected and nitric oxide was measured using Greiss reagent, as described previously (18).
Superoxide anion measurement
Superoxide anion generation was measured with the LumiMax® superoxide anion detection kit (Stratagene, La Jolla, CA), according to the manufacturer’s instructions. Briefly, freshly isolated wound macrophages (1×106 cells) were treated with phorbol 12-myristate 13-acetate (PMA, 1 μg/ml) or control solution for 10 min. Superoxide was measured at 430 nm with a luminometer (model Lumat LB9507; Berthold Technologies, Bad Wildbad, Germany) at 5 min intervals for 30 min.
Assay of DNA-binding activity of NFκB by an enzyme-linked immunosorbent assay
For the assay, a TransAm® NFκB transcription assay ELISA-based kit (Active Motif, Carlsbad, CA) was used. The assay uses 96-well plates to which oligonucleotide containing the 5′-GGGACTTTCC-3′ sequence is immobilized. Homo- and heterodimers of members of the Rel/NFκB family specifically recognize this sequence. The NFκB dimers contained in nuclear extracts bind specifically to this oligonucleotide and are detected by an appropriate antibody. Secondary antibody was conjugated with horseradish peroxidase. The TransAm® NFκB transcription assay is a colorimetric assay which was performed as per manufacturer’s instructions and quantified by a spectrophotometer.
Multiplex assay
Multiplex cytokine assay was performed using a Luminex assay. Luminex assays are based on xMAP technology (multi-analyte profiling beads) enabling the detection and quantification of multiple protein targets simultaneously. TNF-α, IL-4, IL-6 and IL-10 were simultaneously detected from culture media using a 4-plex cytokine assay system (Panomics).
Statistics
Data are reported as mean ± SD of at least three experiments. Comparisons among multiple groups were made by analysis of variance ANOVA. p<0.05 was considered statistically significant.
RESULTS
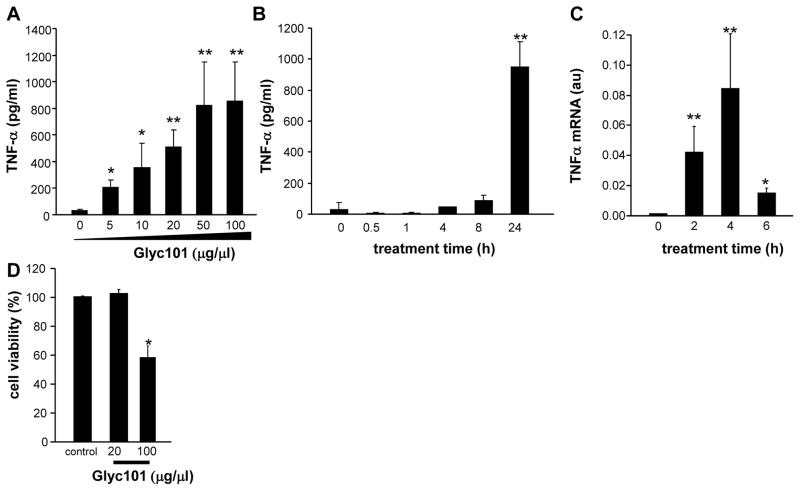
Treatment of murine wound macrophages with varying concentrations (5–100 μg/ml; 24 h) of Glyc101 resulted in a dose-dependent induction of TNFα with a maximum inducible concentration of 50 μg/μl (p<0.05; Figure 2A). Next, the kinetics of TNFα production by wound macrophages was determined following treatment with Glyc101 (20 μg/ml) for 0–24 h. Compared to the earlier time points, the highest concentration of TNFα was noted at 24 h post Gly101 treatment (p<0.05; Figure 2B). TNFα mRNA expression was measured in wound macrophages treated with Glyc101 (20 μg/ml) for 0–6 h using real-time PCR. In macrophages derived from the murine wound, TNFα mRNA levels were elevated at 2, 4, and 6 h post-treatment (p<0.05; Figure 2C) demonstrating that Glyc101 increases TNFα gene transcripts in wound macrophages. Next, to determine the possible toxicity of Glyc101, wound macrophages were treated with Glyc101 (20 and 100 μg/ml) for 24 h. The percent loss in viability of macrophages following Glyc101 treatment was determined using the lactate dehydrogenase (LDH) leakage assay. Glyc101 was observed to be not toxic at 20 μg/ml (p>0.05), whereas 100 μg/ml produced significant cell death (p<0.05; Figure 2D) in wound macrophages.
Figure 2. Glyc101 induced TNFα production and gene expression in macrophages isolated from murine wound.
A. Murine wound macrophages were treated with Glyc101 at varying dosages of 5–100 μg/ml for 24h; B. Kinetics of TNFα production by wound macrophages in culture media was measured following treatment with Glyc101 (20 μg/ml) for 0–24 h; C. TNFα mRNA expression was measured in wound macrophages treated with Glyc101 (20 μg/ml) for 0–6 h using real-time PCR. TNFα mRNA level in cells was normalized against GAPDH (housekeeping gene); D. Murine wound macrophages were treated with Glyc101 for 24 h at the indicated dosages. The % loss in viability of macrophage following GLyc101 treatment was determined using lactate dehydrogenase (LDH) leakage assay. Data are mean ± SD (n=3). *, p<0.05; **, p<0.001 compared to control cells.
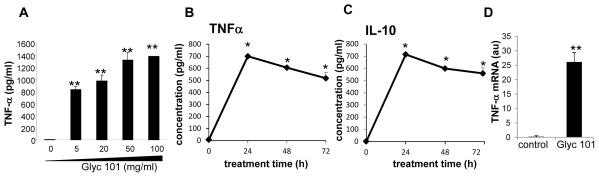
A four-plex cytokine assay was performed to simultaneously detect the levels of IL-4,-6,-10 and TNFα in culture media obtained from human blood monocyte derived macrophages (HBMM) treated with 20μg/ml Glyc101 for 24 h. Among the four cytokines assayed using multiplex assay, Glyc 101 treatment significantly induced production of TNFα and IL-10 levels in HBMM. Glyc101 had no effect on IL-6 or IL-4 production (data not shown). The kinetics of induction in the levels of TNFα and IL-10 was verified using standard ELISA assay (Figure 3B–C). Similar to that observed in mouse wound macrophages, a dose dependent increase in TNFα protein was noted in HBMM treated with Glyc101 (Figure 3A). The increase in TNFα protein was preceded by induction in TNFα mRNA (Fig 3C).
Figure 3. Glyc101 induced TNFα production and gene expression in human blood monocyte derived macrophages (HBMM).
A. Effect of treatment of varying dosages of Glyc101 for 24 h in HBMM TNFα production. Data are mean ± SD (n=5). **, p<0.001 compared to control cells; B. Effect of Glyc101 (20 μg/ml) for 24 h in HBMM IL-10 production. Data are mean ± SD (n=5). **, p<0.001 compared to control cells. C. Effect of Glyc101 (20 μg/ml) treatment for 4 h in HBMM TNFα gene expression measured using real-time PCR. TNFα mRNA level in cells was normalized against β-actin (housekeeping gene). Data are mean ± SD (n=3). **, p<0.001 compared to control cells.
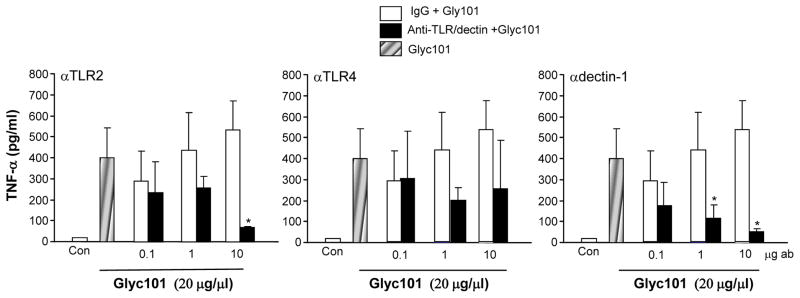
Dectin-1 (Dentritic cell-associated C-typelectin-1; CLECSF12, CLEC7A) is a type-II transmembrane protein and a member of the group V non-classical NK C-type lectin-like (NKCL) receptors (10). Dectin-1 specifically recognizes soluble or particulate β-1,3- and/or β-1,6-glucans, which are found primarily in the cell walls of fungi, but also in plants and some bacteria (10). In macrophages, β-glucans stimulate TNFα production with co-stimulation of Myd88 coupled toll like receptors (TLRs) (19). To determine whether Glyc101 signals through the common innate immune receptors, TLR-2, TLR-4 or dectin-1, HBMMs were pretreated with anti-TLR-2, TLR-4 and dectin-1 antibodies (or corresponding IgG antibody as control) at varying concentrations (0.1–10 μg/ml) for 30 min, followed by activation with Glyc101 (20 μg/ml) for 24 h. Glyc101-induced TNFα production was attenuated in anti-TLR-2 as well as dectin-1 antibody-treated cells, suggesting that Glyc101 signals via TLR-2 and dectin-1 to increase TNFα (p<0.05; Figure 4).
Figure 4. TLR2 and dectin-1 receptors mediate Glyc101 induced TNFα release from macrophages.
HBMM were pretreated with blocking anti-TLR-2, -4 and dectin-1 or corresponding IgG antibody (ab) at varying concentrations (0.1–10 μg/ml) for 30 min followed by activation with Glyc101 (20 μg/ml) for 24 h. TNFα levels were measured using ELISA. Data are mean ± SD (n=3). *, p<0.05 compared corresponding IgG treated cells.
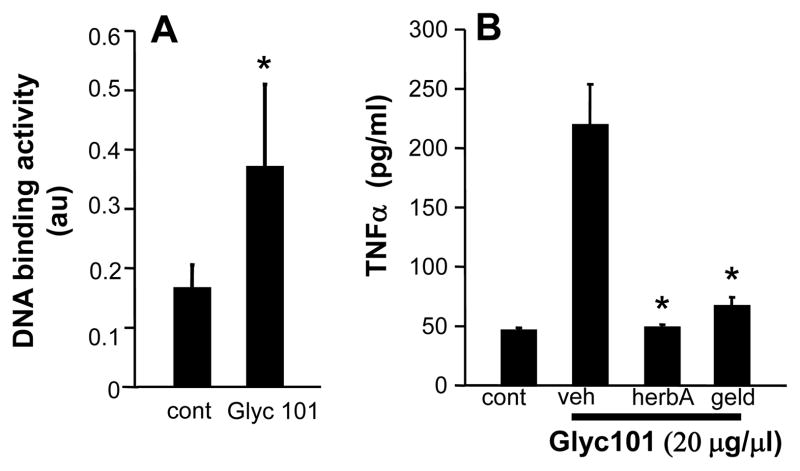
Co-stimulation of Dectin-1 and TLR-2 by β-glucan to produce TNFα is mediated via signaling through the spleen tyrosine kinase (Syk) pathway (20) We determined the role of receptor tyrosine kinase (RTK) signaling in Glyc101-induced TNFα production. Pretreatment of HBMMs with the RTK inhibitors herbimycin A (5 μg/ml) or geldanamycin (1 μg/ml) for 30 min resulted in attenuated production of TNFα by Glyc101 (20 μg/ml, 24 h) (Figure 5A), suggesting a role of RTKs in Glyc101-induced TNFα expression in macrophages.
Figure 5. Involvement of NFκB and tyrosine kinase pathway in Glyc101 induced TNFα production by human macrophages.
A. HBMM were treated with Glyc101 (20 μg/ml) for 1 h. NFκB DNA binding of nuclear extracts was assayed. Data are mean ± SD (n=3). *, p<0.05 compared to untreated cells; B. HBMM were pretreated with RTK inhibitors herbimycin A (5 μg/ml, HerbA) or geldanamycin (1 μg/ml, Geld) for 30 min followed by activation with Glyc101 (20 μg/ml) for 24 h. TNFα levels were measured using ELISA. Data are mean ± SD (n=6). *, p<0.05 compared to control cells. Veh, vehicle.
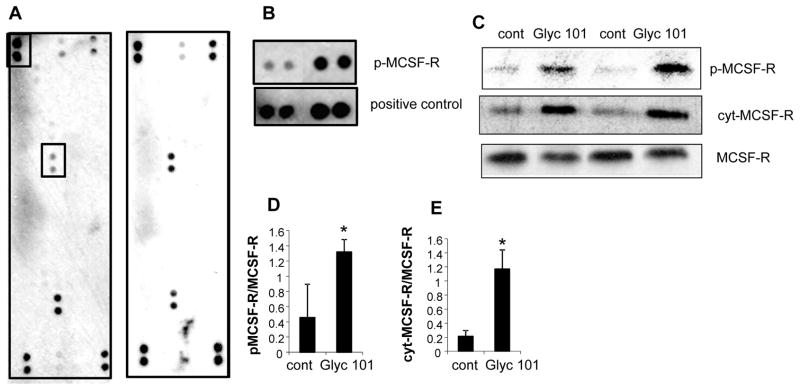
To investigate which specific RTKs are activated as a result of Glyc101 treatment, we used an antibody array that allows simultaneous assessment of the phosphorylation status of 45 RTKs (human phospho-RTK Array, ARY001, R&D Systems). RTK array data demonstrated that phospho-MCSF-R was specifically induced in cells treated with Gyc101 (Figures 6A–B). RTK array data was verified using convention Western blotting using anti-bodies against phospho-MCSF-R and MCSF-R. A potent induction in phosphorylation (Tyr723) of MCSF-R was observed (Figure 6C–D). Binding of M-CSF to its receptor induces receptor dimerization, activation and autophosphorylation of cytoplasmic tyrosine residues followed by intramembrane cleavage and release of the cytoplasmic domain into the cytosol. An increase in the cytoplasmic MCSF-R was also observed (Figure 6C & E). Taken together, the data suggest that phosphorylation of MSCF-R may be a potential mechanism that is involved in Glyc101-induced NFκB activation and subsequent TNFα production pathway.
Figure 6. Induction in phospho-MCSF-R by Glyc101.
A phospho-RTK array reveals that Glyc101 treatment MCSF-R In the array, each RTK is spotted in duplicate. Hybridization signals at the corners serve as controls Each RTK is spotted in duplicate: The pairs of dots in each corner are positive controls. Each pair of positive RTKdots isdenoted by a red numeral, with the identity of the corresponding RTKs listed below the arrays. B. A control and positive dots i.e., (p-MCSF-R) shown as boxed in panel A are shown side by side for better comparison. C. Cells treated with Glyc101 (20 ug/ul) D–E. Densitometer quantification of the blots shown in panel C. data was normalized to native MCSF-R band. Data are mean±SD (n=3). *, p<0.05 compared to non Glyc101 treated control (cont). D. phospho-MCSF-R. E. MCSF-R cytoplasmic (cyt).
Intracellular signaling via dectin-1 has been shown to activate the canonical NFκB pathway in a tyrosine kinase activation-dependent manner (21). DNA binding activity of NFκB in nuclear extracts of Glyc101-treated (20 μg/ml for 1h) HBMM was elevated (p<0.05; Figure 5B), suggesting activation of NFκB mediated signaling by Glyc101.
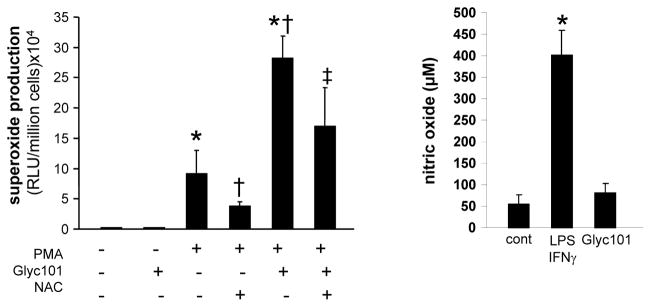
Phagocytosis of β-glucan particles by Dectin-1 activates production of reactive oxygen species (ROS) (22). To investigate the effect of Glyc101 treatment on ROS production, superoxide generation was induced in mouse wound macrophages by PMA (1 μg/ml) in the presence or absence of Glyc101 (20 μg/ml). Superoxide production was detected using Lumimax, a chemiluminiscence based assay system as described in methods. Both PMA and Glyc101-induced superoxide production which was sensitive to the antioxidant N-Acetyl-Cysteine (NAC, 10 mM) treatment (p<0.05; Figure 7A). Co-stimulation of Glyc101 with PMA resulted in the production of superoxide that was higher than treatment of either of these compounds alone (p<0.05). The effect of Glyc101 treatment on another reactive species i.e., nitric oxide (NO) was determined in macrophages stimulated with IFNγ and LPS. Glyc101 treatment did not influence LPS+IFNγ-induced NO production (Figure 7B) suggesting a specific activation of superoxide generating systems following Glyc101 treatment.
Figure 7. Glyc101 promoted inducible superoxide production by macrophages isolated from murine wounds.
A Superoxide production was induced in murine wound macrophages by phorbol 12-myristate 13-acetate (PMA, 1 μg/ml) in the presence or absence of Glyc101 (20 μg/ml). Superoxide production was detected using Lumimax chemiluminiscence based assay system as described in Methods. Inducible superoxide production was sensitive to antioxidant N-Acetyl-Cysteine (NAC, 10 mM) treatment. Data are mean ± SD (n=3). *, p<0.05 compared to control non-PMA treated cells. †, p<0.05 compared to PMA treated cells; ‡, p<0.05 compared to Glyc101 treated cells; B. Nitric oxide (NO) level was measured in culture media using Griess reagent. NO production was induced in mouse macrophages by activating cells with IFNγ and LPS for 24 h. Levels of NO remained unchanged in macrophages treated with Glyc101 (20 μg/ml) for 24 h. Data are mean ± SD (n=3). *, p<0.05 compared to control (cont).
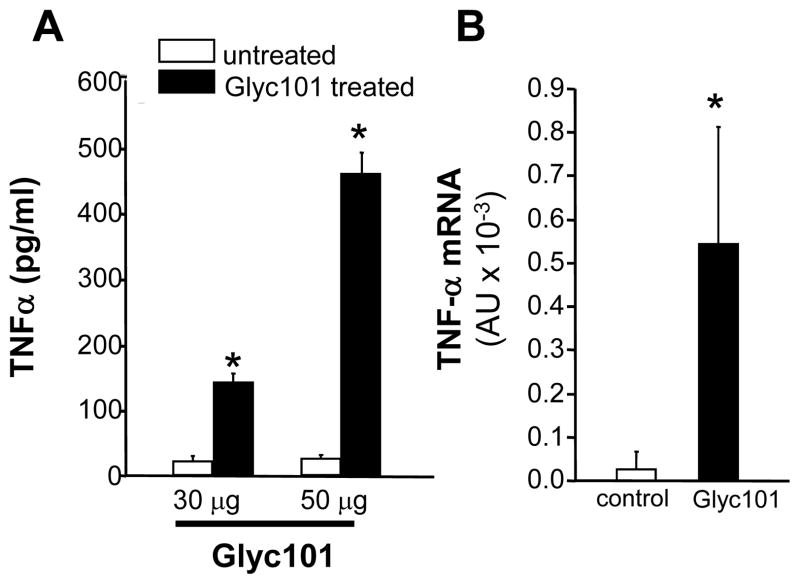
Next, the effect of Glyc101 treatment on macrophage recruitment and function in whole animals was evaluated. PVA sponges containing 30 or 50 μg Glyc101 aqueous suspension were implanted subcutaneously in mice. Wound macrophages were isolated from implanted PVA sponges after 5 days and cultured for 24 h. TNFα production (Figure 8A) as well as mRNA (Figure 8B) levels were significantly (p<0.001) elevated in macrophages derived from sponges treated with Glyc101 in vivo.
Figure 8. Murine wound macrophages harvested from Glyc101 supplemented PVA sponges produced increased TNFα mRNA and protein.
PVA sponges containing 30 or 50 μl of Glyc 101 aqueous suspension (1 mg/ml) were implanted in mice subcutaneously. Wound macrophages were isolated from implanted PVA sponges 5 days after implantation. A. Macrophages were plated in culture plates. TNFα-levels in culture medium was measured using ELISA 24h after plating; B. TNFα mRNA levels in macrophages harvested from Glyc101 treated PVA sponges. Mean ±SD (n=5); *, p<0.001 compared to untreated group.
Finally, to establish the translational significance of data obtained from mouse wound macrophage and cultured human macrophages, we used macrophages isolated from chronic wounds of human subjects undergoing NPWT therapy at The Ohio State University Comprehensive Wound Center. Macrophages were isolated and plated followed by treatment with Glyc101 (20 μg/ml) for 24 h. TNFα production by macrophages from both patients was increased after Glyc101 treatment (Figure 9). These data suggest that Glyc101 is capable of enhancing TNFα levels in macrophages derived from human chronic wounds.
DISCUSSIONS
The present study provides first evidence demonstrating that Glyc101, a novel β-glucan, potently induces TNFα production in wound macrophages via activation of dectin-1 and TLR2 signaling pathways. β-Glucans are major cell wall structural components in fungi and are also found in plants and some bacteria. Among β-glucans, 6-branched 1,3-β-glucan is the best characterized. These polysaccharides represent a heterogeneous group of glucosepolymers, consisting of a backbone of β (1 →3)-linked β-D-glucopyranosyl units with β (1 →6)-linked side chains of varying distribution and length (23). These carbohydrates are considered to be classic pathogen-associated molecular patterns (PAMPs) and are recognized by the innate immune system of vertebrates (24).
Glyc101 enhanced TNFα production by wound and cultured macrophages from humans and mice suggesting that the recognition and signaling pathways for this compound are present in wound site macrophages of both species. TNFα is a pleiotropic cytokine that regulates the inflammatory response to wounding. The overall role of TNFα in wound healing is contradictory (25). Depending on the concentration, length of exposure, and presence of other cytokines, the effect of TNFα can be beneficial or deleterious, ranging from tissue remodeling to inflammation, cytotoxicity, cachexia, shock, and death (25). In vitro, cytotoxic and growth inhibitory effects of TNFα have been demonstrated in endothelial cells and fibroblasts (26). Elevated levels of TNF-α has been observed in non-healing ulcers and associated with impaired healing in type 2 diabetes mouse models (27). A human blister diabetic wound model study recently show that elevated levels of TNFα continued into the resolving phase in diabetic wounds suggesting persistent TNFα levels in wounds may lead inflammatory non healing wounds (28). In addition to deleterious effects of high levels of TNFα, presence of this cytokine in wounds has also been known to have beneficial effects. Subcutaneous injection of TNF-α increases collagen deposition and enhances wound disruption strength (WDS) in adriamycin-treated animals (29). A recent study using TNFα null mice demonstrated that lack of TNFα potentiates Smad-mediated fibrogenic reaction in the healing dermis potentially leading to fibrosis, abnormal contraction and finally organ dysfunction (30). The functional significance of enhanced TNFα production by wound macrophage following treatment with Glyc101 in chronic wounds remains to be investigated.
The fungal β-glucan receptor, Dectin-1, contains an immunoreceptor tyrosine-based activation motif (ITAM)-like motif in its cytoplasmic tail, which is involved in cellular activation (31). Mutagenic analyses have indicated that at least two residues, Trp221 and His223, in dectin-1, are crucial for β-glucan binding (32). Dectin-1 lacks cysteine residues in its stalk region, indicating that it probably does not dimerize (31). Dectin-1 signals via a novel ITAM motif that becomes phosphorylated by Src family tyrosine kinases on receptor engagement. This allows recruitment and activation of the spleen tyrosine kinase (Syk), which then couples with downstream pathways, including those leading to production of ROS and, via the adaptor caspase recruitment domain 9 (CARD9), to the activation of NF-κB (21). While the components of the signaling pathway have yet to be fully elucidated, CARD9 has been identified as an essential downstream adaptor linking Syk-coupled receptors to the canonical NF-κB pathway (21). Macrophage-colony stimulating factor (M-CSF, CSF-1) receptor is an integral membrane tyrosine kinase encoded by the c-fms proto-oncogene. M-CSF receptor is expressed in monocytes (macrophages and their progenitors) and drives growth and development of this blood cell lineage (33). Phosphorylated Tyr723 binds the p85 subunit of PI3 kinase. Such activation is mediated both by binding of the PI3K p85 regulatory subunit to a site in the kinase insert domain in MCSF-R and independently by activation of a Src family kinase (SFK) via the phosphorylation of tyrosine 723 of CSF-1R (34). Activation of PI3K has been results in activation of NFκB (35). Further studies are required to characterize a specific role of MCSF-R in Glyc101 mediated TNFα production by macrophages.
Recent studies highlight the importance of co-operation between Toll-like receptors, the nucleotide oligomerization domain (Nod) proteins, and dectin-1 in regulating inflammatory responses (36). Dectin-1, co-operates with TLR2 to regulate optimal cytokine responses in macrophages (37). Activation of Dectin-1 receptor by β-glucan can induce phagocytosis, phospholipase A2 production and respiratory burst in macrophages (31). Activation of tyrosine kinase Syk is sufficient for the induction of the respiratory burst in macrophages (22). Dectin-1 signaling induced ROS production was NADPH phagocytic oxidase dependent as the ROS production was completely abolished by diphenyliodonium (22).
Glucans have been shown to promote wound repair (38). Likewise, adding activated macrophages prepared from blood was shown to be an efficient treatment of ulcers in elderly patients (39). Impaired wound healing as a result of immune suppression from trauma or other causes is common and increases the risk of infection and other complications (40). Agents that can activate immune cells under such conditions should promote healing. The results in this study indicate that Glyc101 is able to stimulate TNFα production as well as respiratory burst in macrophages derived from wound. Whether Glyc101 is able to activate macrophages from immune suppressed wounds remains to be investigated.
In summary, Glyc101 utilizes classical β-glucan induced dectin-1 and TLR2 signaling pathways for the activation of TNFα transcription and protein in macrophages isolated from both human as well as murine wounds. The signaling pathways involve receptor tyrosine kinases, ROS production and NF-κB activation. The study shows for first time MCSF-1R phopsphorylation by a glucan. Activation of wound macrophages by Glyc101 represents one of the potential mechanisms by which this compound may confer beneficial effects in the treatment of chronic wounds where inefficient immune response is one of the underlying causes of impaired healing.
Acknowledgments
The study was partly supported by NIH DK076566.
Footnotes
Disclosure of conflict. Glycotex Inc. provided GLYC101samples and partial funding for this study.
References
- 1.Lazarus GS, Cooper DM, Knighton DR, Margolis DJ, Pecoraro RE, Rodeheaver G, et al. Definitions and guidelines for assessment of wounds and evaluation of healing. Arch Dermatol. 1994;130(4):489–93. [PubMed] [Google Scholar]
- 2.Sen CK, Gordillo GM, Roy S, Kirsner R, Lambert L, Hunt TK, et al. Human skin wounds: a major and snowballing threat to public health and the economy. Wound Repair Regen. 2009;17(6):763–71. doi: 10.1111/j.1524-475X.2009.00543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer AJ, Clark RA. Cutaneous wound healing. N Engl J Med. 1999;341(10):738–46. doi: 10.1056/NEJM199909023411006. [DOI] [PubMed] [Google Scholar]
- 4.Pierce GF. Inflammation in nonhealing diabetic wounds: the space-time continuum does matter [comment] American Journal of Pathology. 2001;159(2):399–403. doi: 10.1016/S0002-9440(10)61709-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Leibovich SJ, Wiseman DM. Macrophages, wound repair and angiogenesis. Prog Clin Biol Res. 1988;266:131–45. [PubMed] [Google Scholar]
- 6.Chen J, Seviour R. Medicinal importance of fungal beta-(1-->3), (1-->6)-glucans. Mycol Res. 2007;111(Pt 6):635–52. doi: 10.1016/j.mycres.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 7.LeBlanc BW, Albina JE, Reichner JS. The effect of PGG-beta-glucan on neutrophil chemotaxis in vivo. J Leukoc Biol. 2006;79(4):667–75. doi: 10.1189/jlb.0305150. [DOI] [PubMed] [Google Scholar]
- 8.Ley K. Integration of inflammatory signals by rolling neutrophils. Immunol Rev. 2002;186:8–18. doi: 10.1034/j.1600-065x.2002.18602.x. [DOI] [PubMed] [Google Scholar]
- 9.Babineau TJ, Hackford A, Kenler A, Bistrian B, Forse RA, Fairchild PG, et al. A phase II multicenter, double-blind, randomized, placebo-controlled study of three dosages of an immunomodulator (PGG-glucan) in high-risk surgical patients. Arch Surg. 1994;129(11):1204–10. doi: 10.1001/archsurg.1994.01420350102014. [DOI] [PubMed] [Google Scholar]
- 10.Goodridge HS, Wolf AJ, Underhill DM. Beta-glucan recognition by the innate immune system. Immunol Rev. 2009;230(1):38–50. doi: 10.1111/j.1600-065X.2009.00793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khanna S, Biswas S, Shang Y, Collard E, Azad A, Kauh C, et al. Macrophage dysfunction impairs resolution of inflammation in the wounds of diabetic mice. PlosOne. 2010;5(3):e9539. doi: 10.1371/journal.pone.0009539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maree AF, Komba M, Dyck C, Labecki M, Finegood DT, Edelstein-Keshet L. Quantifying macrophage defects in type 1 diabetes. Journal of Theoretical Biology. 2005;233(4):533–51. doi: 10.1016/j.jtbi.2004.10.030. [DOI] [PubMed] [Google Scholar]
- 13.Eming SA, Krieg T, Davidson JM. Inflammation in wound repair: molecular and cellular mechanisms. Journal of Investigative Dermatology. 2007;127(3):514–25. doi: 10.1038/sj.jid.5700701. [DOI] [PubMed] [Google Scholar]
- 14.Efron DT, Barbul A. Subcutaneous sponge models. Methods in Molecular Medicine. 2003;78:83–93. doi: 10.1385/1-59259-332-1:083. [DOI] [PubMed] [Google Scholar]
- 15.Khanna S, Roy S, Ryu H, Bahadduri P, Swaan PW, Ratan RR, et al. Molecular basis of vitamin E action: tocotrienol modulates 12-lipoxygenase, a key mediator of glutamate-induced neurodegeneration. J Biol Chem. 2003;278(44):43508–15. doi: 10.1074/jbc.M307075200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy S, Khanna S, Rink C, Biswas S, Sen CK. Characterization of the acute temporal changes in excisional murine cutaneous wound inflammation by screening of the wound-edge transcriptome. Physiol Genomics. 2008 doi: 10.1152/physiolgenomics.00045.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roy S, Khanna S, Rink T, Radtke J, Williams WT, Biswas S, et al. P21waf1/cip1/sdi1 as a central regulator of inducible smooth muscle actin expression and differentiation of cardiac fibroblasts to myofibroblasts. Molecular Biology of the Cell. 2007;18(12):4837–46. doi: 10.1091/mbc.E07-03-0270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khanna S, Roy S, Packer L, Sen CK. Cytokine-induced glucose uptake in skeletal muscle: redox regulation and the role of alpha-lipoic acid. Am J Physiol. 1999;276(5 Pt 2):R1327–33. doi: 10.1152/ajpregu.1999.276.5.R1327. [DOI] [PubMed] [Google Scholar]
- 19.Tsoni SV, Brown GD. beta-Glucans and dectin-1. Ann N Y Acad Sci. 2008;1143:45–60. doi: 10.1196/annals.1443.019. [DOI] [PubMed] [Google Scholar]
- 20.Ferwerda G, Meyer-Wentrup F, Kullberg BJ, Netea MG, Adema GJ. Dectin-1 synergizes with TLR2 and TLR4 for cytokine production in human primary monocytes and macrophages. Cell Microbiol. 2008;10(10):2058–66. doi: 10.1111/j.1462-5822.2008.01188.x. [DOI] [PubMed] [Google Scholar]
- 21.Reid DM, Gow NA, Brown GD. Pattern recognition: recent insights from Dectin-1. Curr Opin Immunol. 2009;21(1):30–7. doi: 10.1016/j.coi.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Underhill DM, Rossnagle E, Lowell CA, Simmons RM. Dectin-1 activates Syk tyrosine kinase in a dynamic subset of macrophages for reactive oxygen production. Blood. 2005;106(7):2543–50. doi: 10.1182/blood-2005-03-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schmid F, Stone BA, Brownlee RT, McDougall BM, Seviour RJ. Structure and assembly of epiglucan, the extracellular (1-->3;1-->6)-beta-glucan produced by the fungus Epicoccum nigrum strain F19. Carbohydr Res. 2006;341(3):365–73. doi: 10.1016/j.carres.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Harada T, Ohno N. Contribution of dectin-1 and granulocyte macrophage-colony stimulating factor (GM-CSF) to immunomodulating actions of beta-glucan. Int Immunopharmacol. 2008;8(4):556–66. doi: 10.1016/j.intimp.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Sander AL, Henrich D, Muth CM, Marzi I, Barker JH, Frank JM. In vivo effect of hyperbaric oxygen on wound angiogenesis and epithelialization. Wound Repair Regen. 2009;17(2):179–84. doi: 10.1111/j.1524-475X.2009.00455.x. [DOI] [PubMed] [Google Scholar]
- 26.Mauviel A, Daireaux M, Redini F, Galera P, Loyau G, Pujol JP. Tumor necrosis factor inhibits collagen and fibronectin synthesis in human dermal fibroblasts. FEBS Lett. 1988;236(1):47–52. doi: 10.1016/0014-5793(88)80283-7. [DOI] [PubMed] [Google Scholar]
- 27.Siqueira MF, Li J, Chehab L, Desta T, Chino T, Krothpali N, et al. Impaired wound healing in mouse models of diabetes is mediated by TNF-alpha dysregulation and associated with enhanced activation of forkhead box O1 (FOXO1) Diabetologia. 2010;53(2):378–88. doi: 10.1007/s00125-009-1529-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landis RC, Evans BJ, Chaturvedi N, Haskard DO. Persistence of TNFalpha in diabetic wounds. Diabetologia. 2010;53(7):1537–8. doi: 10.1007/s00125-010-1766-0. [DOI] [PubMed] [Google Scholar]
- 29.Mooney DP, O’Reilly M, Gamelli RL. Tumor necrosis factor and wound healing. Ann Surg. 1990;211(2):124–9. doi: 10.1097/00000658-199002000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinozaki M, Okada Y, Kitano A, Ikeda K, Saika S. Impaired cutaneous wound healing with excess granulation tissue formation in TNFalpha-null mice. Arch Dermatol Res. 2009;301(7):531–7. doi: 10.1007/s00403-009-0969-z. [DOI] [PubMed] [Google Scholar]
- 31.Brown GD. Dectin-1: a signalling non-TLR pattern-recognition receptor. Nat Rev Immunol. 2006;6(1):33–43. doi: 10.1038/nri1745. [DOI] [PubMed] [Google Scholar]
- 32.Adachi Y, Ishii T, Ikeda Y, Hoshino A, Tamura H, Aketagawa J, et al. Characterization of beta-glucan recognition site on C-type lectin, dectin 1. Infect Immun. 2004;72(7):4159–71. doi: 10.1128/IAI.72.7.4159-4171.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Byrne PV, Guilbert LJ, Stanley ER. Distribution of cells bearing receptors for a colony-stimulating factor (CSF-1) in murine tissues. J Cell Biol. 1981;91(3 Pt 1):848–53. doi: 10.1083/jcb.91.3.848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee AW, States DJ. Both src-dependent and -independent mechanisms mediate phosphatidylinositol 3-kinase regulation of colony-stimulating factor 1-activated mitogen-activated protein kinases in myeloid progenitors. Mol Cell Biol. 2000;20(18):6779–98. doi: 10.1128/mcb.20.18.6779-6798.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Li Y, Yu M, Chen B, Shen B. Lineage-dependent NF-kappaB activation contributes to the resistance of human macrophages to apoptosis. Hematol J. 2003;4(4):277–84. doi: 10.1038/sj.thj.6200252. [DOI] [PubMed] [Google Scholar]
- 36.Underhill DM. Collaboration between the innate immune receptors dectin-1, TLRs, and Nods. Immunol Rev. 2007;219:75–87. doi: 10.1111/j.1600-065X.2007.00548.x. [DOI] [PubMed] [Google Scholar]
- 37.Dennehy KM, Ferwerda G, Faro-Trindade I, Pyz E, Willment JA, Taylor PR, et al. Syk kinase is required for collaborative cytokine production induced through Dectin-1 and Toll-like receptors. Eur J Immunol. 2008;38(2):500–6. doi: 10.1002/eji.200737741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leibovich SJ, Danon D. Promotion of wound repair in mice by application of glucan. J Reticuloendothel Soc. 1980;27(1):1–11. [PubMed] [Google Scholar]
- 39.Danon D, Madjar J, Edinov E, Knyszynski A, Brill S, Diamantshtein L, et al. Treatment of human ulcers by application of macrophages prepared from a blood unit. Exp Gerontol. 1997;32(6):633–41. doi: 10.1016/s0531-5565(97)00094-6. [DOI] [PubMed] [Google Scholar]
- 40.Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care. 2006;12(4):325–32. doi: 10.1097/01.ccx.0000235210.85073.fc. [DOI] [PubMed] [Google Scholar]