Abstract
Objective
There is a bidirectional relationship between mood disorders (e.g., depression) and altered cardiovascular regulation (e.g., heart disease), however the precise causal and/or common mechanisms underlying this association are unclear. In previous studies we have noted indications of altered sympathetic drive to the heart in rats that exhibit anhedonia, an operational sign of depression induced by subjecting the animals to a series of mild and unpredictable stressors [chronic mild stress (CMS) rodent model of depression]. The purpose of the present study was to more fully characterize baroreceptor reflex function in rats with experimentally-induced depression.
Methods
Male, Sprague-Dawley rats were exposed to either 4 weeks of mild, unpredictable stressors (CMS group) or standard housing conditions (control group). Depression-like behavior, resting hemodynamic and cardiac parameters, and baroreceptor reflex function were investigated in all animals following the CMS period.
Results
CMS produced anhedonia, evidenced by reduced sucrose intake and sucrose preference, as well as elevated resting heart rate (HR), slightly elevated blood pressure, and reduced HR variability. These animals also exhibited significantly attenuated sympathoexcitatory responses to hypotension, and an elevation of basal sympathetic nerve activity.
Conclusions
These findings suggest that CMS is associated with altered sympathoexcitatory responses following baroreceptor unloading and provide further insights into potential common mechanisms underlying the association of depression and altered cardiovascular control.
Keywords: Autonomic nervous system, Baroreflex, Cardiovascular, Heart rate variability, Lumbar sympathetic nervous system activity, Mood disorders
INTRODUCTION
The syndrome of depression is bidirectionally associated with cardiovascular disease, indicating that the presence of one of these conditions increases an individual’s likelihood of developing the other disorder (1–5). While the co-morbidity of depression and heart disease has received increased attention in recent studies involving human subjects, understanding the precise causal and common mechanisms that underlie this association requires further experimental research. The link between depression and cardiovascular dysregulation may be mediated in part by autonomic mechanisms that contribute to the regulation of cardiovascular function. The arterial baroreceptor reflex (i.e., baroreflex) regulates blood pressure through reflex changes in autonomic activity in order to maintain cardiac output and peripheral vascular resistance. The arterial baroreflex relies on alterations of the combined influence of sympathetic and parasympathetic (vagal) nervous system activity on the heart as well as modulation of sympathetic tone to peripheral vascular beds such as the heart, kidneys, and brain (6–8). Therefore, study of the arterial baroreflex is ideally suited for characterizing cardiovascular autonomic reflex function of a particular animal or individual.
Cardiac reflexes are important in the pathogenesis of cardiovascular disease. Early studies suggested that arterial baroreflex dysfunction may contribute to the pathogenesis of hypertension (see 9). Also, in animal models of heart disease, a reduced baroreflex gain is associated with an increased risk of ventricular fibrillation during a brief ischemic episode (10, 11), and coronary artery occlusion has been shown to attenuate the baroreflex control of heart rate (HR) both in anesthetized dogs and in humans (12, 13). Reduced baroreflex sensitivity has also been shown to differentiate high- from low-risk patients recovering from myocardial infarction and heart failure (14). A reduction in the gain of the arterial baroreflex can therefore contribute to cardiovascular morbidity and mortality via a reduction in parasympathetic activity, an increase in sympathetic activity, or both.
Recent studies suggest that depression in humans may be associated with reduced baroreflex sensitivity. Using power spectral analysis to assess the magnitude of R-R interval changes associated with systolic blood pressure alterations, Watkins and Grossman (15) reported altered baroreflex sensitivity in cardiac patients with depression. Pitzalis et al. (16) observed reduced baroreflex sensitivity, measured by a noninvasive method, in post-myocardial infarction patients with self-reported depression. Furthermore, Broadley and colleagues (17) reported reduced baroreflex sensitivity in treated (remitted) depressed patients with no apparent heart disease or conventional cardiovascular risk factors. However, baroreflex dysfunction in depressed individuals has not been consistently demonstrated in all studies; a recent study found no association of baroreflex sensitivity with symptoms of depression in patients following acute myocardial infarction, but symptoms of anxiety were associated with reduced baroreflex sensitivity in this patient sample (18). Considered together, these findings indicate a need for using validated experimental methods to study more directly the potential changes in autonomic and baroreflex function in the context of depression.
Currently it is unclear whether the association of depression and cardiovascular dysregulation involves specific baroreceptor reflex changes or generalized sympathetic nervous system dysfunction that in turn may affect cardiovascular function. The study of autonomic function and cardiac reflexes in an animal model of depression can provide valuable insight into the precise physiological changes that are associated with depressive signs and symptoms. Therefore, the current study is an investigation of baroreceptor reflex function using direct measurements of basal cardiovascular function and sympathetic nerve activity in the chronic mild stress (CMS) rodent model of depression. The CMS model involves exposing rodents to a period of unpredictable, mild stressors, such as strobe light, white noise, and damp bedding. This model is a widely studied, valid, and reliable animal analog of depression, and has been shown to produce several behavioral changes that mirror the mood disorder (19–22). In addition to its utility for investigating depression-relevant behaviors, our laboratory has demonstrated that CMS is a useful model for studying cardiovascular changes associated with depression and potential causal and common mechanisms that underlie the association of depression and cardiovascular disease (23–26). For instance, CMS in rats has been shown to produce elevated resting HR, reduced resting HR variability, exaggerated pressor and HR responses to a novel acute stressor, sympathoexcitation, and increased susceptibility to ventricular arrhythmias (23, 24, 26).
The findings from human subjects and the CMS model of depression suggest that alterations in sympathetic function may underlie the association of depression-like behaviors and cardiovascular dysregulation. In the current study, behavioral and cardiovascular changes were investigated in the CMS model of depression with a focus on autonomic and baroreceptor reflex function determined by direct sympathetic nerve recordings and pharmacological manipulation of arterial pressure with vasoactive drugs. We predicted that exposure to CMS would induce behaviors relevant to depression, basal changes in cardiovascular function and dysfunction of the sympathetic nervous system and arterial baroreflex.
METHODS
Animals
Sixteen male Sprague-Dawley rats (Harlan, Indianapolis, IN), weighing 250–300 grams, were used for the experimental procedures. Rats were allowed one week to acclimate to the laboratory surroundings before beginning experimentation. All animals were housed in individual plastic cages with bedding for the duration of the experimental procedures unless otherwise noted. Food (Purina Rat Chow 5012) and tap water were available ad libitum for the duration of the experiments unless otherwise noted. Sucrose solution (1%) was available ad libitum for one week preceding the experimental procedures to allow for adaptation to its taste. The temperature was maintained at 22 ± 2° C. A 12/12 hour light/dark cycle was maintained, with lights on at 0600, unless otherwise noted. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and approved by the University of Iowa Animal Care and Use Committee.
General Experimental Design
Details for the experimental design and procedures are discussed in the following sections. Following adaptation to the laboratory environment and baseline sucrose tests, rats were randomly assigned to either the CMS (n = 8) or control group (n = 8). The CMS group was exposed to mild and unpredictable stressors for 4 weeks. Following the CMS period with weekly sucrose preference tests, all rats (CMS and control) were implanted with arterial and venous femoral catheters and a lumbar sympathetic nerve activity (LSNA) recording electrode under halothane anesthetic. Following recovery, baseline resting cardiovascular parameters [mean arterial pressure (MAP), HR and HR variability] and assessment of baroreflex responses to pharmacological stimuli were measured in all animals in an unanesthetized state.
Chronic Mild Stress
Animals were randomly separated into two groups, CMS (n = 8) and control (n = 8). The CMS procedure employed here was designed to maximize the unpredictable nature of the stressors while minimizing pain and discomfort; details of this procedure, including reliability and validity of the model, have been described in detail elsewhere (19, 20, 23, 27). Briefly, the CMS group was exposed to the following stressors in a randomized fashion during a one-week period: continuous overnight illumination (two 12-hour periods), 40° cage tilt (one 7-hour period), paired housing (one 16-hour period and one 20-hour period), damp bedding (300 ml water spilled onto bedding; one 16-hour period), acute water deprivation (one 16-hour period) exposure to an empty water bottle immediately following the period of acute water deprivation (one 1-hour period), stroboscopic illumination (300 flashes/minute; one 6-hour period and one 4-hour period), and continuous white noise (approx. 90 dB; one 4-hour period and one 3-hour period). The CMS procedure was carried out for a total of 4 weeks. Control animals were left undisturbed in their home cages with the exception of general handling (i.e., cage cleaning and measuring body weight), which was matched to that of the CMS group.
Sucrose Preference Tests
The methods for acute sucrose preference tests, used to operationally define anhedonia, have been described previously (23, 24). Anhedonia was defined specifically as a reduction in sucrose intake and sucrose preference relative to the control group and baseline values. Two baseline preference tests were performed (results were averaged), and preference tests were then conducted once weekly throughout the CMS period.
Catheter and Lumbar Sympathetic Nerve Recording
Electrode Surgical Implantation
Surgical procedures for the placement of femoral catheters and lumbar sympathetic recording electrodes were conducted under halothane anesthesia, using aseptic surgical techniques, similar to procedures described previously (28). Polyethylene (PE 10 fused to PE 50) catheters were inserted into the aorta and abdominal vena cava via the left femoral artery and vein for measurement of arterial pressure and administration of vasoactive drugs, respectively.
Using a midline abdominal incision, a branch of the lumbar sympathetic chain was dissected free. A bipolar Teflon insulated silver wire electrode (Medwire, 0.005 internal diameter, 36 gauge) threaded through silastic tubing (0.25 internal diameter) was placed around the isolated nerve. The nerve–electrode complex was covered with a polyvinylsiloxane gel (Coltene President, Mahwah, NJ) which was allowed to harden before closure. A ground wire was sutured to the abdominal wall, and incision sites were closed with suture. The catheters and the wire of the lumbar sympathetic recording electrode were tunneled subcutaneously and exteriorized at the dorsal cervical region. Catheters were filled with 10 U/ml of heparinized saline and capped with an airtight plug until the experiment. Animals were given subcutaneous fluids and Stadol (2 mg/kg; Bristol Myers Squibb, Princeton, NJ) for postoperative analgesia, and were monitored closely until they were fully awake and mobile. Following awakening from anesthesia, animals were returned to the home cages for 24 hours of recovery before any additional manipulations were conducted.
Measurement of Arterial Pressure and Heart Rate
Direct MAP and HR were recorded in unrestrained, conscious rats (CMS and control groups). On the morning of the test, animals were removed from their home cages and placed into an experimental cage that was located inside a Faraday cage to reduce electrical noise. All animals were allowed to adapt to the surroundings for several hours to allow for stabilization of cardiovascular parameters. Catheters were connected to a pressure transducer (Maxxim Medical, Athens, TX) coupled to a multi-channel recorder via a custom-designed amplifier (University of Iowa, Iowa City, IA). The analog input was converted into a digital signal using a PowerLab data acquisition system (ADInstruments, Mountain View, CA). MAP was derived electronically using a low-pass filter. HR was determined by measuring the number of heartbeats triggered from the arterial pressure pulse, and was calculated online.
Sympathetic Nerve Recordings
Sympathetic nerve and cardiac baroreflex activity were assessed according to procedures described elsewhere (29, 30). LSNA was amplified 2,000 times using a Grass preamplifier (P511), and filtered using a high-pass frequency level of 100 Hz and a low-pass frequency level of 3 kHz. Action potentials were monitored using a Tektronix oscilloscope and a custom designed audio monitor (Bioengineering, University of Iowa, Iowa City, IA). Nerve activity was rectified and integrated using a RMS converter with a time constant of 28 ms. The rectified, integrated signal was then electronically averaged, and this mean signal used as the relative measure of LSNA. Data signals were gathered and analyzed with a PowerLab data acquisition system (ADInstruments, Mountain View, CA). Background noise was determined at the end of the experiment after the rat was euthanized. The rectified, integrated, and amplified LSNA signal (in mV) was converted to absolute LSNA (in μV·s) using the following methods: 1) calibration of the signal via a known microvolt input, 2) dividing the amplified signal by the amplification (x2,000), and 3) measuring and subtracting out background electrical noise after each animal was euthanized. These methods were similar to those previously employed to measure absolute baseline sympathetic nervous system activity (30).
Baroreceptor Reflex Testing
Following adaptation to the testing cage, hemodynamic parameters were monitored for 30–60 minutes to ensure stabilization of MAP, HR and LSNA. After the collection of baseline parameters, arterial baroreflex curves were generated by producing ramp changes in arterial pressure over approximately 2–3 min. Initially, MAP was increased to 170–180 mmHg by infusion of the α1-adrenergic receptor agonist, phenylephrine (Sigma, St. Louis, MO), at increasing rates (2–25 μg/kg/min). MAP, HR and LSNA were allowed to return to within 10% of baseline values before proceeding with the experimental protocol. Arterial pressure was then decreased to 50–60 mmHg within 2–3 min by infusion of the vasodilator, sodium nitroprusside (Sigma), at sequentially increasing rates (10–100 μg/kg/min). The rate of change of arterial pressure was held constant by observing the recorded pressure alteration and varying the rate of infusion to produce a smooth ramp of pressure increase or decrease. Care was taken to keep the rate of change of arterial pressure similar in all animals, at approximately 1–2 mmHg/sec. Volumes infused did not exceed 100 μl. Baroreceptors were always activated first (phenylephrine infusion) prior to unloading (nitroprusside infusion) in order to minimize any potential effects of reflexly released humoral agents, such as vasopressin or angiotensin II, on baroreflex function.
Data Analysis
Values are presented as means ± standard error of the mean (SEM) for the indicated analyses, tables and figures. Follow-up analyses were conducted using Student’s t-tests (hypothesis-driven, statistically justified comparisons only) using a Bonferroni correction for all multiple comparisons. A probability value of P < 0.05 was considered to be statistically significant.
For sucrose preference tests, absolute water and sucrose intake were calculated for each group. The preference for sucrose, relative to water, was calculated using the following formula:
Absolute sucrose intake, sucrose preference, and body weight data were analyzed using mixed-design analyses of variance (ANOVA; one factor for independent groups and one repeated measures factor) and a priori Student’s t-tests where appropriate.
Cardiovascular data were analyzed using mixed-design ANOVAs and independent Student’s t-tests where appropriate. For HR variability, the systolic pulse recording was statistically analyzed by taking the standard deviation of all normal-to-normal intervals (SDNN index) from the systolic pulse waveform, from a 5-minute segment of data in each individual rat [as described by the Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology (31)]. This parameter was averaged within a group.
Data relating changes in HR to MAP and LSNA were fit to a sigmoidal logistic function using the following equation:
Parameters (P1–P4), which are used to describe basic baroreflex function, were generated from data fit to the logistic function. These parameters were: 1) the maximum LSNA or HR during decreases in arterial pressure (P1); 2) the coefficient used to calculate the gain as a function of pressure (P2); 3) the inflection point (MAP at the midpoint of the curve; P3); and 4) the minimum LSNA or HR during increases in arterial pressure (P4). In addition, the gain (G) of the reflex at each level of arterial pressure was calculated for the entire baroreflex curve using the following equation:
For each individual animal’s fit curve, the four parameters (P1–P4) and gain were derived. These parameters were averaged within a group. The average parameters were then statistically compared using independent Student’s t-tests. The mean parameters and gain were used to generate an average baroreflex curve for each group.
Baroreflex control of LSNA was expressed as a percentage of baseline or control LSNA before experimental interventions, or on an absolute (μV·s) basis. Relative quantification of baseline or control LSNA was considered to be 100%. This analysis allows for direct evaluation of the animal’s ability to reflexly increase or decrease LSNA relative to its basal level. Absolute quantification of LSNA was performed using the methods described above.
To determine the specific relation between anhedonia and sympathetic nervous system function, the levels of basal LSNA and maximum LSNA responses to decreases in arterial pressure were correlated with sucrose intake (CMS group only) using Pearson’s r correlation coefficient.
RESULTS
Sucrose Preference Tests
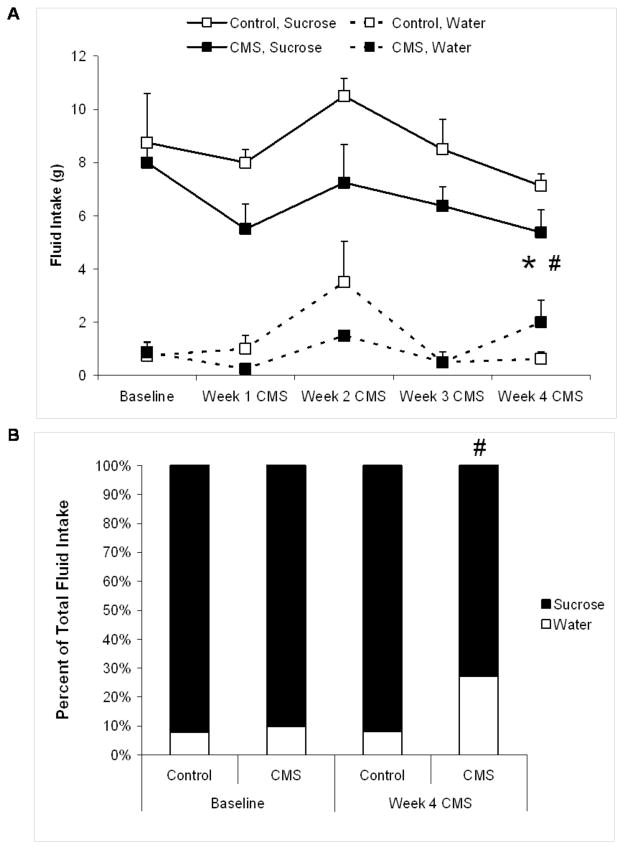
Figure 1 displays the fluid intake during the sucrose preference tests used to define anhedonia in the CMS and control groups at baseline and throughout the CMS period. Figure 1A presents absolute water and sucrose intake in the two groups. Mixed-design ANOVAs were performed on water and sucrose intake separately, across time (baseline through 4 weeks of CMS). For water intake there was a significant main effect of time [F(4,70) = 5.36, P < 0.05] and a significant group by time interaction [F(4,70) = 3.10, P < 0.05] however, Student’s t-tests showed no significant difference in water intake between CMS and control groups at baseline or following 4 weeks of CMS (P > 0.05 for both comparisons).
Figure 1.
Fluid intake and sucrose preference in chronic mild stress (CMS) and control groups. Panel A displays mean (+ SEM) water and sucrose intake at baseline and during 4 weeks of CMS. Panel B displays percent preference for sucrose at baseline and following 4 weeks of CMS. For absolute sucrose values and sucrose preference (hypothesis-driven, statistically justified comparisons only): *P < 0.05 vs. respective control value; #P < 0.05 vs. respective baseline value.
An ANOVA performed on sucrose intake yielded main effects of time [F(4,70) = 2.83, P < 0.05] and group [F(1,70) = 12.75, P < 0.05]. There was no difference in sucrose intake between the two groups at baseline (P > 0.05). Both groups showed a slight, but non-sigificant, reduction in sucrose intake following 1 week of CMS. Following 4 weeks of CMS, the CMS group consumed significantly less sucrose than its respective baseline value [t(7) = 2.12, P < 0.05] and the control group [t(14) = 1.80, P < 0.05]. Sucrose intake following 4 weeks of CMS in the control group was not significantly different from its respective baseline value (P > 0.05).
Figure 1B displays the percent preference for sucrose in CMS and control groups at baseline and following 4 weeks of CMS. An ANOVA yielded a significant main effect of time [F(4,70) = 3,58, P < 0.05]. The baseline preference for sucrose did not differ between CMS and control animals (P < 0.05). Following 4 weeks of CMS, the preference for sucrose was significantly reduced in the CMS group compared to its respective baseline value [t(7) = 1.93, P < 0.05] and reduced compared to the control group [t(14) = 1.68, P = 0.05].
Body Weight
To determine whether the CMS procedure significantly affected the body weight of animals in the present experiments, a mixed-design ANOVA was performed on the body weight of both groups (baseline through 4 weeks CMS). Baseline body weight was 317 ± 4 and 325 ± 4 grams in the control and CMS groups, respectively; body weight following 4 weeks of CMS was 370 ± 3 and 369 ± 3 grams in the control and CMS groups, respectively. As expected, a main effect of time was found [F(4,70) = 40.85, P < 0.05], but the main effect of group and the interaction were not significant (P > 0.05). No follow-up t-tests were performed.
Baseline Resting Hemodynamic Parameters
Baseline resting MAP, HR and HR variability were calculated in the CMS and control groups following 4 weeks of the CMS procedure (Figure 2). MAP was slightly, but significantly, elevated in the CMS group relative to the control group [t(12) = 1,78, P < 0.05]. HR was also significantly elevated in CMS rats [t(12) = 2.26, P < 0.05]. Resting HR variability (SDNN index) was slightly lower in the CMS group versus the control group [t(12) = 2.00, P = 0.05].
Figure 2.
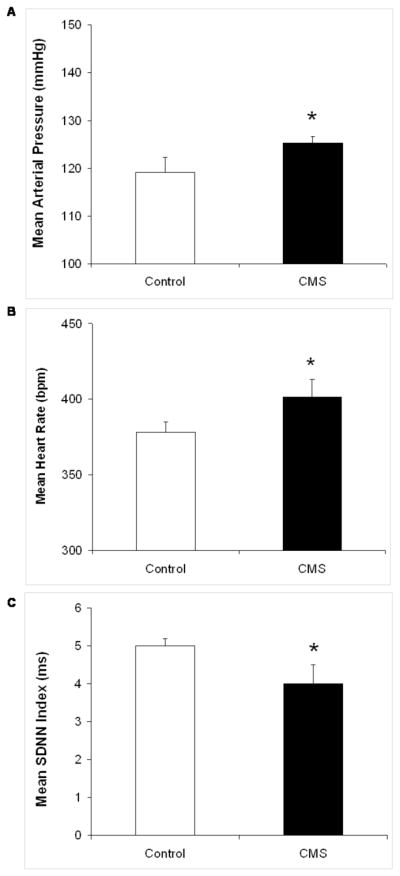
Mean (+ SEM) resting mean arterial pressure (MAP; Panel A), heart rate (HR; Panel B), and HR variability represented by the standard deviation of normal-to-normal (N-N) intervals (SDNN index; Panel C) in chronic mild stress (CMS) and control groups following 4 weeks of CMS. *P < 0.05 vs. control value.
Baroreceptor Reflex Responses
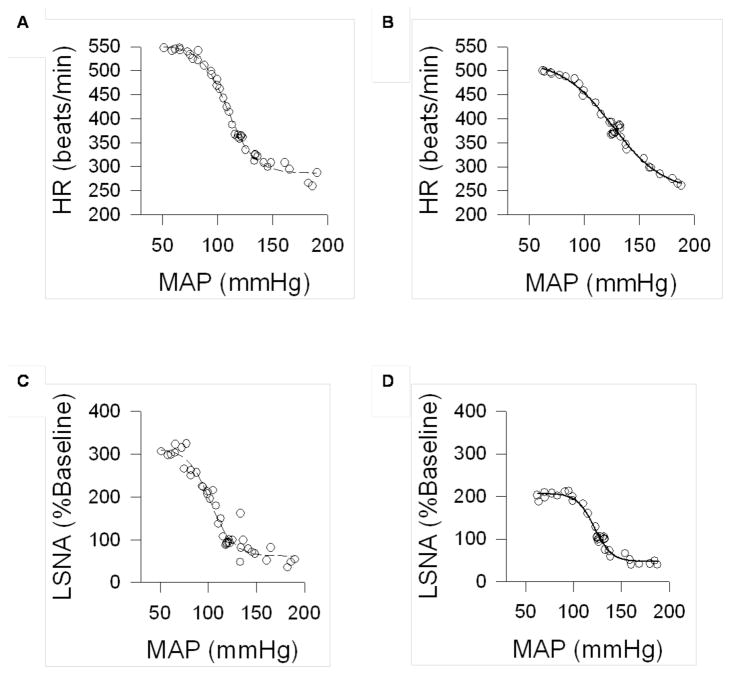
Increases in MAP through ramp infusions of phenylephrine elicited reflex reductions in HR and LSNA in both groups. Conversely, infusion of sodium nitroprusside elicited an increase in LSNA and a tachycardic response in the CMS and control groups. The change in MAP was controlled to approximately similar rates (1–2 mmHg/sec) in both groups. Sigmoid baroreflex curves demonstrating the goodness of fit from representative CMS and control rats are shown in Figure 3.
Figure 3.
Sigmoid curves showing baroreflex control of heart rate (HR; Panels A and B) and lumbar sympathetic nerve activity (LSNA, calculated as a percentage of baseline nerve activity; Panels C and D), illustrating goodness of fit for a control (Panels A and C) and chronic mild stress (CMS; Panels B and D) rat. Circles represent recorded data points, and the line represents the fit curve.
Baroreceptor Reflex Control of Heart Rate
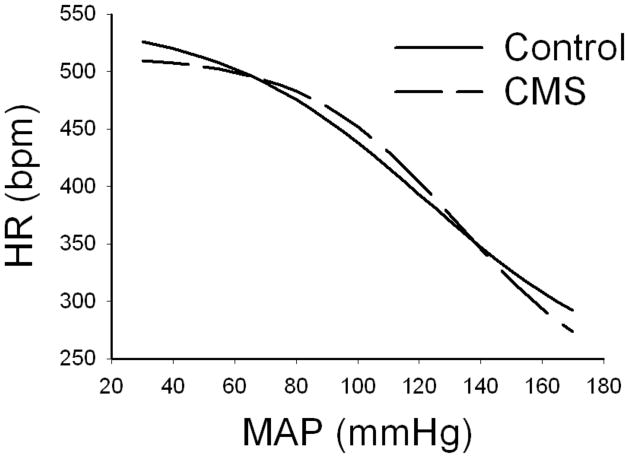
Mean sigmoid curves showing the baroreflex control of HR in CMS and control groups are depicted in Figure 4, with corresponding curve parameters listed in Table 1. There were no changes in baroreflex control of HR following CMS. This was further evidenced by no significant differences in any of the parameters describing the baroreflex curves (P > 0.05 for all comparisons). In addition, the peak gain (slope at the inflection point) of the HR baroreflex curves was similar between CMS and control animals (P > 0.05).
Figure 4.
Mean sigmoid curves showing baroreflex control of heart rate (HR) in chronic mild stress (CMS) and control groups during intravenous infusion of phenylephrine and sodium nitroprusside.
Table 1.
Curve parameters for baroreflex control of HR or LSNA in CMS and control rats
| Baroreflex Control of HR | ||||
|---|---|---|---|---|
| Maximum (bpm) | Midpoint (mmHg) | Minimum (bpm) | Peak Gain (bpm/mmHg) | |
| Control | 544.3 ± 11.7 | 122.1 ± 5.1 | 232.0 ± 11.6 | −2.8 ± 0.1 |
| CMS | 514.4 ± 23.8 | 133.0 ± 7.8 | 218.3 ± 34.0 | −3.0 ± 0.1 |
| Baroreflex Control of LSNA (% of Baseline Activity) | ||||
|---|---|---|---|---|
| Maximum (%LSNA) | Midpoint (mmHg) | Minimum (%LSNA) | Peak Gain (%LSNA/mmHg) | |
| Control | 300.4 ± 10.4 | 104.3 ± 3.4 | 67.0 ± 6.4 | −6.0 ± 1.0 |
| CMS | 233.3 ± 24.9* | 111.9 ± 2.9 | 58.7 ± 8.7 | −4.2 ± 0.9# |
| Baroreflex Control of LSNA (absolute μV·s activity) | ||||
|---|---|---|---|---|
| Maximum (μV·s) | Midpoint (mmHg) | Minimum (μV·s) | Peak Gain (μV·s/mmHg) | |
| Control | 5.5 ± 1.0 | 105.7 ± 3.8 | 1.6 ± 0.5 | −0.1 ± 0.02 |
| CMS | 6.3 ± 0.7 | 113.1 ± 1.2 | 1.1 ± 0.1 | −0.1 ± 0.02 |
Note: Values are means ± SEM.
P < 0.05 vs. respective control value.
P < 0.1 vs. respective control value. CMS, Chronic Mild Stress; HR, Heart Rate; LSNA, Lumbar Sympathetic Nerve Activity.
Baroreceptor Reflex Control of Lumbar Sympathetic Nerve Activity
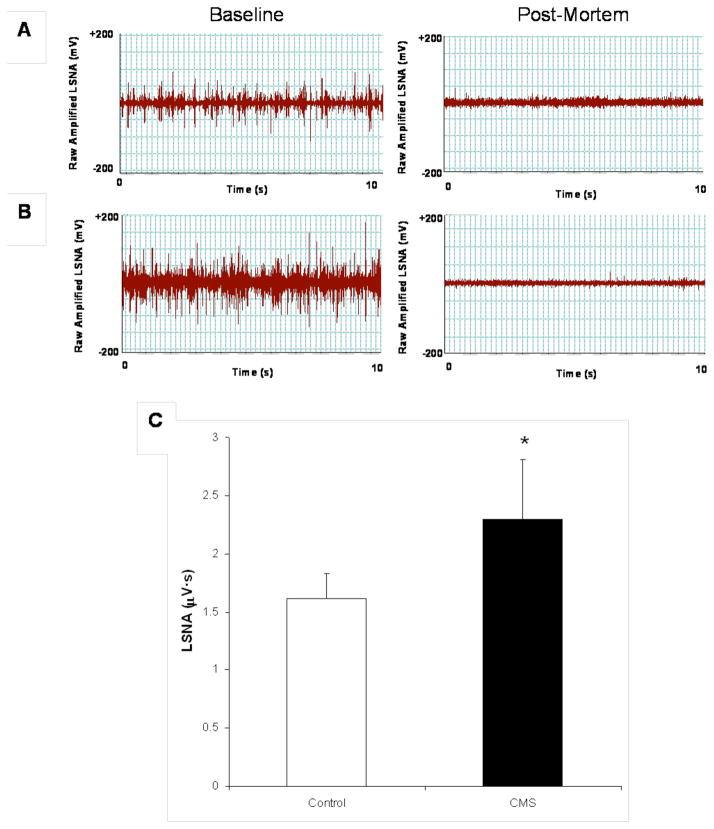
Basal LSNA was significantly elevated in the CMS group, compared to the control group [t(12) = 1.88, P < 0.05] (Figure 5). Mean data displaying the effects of CMS on baroreflex control of LSNA, calculated as a percentage of baseline nerve activity, are illustrated in Figure 6. Corresponding curve parameters for these figures are listed in Table 1. Both groups exhibited similar reductions in LSNA in response to an increase in MAP. However, when MAP was lowered through infusion of sodium nitroprusside, the maximal increase in LSNA was significantly attenuated in CMS rats relative to control rats [t(9) = 2.61, P < 0.05]. Baroreflex gain tended to be reduced in CMS animals (see Figure 6), however the peak gain was not significantly different between groups (P > 0.05).
Figure 5.
Lumbar sympathetic nerve activity (LSNA) following 4 weeks of chronic mild stress (CMS). Panels A and B display neurograms from representative control and CMS rats, respectively, showing raw, filtered, amplified LSNA (10 s segment) at baseline (left panels) and following sacrifice (right panel). Panel C displays mean (+ SEM) resting LSNA in CMS and control groups in absolute microvoltage activity (Panel C). *P < 0.05 vs. control value.
Figure 6.
Mean sigmoid curves showing baroreflex control of lumbar sympathetic nerve activity (LSNA), calculated as a percentage of baseline nerve activity in chronic mild stress (CMS) and control groups during intravenous infusion of phenylephrine and sodium nitroprusside. See text and Table 1 for results of statistical tests.
Relationship Between Behavior and Sympathetic Nervous System Function
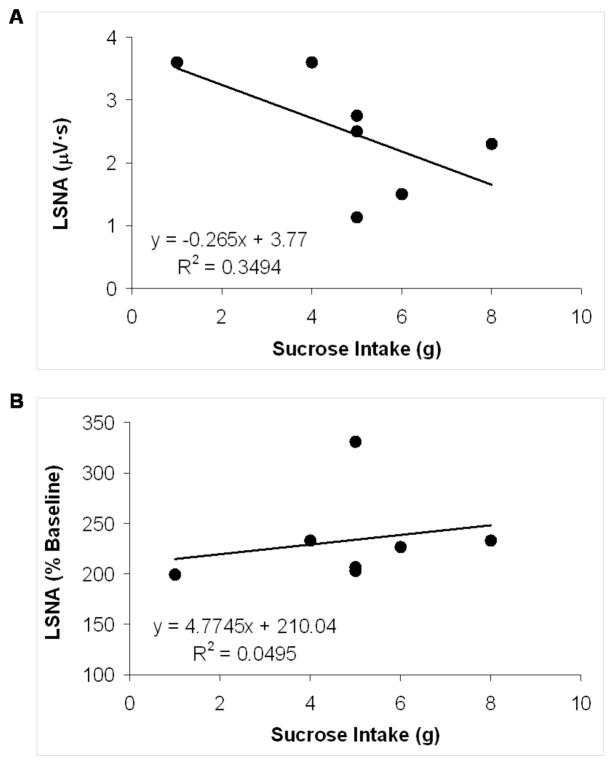
Pearson’s r correlation coefficients were calculated to determine the specific relations between degree of anhedonia and degree of sympathetic dysfunction in the CMS group. These correlations indicated predictable inter-relations between sucrose intake and basal LSNA levels (negative relationship) and between sucrose intake and the maximum LSNA response to the hypotensive challenge (positive relationship) (Figure 7). Due to the small sample sizes, statistical significance of the correlations was not computed here.
Figure 7.
Scatterplots showing the correlation of 4-week sucrose intake with maximal lumbar sympathetic nerve activity (LSNA) in response to baroreceptor unloading (Panel A), and 4-week sucrose intake with basal LSNA (Panel B), in the chronic mild stress (CMS) group. Linear trendlines, corresponding line equations, and R2 values are also shown. See text for a further description of results.
DISCUSSION
The present study was undertaken to determine whether behaviors relevant to depression, resting cardiovascular parameters, and arterial baroreflex function were altered in the CMS model of depression. To investigate these experimental questions, the current series of experiments measured anhedonia (sucrose intake), baseline cardiovascular parameters (MAP, HR, and HR variability), and baroreflex control of HR and LSNA in response to pharmacologically manipulated changes in arterial pressure. Sucrose intake and basal cardiovascular function were altered following exposure to CMS. Additionally, CMS was associated with an attenuated maximal %LSNA response to baroreceptor unloading and an increase in basal sympathetic nerve activity (without a corresponding change in the gain of the arterial baroreflex). The present results, involving direct sympathetic nerve recording and pharmacological manipulation of arterial pressure in a valid and reliable animal model of depression, provide additional evidence for sympathetic nervous system dysfunction as a potential common mechanism underlying mood and cardiovascular dysfunction.
Similar to previous studies from our laboratory and others (22–24), the CMS group displayed a significant reduction in sucrose intake and sucrose preference following 4 weeks of CMS, indicative of the reduced responsiveness to pleasurable stimuli (i.e., anhedonia) often observed in human depression. The reduction in sucrose consumption is a direct result of CMS, as the CMS group did not alter its water intake during the study and it did not differ in body weight from the control group. Thus, these responses represent a specific hedonic deficit in the CMS group, rather than a generalized attenuation of ingestive behavior, and provide further evidence for the utility of CMS as a rodent model of depression. Furthermore, the presence of anhedonia in the CMS model is consistent with behavioral signs of depression in humans. Anhedonia is estimated to be present in approximately 95% of all depressed patients (32), and this symptom is an essential feature of Major Depressive Disorder according to Diagnostic and Statistical Manual of Mental Disorder, 4th Edition, Text Revision (DSM-IV®-TR) criteria (33).
Following 4 weeks of CMS, resting cardiovascular function was altered in rats exposed to CMS. Basal MAP and HR were elevated, and HR variability was reduced, in the CMS group relative to the control group. The increased HR and reduced HR variability shown here are similar to the changes described in our previous studies with the CMS model (23, 24) and may indicate an increased risk of cardiovascular pathophysiology. The significant elevation of MAP in the CMS group is a deviation from earlier reports from our laboratory (23, 24); however, the absolute difference between the two groups was only 6 mmHg. Further investigation of blood pressure and vascular tone is required to determine the physiological (and potential clinical) significance of this elevation of MAP in the CMS model. It is possible that MAP recovers over a different time course in CMS and control animals following implantation of indwelling catheters and the LSNA recording electrode. Therefore, it would be ideal if in future studies a new technology could be developed that would allow measurement of autonomic function after a longer recovery period following surgical procedures.
Maximal baroreflex-mediated increases in LSNA were significantly attenuated in CMS rats, however peak gain, although reduced, did not reach statistical significance. Evidence from Pitzalis and colleagues (16), showing a reduction in baroreflex function in post-myocardial infarct unmedicated depressed patients but not in depressed patients treated with β-adrenergic receptor antagonists, suggests that cardiac sympathetic tone plays a major role in mediating baroreflex changes associated with depression. Similarly, previous findings from our laboratory have shown that sympathetic tone to the heart is elevated in animals exposed to CMS, demonstrated with selective pharmacological autonomic blockade protocols (23, 24). Sympathoexcitation, often manifest as increased basal HR, reduced HR variability, and increased arrhythmias, may be a mediating factor in the cardiovascular changes associated with depression and the CMS model. Consistent with this hypothesis, absolute basal LSNA (μV·s) was significantly elevated in animals exposed to CMS, versus control animals. The fact that a significant increase in absolute LSNA was detected in the CMS group (Figure 5) despite extraneous background noise and variability in the nerve-electrode interface indicates that this finding has particularly important physiological relevance. Although it is possible that the attenuated maximal LSNA responses to baroreceptor unloading in the CMS group are due to an initial basal elevation of LSNA rather than a specific alteration in the baroreflex, this could reduce the sympathetic reserve which could be called upon to compensate for a hypotensive challenge, a physiological significant effect.
The findings from the current study are consistent with many previous studies in humans and in animal models of depression. For example, a previous study from our laboratory found that the olfactory bulbectomy rodent model of depression was associated with a generalized attenuation in sympathoexcitatory responses, but no specific change in the gain of the baroreflex (30). Similarly, Broadley et al. (34) found no baroreflex sensitivity differences between normal control subjects and patients who met DSM-IV diagnostic criteria for depression. Also, Watkins et al. (18) reported that symptoms of anxiety, but not symptoms of depression, were associated with reduced baroreflex sensitivity in a sample of patients following acute myocardial infarction, suggesting that anxiety may be a mediating factor in this group’s previously reported cardiac changes associated with depression.
The results described here suggest that sympathetic activation, represented by elevated MAP and HR, reduced HR variability, increased sympathetic nerve activity, and attenuated sympathoexcitatory responses to baroreceptor unloading, may contribute to the increased risk of cardiovascular pathophysiology associated with depression. It is possible that the presence of exogenous stressors produces both the depressive signs and the sympathetic dysfunction that is observed in depressed individuals and the CMS model. The sympathetic dysfunction described here appears to be directly related to the degree of anhedonia in the CMS model; correlational analyses indicated that a greater degree of anhedonia (i.e., lower sucrose intake) was related to higher basal LSNA, and that a greater degree of anhedonia was related to a greater attenuation of LSNA responses to hypotension, following 4 weeks of CMS (refer to Figure 7). Although the small sample sizes in the current study do not warrant statistical analyses to determine the significant difference between these correlation coefficients and zero, the findings suggest that anhedonia is predictably related to sympathetic dysfunction in the CMS group, and highlight the need for studying potential individual differences in the behavioral and cardiovascular responses to environmental stressors in the CMS model.
Further experimental investigations are required to understand the precise autonomic and cardiovascular reflex mechanisms in patients with depression and heart disease. A greater understanding of the central nervous system mechanisms that underlie autonomic function may provide insight into peripheral cardiovascular control mechanisms related to depression. For instance, direct investigation of cortical, limbic and brainstem regions implicated in autonomic functions may provide insight into the precise changes in the neural control of cardiac tone that are associated with depressive syndromes. Continued experimental investigations using valid and reliable animal models of depression, such as the studies described here, will increase our understanding of causal and common mechanisms and may lead to improved treatment strategies for patients with co-morbid depression and heart disease.
Acknowledgments
The authors are grateful to Mr. Terry Beltz, Mr. Lloyd Frei, and Mr. Keith Miller for technical assistance. This research was supported by National Institute of Mental Health MH-65839 (AJG), National Heart, Lung, and Blood Institute HL-14388 (AKJ) and HL-57472 (AKJ), National Institute of Diabetes and Digestive and Kidney Diseases DK-66086 (AKJ).
Acronyms
- ANOVA
analysis of variance
- CMS
chronic mild stress
- DSM-IV®-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision
- HR
heart rate
- LSNA
lumbar sympathetic nerve activity
- MAP
mean arterial pressure
- SEM
standard error of the mean
- SDNN
standard deviation of normal-to-normal intervals
Reference List
- 1.Johnson AK, Grippo AJ. Sadness and broken hearts: neurohumoral mechanisms and co-morbidity of ischemic heart disease and psychological depression. J Physiol Pharmacol. 2006;57 (Suppl 11):5–29. [PubMed] [Google Scholar]
- 2.Grippo AJ, Johnson AK. Biological mechanisms in the relationship between depression and heart disease. Neurosci Biobehav Rev. 2002;26:941–62. doi: 10.1016/s0149-7634(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 3.Rudisch B, Nemeroff CB. Epidemiology of comorbid coronary artery disease and depression. Biol Psychiatry. 2003;54:227–40. doi: 10.1016/s0006-3223(03)00587-0. [DOI] [PubMed] [Google Scholar]
- 4.Frasure-Smith N, Lespérance F. Depression and other psychological risks following myocardial infarction. Arch Gen Psychiatry. 2003;60:627–36. doi: 10.1001/archpsyc.60.6.627. [DOI] [PubMed] [Google Scholar]
- 5.Carney RM, Freedland KE. Depression, mortality, and medical morbidity in patients with coronary heart disease. Biol Psychiatry. 2003;54:241–7. doi: 10.1016/s0006-3223(03)00111-2. [DOI] [PubMed] [Google Scholar]
- 6.Rowell LB. Human cardiovascular control. New York: Oxford University Press; 1993. [Google Scholar]
- 7.Stauss HM. Baroreceptor reflex function. Am J Physiol Regul Integr Comp Physiol. 2002;283:R284–R286. doi: 10.1152/ajpregu.00219.2002. [DOI] [PubMed] [Google Scholar]
- 8.Head GA, Mayorov DN. Central angiotensin and baroreceptor control of circulation. Ann NY Acad Sci. 2001;940:361–79. doi: 10.1111/j.1749-6632.2001.tb03691.x. [DOI] [PubMed] [Google Scholar]
- 9.Sleight P. The importance of the autonomic nervous system in health and disease. Aust NZ J Med. 1997;27:467–73. doi: 10.1111/j.1445-5994.1997.tb02220.x. [DOI] [PubMed] [Google Scholar]
- 10.Schwartz PJ, Vanoli E, Stramba-Badiale M, De Ferrari GM, Billman GE, Foreman RD. Autonomic mechanisms and sudden death. New insights from analysis of baroreceptor reflexes in conscious dogs with and without a myocardial infarction. Circulation. 1988;78:969–79. doi: 10.1161/01.cir.78.4.969. [DOI] [PubMed] [Google Scholar]
- 11.Billman GE, Schwartz PJ, Stone HL. Baroreceptor reflex control of heart rate: a predictor of sudden cardiac death. Circulation. 1982;66:874–80. doi: 10.1161/01.cir.66.4.874. [DOI] [PubMed] [Google Scholar]
- 12.Trimarco B, Ricciardelli B, Cuocolo A, Volpe M, De Luca N, Mele AF, Condorelli M. Effects of coronary occlusion on arterial baroreflex control of heart rate and vascular resistance. Am J Physiol Heart Circ Physiol. 1987;252:H749–H759. doi: 10.1152/ajpheart.1987.252.4.H749. [DOI] [PubMed] [Google Scholar]
- 13.Airaksinen KE. Autonomic mechanisms and sudden death after abrupt coronary occlusion. Ann Med. 1999;31:240–5. doi: 10.3109/07853899908995886. [DOI] [PubMed] [Google Scholar]
- 14.Mortara A, La Rovere MT, Pinna GD, Prpa A, Maestri R, Febo O, Pozzoli M, Opasich C, Tavazzi L. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–8. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 15.Watkins LL, Grossman P. Association of depressive symptoms with reduced baroreflex cardiac control in coronary artery disease. Am Heart J. 1999;137:453–7. doi: 10.1016/s0002-8703(99)70491-6. [DOI] [PubMed] [Google Scholar]
- 16.Pitzalis MV, Iacoviello M, Todarello O, Fioretti A, Guida P, Massari F, Mastropasqua F, Russo GD, Rizzon P. Depression but not anxiety influences the autonomic control of heart rate after myocardial infarction. Am Heart J. 2001;141:765–71. doi: 10.1067/mhj.2001.114806. [DOI] [PubMed] [Google Scholar]
- 17.Broadley AJM, Frenneaux MP, Moskvina V, Jones CJH, Korszun A. Baroreflex sensitivity is reduced in depression. Psychosom Med. 2005;67:648–51. doi: 10.1097/01.psy.0000170829.91643.24. [DOI] [PubMed] [Google Scholar]
- 18.Watkins LL, Blumenthal JA, Carney RM. Association of anxiety with reduced baroreflex cardiac control in patients after acute myocardial infarction. Am Heart J. 2002;143:460–6. doi: 10.1067/mhj.2002.120404. [DOI] [PubMed] [Google Scholar]
- 19.Willner P. Chronic mild stress (CMS) revisited: consistency and behavioural-neurobiological concordance in the effects of CMS. Neuropsychobiology. 2005;52:90–110. doi: 10.1159/000087097. [DOI] [PubMed] [Google Scholar]
- 20.Willner P. The chronic mild stress procedure as an animal model of depression: valid, reasonably reliable, and useful. Psychopharmacology. 1997;134:371–7. [Google Scholar]
- 21.Willner P, Sampson D, Papp M, Phillips G, Muscat R. Animal models of anhedonia. In: Soubrié P, editor. Anxiety, depression, and mania. Animal models of psychiatric disorders. Basel: Karger; 1991. pp. 71–99. [Google Scholar]
- 22.Willner P, Towell A, Sampson D, Sophokleous S, Muscat R. Reduction of sucrose preference by chronic unpredictable mild stress, and its restoration by a tricyclic antidepressant. Psychopharmacology. 1987;93:358–64. doi: 10.1007/BF00187257. [DOI] [PubMed] [Google Scholar]
- 23.Grippo AJ, Moffitt JA, Johnson AK. Cardiovascular alterations and autonomic imbalance in an experimental model of depression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1333–R1341. doi: 10.1152/ajpregu.00614.2001. [DOI] [PubMed] [Google Scholar]
- 24.Grippo AJ, Beltz TG, Johnson AK. Behavioral and cardiovascular changes in the chronic mild stress model of depression. Physiol Behav. 2003;78:703–10. doi: 10.1016/s0031-9384(03)00050-7. [DOI] [PubMed] [Google Scholar]
- 25.Grippo AJ, Beltz TG, Weiss RM, Johnson AK. The effects of chronic fluoxetine treatment on chronic mild stress-induced cardiovascular changes and anhedonia. Biol Psychiatry. 2006;59:309–16. doi: 10.1016/j.biopsych.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Grippo AJ, Santos CM, Johnson RF, Beltz TG, Martins JB, Felder RB, Johnson AK. Increased susceptibility to ventricular arrhythmias in a rodent model of experimental depression. Am J Physiol Heart Circ Physiol. 2004;286:H619–H626. doi: 10.1152/ajpheart.00450.2003. [DOI] [PubMed] [Google Scholar]
- 27.Grippo AJ, Francis J, Beltz TG, Felder RB, Johnson AK. Neuroendocrine and cytokine profile of chronic mild stress-induced anhedonia. Physiol Behav. 2005;84:697–706. doi: 10.1016/j.physbeh.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 28.Moffitt JA, Foley CM, Schadt JC, Laughlin MH, Hasser EM. Attenuated baroreflex control of sympathetic nerve activity after cardiovascular deconditioning in rats. Am J Physiol Regul Integr Comp Physiol. 1998;274:R1397–R1405. doi: 10.1152/ajpregu.1998.274.5.r1397. [DOI] [PubMed] [Google Scholar]
- 29.Moffitt JA, Schadt JC, Hasser EM. Altered central nervous system processing of baroreceptor input following hindlimb unloading in rats. Am J Physiol Heart Circ Physiol. 1999;277:H2272–H2279. doi: 10.1152/ajpheart.1999.277.6.h2272. [DOI] [PubMed] [Google Scholar]
- 30.Moffitt JA, Grippo AJ, Holmes PV, Johnson AK. Olfactory bulbectomy attenuates cardiovascular sympathoexcitatory reflexes in rats. Am J Physiol Heart Circ Physiol. 2002;283:H2575–H2583. doi: 10.1152/ajpheart.00164.2002. [DOI] [PubMed] [Google Scholar]
- 31.Task Force of the European Society of Cardiology, North American Society of Pacing and Electrophysiology. Heart rate variability: standards of measurement, physiological interpretation, and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 32.Keller MB, Klein DN, Hirschfeld RM, Kocsis JH, McCullough JP, Miller I, First MB, Holzer CP, Keitner GI, Marin DB. Results of the DSM-IV mood disorder field trial. Am J Psychiatry. 1995;152:843–9. doi: 10.1176/ajp.152.6.843. [DOI] [PubMed] [Google Scholar]
- 33.American Psychiatric Association. Diagnostic and statistical manual of mental disorders, fourth edition, text revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 34.Broadley AJ, Korszun A, Jones CJ, Frenneaux MP. Arterial endothelial function is impaired in treated depression. Heart. 2002;88:521–3. doi: 10.1136/heart.88.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]