Abstract
Stem cells are found in naturally occurring 3D microenvironments in vivo, which are often referred to as the stem cell niche 1. Culturing stem cells inside of 3D biomaterial scaffolds provides a way to accurately mimic these microenvironments, providing an advantage over traditional 2D culture methods using polystyrene as well as a method for engineering replacement tissues 2. While 2D tissue culture polystrene has been used for the majority of cell culture experiments, 3D biomaterial scaffolds can more closely replicate the microenvironments found in vivo by enabling more accurate establishment of cell polarity in the environment and possessing biochemical and mechanical properties similar to soft tissue.3 A variety of naturally derived and synthetic biomaterial scaffolds have been investigated as 3D environments for supporting stem cell growth. While synthetic scaffolds can be synthesized to have a greater range of mechanical and chemical properties and often have greater reproducibility, natural biomaterials are often composed of proteins and polysaccharides found in the extracelluar matrix and as a result contain binding sites for cell adhesion and readily support cell culture. Fibrin scaffolds, produced by polymerizing the protein fibrinogen obtained from plasma, have been widely investigated for a variety of tissue engineering applications both in vitro and in vivo4. Such scaffolds can be modified using a variety of methods to incorporate controlled release systems for delivering therapeutic factors 5. Previous work has shown that such scaffolds can be used to successfully culture embryonic stem cells and this scaffold-based culture system can be used to screen the effects of various growth factors on the differentiation of the stem cells seeded inside 6,7.
This protocol details the process of polymerizing fibrin scaffolds from fibrinogen solutions using the enzymatic activity of thrombin. The process takes 2 days to complete, including an overnight dialysis step for the fibrinogen solution to remove citrates that inhibit polymerization. These detailed methods rely on fibrinogen concentrations determined to be optimal for embryonic and induced pluripotent stem cell culture. Other groups have further investigated fibrin scaffolds for a wide range of cell types and applications - demonstrating the versatility of this approach 8-12.
Keywords: Bioengineering, Issue 61, Extracellular matrix, stem cells, biomaterials, drug delivery, cell culture
Protocol
Notes before starting protocol: Fibrinogen is a blood derived protein and thus appropriate safety training must be completed before handling. This protocol requires 2 days to complete so time the desired stem cultures appropriately to ensure they are ready for seeding. In terms of calculating how much fibrinogen to weigh out, three 35 mm petri dishes of tris buffered saline (TBS, pH 7.4) containing 110-130 mg of fibrinogen dissolved in 3 mL of TBS will be sufficient to produce 1 24 well plate containing 400 μl fibrin scaffolds in each well.
1. Day One: Making fibrinogen solutions and overnight dialysis
Take lyophilized fibrinogen out of the refrigerator and let it sit for 20 minutes to allow it to come to room temperature.
Add 3 mL of Tris buffered saline (TBS) to each 35 mm petri dish. Each plate of fibrinogen yields 2 to 3 mL of fibrinogen solution ranging in concentration from 12 to 20 mg/mL and the total amount of fibrinogen needed can be calculated based on these approximations.
Weigh out approximately 100-130 mg fibrinogen (Fg) and sprinkle onto the surface of the TBS present in each dish. Wait 5 minutes for the fibrinogen to begin to go into solution. Cover petri dish with lid.
Incubate petri dishes at 37°C for 2 hours to allow the fibrinogen to fully go into the TBS solution.
Wet dialysis tubing (7000 MW cut off) with TBS. Fold bottom end of the tubing and clamp end shut with a dialysis clamp.
Pipette the fibrinogen solutions from dishes into dialysis tubing. Fold over top end and clamp shut with a dialysis clamp.
Place dialysis tubing containing fibrinogen solution into a 4 liter container of TBS. Mix solution using stir plate set to low speed (100 rpm).
Dialyze solution overnight on stir plate for at least 12 hours. The TBS solution does not need to be changed over this time period.
2. Day 2: Polymerization of fibrin scaffolds and seeding of stem cells into scaffolds
All steps described for this process should be performed in a sterile tissue culture hood as these scaffolds will be seeded with stem cell cultures.
Remove the fibrinogen solution from dialysis tubing and place into an appropriate sized conical tube (either 15 mL or 50 mL).
Filter fibrinogen solution with a 5.0 μm syringe filter to remove large impurities in the solution. Depending on the volume of the solution, the use of multiple filters may be necessary to process the entire solution.
Sterile filter the fibrinogen solution with a 0.22 μm filter into a 50mL conical tube. Using an appropriate size pipette, determine the volume of the filtered fibrinogen solution to use later when doing calculations. Depending on the volume of the solution, the use of multiple filters may be necessary to process the entire solution.
Measure absorbance of the fibrinogen solution at 280 nm using the spectrophotometer. A dilution factor of 50 is often necessary to obtain an absorbance under 1.
Calculate the concentration of protein present in the fibrinogen solution using the following equation: [Fibrinogen in mg/mL] = (A280 × Dilution Factor) ÷ 1.55 where 1.55 is the extinction coefficient at A280 for human fibrinogen 13. Next calculate the amount of TBS needed to dilute the concentration of the solution to 11.1 mg/mL of fibrinogen based on the initial volume. The final concentration of protein in the fibrin scaffolds will be 10 mg/mL after polymerization.
Obtain sterile thrombin and calcium chloride solutions. First, add 15 μL thrombin solution (concentration: 40 U/mL - final concentration in scaffold will be 2 U/mL) to right side of each well in the first row of the 24 well plate. Next, add 15 μL of 50 mM calcium chloride solution to left side of each well. Finally, add 270 μL of the fibrinogen solution to the plate. Shake plate from side to side followed by shaking back to front to ensure mixing of solutions.
Let the row containing the mixtures polymerize for 5 minutes at room temperature. The scaffolds become more opaque as they polymerize.
Repeat steps 2.6 and 2.7 until the desired number of fibrin scaffolds have been polymerized.
After the last 5 minute incubation, place the plates inside of a 37°C incubator to allow for complete polymerization of scaffolds.
After the one hour incubation is complete, the next step is to seed the scaffold with embryoid bodies. Using a 20 μL pipettemen, select an individual embryoid body and place in the center of the fibrin scaffold. Check with microscope that each well contains a single embryoid body.
Add 5 μL of thrombin solution (concentration: 40 U/mL) and 5 μL of 50 mM calcium chloride solution on top of each embryoid body followed by 90 μL of fibrinogen solution to each well. The solution should form a bubble encapsulating the embryoid bodies.
Place plate back in 37°C incubator for an additional hour to ensure polymerization.
After incubation, add 1 mL of the appropriate cell culture media to each well and place plate back into 37°C incubator.
3. Representative Results
Figure 1 shows a schematic of the side view of an individual well containing the 3D fibrin scaffold culture system seeded with an embryoid body. Figure 2A shows representative images of mouse induced pluripotent stem cells being cultured on mouse embryonic fibroblast feeder layers. These cells are then induced to form embryoid bodies, aggregates of cells containing neural progenitors, using an 8 day retinoic acid treatment protocol (Figure 2B) as previously described 14 while Figure 3 shows the appearance of an iPS-derived embryoid body after 3 days of culture inside of 3D fibrin scaffolds. Similar results have been obtained previously using mouse embryonic stem cells 7. Additionally, this method has been used to culture iPS-derived embryoid bodies produced using a different protocol involving retinoic acid and purmorphamine 15 inside of 3D fibrin scaffolds, demonstrating the versatility of this culture system.
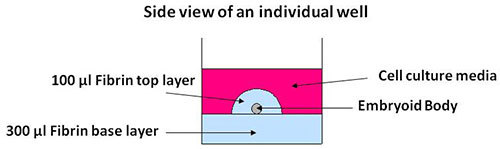 Figure 1. Schematic showing the side view of an individual well containing a 3D fibrin scaffold seeded with an embryoid body.
Figure 1. Schematic showing the side view of an individual well containing a 3D fibrin scaffold seeded with an embryoid body.
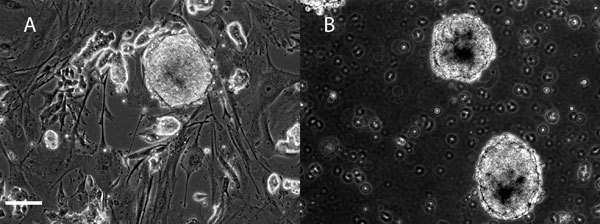 Figure 2. Mouse induced pluripotent stem (iPS) cell culture and differentiation . To differentiate these cells into neural phenotypes, the iPS cells are cultured in suspension to produce aggregates of cells called embryoid bodies (EBs). These EBs are then treated with retinoic acid to induce neural differentiation and this protocol was previously used to induce neural differentiation of embryonic stem cells. A) Undifferentiated mouse iPS cell colony cultured on a mouse embryonic fibroblast feeder layer. B) Embryoid bodies derived from mouse iPS cells in suspension culture taken on day 8 after treatment with retinoic acid to induce neural differentiation. Scale bar is 100 μm.
Figure 2. Mouse induced pluripotent stem (iPS) cell culture and differentiation . To differentiate these cells into neural phenotypes, the iPS cells are cultured in suspension to produce aggregates of cells called embryoid bodies (EBs). These EBs are then treated with retinoic acid to induce neural differentiation and this protocol was previously used to induce neural differentiation of embryonic stem cells. A) Undifferentiated mouse iPS cell colony cultured on a mouse embryonic fibroblast feeder layer. B) Embryoid bodies derived from mouse iPS cells in suspension culture taken on day 8 after treatment with retinoic acid to induce neural differentiation. Scale bar is 100 μm.
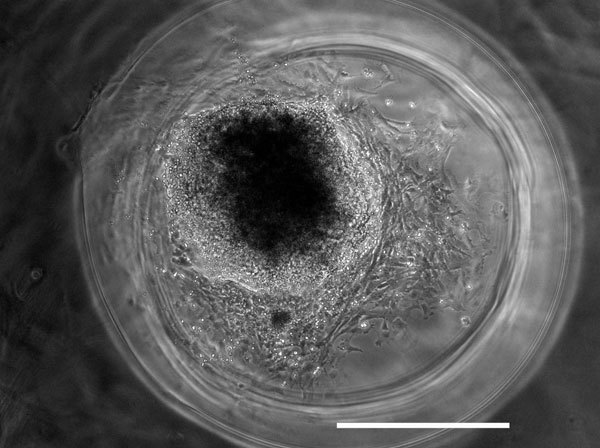 Figure 3. Example results of a mouse iPS embryoid body after 3 days of culture inside of a 3D fibrin scaffold. Cells have begun to migrate and differentiate inside of the fibrin scaffold. Scale bar is 500 μm.
Figure 3. Example results of a mouse iPS embryoid body after 3 days of culture inside of a 3D fibrin scaffold. Cells have begun to migrate and differentiate inside of the fibrin scaffold. Scale bar is 500 μm.
Discussion
This protocol detailed above provides a method for generating 3D fibrin scaffolds for pluripotent stem cell culture, specifically for mouse embryonic and induced pluripotent stem cells. This 3D biomaterial based culture system more accurately mimics the stem cell niche found in vivo and as a result, it can be used to screen biological cues to determine their effects on stem cell differentiation 6. Our observations have shown that these scaffolds when seeded with stem cell derived embryoid bodies remain for 2 weeks in vitro before becoming completely degraded. To further increase the stability of these scaffolds, aprotinin (a protease inhibitor) can be used to slow degradation through addition to the cell culture media.7,16 These scaffolds have also been used successfully for neural tissue engineering applications, specifically the generation of tissue similar to that found in the central nervous system17. While other groups have combined iPS cells with fibrin glue for treating Ischemic stroke18, this work represents the first known report of combining iPS cells with fibrin scaffolds for neural tissue engineering applications. This work also served as a starting point for combining a range of stem cell types with fibrin scaffolds for other tissue engineering applications.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors would like to acknowledge NSERC Discovery Grant 402462 "Tissue engineered scaffolds for controlling induced pluripotent stem cell behavior".
References
- Keung AJ, Healy KE, Kumar S, Schaffer DV. Biophysics and dynamics of natural and engineered stem cell microenvironments. Wiley Interdiscip. Rev. Syst. Biol. Med. 2010;2:49–64. doi: 10.1002/wsbm.46. [DOI] [PubMed] [Google Scholar]
- Willerth SM, Sakiyama-Elbert SE. Combining stem cells and biomaterial scaffolds for constructing tissues and cell delivery. StemBook. 2008. [PubMed]
- Lee J, Cuddihy MJ, Kotov NA. Three-dimensional cell culture matrices: state of the art. Tissue Eng. Part B Rev. 2008;14:61–86. doi: 10.1089/teb.2007.0150. [DOI] [PubMed] [Google Scholar]
- Ahmed TA, Dare EV, Hincke M. Fibrin: a versatile scaffold for tissue engineering applications. Tissue Eng. Part B Rev. 2008;14:199–215. doi: 10.1089/ten.teb.2007.0435. [DOI] [PubMed] [Google Scholar]
- Breen A, O'Brien T, Pandit A. Fibrin as a delivery system for therapeutic drugs and biomolecules. Tissue Eng. Part B Rev. 2009;15:201–214. doi: 10.1089/ten.TEB.2008.0527. [DOI] [PubMed] [Google Scholar]
- Willerth SM, Faxel TE, Gottlieb DI, Sakiyama-Elbert SE. The effects of soluble growth factors on embryonic stem cell differentiation inside of fibrin scaffolds. Stem Cells. 2007;25:2235–2244. doi: 10.1634/stemcells.2007-0111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willerth SM, Arendas KJ, Gottlieb DI, Sakiyama-Elbert SE. Optimization of fibrin scaffolds for differentiation of murine embryonic stem cells into neural lineage cells. Biomaterials. 2006;27:5990–6003. doi: 10.1016/j.biomaterials.2006.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorodetsky R. Fibrin Microbeads Loaded with Mesenchymal Cells Support Their Long-Term Survival While Sealed at Room Temperature. Tissue Eng. Part C Methods. 2011. [DOI] [PMC free article] [PubMed]
- Barsotti MC. Fibrin acts as biomimetic niche inducing both differentiation and stem cell marker expression of early human endothelial progenitor cells. Cell Prolif. 2011;44:33–48. doi: 10.1111/j.1365-2184.2010.00715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park JS, Yang HN, Woo DG, Jeon SY, Park KH. Chondrogenesis of human mesenchymal stem cells in fibrin constructs evaluated in vitro and in nude mouse and rabbit defects models. Biomaterials. 2011;32:1495–1507. doi: 10.1016/j.biomaterials.2010.11.003. [DOI] [PubMed] [Google Scholar]
- Mooney R, Tawil B, Mahoney M. Specific fibrinogen and thrombin concentrations promote neuronal rather than glial growth when primary neural cells are seeded within plasma-derived fibrin gels. Tissue Eng. Part A. 2010;16:1607–1619. doi: 10.1089/ten.TEA.2009.0372. [DOI] [PubMed] [Google Scholar]
- Liu H, Collins SF, Suggs LJ. Three-dimensional culture for expansion and differentiation of mouse embryonic stem cells. Biomaterials. 2006;27:6004–6014. doi: 10.1016/j.biomaterials.2006.06.016. [DOI] [PubMed] [Google Scholar]
- Dellenback RJ, Chien S. The extinction coefficient of fibrinogen from man, dog, elephant, sheep, and goat at 280 mmu. Proc. Soc. Exp. Biol. Med. 1970;134:353–355. doi: 10.3181/00379727-134-34792. [DOI] [PubMed] [Google Scholar]
- Bain G, Kitchens D, Yao M, Huettner JE, Gottlieb DI. Embryonic stem cells express neuronal properties in vitro. Dev. Biol. 1995;168:342–357. doi: 10.1006/dbio.1995.1085. [DOI] [PubMed] [Google Scholar]
- Li XJ. Directed differentiation of ventral spinal progenitors and motor neurons from human embryonic stem cells by small molecules. Stem Cells. 2008;26:886–893. doi: 10.1634/stemcells.2007-0620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorentz KM, Kontos S, Frey P, Hubbell JA. Engineered aprotinin for improved stability of fibrin biomaterials. Biomaterials. 2011;32:430–438. doi: 10.1016/j.biomaterials.2010.08.109. [DOI] [PubMed] [Google Scholar]
- Willerth SM, Rader A, Sakiyama-Elbert SE. The effect of controlled growth factor delivery on embryonic stem cell differentiation inside fibrin scaffolds. Stem Cell Res. 2008;1:205–218. doi: 10.1016/j.scr.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ. Functional improvement of focal cerebral ischemia injury by subdural transplantation of induced pluripotent stem cells with fibrin glue. Stem Cells Dev. 2010;19:1757–1767. doi: 10.1089/scd.2009.0452. [DOI] [PubMed] [Google Scholar]


