Abstract
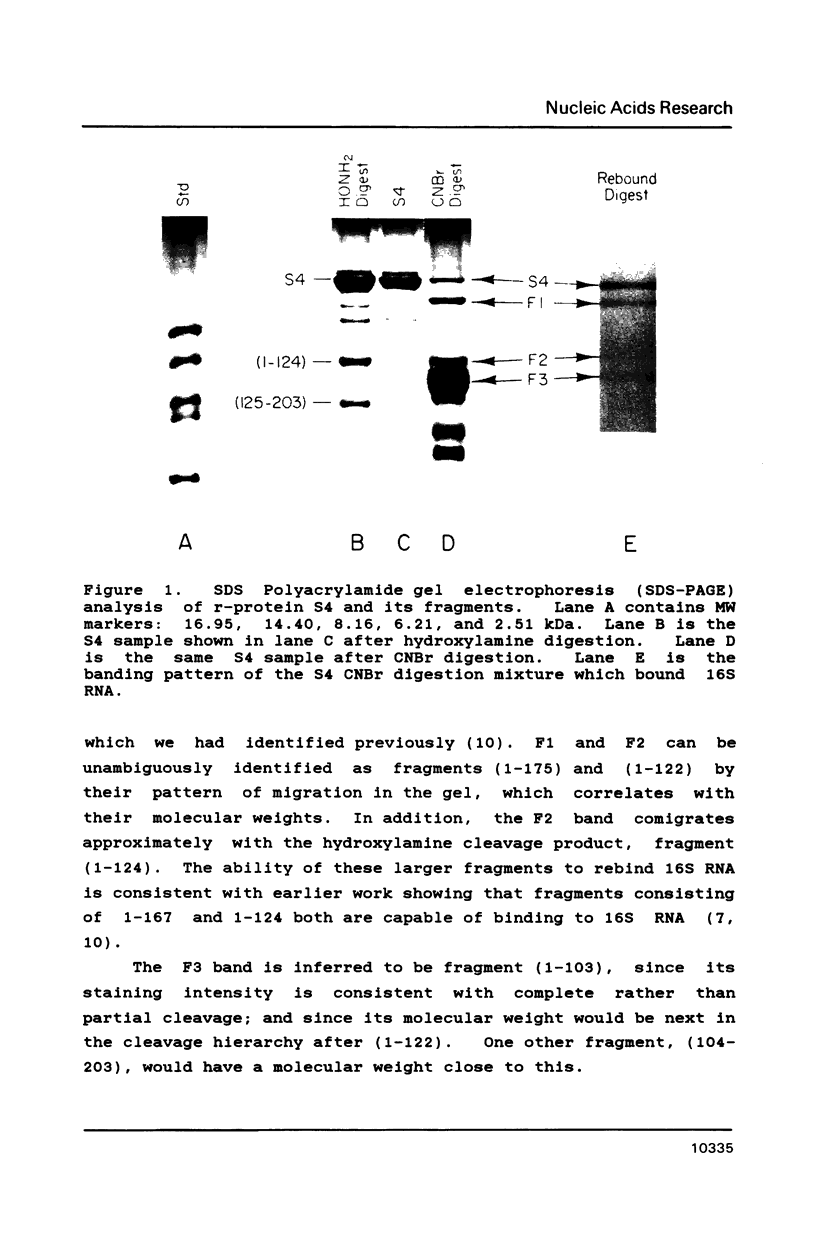
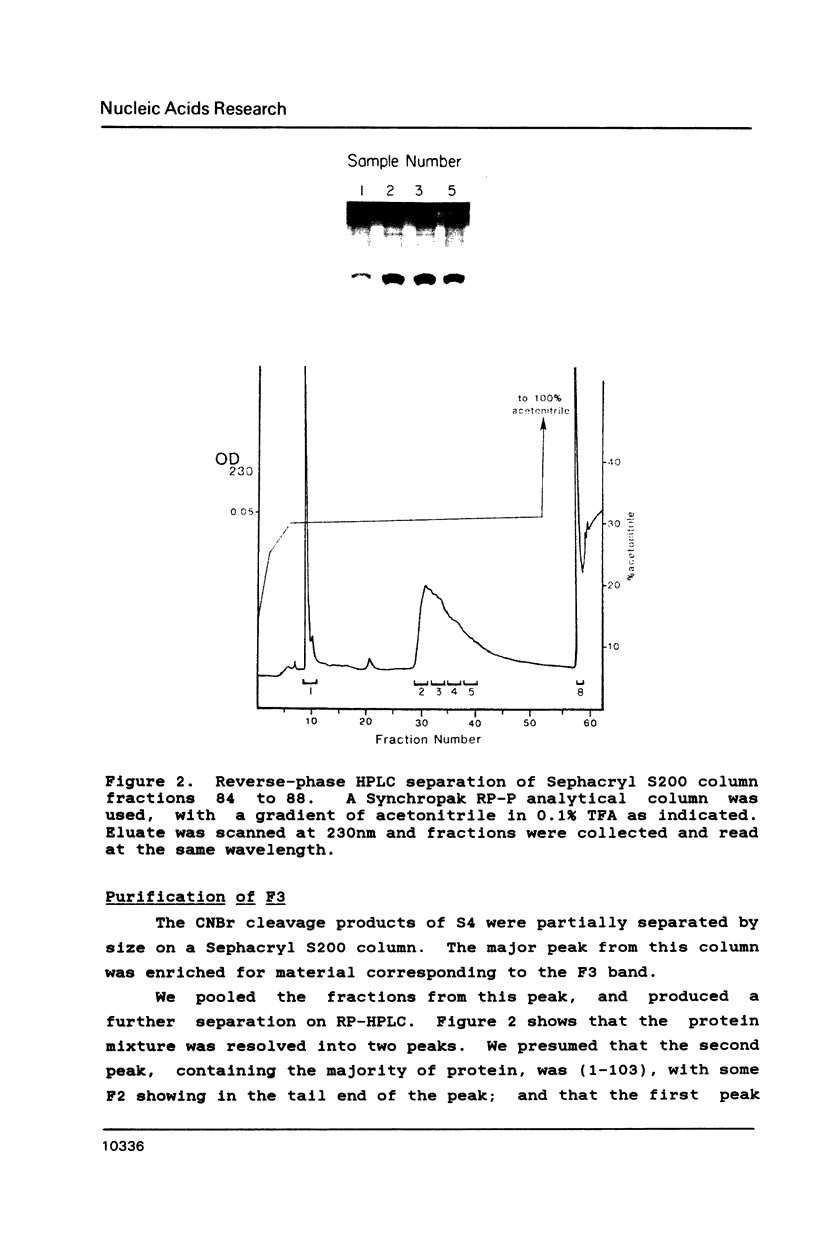
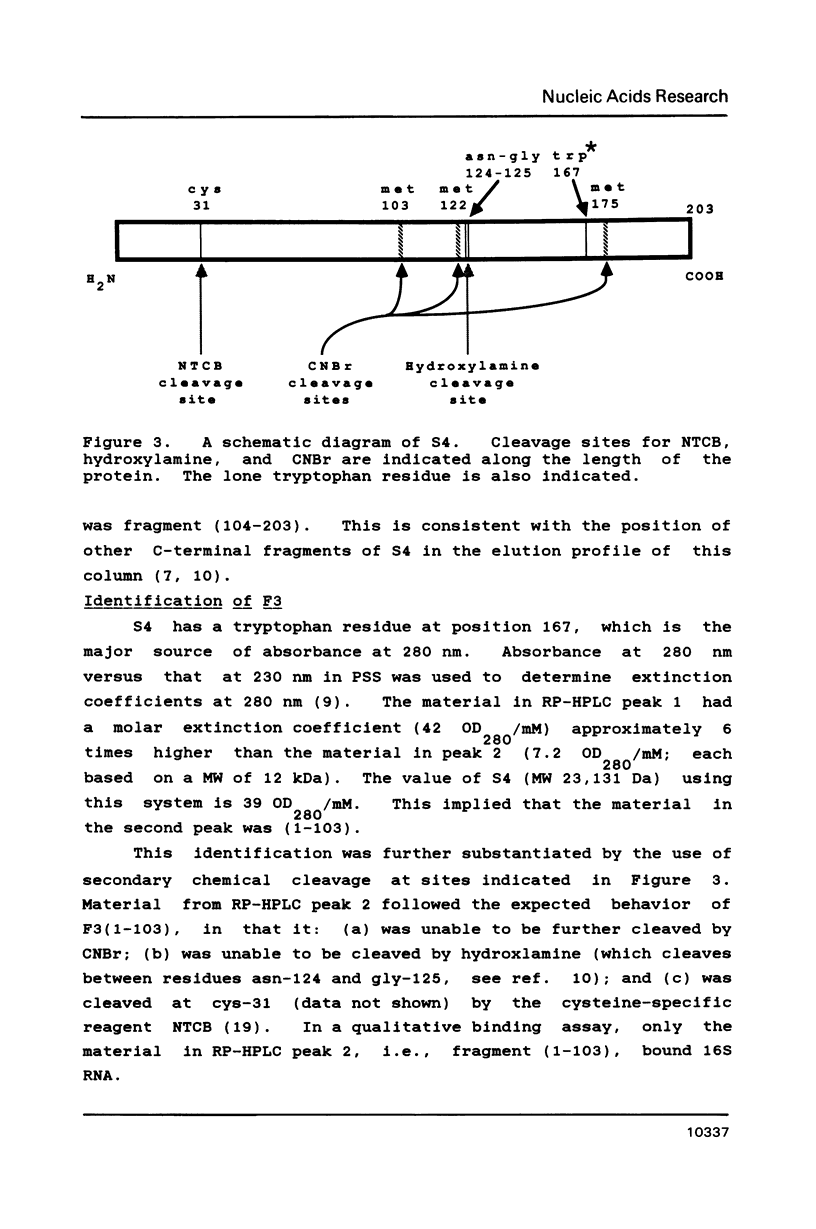
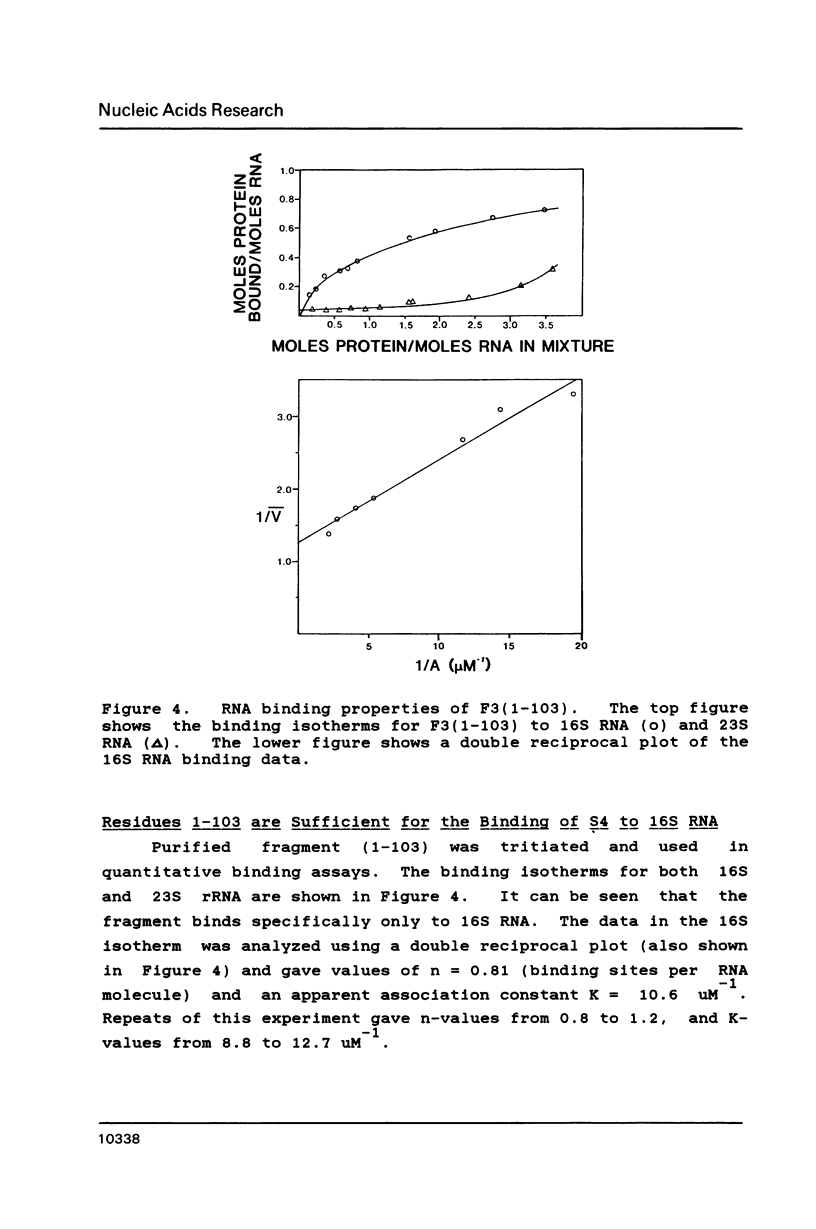
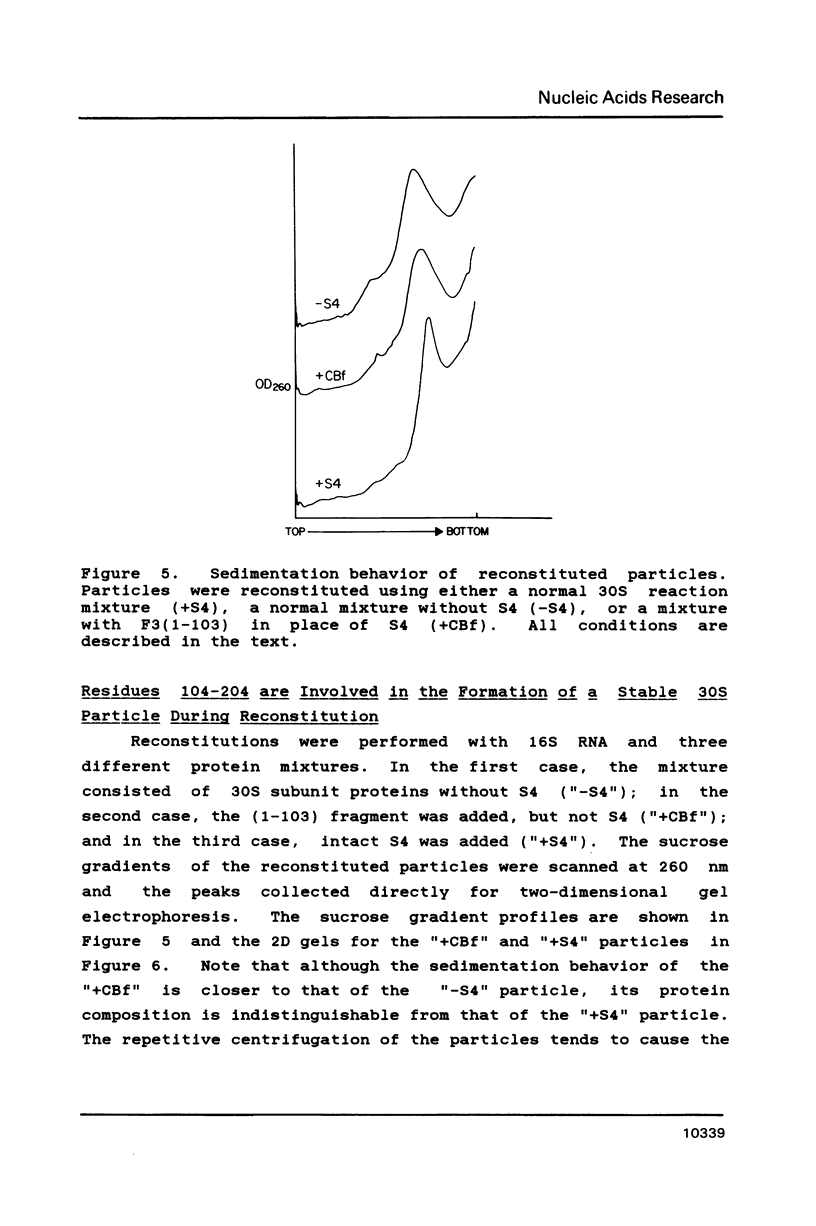
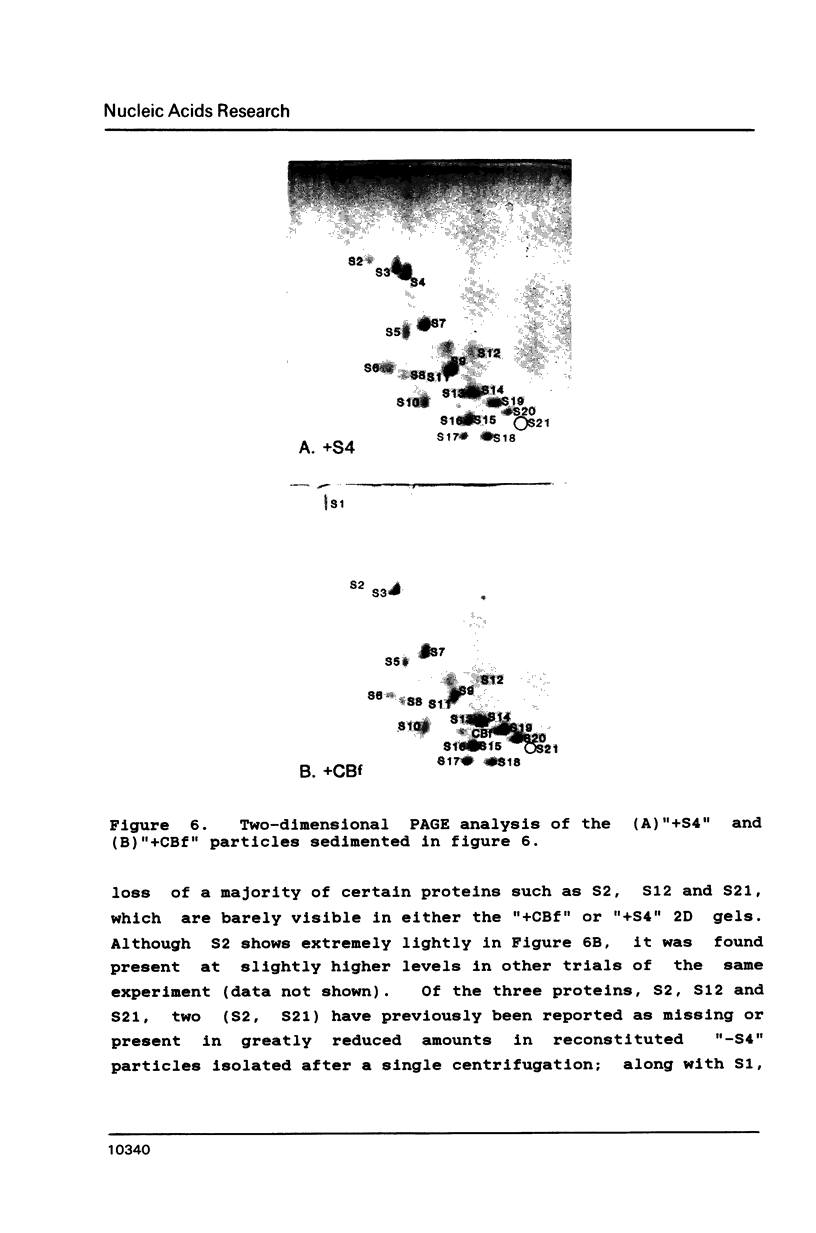
Escherichia coli ribosomal protein S4 was subjected to cyanogen bromide cleavage and was found to generate a complete cleavage product capable of rebinding 16S rRNA. This fragment, consisting of residues 1-103, was found to bind with an apparent association constant of 11 microM-1. This fragment was used in place of S4 in an in vitro reconstitution experiment. Although the particles formed had a protein composition not significantly different from reconstituted 30S ribosomal subunits, their sedimentation behavior was more like that of particles reconstituted without S4. These results indicate to us that, although residues 104-203 of S4 are involved in the assembly of the 30S ribosome, they are not necessary for the binding of S4 to 16S RNA. Taken with previous results, the domain of S4 involved in specific binding of 16S RNA can be confined to residues 47-103.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amons R., Möller W., Schiltz E., Reinbolt J. Studies on the binding sites of protein S4 to 16S RNA in Escherichia coli ribosomes. FEBS Lett. 1974 Apr 15;41(1):135–138. doi: 10.1016/0014-5793(74)80972-5. [DOI] [PubMed] [Google Scholar]
- Bruce J., Firpo E. J., Schaup H. W. Ribosomal protein-nucleic acid interactions. I. Isolation of a polypeptide fragment from 30S protein S8 which binds to 16S rRNA. Nucleic Acids Res. 1977 Oct;4(10):3327–3340. doi: 10.1093/nar/4.10.3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel M., Datta D., Nierras C. R., Craven G. R. Preparative ion-exchange high-performance liquid chromatography of bacterial ribosomal proteins. Anal Biochem. 1986 Oct;158(1):179–188. doi: 10.1016/0003-2697(86)90607-x. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Conrad R. C., Craven G. R. Chemical and functional characterization of an altered form of ribosomal protein S4 derived from a strain of E. coli defective in auto-regulation of the alpha operon. Nucleic Acids Res. 1986 Sep 11;14(17):6929–6944. doi: 10.1093/nar/14.17.6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changchien L. M., Craven G. R. Specific ribosomal RNA recognition by a fragment of E. coli ribosomal protein S4 missing the C-terminal 36 amino acid residues. Nucleic Acids Res. 1985 Sep 11;13(17):6343–6360. doi: 10.1093/nar/13.17.6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changchien L. M., Craven G. R. Studies on the role of amino acid residues 31 through 46 of ribosomal protein S4 in the mechanism of 30 S ribosome assembly. J Mol Biol. 1978 Oct 15;125(1):43–56. doi: 10.1016/0022-2836(78)90253-x. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Craven G. R. The function of the N-terminal region of ribosomal protein S4. J Mol Biol. 1976 Dec;108(2):381–401. doi: 10.1016/s0022-2836(76)80126-x. [DOI] [PubMed] [Google Scholar]
- Changchien L. M., Craven G. R. The use of hydroxylamine cleavage to produce a fragment of ribosomal protein S4 which retains the capacity to specifically bind 16S ribosomal RNA. Nucleic Acids Res. 1986 Mar 11;14(5):1957–1966. doi: 10.1093/nar/14.5.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craven G. R., Gupta V. Three-dimensional organization of the 30S ribosomal proteins from Escherichia coli. I. Preliminary classification of the proteins. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1329–1336. doi: 10.1073/pnas.67.3.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehresmann B., Reinbolt J., Ebel J. P. Studies of the binding of E. coli ribosomal protein S4 to 16S rRNA after UV irradiation of the S4--16S rRNA complex. FEBS Lett. 1975 Oct 15;58(1):106–111. doi: 10.1016/0014-5793(75)80236-5. [DOI] [PubMed] [Google Scholar]
- Giorginis S., Subramanian A. R. The major ribosome binding site of Escherichia coli ribosomal protein S1 is located in its N-terminal segment. J Mol Biol. 1980 Aug 25;141(4):393–408. doi: 10.1016/0022-2836(80)90253-3. [DOI] [PubMed] [Google Scholar]
- Giri L., Hill W. E., Wittmann H. G., Wittmann-Liebold B. Ribosomal proteins: their structure and spatial arrangement in prokaryotic ribosomes. Adv Protein Chem. 1984;36:1–78. doi: 10.1016/s0065-3233(08)60295-8. [DOI] [PubMed] [Google Scholar]
- Hardy S. J., Kurland C. G., Voynow P., Mora G. The ribosomal proteins of Escherichia coli. I. Purification of the 30S ribosomal proteins. Biochemistry. 1969 Jul;8(7):2897–2905. doi: 10.1021/bi00835a031. [DOI] [PubMed] [Google Scholar]
- Held W. A., Mizushima S., Nomura M. Reconstitution of Escherichia coli 30 S ribosomal subunits from purified molecular components. J Biol Chem. 1973 Aug 25;248(16):5720–5730. [PubMed] [Google Scholar]
- Houghten R. A., Li C. H. Reduction of sulfoxides in peptides and proteins. Anal Biochem. 1979 Sep 15;98(1):36–46. doi: 10.1016/0003-2697(79)90702-4. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Newberry V., Brosius J., Garrett R. Fragment of protein L18 from the Escherichia coli ribosome that contains the 5S RNA binding site. Nucleic Acids Res. 1978 Jun;5(6):1753–1766. doi: 10.1093/nar/5.6.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry V., Yaguchi M., Garrett R. A. A trypsin-resistant fragment from complexes of ribosomal protein S4 with 16-S RNA of Escherichia coli and from the uncomplexed protein. Eur J Biochem. 1977 Jun 1;76(1):51–61. doi: 10.1111/j.1432-1033.1977.tb11569.x. [DOI] [PubMed] [Google Scholar]
- Schwarzbauer J., Craven G. R. Apparent association constants for E. coli ribosomal proteins S4, S7, S8, S15, S17 and S20 binding to 16S RNA. Nucleic Acids Res. 1981 May 11;9(9):2223–2237. doi: 10.1093/nar/9.9.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarzbauer J., Craven G. R. The use of membrane filtration to determine apparent association constants for ribosomal protein-RNA complex formation. Methods Enzymol. 1979;59:583–591. doi: 10.1016/0076-6879(79)59114-9. [DOI] [PubMed] [Google Scholar]
- Sedmak J. J., Grossberg S. E. A rapid, sensitive, and versatile assay for protein using Coomassie brilliant blue G250. Anal Biochem. 1977 May 1;79(1-2):544–552. doi: 10.1016/0003-2697(77)90428-6. [DOI] [PubMed] [Google Scholar]
- Stark G. R. Cleavage at cysteine after cyanylation. Methods Enzymol. 1977;47:129–132. doi: 10.1016/0076-6879(77)47015-0. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Yates J. L., Arfsten A. E., Nomura M. In vitro expression of Escherichia coli ribosomal protein genes: autogenous inhibition of translation. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1837–1841. doi: 10.1073/pnas.77.4.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]