Abstract
In viruses with positive-sense RNA genomes pathogenic to humans, animals and plants, progeny encapsidation into mature and stable virions is a cardinal phase during establishment of infection in a given host. Consequently, study of encapsidation deciphers the information regarding the know-how of the mechanism regulating virus assembly to form infectious virions. Such information is vital in formulating novel methods of curbing virus spread and disease control. Virus encapsidation can be studied in vivo and in vitro. Genome encapsidation in vivo is a highly regulated selective process involving macromolecular interactions and subcellular compartmentalization. Therefore, study leading to dissect events encompassing virus encapsidation in vivo would provide basic knowledge to understand how viruses proliferate and assemble. Recently in vitro encapsidation has been exploited for the research in the area of biomedical imaging and therapeutic applications. Non-enveloped plant viruses stand far ahead in the venture of in vitro encapsidation of the negatively charged foreign material. Brome mosaic virus (BMV), a non-enveloped multicomponent RNA virus pathogenic to plants, has been used as a model system for studying genome packaging in vivo and in vitro. For encapsidation assays in Nicotiana benthamiana plants, Agrobacterium -mediated transient expression, refer to as agroinfiltration, is an efficient and robust technique for the synchronized delivery and expression of multiple components to the same cell. In this approach, a suspension of Agrobacterium tumefaciens cells carrying binary plasmid vectors carrying cDNAs of desiredviral mRNAs is infiltrated into the intercellular space withina leaf using nothing more sophisticated than a 1 ml disposable syringe (without needle). This process results in the transfer of DNA insert into plant cells; the T-DNA insert remains transiently in the nucleus and is then transcribed by the host polymerase II, leading to the transient expression. The resulting mRNA transcript (capped and polyadenylated) is then exported to the cytoplasm for translation. After approximately 24 to 48 hours of incubation,sections of infiltrated leaves can be sampled for microscopyor biochemical analyses. Agroinfiltration permits large numbers (hundreds to thousands) of cells to be transfected simultaneously. For in vitro encapsidation, purified virions of BMV are dissociated into capsid protein by dialyzing against dissociation buffer containing calcium chloride followed by removal of RNA and un-dissociated virions by centrifugation. Genome depleted capsid protein subunits are then reassembled with desired viral genome components or non-viral components such as indocyanine dye.
Keywords: Immunology, Issue 61, Agrobacterium, Brome mosaic virus, Nicotiana benthamiana, encapsidation, dissociation, in vitro assembly, Nano technology
Protocol
1. Plant material
Nicotiana benthamiana plants to be used in the encapsidation assay should be at 4 leaves stage (approximately 3-4 week old plants).
2. Delivery and expression of functional viral genome components to plant cells by agroinfiltration
Day 1: The Agrobacterium strain (eg. EH 105 or GV 3101) harboring the pCass- BMV RNA 1, BMV RNA 2 and BMV RNA 31,2 are grown on LB agar plates supplemented with 50 mg/ml of kanamycin (selects for the pCASS vector) and 100mg/ml of rifampicin (selects for the Agrobacterium). Incubation is being carried out at 28 °C for two days.
Day 3: Inoculate single colony from the LB agar plate into 2 ml of LB broth containing 50 mg/ml of kanamycin and 100 mg/ml of rifampicin for 1 day at 28 °C in orbital shaker set at 250 RPM.
Day 4: Inoculate 50 ml of the LB broth supplemented with 50 mg/ml of kanamycin and 100 mg/ml of rifampicin, 10 mM MES pH 5.6 and 100 mM acetosyringone with one ml of culture in a 250 ml of Erlenmeyer flask for 16 hours at 28 °C on orbital shaker at 250 RPM.
Day 5: Transfer the culture (when OD600 reached 1.0) to an oak ridge tube or sterile Falcon tube and centrifuge for 10 minutes at 5000 rpm at 4 °C.
Dissolve the pellet in 10 ml of 10 mM MgCl2 and centrifuge for 10 minutes at 5000 RPM at 4 °C, Repeat this step one more time to ensure complete removal of the antibiotic.
Suspend the pellet in 10 ml buffer containing 10 mM MgCl2 and 10 mM MES (pH 5.6).
Measure the OD600 for each culture of BMV RNA 1, RNA 2 and RNA 3 and adjust to 0.1 OD600 using 10 mM MgCl2 and 10mM MES (pH 5.6).
Mix 1 ml each all three 0.1 OD600 culture suspensions.
Add 100 mM of acetosyringone, mix gently and keep the mixture undisturbed at room temperature for 3 hours.
Draw the culture suspension into 1 ml. syringe without needle.
Infiltrate the above culture suspension into abaxial side of 2-3 well-expanded leaves of N. benthamiana (5 leaves stage, 3-4 week old plants) by gently pressing the syringe to one half of the abaxial surface of leaf.
Repeat this infiltration procedure to other leaves.
Keep infiltrated plants in the green house with 24 °C.
Harvest infiltrated leaves 3 to 4 days post infiltration (dpi).
3. Purification of BMV virions
Collect N. benthamiana leaves agroinfiltrated with a mixture containing all three wild type BMV agrotransformants.
Grind leaves in a sterile pestle and mortar with equal volume (w/v) of BMV extraction buffer (0.5 M NaAc; 0.08 M MgAc, pH 4.5 and 1/100 volume of β-mercaptoethanol which is to be added just before use)3. To maximize virus yield, it is recommended to add acid washed sand (~0.5-1 g) that facilitates easy grinding and efficient cell disruption.
Filter the leaf extract through 2-3 layers of muslin cloth and collect the flurry.
Again grind the retained portion on muslin cloth with equal volume of BMV extraction buffer to the initial weight of leaf material.
Repeat the filtration procedure using muslin cloth. These steps should be carried out at 4 °C.
Transfer the filtrate solution to a centrifuge tube and add equal volume of pre-chilled chloroform and vortex (or shake) for 5 minutes at the room temperature till the color of the suspension turns light green.
Centrifuge the emulsified solution for 15 minutes at 12,000 RPM at 4 °C.
Collect the aqueous phase and stir for 30 minutes on magnetic stirrer to remove any residual chloroform.
Transfer the suspension to a sterile ultracentrifuge tube.
Underlay 1 ml. 10% sucrose (prepared in virus extraction buffer) as a cushion3.
Centrifuge at 30,000 rpm for 3 hours in a Beckman ultracentrifuge at 4 °C.
Discard the supernatant and suspend pellet in desired volume of BMV suspension buffer (to prepare suspension buffer dilute BMV extraction buffer to 1/10 with sterile distilled water).
The partially purified virion preparation from above step is subjected to 10-40% sucrose density gradient centrifugation for 150 min at 28000 rpm at 4 °C.
Collect the virus band either manually or by a fractionator. The virus band will appear blue under white light illumination.
Dilute the sucrose solution containing virus sample at least 50% with virus suspension buffer.
Centrifuge diluted virus containing sucrose solution for 3 hours at 30,000 rpm in a Beckman ultracentrifuge at 4 °C.
Finally suspend the highly purified virus pellet in a desired volume of virus suspension buffer.
Measure the concentration of the virion (mg/ml) using spectrophotometer at OD260.
![]()
Note: Based on RNA content, the extinction coefficient for BMV is 54.
Verify the purity of virions by viewing under TEM (Fig. A)
4. Preparation of capsid protein subunits for in vitro assembly
Prepare 1 Liter of 1x virus dissociation buffer (0.5 M CaCl2; 50 mM Tris HCl, pH 7.5; 1.0 mM EDTA; 1.0 mM DTT and 0.5 mM PMSF)5.
Prepare dialysis membrane according to Sambrook et al6.
Dispense required concentration of purified virus into a dialysis bag.
Place the virus containing dialysis bag into a beaker containing the 1x virus dissociation buffer.
Dialyze 24 hours at 4°C while stirring.
Collect the solution from the dialysis bag after 24 hours and centrifuge at 12,000x g for 15 min at 4 °C. This will separate the BMV RNA from the dissociated virions.
Collect the supernatant and centrifuge at 35,000 rpm in Beckman centrifuge for 3 hours at 4°C to pellet any un-dissociated virus particle.
Collect the supernatant.
At this stage it is imperative to remove any contaminating virion RNA from dissociated capsid protein subunits. To do this, the supernatant from step 8 should be subjected to another round of over night reassembly of capsid protein subunits without adding any RNA (in RNA 1x assembly buffer, see below) followed by an high speed centrifugation (30,000 rpm for 3 hrs at 4 °C) to pellet any assembled virions. After this reassembly step, the supernatant containing coat protein subunits would be free of any residual contaminating RNA. However, to ensure this, it is advisable to perform an additional in vitro reassembly with only coat protein subunits using RNA 1x assembly buffer. After over night reassembly, the preparation must be free of assembled virions (verify using TEM).
Determine the concentration of capsid protein subunits by measuring at OD 254 and 280 nm or by other methods such as Bradford assay.
Perform 12-15% SDS-PAGE followed by western blot to determine the integrity of the dissociated capsid protein subunits.
5. In vitro assembly of RNA containing virions
Prepare RNA transcripts to be re-assembled into virions and calculate the concentration7.
Mix capsid protein subunits and RNA transcript at a ratio of 1:5 (wt/wt)5.
Dispense the above mixture to a dialysis bag and properly secure to avoid any leaks.
Prepare 1,000 ml. of RNA 1x assembly buffer (50 mM NaCl; 50 mM Tris-HCl, pH 7.2; 10 mM KCl; 5 mM MgCl2, 1.0 mM DTT)5.
Place the dialysis bag containing the reaction mixture against 1x assembly buffer into the beaker and stir.
Dialyze the re-assembly reaction at 4 °C for 24 hours by gentle stirring.
Collect the mixture from the dialysis bag after 24 hours and add 1.5 ml of RNA assembly buffer.
Pass this mixture through a Centricon-100 column by centrifuging at low speed (2,000x g) for 30 minutes.
Wash the column once with 1.5 ml of assembly buffer at 4 °C for 30 min.
Repeat the above washing step twice.
Finally, elute the re-assembled virions by centrifugation at 10,000g at 4 °C for 5 min.
Estimate the virion concentration at OD 260 nm.
6. Fabrication of Optical Viral Ghosts (OVGs)
Add Indocyanine green (ICG)8 to the purified capsid protein at desired concentration ratio(the weight ratio of Indocyanine green and capsid protein used in this report was 1:10) and mix thoroughly by pipetting.
Dialyze the capsid protein and ICG solution against the OVG assembly buffer (1 M NaCl; 50 mM NaAc; 1 mM EDTA; and 1 mM DTT, pH4.8) at 4 °C for 24 hours.
After 24 hours, collect the solution from the dialysis bag (12 kDa pore size) and centrifuge at 90,000 rpm for 1 hour at 4 °C (Fig. B)
Remove the supernatant and suspend the pellet in BMV suspension buffer by vortexing or allowing it to suspend by itself in BMV suspension buffer overnight at 4 °C.
Verify the morphology of OVGs by TEM (Fig. C).
Measure the absorbance from 240 nm to 950 nm using spectrophotometer (Cary 50, Varian Inc.); the presence of ICG is confirmed by signature absorbance peaks around 700 nm and 790 nm8. (Fig. D)
The typical ICG fluorescence peaks at ~700 nm and ~800 nm can be seen upon 620 nm excitation of OVG solution (Fig. D) using spectrofluorometer (Fluorolog 3, Jobin-Yvon).
7. Representative Results
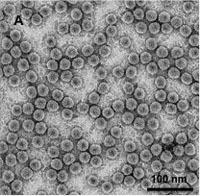 Figure A. TEM image of negatively stained BMV virions purified from N. benthamiana plants agroinfiltrated with a mixture of all three wild type BMV agroconstructs (scale bar = 100 nm).
Figure A. TEM image of negatively stained BMV virions purified from N. benthamiana plants agroinfiltrated with a mixture of all three wild type BMV agroconstructs (scale bar = 100 nm).
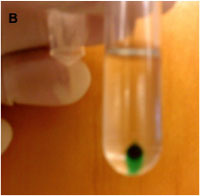 Figure B. Pellet of in vitro assembled ICG containing OVGs following high-speed centrifugation.
Figure B. Pellet of in vitro assembled ICG containing OVGs following high-speed centrifugation.
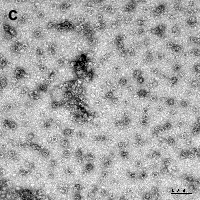 Figure C. TEM image of negatively stained ICG containing OVGs (scale bar = 100 nm).
Figure C. TEM image of negatively stained ICG containing OVGs (scale bar = 100 nm).
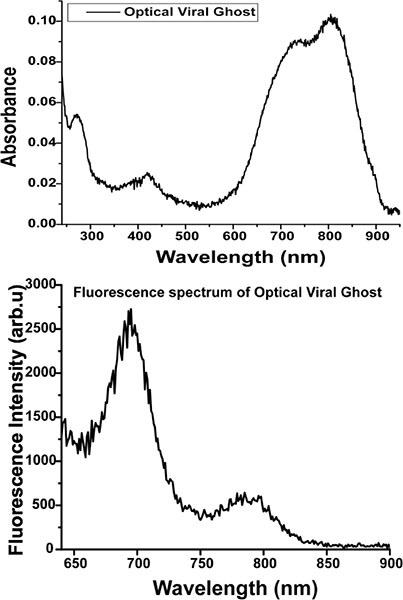 Figure D. Absorbance (Top) and fluorescence (bottom) spectra of OVGs. The excitation wavelength used for OVG emission was 620 nm.
Figure D. Absorbance (Top) and fluorescence (bottom) spectra of OVGs. The excitation wavelength used for OVG emission was 620 nm.
Discussion
Agroinfiltration approach presented here can widely be applicable to a wide range of plant viruses. A hallmark feature of this approach is synchronized delivery of multiple agroconstruct to the same cell-a major drawback commonly associated with routinely used mechanical inoculation of plant viruses. In vivo and in vitro assembly studies using brome mosaic virus as a model can be conducted efficiently by following few tips.(i)For successful infiltration of N. benthamiana leaves, do not water the plants for 24 hours before infiltration; (ii) Agrobacterium suspension culture would spread into intracellular spaces with ease, if infiltration were performed between 4-5 PM; (iii) The optical density of the culture should not exceed 1.0 at OD600. Infiltration of agrocultures with higher cell density is known to induce cell toxicity and leaf senescence9,10 that could severely affect viral replication and subsequent virion formation; (iv) During infiltration gentle pressure should be applied on the abaxial side of the leaf to prevent extensive damage to the leaf, which might trigger immune response in plant; (v) Sucrose density gradient from 10% to 40% can be made in a tube by making 25% of sucrose in BMV suspension buffer (by adding the highest concentration and lowest concentration and dividing it by 2 i.e., 10%+ 40% / 2 = 25%) and immediately freeze at -80 °C for 2-4 hr and further allowing the sucrose to thaw slowly by keeping it overnight at 4 °C in an straight position.
Disclosures
We have nothing to disclose.
Acknowledgments
The authors would like to thank several members of the lab for their valuable suggestions in the development of agroinfiltration and in vitro assembly assays. This work was funded by a grant from University of California. This study was supported in parts by a grant from the National Science Foundation (CBET-1144237).
References
- Annamalai P, Rao AL. Replication-independent expression of genome components and capsid protein of brome mosaic virus in planta: a functional role for viral replicase in RNA packaging. Virology. 2005;338:96–111. doi: 10.1016/j.virol.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Annamalai P, Rofail F, Demason DA, Rao AL. Replication-coupled packaging mechanism in positive-strand RNA viruses: synchronized coexpression of functional multigenome RNA components of an animal and a plant virus in Nicotiana benthamiana cells by agroinfiltration. J. Virol. 2008;82:1484–1495. doi: 10.1128/JVI.01540-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annamalai P, Rao AL. In: Plant Virology Protocols. Foster N, Johansen H, editors. Vol. 451. Humana Press; 2008. pp. 251–264. [Google Scholar]
- Lane LC. The bromoviruses. Adv. Virus Res. 1974;19:151–220. doi: 10.1016/s0065-3527(08)60660-0. [DOI] [PubMed] [Google Scholar]
- Choi YG, Rao AL. Molecular studies on bromovirus capsid protein. VII. Selective packaging on BMV RNA4 by specific N-terminal arginine residuals. Virology. 2000;275:207–217. doi: 10.1006/viro.2000.0513. [DOI] [PubMed] [Google Scholar]
- Sambrrok J, Russel DW. Molecular Cloning. A Laboratory Manual. Cold Spring Harbor Laboratory Press; 2001. [Google Scholar]
- Dreher TW, Rao AL, Hall TC. Replication in vivo of mutant brome mosaic virus RNAs defective in aminoacylation. J. Mol. Biol. 1989;206:425–438. doi: 10.1016/0022-2836(89)90491-9. [DOI] [PubMed] [Google Scholar]
- Jung B, Rao AL, Anvari B. Optical nano-constructs composed of genome-depleted brome mosaic virus doped with a near infrared chromophore for potential biomedical applications. A.C.S. Nano. 2011;5:1243–1252. doi: 10.1021/nn1028696. [DOI] [PubMed] [Google Scholar]
- Voinnet O, Lederer C, Baulcombe DC. A viral movement protein prevents spread of the gene silencing signal in Nicotiana benthamiana. Cell. 2000;103:157–167. doi: 10.1016/s0092-8674(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Wroblewski T, Tomczak A, Michelmore R. Optimization of Agrobacterium-mediated transient assays of gene expression in lettuce, tomato and Arabidopsis. Plant Biotechnol. J. 2005;3:259–273. doi: 10.1111/j.1467-7652.2005.00123.x. [DOI] [PubMed] [Google Scholar]


