Abstract
What causes the variations in evolutionary rates is fundamental to molecular evolution. However, in plants, the causes of within-gene evolutionary rate variations remain underexplored. Here we use the principal component regression to examine the contributions of eleven exon features to the within-gene variations in nonsynonymous substitution rate (dN), synonymous substitution rate (dS), and the dN/dS ratio in Arabidopsis species. We demonstrate that exon features related to protein structural-functional constraints and mRNA splicing account for the largest proportions of within-gene variations in dN/dS and dN. Meanwhile, for dS, a combination of expression level, exon length, and structural-functional features explains the largest proportion of within-gene variances. Our results suggest that the determinants of within-gene variations differ from those of between-gene variations in evolutionary rates. Furthermore, the relative importance of different exon features also differs between plants and animals. Our study thus may shed a new light on the evolution of plant genes.
Keywords: exonic evolutionary rates, nonsynonymous substitution rate, synonymous substitution rate, principal component regression, Arabidopsis thaliana
Introduction
Evolutionary rates are known to vary significantly between different genes.1,2 The genomic determinants of evolutionary rates have been extensively studied.2 Gene expression level is presently considered as a major determinant of evolution rate—highly expressed genes tend to have a lower nonsynonymous substitution rate (dN) and a lower nonsynonymous-to-synonymous rate (dN/dS) ratio than lowly expressed genes.3,4 Meanwhile, other biological features are also reported to be important for determining evolutionary rates, including tissue specificity of gene expression,5,6 gene compactness,7 protein extracellularity,8 presence of paralogous genes,9,10 and G+C content.11,12 Notably, evolutionary rates also vary within genes. For example, the determinants of evolutionary rates, such as the number of solvent-accessible amino acid residues,13,14 proportion of intrinsically disordered regions (IDRs),15–17 and proportion of functional domains,18–20 may differ significantly between exons, leading to significant variations in evolutionary rates even for different exons of the same genes.
Recently, it has been shown that alternative splicing (AS), an important mechanism to increase proteome diversity,21,22 has significant effects on within-gene variations in evolutionary rates in mammals.17,23,24 Particularly, the protein regions encoded by alternatively spliced exons (ASEs) evolve more rapidly than those encoded by constitutively spliced exons (CSEs).23,24 Notably, however, the contribution of AS to the diversity of transcriptome and proteome actually differs between animals and plants, with the former having more alternatively spliced transcript isoforms than the latter.22,25–27 Furthermore, the patterns of AS also differ between the two lineages. Genome-wide studies showed that the major AS type in plants is intron retention,28,29 which is the rarest event in animals.22,26,27 Therefore, whether AS also plays an important role in plant exon evolution remains unknown.
Another difference between plants and animals is the higher rates of gene duplication in plant genomes. Particularly, whole-genome duplications (WGDs) occur more frequently in plants than in animals.30,31 The genome of Arabidopsis thaliana, for example, has experienced at least four WGD events.32 In addition, tandem gene duplicates also occur more frequently in plants than in animals.33 Accordingly, gene duplication may have profound impacts on the functions and evolution of plant genes. Since both of AS and gene duplication can increase proteome diversity, the higher level of gene duplication may have compensated for the lower level of AS in plants, thus decreasing the importance of AS. In this vein, it is likely that the influences of AS on exon evolution are less significant in plants than in animals. This hypothesis, nevertheless, has not been examined.
In this study, we systematically examined the contributions of eleven exon features to the evolutionary rates of plant exons: (1) exonic expression level; (2) the ASE/CSE exon types; (3) weighted exon frequency (WEF, which is an mRNA splicing-related feature; see Materials and Methods); (4) proportion of solvent-accessible amino acid residues (PSA); (5) proportion of Pfam domain (PD); (6) proportion of intrinsically disordered regions (PIDR); (7) exon length; (8) 5′ intron length; (9) 3′ intron length; (10) exon duplicability; and (11) G+C content. Note that some of these features are correlated with each other. One obvious example is PSA, PD, and PIDR, all of which are structural-functional features. Intrinsically disordered regions tend to lack functionally structured domains and be solvent-accessible.34–36 In addition, 5′ and 3′ intron length are both related to the “compactness” of the interested exons, and thus are collectively termed “compactness features”. Other correlations are less obvious. For example, G+C content was suggested to be positively correlated with the level of gene duplication.10 Gene compactness was also reported to be positively correlated with G+C content and gene expression level, but negatively correlated with protein evolutionary rate.9 Furthermore, gene compactness correlates with gene expression level in contradictory directions between mammals and plants.37,38 In lieu of the correlations between the analyzed exon features, an appropriate analysis able to control for the confounding effects of intercorrelated variables is required for the purpose of this study. Here we use principal component regression (PCR) analysis to delineate the relative contributions of the eleven exon features to the variations in exonic evolutionary rates in Arabidopsis species (Arabidopsis thaliana and A. lyrata). PCR has been shown to be appropriate for analyzing interacting variables on noisy data.39,40
Our results suggest that for Arabidopsis, structural-functional features constitute a single dominant component in affecting the variances in exonic dN/dS and dN. For dS, however, a combination of multiple features, including exonic expression level, exon length, and structural-functional features, consist of the most important component in determining the variance in dS. Our results suggest that the determinants of exon-level evolutionary rates are fairly different from those of the gene-level evolutionary rates. Furthermore, the determinants of exonic evolutionary rates also differ between animals and plants. Our analysis thus has provided new insights into plant exon evolution.
Materials and Methods
Data sources and sequence alignments
The genomic sequences, transcript sequences, gene annotations, transcript structures, and gene orthology between A. thaliana and A. lyrata were retrieved from the EnsemblPlants website (http://plants.ensembl.org/index.html) (Release 11, TAIR10) via the BioMart interface.41,42 To ensure data quality, only known transcripts with known protein products were analyzed. Since AS is one of the focus of this study, we retained the genes that have at least 2 transcripts and obtained 52,840 orthologous exon pairs from 4,926 one-to-one orthologous gene pairs, which correspond to 11,723 A. thaliana transcripts. We defined the CSE/ASE exon type according to the transcripts of A. thaliana because it is better annotated and has a larger number of alternatively spliced transcripts than A. lyrata. Exons lacking information of any of the analyzed features were filtered out. In the end, we generated an integrated dataset of 28,173 within-gene exon pairs based on the 9,412 exons of 2,102 transcripts.
For each orthologous gene pair, the peptide sequences of all transcript isoforms were aligned by using the MUSCLE program.43 We then chose the longest alignable pair of orthologous peptide sequences for each gene pair. These sequences were then back- translated to nucleotide sequences, and divided into exons with reference to A. thaliana annotations for calculation of evolutionary rates and measurements of exon features. For simplicity, the codons that span an exon-exon boundary were excluded from our analysis.
Measurements of exonic expression level
The RNA-seq data were retrieved from the Sequence Read Archive (SRA)44 website (http://trace.ncbi.nlm.nih.gov/Traces/sra/). We retrieved a series of raw data submitted by Filichkin et al (SRP000935)29 and Schimidt’s lab (SRP007763). These RNA-seq data cover several experimental conditions, including abiotic stresses (cold, drought, heat, highlight, and salt), aging stress, and response to different iron concentrations, in addition to normal growth conditions. To measure exonic expression level, we first identified the unique sequences in the analyzed exons using inhouse PERL scripts, and then calculated the number of RNA-seq short reads that can be mapped to these unique regions. The RNA-seq raw data were processed to output the fastq files by using sratoolkit 2.1.6 (available at: http://trace.ncbi.nlm.nih.gov/Traces/sra/sra.cgi?view=software). The fastq files were further processed and filtered by the Bowtie-TopHat-Cufflinks-SAMtools toolset45–48 (available at: http://bowtie-bio.sourceforge.net/index.shtml; http://tophat.cbcb.umd.edu/; http://cufflinks.cbcb.umd.edu/; http://samtools.sourceforge.net/) to generate the FPKM (fragments per kilobase of exon per million fragments mapped) value for each exon of interest. The expression level of an exon (expL) is the average FPKM value.
Estimations of exonic evolutionary rates
We calculated dN, dS, and dN/dS of all orthologous exon pairs by using the YN00 program of the PAML 4 package.49,50 To avoid biases in evolutionary rate estimations, we excluded exons whose lengths are shorter than 81 bp.17,51
Computation of splicing features
The analyzed exons were divided into five different classes: complex ASE, multiple ASE, single ASE, simple CSE, and complex CSE (see Supplementary Fig. S1A for more details). Briefly, ASEs are the exons that are occasionally skipped in the transcript, whereas CSEs are those that are always present in the transcript. This classification, however, is apparently oversimplified because exons from different transcript isoforms usually partially overlap with each other (Supplementary Fig. S1A). We thus divided ASEs into three different types. A single ASE is one that occurs only once in all of the transcript isoforms. By contrast, a multiple ASE occurs multiple times, and the boundary of this ASE remains unchanged in different transcript isoforms. If a multiple ASE changes its boundary in different transcripts, it is termed a complex ASE. Meanwhile, a simple CSE is one that does not change its boundaries in transcript isoforms. In the case where the boundaries of a CSE do change, or the CSE becomes discontinuous in another transcript isoform, the CSE is termed a complex CSE (Supplementary Fig. S1A). To incorporate this exon classification into our PCR analysis, we assigned an integer value to each of the five types of exons: “1” for complex ASE, “2” for multiple ASE, “3” for single ASE, “4” for complex CSE, and “5” for simple CSE.
We also calculated the weighted exon frequency (WEF) as a quantitative measurement of the relative importance of exons in AS events. The “frequency” of an exon is the proportion of transcript isoforms that include this specific exon. For example, if a gene contains four AS isoforms, and a certain exon is included in three of the four isoforms, the frequency of this exon is 3/4. However, since exons from different isoforms can partially overlap with each other, it is sometimes difficult to clearly define the “frequency” of an exon. Therefore, we use WEF instead, which is the length-weighted frequency of an exon. Supplementary Figure S1B gives examples of WEF calculation. Briefly, an exon was divided into several sub-regions according to how it overlaps with exons from other transcript isoforms. The frequency of each sub-region can be calculated and then weighted by the length of each sub-region to yield WEF.
Measurements of structural-functional features
The structural-functional features analyzed in this study include the proportion of solvent-accessible amino acid residues (PSA), proportion of Pfam domain sites (PD), and proportion of intrinsically disordered regions (PIDR).
Solvent-accessible residues were predicted by using the SSpro/ACCpro 4.1 program52 with a 30% exposure threshold. Intrinsically disordered regions were predicted by using DISOPRED V253 with default parameters. The Pfam domain information54 was retrieved from the EnsmblPlant databases.
Measurements of compactness features and other features
The exon length, 5′/3′ intron length, and G+C content were calculated using an in-house PERL script with reference to the annotations of EnsemblPlants. Note that the first and the last coding exons, which do not have 5′ or 3′ intron, respectively, were excluded. Exon duplicability (ED) was defined as the “copy number” of an exon in the A. thaliana transcriptome. We BLASTN-aligned55 the exon of interest against the A. thaliana exome using default parameters. A BLAST hit was considered as a potential duplicate of the exon of interest if it satisfies the following criteria: (1) the alignable length is ≧90% of the query exon length; (2) the alignable region has a ≧90% similarity with the query exon.
Statistical analysis
The PCR analyses were conducted by using the program provided by Drummond et al39 under the R environments.56 In this study, we calculated the differences in evolutionary rates (dN, dS, and dN/dS) and the eleven exon features for pairs of exons from the same transcripts (the total number of within-gene exon pairs analyzed here is 28,173). These differences were then analyzed using PCR to delineate the contributions of the exon features to the variances in evolutionary rates.
We also compared exons from different genes by randomly generating 500 datasets, with each dataset containing 28,173 between-gene exon pairs, which is the number of exon pairs in the within-gene analysis. We first randomly selected one exon from the analyzed transcripts, and then selected a second exon that belongs to a gene other than where the first exon is from. By doing so we could be sure that the two exons are from different genes. This process was iterated 28,173 times to derive 28,173 between-gene exon pairs for each of the 500 random datasets. A PCR analysis was then conducted for each of these 500 random datasets. The results of the 500 random sampling experiments were then averaged to represent the effects of between-gene differences in exon features on variances in evolutionary rates.
Results and Discussions
The correlations between exon features and exonic evolutionary rates
To confirm whether the eleven analyzed exon features are correlated with exonic evolutionary rates in Arabidopsis, we pooled all of the exons together and conducted simple Pearson’s correlations separately for dN, dS and dN/dS ratio against each individual exon feature. As shown in Table 1, exonic dN/dS is significantly correlated with each of the eleven exon features. Similar results are also observed for dN, except that the correlation between 3′ intron length and dN is statistically insignificant. By contrast, only five of the eleven features (exon length, expression level, exon duplicability, 3′ intron length, and ASE/CSE exon type) are significantly correlated with dS. Notably, exon features related to structural-functional constraints (PD, PSA, and PIDR) have the highest correlations with exonic dN/dS. For dN and dS, unexpectedly, exon length seems to be the most important determinants. Another unexpected observation is that expression level ranks only the fifth and the fourth, respectively, in terms of coefficient of correlation with dN/dS and dN, although it ranks the second in the case of dS. These results, however, are oversimplified because the interactions between different features are not controlled. Accordingly, we conducted PCR analyses to control for the confounding effects of inter-correlations between the analyzed features.
Table 1.
The Pearson’s coefficient of correlation between each of the eleven exon features and dN/dS, dN, and dS.
| Exon feature | Pearson’s coefficient of correlation (rank) | ||
|---|---|---|---|
|
|
|||
| dN/dS | dN | dS | |
| % Pfam domain | −0.2338 (3)a,b,**** | −0.2292 (5)**** | 0.0126 (8) |
| % solvent-accessible amino acid residues | 0.2372 (2)**** | 0.2434 (3)**** | 0.0055 (10) |
| % intrinsically disordered regions | 0.2694 (1)**** | 0.2842 (2)**** | 0.0165 (7) |
| ASE/CSE exon type | −0.0294 (11)* | −0.0210 (10)* | 0.0171 (5)* |
| Weighted exon frequency | −0.0309 (10)* | −0.0232 (9)* | 0.0169 (6) |
| 5′ intron length | 0.0496 (7)*** | 0.0475 (7)** | −0.0068 (9) |
| 3′ intron length | 0.0421 (8)** | 0.0096 (11) | −0.0537 (3)*** |
| Exon length | 0.2180 (4)**** | 0.3133 (1)**** | 0.1560 (1)**** |
| Exonic expression level | −0.1852 (5)**** | −0.2382 (4)**** | −0.0892 (2)**** |
| Exon duplicability | −0.0625 (6)**** | −0.0898 (6)**** | −0.0394 (4)** |
| G+C content | −0.0349 (9)** | −0.0356 (8)** | −0.0016 (11) |
Notes:
The number in the parenthesis indicates the “rank” of the specific exon feature according to the absolute value of the coefficient of correlation;
statistical significance.
P < 0.05;
P < 10−3;
P < 10−6;
P < 10−9.
The exon features are grouped into biologically sensible components
Considering that some of the differences in exon features may result largely from the gene-level differences (e.g., expression level), we calculated the within-gene differences in the eleven exon features (i.e, differences between exons from the same transcript) and exonic evolutionary rates (ΔdN, ΔdS, and ΔdN/dS ratio). We then conducted PCR separately for ΔdN, ΔdS, and ΔdN/dS ratio against the within- gene differences in the eleven exon features. To probe the effect of between-gene differences in exon features on the variances in evolutionary rates, we also performed the same PCR analysis by analyzing exons from different genes on 500 random datasets and averaged the results for comparison with the within-gene analysis (see Materials and Methods).
The principal components derived from the within-gene analysis (Table 2) and the between-gene analysis (Table 3) give similar, biologically meaningful groupings for the eleven exon features: (1) structural-functional features: PSA, PIDR, and PD; (2) mRNA splicing features: weighted exon frequency (WEF) and ASE/CSE exon type; (3) compactness features: 3′ and 5′ intron lengths; (4) exonic expression level; (5) exon length; and (6) other features: exon duplicability and G+C content. These results reflect the strength of PCR in identifying biologically relevant variables. Furthermore, the similar results between within-gene (Table 2) and between-gene (Table 3) analyses indicate the consistency and reliability of the PCR approach. Notably, exon length and exonic expression level usually occur in the same components (components 3 and 9 in both Tables 2 and 3). It was previously reported that gene expression level is negatively correlated with gene size57,58 or transcript size58,59 in plants. However, whether such correlations apply to exon length and exonic expression level remains unclear. Therefore, we present exon length and exonic expression level as different features in our results, although these two features tend to occur in the same components. The projection patterns of the
Table 2.
The percent contributions of individual features to the principal components in the within-gene analysis.
| Exon features | Comp 1 | Comp 2 | Comp 3 | Comp 4 | Comp 5 | Comp 6 | Comp 7 | Comp 8 | Comp 9 | Comp 10 | Comp 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASE/CSE exon type | 0.070 | 0.421 | 0.002 | 0.001 | 0.003 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.501 |
| Weighted exon frequency (WEF) | 0.070 | 0.418 | 0.002 | 0.001 | 0.007 | 0.002 | 0.001 | 0.000 | 0.000 | 0.000 | 0.498 |
| Average exonic expression level | 0.042 | 0.018 | 0.454 | 0.000 | 0.004 | 0.006 | 0.000 | 0.000 | 0.462 | 0.013 | 0.000 |
| 5′ intron length | 0.014 | 0.000 | 0.017 | 0.088 | 0.330 | 0.161 | 0.362 | 0.025 | 0.002 | 0.001 | 0.000 |
| 3′ intron length | 0.001 | 0.000 | 0.003 | 0.422 | 0.161 | 0.001 | 0.389 | 0.010 | 0.012 | 0.001 | 0.000 |
| Exon length | 0.092 | 0.028 | 0.330 | 0.005 | 0.001 | 0.000 | 0.063 | 0.002 | 0.434 | 0.046 | 0.000 |
| G+C content | 0.005 | 0.001 | 0.017 | 0.428 | 0.252 | 0.089 | 0.133 | 0.017 | 0.038 | 0.020 | 0.000 |
| Exon duplicability | 0.000 | 0.013 | 0.001 | 0.022 | 0.216 | 0.708 | 0.035 | 0.000 | 0.002 | 0.000 | 0.001 |
| % solvent-accessible amino acid residues | 0.208a | 0.031 | 0.087 | 0.030 | 0.024 | 0.029 | 0.000 | 0.270 | 0.032 | 0.289 | 0.000 |
| % intrinsically disordered regions | 0.298 | 0.042 | 0.053 | 0.002 | 0.001 | 0.002 | 0.002 | 0.022 | 0.012 | 0.566 | 0.000 |
| % Pfam domain | 0.198 | 0.027 | 0.035 | 0.000 | 0.001 | 0.001 | 0.013 | 0.655 | 0.006 | 0.063 | 0.000 |
Notes:
The numbers larger than 0.2 are color-shaded. The color code in the table is the same as that used in Figure 1. eleven principal components in the within-gene analysis are given in Supplementary Figure S2.
Table 3.
The percent contributions of individual features to the principal components in the between-gene analysis.
| Exon features | Comp 1 | Comp 2 | Comp 3 | Comp 4 | Comp 5 | Comp 6 | Comp 7 | Comp 8 | Comp 9 | Comp 10 | Comp 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ASE/CSE exon type | 0.003 | 0.485 | 0.000 | 0.005 | 0.001 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.500 |
| Weighted exon frequency (WEF) | 0.003 | 0.483 | 0.001 | 0.006 | 0.000 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.500 |
| Average exonic expression level | 0.094 | 0.001 | 0.409 | 0.004 | 0.003 | 0.003 | 0.003 | 0.001 | 0.481 | 0.002 | 0.000 |
| 5′ intron length | 0.018 | 0.009 | 0.002 | 0.301 | 0.080 | 0.058 | 0.525 | 0.001 | 0.000 | 0.000 | 0.000 |
| 3′ intron length | 0.008 | 0.004 | 0.003 | 0.385 | 0.097 | 0.035 | 0.458 | 0.005 | 0.008 | 0.000 | 0.000 |
| Exon length | 0.137 | 0.006 | 0.259 | 0.008 | 0.071 | 0.026 | 0.001 | 0.050 | 0.422 | 0.025 | 0.000 |
| G+C content | 0.000 | 0.000 | 0.094 | 0.207 | 0.267 | 0.355 | 0.004 | 0.014 | 0.061 | 0.014 | 0.000 |
| Exon duplicability | 0.010 | 0.004 | 0.006 | 0.014 | 0.462 | 0.480 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 |
| % solvent accessible amino acid residues | 0.187a | 0.003 | 0.127 | 0.062 | 0.013 | 0.035 | 0.001 | 0.300 | 0.025 | 0.248 | 0.000 |
| % intrinsically disordered regions | 0.302 | 0.003 | 0.076 | 0.008 | 0.005 | 0.000 | 0.000 | 0.006 | 0.002 | 0.600 | 0.000 |
| % Pfam domain | 0.237 | 0.002 | 0.024 | 0.000 | 0.002 | 0.000 | 0.002 | 0.622 | 0.001 | 0.109 | 0.000 |
Notes:
The numbers larger than 0.2 are color-shaded. The color code in the table is the same as that used in Figure 1.
Structural-functional features are the single dominant feature category in explaining the variances in exonic dN/dS and dN in Arabidopsis
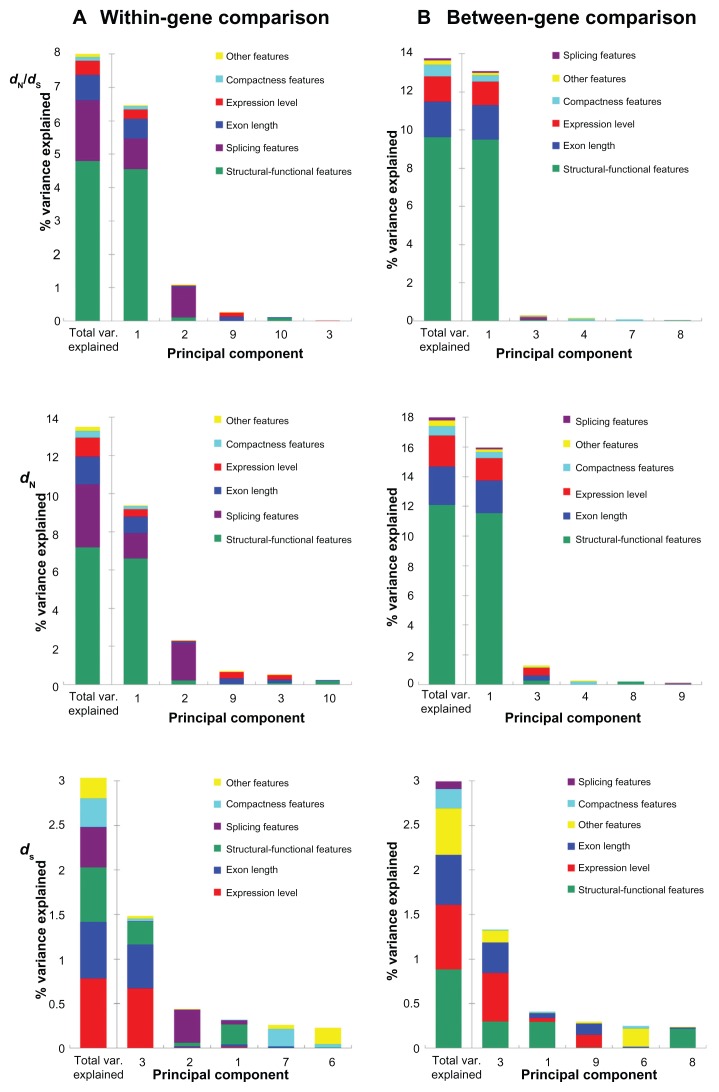
The variances in evolutionary rates that can be accounted for by the six aforementioned feature categories are shown in Figure 1 (note that only the five most important components are demonstrated). For the dN/dS ratio in the within-gene analysis (Fig. 1A, upper panel), structural-functional features dominate the most influential component (Component 1), which explains 6.47% of the variance in exonic dN/dS. Interestingly, the second most important component (Component 2) is dominated by splicing features, which explain only 1.09% of exonic dN/dS variance. None of the rest of the components explains more than 0.3% of the exonic dN/dS variance (Supplementary Table S1). In other words, structural-functional features appear to be the single dominant feature category in explaining exonic dN/dS in Arabidopsis. The total variance in dN/dS explained by all of the eleven exon features is 8.00%, among which structural- functional features account for 60% (4.80/8.00) (Fig. 1A, upper panel and Supplementary Table S1). Similar results are also observed in the PCR analysis of dN, where structural-functional features account for 53% (7.20/13.49) of the total variance explained (Fig. 1A, middle panel and Supplementary Table S2). Meanwhile, splicing features, which dominate the second most important component (Component 2) in the within-gene analysis, account for 22.8% (1.82/8.00) and 24.4% (3.29/13.49) of total variances in dN/dS and dN explained, respectively (upper and middle panel of Fig. 1A; Supplementary Tables S1 and S2). Exon length is the third most important determinant, accounting for 9.4% (0.75/8.00) and 10.6% (1.43/13.49) of the total within-gene variances in dN/dS and dN explained, respectively (upper and middle panel of Fig. 1A; Supplementary Tables S1 and S2). Notably, expression level ranks only the fourth in this within-gene analysis, accounting for a fairly small percentage of total variances in dN/dS (5.3% = 0.42/8.00) and dN (7.4% = 1.00/13.49) explained. In addition, the contributions of compactness features are even smaller than those of expression level. This feature category explains only 1.5% and 2.6% in the variance in dN/dS and dN, respectively (Supplementary Tables S1 and S2).
Figure 1.
The percent of variances in dN/dS (upper panel), dN (middle panel), and dS (lower panel) explained by the principal components for (A) the within-gene comparison; and (B) the between-gene comparison.
Notes: Only the five most important components are shown here. The leftmost bar in each panel indicates the total variances explained by all of the eleven components.
To examine the influences of gene-level feature differences on exonic evolutionary rates, we also conducted between-gene PCR analyses by comparing exons from different genes (see Materials and Methods for more details). Unexpectedly, structural-functional features remain the most important factor in affecting the variances in exonic dN/dS and dN, although the importance of splicing features has significantly decreased in this between-gene analysis (upper and middle panel of Figure 1B and Supplementary Tables S4 and S5). In fact, structural-functional features account for 70% (9.63/13.68) and 67% (12.11/17.97) of the total variance explained in exonic dN/dS or dN, respectively (upper and middle panel of Fig. 1B; Supplementary Tables S4 and S5). Interestingly, exon length is the second most important determinant for between-gene variances in dN/dS and dN, accounting for 13.6% (1.86/13.68) and 14.4% (2.58/17.97) of the total variance explained, respectively. By contrast, expression level, the major determinant of gene-level dN/dS, ranks only the third, and accounting for 9.7% (1.33/13.68) and 11.6% (2.08/17.97) of the total variance explained in exonic dN/dS and dN, respectively (upper and middle panel of Fig. 1B; Supplementary Tables S4 and S5). Compactness features again have only minor contributions in this between-gene analysis, accounting for 4.3% (0.59/13.68) and 3.5% (0.62/17.97), respectively, of the total variance in dN/dS and dN explained.
One notable observation in Figure 1 is that the total variances in dN/dS and dN explained by the eleven analyzed exon features are larger in the between-gene analysis than in the within-gene analysis. This is probably because the between-gene differences are larger than within-gene differences in evolutionary rates and the analyzed exon features. The larger variations may have led to a better resolution of the PCR analyses. Furthermore, the total variances explainable by the exon features are larger for dN than for dN/dS in both of the within-gene and between-gene analyses. One possible explanation is that the exon features that have major effects on dN also significantly affect dS. In other words, the explainable variances in dN may be partly cancelled out by the variances in dS, leading to decreased explainable variances in dN/dS. Also note that the variances in exonic dN/dS explained by the major component (6.47% and 13.09%, Supplementary Tables S1 and S4) in this study are smaller than that reported for yeast39 (~40%) but larger than that reported for mammals7 (~5%). Note that both of the latter two studies measured the dN/dS ratio for genes as a whole, whereas in this study we measured the dN/dS for individual exons. The differences in methodology and target of study (gene or exon) may be one of the reasons that cause the differences in explainable dN/dS variance. Another possible reason for the differences among the three studies is that the three examined lineages (unicellular eukaryotes, plants and mammals) may have very different effective population size (Ne). The efficiency of natural selection is known to be positively correlated with Ne.60,61 For the three lineages being compared here, yeast has the largest,62 and mammals may have the smallest Ne.60,63 Therefore, the order of explainable variance in dN/dS may have reflected the order of efficiency of selection. This speculation, however, requires further supporting evidence because the Ne of Arabidopsis thaliana remains to be determined.
One unexpected result in this study is that expression level accounts for a relatively small variance in dN/dS. Expression level was reported to be the most dominant dN/dS determinant at the gene level.2,6,64,65 There are several possible explanations for the reduced importance of expression level in affecting dN/dS in this study. First, the RNA-seq data used in this study were derived from several stress conditions (see Materials and Methods). It is likely that overall gene (and exon) expression levels are suppressed in these conditions, leading to lack of resolution in expression level even between different genes. Second, the sequencing depth in the analyzed RNA-seq dataset may be insufficient to yield reliable exonic expression level. Third, our PCR analysis indicates that exonic expression level and exon length usually occur in the same components (Tables 2 and 3). The reason for this correlation remains unclear. However, if we merge exon length and exonic expression level into one feature category, this category would be the second most influential feature category for explaining the variances in dN/dS and dN in the between-gene analysis but not in the within-gene analysis, where splicing features would remain the second most important feature category (Supplementary Tables S1, S2, S4, and S5).
An interesting observation in Figure 1 is that splicing features play a relatively important role in determining within-gene variances in dN/dS and dN, but not in the case of between-gene analysis. Note that splicing features are specific to individual exons (rather than transcripts or genes as a whole). Unlike expression level, splicing features may differ significantly within the same gene (e.g., WEF = 1.0 versus WEF = 0.5) but be similar for exons from different genes (e.g., two exons from different genes are both simple CSEs; both WEF = 1.0). This is especially true considering that the majority (97.5%) of the analyzed exons are CSEs. Therefore, within-gene variations in splicing features may better explain within-gene variances in dN/dS and dN than between-gene variations. Notably, however, the contributions of splicing features to within-gene variances in exonic dN/dS and dN are only a fraction of those made by structural-functional features (Fig. 1 and Supplementary Tables S1 and S2). This observation appears to support our hypothesis that alternative splicing plays a relatively minor role in affecting exon evolution in plants. In fact, our recent study indicates that in mammals, alternative splicing and structural-functional features have similar contributions to within-gene variances in dN/dS and dN.66
The variances in exonic dS can be explained by a combination of different exon features
For the PCR analysis of within-gene variance in dS, however, we obtained quite different results from what was observed for dN/dS and dN: the major component (Component 3) is composed of multiple exon features (exonic expression level, exon length, and structural-functional features), and none of the features accounts for more than half of the component (Fig. 1A, lower panel and Table 2). Interestingly, the second most important component remains Component 2 (as in the case of dN/dS and dN), which is composed mainly of splicing features. These observations suggest that the variance in within-gene dS is determined by a combination of different biological factors. By contrast, in the case of within-gene variances in dN/dS and dN, one feature category (structural-functional features) seems to be the single dominant factor (upper and middle panel of Fig. 1A). When the contributions of each feature category are summed up, exonic expression level accounts for the largest percent (25.1% = 0.78/3.12) of variance in within-gene dS explained, followed by exon length (20.3% = 0.63/3.12), structural-functional features (19.8% = 0.62/3.12), splicing features (14.5% = 0.45/3.12), compactness features (10.3% = 0.32/3.12), and finally by other features (10.1% = 0.32/3.12) (Supplementary Table S3).
The between-gene analysis of variance in dS again yields fairly different results from what were observed for dN/dS and dN. Particularly, none of the analyzed exon features (or feature categories) comprises a single dominant factor. As shown in Figure 1B (lower panel), the constituents of the major component (Component 3) in this analysis are similar to those in the within-gene dS analysis (Fig. 1A, lower panel) but with somewhat different weightings. In this analysis, Component 3 is composed mainly of structural-functional features, expression level, exon length, and other features, with the last feature category accounting for a larger proportion of dS variance (22.8%) than in the within-gene analysis (17.5%). The total variance in between-gene dS explained by each category is, in a descending order, structural-functional features (29.6% = 0.88/2.99), expression level (24.3% = 0.73/2.99), exon length (18.7% = 0.56/2.99), other features (17.4% = 0.52/2.99), compactness features (7.4% = 0.22/2.99), and splicing features (2.7% = 0.08/2.99) (Supplementary Table S6).
A noticeable observation here is that exonic expression level and exon length, if considered together as one biological factor, could account for the largest proportion of the total variance in exonic dS explained in both of the within-gene analysis (Fig. 1A, lower panel) and the between-gene analysis (Fig. 1B, lower panel). Recall that these two exon features usually occur in the same components (Tables 2 and 3), even though we do not know the biological connection between them. In fact, these two exon features have a significantly negative correlation with each other (Pearson’s r = −0.5278, P ~ 0). If we consider these two exon features as expression level-related features, the high proportions of variance in dS accounted for by these features may be explained as the requirement for translational efficiency—highly expressed genes are known to have more biased codon usages,67–69 which may lead to increased dS. This speculation, however, remains to be validated. The Pearson’s correlation coefficient between each principal component and the target variables (dN/dS, dN and dS) in the within-gene analysis are given in Supplementary Table S7.
The relative importance of exonic expression level and structural-functional features in affecting between-gene dN/dS variance
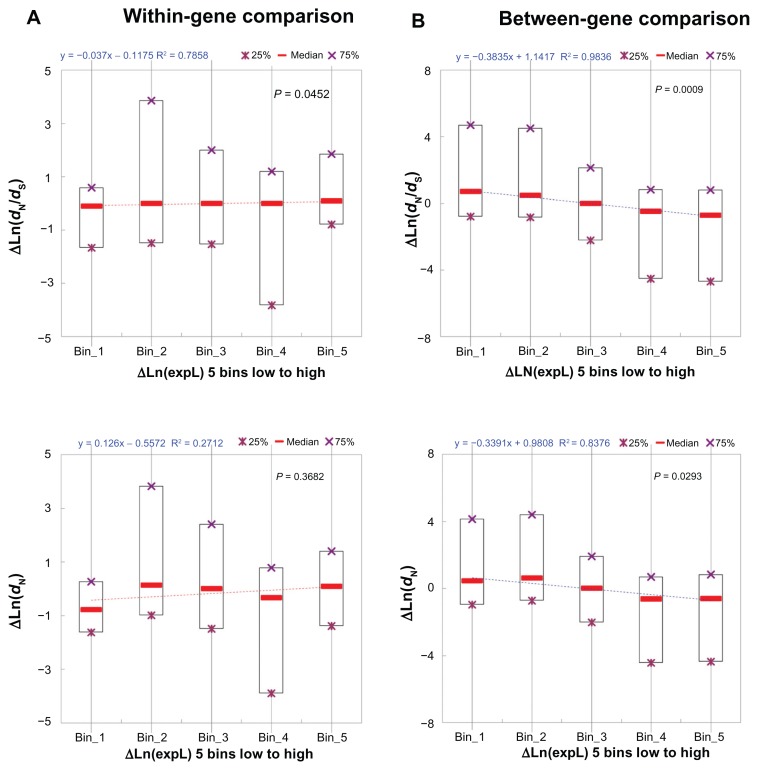
Somewhat surprising in Figure 1B is the observation that structural-functional features constitute the single most important feature category in affecting between-gene variances in dN/dS and dN. It is unexpected because exonic expression level is expected to diverge more significantly between genes than between exons of the same genes. However, this comment does not apply to structural-functional features. To examine whether our analyses contain certain technical errors, we re-examined our data by dividing the within-gene and between-gene exon pairs, separately, into five groups according to two different criteria: (1) the difference in exonic expression level (ΔexpL); and (2) the difference in PSA (ΔPSA, a representative structural- functional feature). We then conducted simple regressions between ΔdN/dS (and ΔdN) and the two exon features separately.
We first examined the correlations between ΔexpL and ΔdN/dS (and ΔdN). As shown in Figure 2A, the correlations are marginally significant or statistically insignificant (P = 0.0452 for ΔdN/dS; P = 0.3682 for ΔdN) in the case of within-gene exon pairs. By contrast, the correlations are statistically significant (P = 0.0009 for ΔdN/dS; P = 0.0293 for ΔdN), and the regression coefficients are negative in the case of between-gene exon pairs (Fig. 2B). Therefore, the general concept that highly expressed genes (and thus the exons within) tend to evolve slower is supported in our between-gene dataset (Fig. 2B). This (ΔexpL-ΔdN/dS) negative correlation, however, is not observed in the within-gene dataset (Fig. 2A). These observations appear to support our hypothesis that expression level differ by a greater extent between genes than between exons from the same genes. Therefore, exonic expression level may not have major contributions to within-gene variations in dN/dS. Nevertheless, this cannot explain why structural-functional features are more influential than exonic expression level in the between-gene analysis (Fig. 1B). We then examined the correlations between ΔPSA and ΔdN/dS (and ΔdN).
Figure 2.
The linear correlation between difference in exonic expression level (ΔLn(expL)) and difference in dN/dS (ΔLn(dN/dS), upper panel) or difference in dN (ΔLn(dN), lower panel) for (A) the within-gene comparison; and (B) the between-gene comparison.
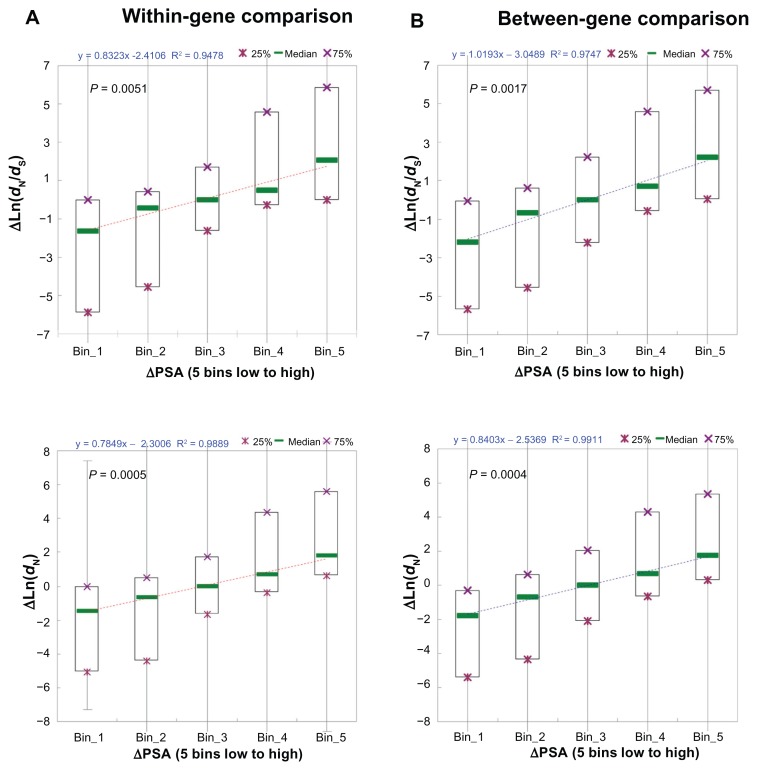
Interestingly, as shown in Figure 3, for both within- gene dataset (Fig. 3A) and between-gene dataset (Fig. 3B), ΔPSA is significantly positively correlated with ΔdN/dS and ΔdN (P = 0.0051 and 0.0017 for within-gene and between-gene ΔdN/dS, respectively; P = 0.0005 and 0.0004 for within-gene and between-gene ΔdN, respectively). Notably, the regression coefficients are larger for between-gene dataset than for the within-gene dataset. In other words, between-gene divergences in PSA may better explain differences in exonic dN/dS (and dN) than within-gene divergences. Similar trends are also observed for the other two structural-functional features (PD and PIDR, Supplementary Figs. S3 and S4). Although it is unexpected, this observation is likely to be true because genes may diverge significantly in the densities of structural-functional domains, which can be reflected also at the exon level. Therefore, our previous result in Figure 1 appears to be correct that structural- functional features account for a larger proportion of total variances in dN/dS and dN explained in the between-gene analysis than in the within-gene analysis.
Figure 3.
The linear correlation between difference in percent of solvent-accessible amino acid residues (ΔPSA) and difference in dN/dS (ΔLn(dN/dS), upper panel) or difference in dN (ΔLn(dN), lower panel) for (A) the within-gene comparison; and (B) the between-gene comparison.
Notably, the absolute values of regression coefficients are larger for all of the three structural-functional features (Fig. 3B and Supplementary Figs. S3B and S4B) than for exonic expression level (Fig. 2B). This is probably the reason why we observe larger contributions by structural-functional features to between-gene variances in dN/dS and dN than by exonic expression level. However, as we will discuss in the next section, the relative importance of exonic expression level and structural-functional features in affecting exonic dN/dS needs further investigations. In fact, in mammals, exonic expression level plays a more important role than structural-functional features in affecting between-gene variations in exonic dN/dS.66
Gene level versus exon level— the differences in evolutionary rate determinants
Recently, Yang and Gaut also used PCR to systematically examine the determinants of gene-level dN (Ka) and dS (Ks) in Arabidopsis species.6 The authors reported that all of the analyzed features could explain 21.4% and 11.1% of the variance in dN and dS, respectively. By contrast, in this study, all of the exon features can explain 13.5% and 3.12% of within-gene variance in dN and dS, respectively. For the between-gene analysis, the percentages are 18.0% and 3.0%, respectively. Obviously, the total explainable variances in our study are smaller than those observed in Yang and Gaut’s study. Notably, in the current study, the measurements of exon features and evolutionary rates may be somewhat noisy because of the relatively short length of exons (as compared with genes or transcripts as a whole). Although we excluded short exons (see Materials and Methods), the variations in the exon features are unavoidably larger than the biological feature measurements obtained for complete genes.66 This is perhaps one of the reasons why the explainable variances in this study are smaller than in the gene-level analysis.
Meanwhile, the major determinants of variances in dN and dS also differ significantly between the two studies. In Yang and Gaut’s study,6 the most important factor in affecting dN appears to be gene expression-related features (gene expression level and expression breadth), whereas in this study, the most important determinant is related to the structural-functional constraints (PIDR, PSA, and PD). Note that we did not include expression breadth in our analysis because the RNA-seq data cover only an inadequate number of tissues. In the case of dS, the most important gene-level determinants are gene length and intron number. But at the exon level, the most important determinants are expression level, exon length, and structural-functional features. There are several possible reasons for this difference. Firstly, in Yang and Gaut’s study, gene expression level was measured by using microarray, whereas in this study RNA-seq data were used to derive exonic expression level. The microarray approach has some potential caveats (e.g., cross-hybridization and differential probe affinity). Nevertheless, the RNA-seq data used in this study also suffer some potential problems (e.g., insufficient sequencing depth and stress condition-based RNA expression data). The differences in data source and the limitations of the data may have led to difference in major determinants of evolutionary rates between the two studies. Secondly, the two studies actually explore similar topics (i.e., determinants of evolutionary rates) at two different levels (gene and exon level). Therefore, the analyzed biological features differ because of the nature of the studies. For example, splicing features, which are unique to this study, cannot be incorporated into the gene-level study. Another example is the lengths of untranslated regions, which are analyzed in Yang and Gaut’s study but seem to be less relevant (and thus not included) in this study. The differences in nature and the analyzed biological features may be part of the reasons why the two studies yielded different results.
In addition, one noticeable feature of PCR is that the percent of variance explained by each variable may change when different numbers of variables are included. For example, in our analysis the three structural-functional features (PSA, PIDR, and PD) are correlated with each other. If we remove PIDR and PD from our analysis, the results become somewhat different (Supplementary Fig. S5)—the relative importance of expression level increases while that of structural-functional features (PSA) decreases in both of the within-gene and between-gene analyses. However, the total variance explained by all of the components also decrease by ~2% for dN and dN/dS. This result implies that although the three structural-functional features are correlated with each other, they might still have some independent effects on exonic dN and dN/dS. Furthermore, given adequate RNA-seq-based expression data, we might be able to include other expression-related features (such as expression breadth and tissue specificity of expression) in the future. The relative importance between expression features and structural-functional features in affecting plant exon evolution can then be re-evaluated.
Potential caveats
A potential caveat in this study is that we classify exons into different types (CSEs or ASEs) according to the transcript structures of A. thaliana. It is likely that the classification of exons may be different for A. lyrata. However, as the majority of A. lyrata genes encode only one annotated transcript, we do not have sufficient information for exon classifications for this species. That said, we speculate that we may obtain similar results even if we have sufficient information for A. lyrata. This is because a previous study indicated that exon classifications according to annotations of different mammalian species (i.e., human or mouse) yielded similar results in the analyses of exonic evolutionary rates.24
Still another limitation of this study is that we do not know all of the determinants of evolutionary rates in plants. Particularly, complex biological interactions are common in multicellular organisms. The “interaction terms” and the spatio-temporal regulations of biological functions are obviously omitted in this study due to lack of information. This lack of knowledge may lead to input of insufficient determinants, and thus a reduction in the variance explained in the PCR analyses.
Concluding remarks
To our knowledge, this is the first study to systematically examine the determinants of within-gene variations in evolutionary rates in plants. We not only confirmed the findings in a similar study in animals (that the determinants of exon-level and gene-level evolutionary rates differ from each other), but also demonstrated important differences in the relative importance of biological features in affecting exonic evolutionary rates between animal and plants. Particularly, the importance of alternative splicing in plants seems to be less significant in plants than in animals. Notably, alternative splicing is an important regulatory mechanism in multicellular organisms, and more so in animals than in plants. Our study shows the importance of alternative splicing from another angle. That is, alternative splicing has major but differential effects on exon evolution in animals and plants. Many important questions remain unanswered, however. For example, some important regulatory mechanisms, such as DNA methylation and nucleosome occupancy, are not included in this study. In addition, it will be interesting to conduct similar analyses for more species from major taxa for comparison (e.g., gymnosperms vs. angiosperms; monocots vs. dicots; algae vs. fungi vs. seed plants… etc.) when adequate information is available.
Supplementary Data
(A) The classification of the ASE/CSE exon type; (B) Two examples of how the weighted exon frequency is calculated.
The projection patterns of the eleven principal components in the within-gene analysis.
The linear correlation between difference in the percent of Pfam domain ( ΔPD) and difference in d
The linear correlation between difference in the percent of intrinsically disordered region (ΔPIDR) and difference in dN/dS (ΔLn(dN/dS), upper panel) or difference in dN (ΔLn (dN), lower panel) for (A) the within-gene comparison; and (B) the between-gene comparison.
The percent of variances in dN/dS (upper panel), dN (middle panel), and dS (lower panel) explained by the principal components for (A) the within-gene comparison; and (B) the between-gene comparison.
Notes: Only the five most important components are shown here. The leftmost bar in each panel indicates the total variances explained by all of the nine components. Note that in this analysis, percent of intrinsically disordered region (PIDR) and percent of domain (PD) are excluded.
Table S1.
The within-gene variance in exonic dN/dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 2 | Comp 9 | Comp 10 | Comp 3 | Comp 4 | Comp 5 | Comp 6 | Comp 7 | Comp 8 | Comp 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 8.00 | 6.47 | 1.09 | 0.27 | 0.11 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | |
| ASE/CSE exon type | 0.913 | 0.4536 | 0.4589 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Weighted exon frequency (WEF) | 0.909 | 0.4536 | 0.4556 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Average exonic expression level | 0.423 | 0.2722 | 0.0196 | 0.1247 | 0.0014 | 0.0045 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| 5′ intron length | 0.101 | 0.0907 | 0.0000 | 0.0005 | 0.0001 | 0.0002 | 0.0009 | 0.0033 | 0.0016 | 0.0036 | 0.0003 | 0.0000 |
| 3′ intron length | 0.020 | 0.0065 | 0.0000 | 0.0032 | 0.0001 | 0.0000 | 0.0042 | 0.0016 | 0.0000 | 0.0039 | 0.0001 | 0.0000 |
| Exon length | 0.753 | 0.5962 | 0.0305 | 0.1172 | 0.0051 | 0.0033 | 0.0001 | 0.0000 | 0.0000 | 0.0006 | 0.0000 | 0.0000 |
| G+C content | 0.055 | 0.0324 | 0.0011 | 0.0103 | 0.0022 | 0.0002 | 0.0043 | 0.0025 | 0.0009 | 0.0013 | 0.0002 | 0.0000 |
| Exon duplicability | 0.025 | 0.0000 | 0.0142 | 0.0005 | 0.0000 | 0.0000 | 0.0002 | 0.0022 | 0.0071 | 0.0004 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 1.426 | 1.3478 | 0.0338 | 0.0086 | 0.0318 | 0.0009 | 0.0003 | 0.0002 | 0.0003 | 0.0000 | 0.0027 | 0.0000 |
| % intrinsically disordered regions | 2.043 | 1.9310 | 0.0458 | 0.0032 | 0.0623 | 0.0005 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 |
| % Pfam domain | 1.328 | 1.2830 | 0.0294 | 0.0016 | 0.0069 | 0.0004 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0066 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 4.798 | 4.562 | 0.109 | 0.014 | 0.101 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 |
| Splicing features | 1.822 | 0.907 | 0.915 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Exon length | 0.753 | 0.596 | 0.031 | 0.117 | 0.005 | 0.003 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 |
| Expression level | 0.423 | 0.272 | 0.020 | 0.125 | 0.001 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Compactness features | 0.121 | 0.097 | 0.000 | 0.004 | 0.000 | 0.000 | 0.005 | 0.005 | 0.002 | 0.008 | 0.000 | 0.000 |
| Other features | 0.080 | 0.032 | 0.015 | 0.011 | 0.002 | 0.000 | 0.005 | 0.005 | 0.008 | 0.002 | 0.000 | 0.000 |
Table S2.
The within-gene variance in exonic dN explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 2 | Comp 9 | Comp 3 | Comp 10 | Comp 7 | Comp 6 | Comp 4 | Comp 5 | Comp 11 | Comp 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 13.49 | 9.38 | 2.33 | 0.7 | 0.52 | 0.24 | 0.21 | 0.05 | 0.02 | 0.02 | 0.02 | 0.00 | |
| ASE/CSE exon type | 1.650 | 0.6580 | 0.9809 | 0.0000 | 0.0010 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0001 | 0.0100 | 0.0000 |
| Weighted exon frequency (WEF) | 1.643 | 0.6580 | 0.9739 | 0.0000 | 0.0010 | 0.0000 | 0.0002 | 0.0001 | 0.0000 | 0.0001 | 0.0100 | 0.0000 |
| Average exonic expression level | 1.000 | 0.3948 | 0.0419 | 0.3234 | 0.2361 | 0.0031 | 0.0000 | 0.0003 | 0.0000 | 0.0001 | 0.0000 | 0.0000 |
| 5′ intron length | 0.235 | 0.1316 | 0.0000 | 0.0014 | 0.0088 | 0.0002 | 0.0760 | 0.0081 | 0.0018 | 0.0066 | 0.0000 | 0.0000 |
| 3′ intron length | 0.113 | 0.0094 | 0.0000 | 0.0084 | 0.0016 | 0.0002 | 0.0817 | 0.0001 | 0.0084 | 0.0032 | 0.0000 | 0.0000 |
| Exon length | 1.430 | 0.8648 | 0.0652 | 0.3038 | 0.1716 | 0.0110 | 0.0132 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| G+C content | 0.136 | 0.0470 | 0.0023 | 0.0266 | 0.0088 | 0.0048 | 0.0279 | 0.0045 | 0.0086 | 0.0050 | 0.0000 | 0.0000 |
| Exon duplicability | 0.080 | 0.0000 | 0.0303 | 0.0014 | 0.0005 | 0.0000 | 0.0074 | 0.0354 | 0.0004 | 0.0043 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 2.167 | 1.9552 | 0.0722 | 0.0224 | 0.0452 | 0.0694 | 0.0000 | 0.0015 | 0.0006 | 0.0005 | 0.0000 | 0.0000 |
| % intrinsically disordered regions | 3.071 | 2.8012 | 0.0979 | 0.0084 | 0.0276 | 0.1358 | 0.0004 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 1.964 | 1.8612 | 0.0629 | 0.0042 | 0.0182 | 0.0151 | 0.0027 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 7.203 | 6.618 | 0.233 | 0.035 | 0.091 | 0.220 | 0.003 | 0.002 | 0.001 | 0.001 | 0.000 | 0.000 |
| Splicing features | 3.294 | 1.316 | 1.955 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 |
| Exon length | 1.430 | 0.865 | 0.065 | 0.304 | 0.172 | 0.011 | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Expression level | 1.000 | 0.395 | 0.042 | 0.323 | 0.236 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Compactness features | 0.348 | 0.141 | 0.000 | 0.010 | 0.010 | 0.000 | 0.158 | 0.008 | 0.010 | 0.010 | 0.000 | 0.000 |
| Other features | 0.215 | 0.047 | 0.033 | 0.028 | 0.009 | 0.005 | 0.035 | 0.040 | 0.009 | 0.009 | 0.000 | 0.000 |
Table S3.
The within-gene variance in exonic dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 3 | Comp 2 | Comp 1 | Comp 7 | Comp 6 | Comp 9 | Comp 4 | Comp 10 | Comp 11 | Comp 5 | Comp 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 3.12 | 1.48 | 0.44 | 0.32 | 0.26 | 0.23 | 0.19 | 0.1 | 0.07 | 0.03 | 0.00 | 0.00 | |
| ASE/CSE exon type | 0.226 | 0.0030 | 0.1852 | 0.0224 | 0.0003 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0150 | 0.0000 | 0.0000 |
| Weighted exon frequency (WEF) | 0.225 | 0.0030 | 0.1839 | 0.0224 | 0.0003 | 0.0005 | 0.0000 | 0.0001 | 0.0000 | 0.0149 | 0.0000 | 0.0000 |
| Average exonic expression level | 0.783 | 0.6719 | 0.0079 | 0.0134 | 0.0000 | 0.0014 | 0.0878 | 0.0000 | 0.0009 | 0.0000 | 0.0000 | 0.0000 |
| 5′ intron length | 0.170 | 0.0252 | 0.0000 | 0.0045 | 0.0941 | 0.0370 | 0.0004 | 0.0088 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| 3′ intron length | 0.151 | 0.0044 | 0.0000 | 0.0003 | 0.1011 | 0.0002 | 0.0023 | 0.0422 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| Exon length | 0.633 | 0.4884 | 0.0123 | 0.0294 | 0.0164 | 0.0000 | 0.0825 | 0.0005 | 0.0032 | 0.0000 | 0.0000 | 0.0000 |
| G+C content | 0.134 | 0.0252 | 0.0004 | 0.0016 | 0.0346 | 0.0205 | 0.0072 | 0.0428 | 0.0014 | 0.0000 | 0.0000 | 0.0000 |
| Exon duplicability | 0.182 | 0.0015 | 0.0057 | 0.0000 | 0.0091 | 0.1628 | 0.0004 | 0.0022 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 0.245 | 0.1288 | 0.0136 | 0.0666 | 0.0000 | 0.0067 | 0.0061 | 0.0030 | 0.0202 | 0.0000 | 0.0000 | 0.0000 |
| % intrinsically disordered regions | 0.235 | 0.0784 | 0.0185 | 0.0954 | 0.0005 | 0.0005 | 0.0023 | 0.0002 | 0.0396 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 0.136 | 0.0518 | 0.0119 | 0.0634 | 0.0034 | 0.0002 | 0.0011 | 0.0000 | 0.0044 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Expression level | 0.783 | 0.672 | 0.008 | 0.013 | 0.000 | 0.001 | 0.088 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| Exon length | 0.633 | 0.488 | 0.012 | 0.029 | 0.016 | 0.000 | 0.082 | 0.001 | 0.003 | 0.000 | 0.000 | 0.000 |
| Structural-functional features | 0.617 | 0.259 | 0.044 | 0.225 | 0.004 | 0.007 | 0.010 | 0.003 | 0.064 | 0.000 | 0.000 | 0.000 |
| Splicing features | 0.451 | 0.006 | 0.369 | 0.045 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 |
| Compactness features | 0.321 | 0.030 | 0.000 | 0.005 | 0.195 | 0.037 | 0.003 | 0.051 | 0.000 | 0.000 | 0.000 | 0.000 |
| Other features | 0.315 | 0.027 | 0.006 | 0.002 | 0.044 | 0.183 | 0.008 | 0.045 | 0.001 | 0.000 | 0.000 | 0.000 |
Table S4.
The between-gene variance in exonic dN/dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 3 | Comp 4 | Comp 7 | Comp 8 | Comp 5 | Comp 2 | Comp 10 | Comp 6 | Comp 11 | Comp 9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 13.678 | 13.088 | 0.237 | 0.157 | 0.070 | 0.039 | 0.030 | 0.020 | 0.017 | 0.015 | 0.003 | 0.002 | |
| ASE/CSE exon type | 0.046 | 0.0340 | 0.0001 | 0.0008 | 0.0000 | 0.0000 | 0.0000 | 0.0097 | 0.0000 | 0.0000 | 0.0015 | 0.0000 |
| Weighted exon frequency (WEF) | 0.057 | 0.0446 | 0.0002 | 0.0009 | 0.0000 | 0.0000 | 0.0000 | 0.0097 | 0.0000 | 0.0001 | 0.0015 | 0.0000 |
| Average exonic expression level | 1.332 | 1.2329 | 0.0967 | 0.0006 | 0.0002 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0011 |
| 5′ intron length | 0.324 | 0.2365 | 0.0004 | 0.0471 | 0.0367 | 0.0000 | 0.0024 | 0.0002 | 0.0000 | 0.0008 | 0.0000 | 0.0000 |
| 3′ intron length | 0.201 | 0.1036 | 0.0008 | 0.0604 | 0.0320 | 0.0002 | 0.0029 | 0.0001 | 0.0000 | 0.0005 | 0.0000 | 0.0000 |
| Exon length | 1.863 | 1.7945 | 0.0612 | 0.0012 | 0.0001 | 0.0019 | 0.0021 | 0.0001 | 0.0004 | 0.0004 | 0.0000 | 0.0010 |
| G+C content | 0.070 | 0.0004 | 0.0222 | 0.0324 | 0.0003 | 0.0006 | 0.0081 | 0.0000 | 0.0002 | 0.0052 | 0.0000 | 0.0001 |
| Exon duplicability | 0.160 | 0.1354 | 0.0013 | 0.0023 | 0.0003 | 0.0000 | 0.0141 | 0.0001 | 0.0000 | 0.0070 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 2.509 | 2.4524 | 0.0302 | 0.0098 | 0.0001 | 0.0116 | 0.0004 | 0.0001 | 0.0042 | 0.0005 | 0.0000 | 0.0001 |
| % intrinsically disordered regions | 3.983 | 3.9533 | 0.0179 | 0.0012 | 0.0000 | 0.0002 | 0.0001 | 0.0001 | 0.0101 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 3.133 | 3.1008 | 0.0057 | 0.0001 | 0.0002 | 0.0242 | 0.0001 | 0.0000 | 0.0018 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 9.625 | 9.507 | 0.054 | 0.011 | 0.000 | 0.036 | 0.001 | 0.000 | 0.016 | 0.001 | 0.000 | 0.000 |
| Exon length | 1.863 | 1.794 | 0.061 | 0.001 | 0.000 | 0.002 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Expression level | 1.332 | 1.233 | 0.097 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Compactness features | 0.593 | 0.340 | 0.062 | 0.109 | 0.069 | 0.002 | 0.008 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 |
| Other features | 0.230 | 0.136 | 0.024 | 0.035 | 0.001 | 0.001 | 0.022 | 0.000 | 0.000 | 0.012 | 0.000 | 0.000 |
| Splicing features | 0.103 | 0.079 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.019 | 0.000 | 0.000 | 0.003 | 0.000 |
Table S5.
The between-gene variance in exonic dN explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 3 | Comp 4 | Comp 8 | Comp 9 | Comp 2 | Comp 6 | Comp 10 | Comp 5 | Comp 7 | Comp 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 17.973 | 15.941 | 1.269 | 0.267 | 0.207 | 0.121 | 0.059 | 0.050 | 0.042 | 0.008 | 0.005 | 0.003 | |
| ASE/CSE exon type | 0.077 | 0.0457 | 0.0003 | 0.0014 | 0.0001 | 0.0000 | 0.0286 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0013 |
| Weighted exon frequency (WEF) | 0.091 | 0.0590 | 0.0010 | 0.0016 | 0.0000 | 0.0000 | 0.0285 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0013 |
| Average exonic expression level | 2.082 | 1.5039 | 0.5183 | 0.0011 | 0.0002 | 0.0583 | 0.0001 | 0.0001 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| 5′ intron length | 0.380 | 0.2908 | 0.0022 | 0.0797 | 0.0002 | 0.0000 | 0.0005 | 0.0033 | 0.0000 | 0.0008 | 0.0025 | 0.0000 |
| 3′ intron length | 0.241 | 0.1272 | 0.0042 | 0.1027 | 0.0010 | 0.0010 | 0.0002 | 0.0015 | 0.0000 | 0.0009 | 0.0022 | 0.0000 |
| Exon length | 2.583 | 2.1880 | 0.3275 | 0.0021 | 0.0105 | 0.0511 | 0.0004 | 0.0013 | 0.0011 | 0.0006 | 0.0000 | 0.0000 |
| G+C content | 0.206 | 0.0005 | 0.1197 | 0.0553 | 0.0029 | 0.0075 | 0.0000 | 0.0175 | 0.0006 | 0.0022 | 0.0000 | 0.0000 |
| Exon duplicability | 0.204 | 0.1647 | 0.0072 | 0.0040 | 0.0000 | 0.0000 | 0.0002 | 0.0242 | 0.0000 | 0.0038 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 3.239 | 2.9830 | 0.1619 | 0.0167 | 0.0622 | 0.0031 | 0.0003 | 0.0017 | 0.0104 | 0.0001 | 0.0000 | 0.0000 |
| % intrinsically disordered regions | 4.933 | 4.8077 | 0.0961 | 0.0021 | 0.0012 | 0.0002 | 0.0002 | 0.0000 | 0.0252 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 3.935 | 3.7704 | 0.0307 | 0.0001 | 0.1290 | 0.0001 | 0.0001 | 0.0000 | 0.0046 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 12.107 | 11.561 | 0.289 | 0.019 | 0.192 | 0.003 | 0.001 | 0.002 | 0.040 | 0.000 | 0.000 | 0.000 |
| exon length | 2.583 | 2.188 | 0.328 | 0.002 | 0.010 | 0.051 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 |
| expression level | 2.082 | 1.504 | 0.518 | 0.001 | 0.000 | 0.058 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Compactness features | 0.621 | 0.418 | 0.006 | 0.182 | 0.001 | 0.001 | 0.001 | 0.005 | 0.000 | 0.002 | 0.005 | 0.000 |
| Other features | 0.410 | 0.165 | 0.127 | 0.059 | 0.003 | 0.007 | 0.000 | 0.042 | 0.001 | 0.006 | 0.000 | 0.000 |
| Splicing features | 0.169 | 0.105 | 0.001 | 0.003 | 0.000 | 0.000 | 0.057 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 |
Table S6.
The between-gene variance in exonic dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 3 | Comp 1 | Comp 9 | Comp 6 | Comp 8 | Comp 5 | Comp 7 | Comp 2 | Comp 4 | Comp 10 | Comp 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 2.991 | 1.329 | 0.411 | 0.298 | 0.247 | 0.239 | 0.198 | 0.112 | 0.073 | 0.043 | 0.038 | 0.004 | |
| ASE/CSE exon type | 0.040 | 0.0003 | 0.0011 | 0.0000 | 0.0008 | 0.0001 | 0.0002 | 0.0000 | 0.0355 | 0.0002 | 0.0000 | 0.0018 |
| Weighted exon frequency (WEF) | 0.041 | 0.0011 | 0.0014 | 0.0000 | 0.0009 | 0.0000 | 0.0000 | 0.0000 | 0.0354 | 0.0002 | 0.0000 | 0.0018 |
| Average exonic expression level | 0.726 | 0.5426 | 0.0388 | 0.1430 | 0.0006 | 0.0003 | 0.0005 | 0.0003 | 0.0001 | 0.0002 | 0.0001 | 0.0000 |
| 5′ intron length | 0.113 | 0.0023 | 0.0074 | 0.0001 | 0.0153 | 0.0002 | 0.0160 | 0.0584 | 0.0006 | 0.0133 | 0.0000 | 0.0000 |
| 3′ intron length | 0.107 | 0.0043 | 0.0033 | 0.0024 | 0.0082 | 0.0011 | 0.0197 | 0.0513 | 0.0003 | 0.0169 | 0.0000 | 0.0000 |
| Exon length | 0.559 | 0.3432 | 0.0564 | 0.1254 | 0.0063 | 0.0121 | 0.0141 | 0.0002 | 0.0004 | 0.0003 | 0.0010 | 0.0000 |
| G+C content | 0.296 | 0.1248 | 0.0000 | 0.0183 | 0.0872 | 0.0034 | 0.0529 | 0.0004 | 0.0000 | 0.0087 | 0.0005 | 0.0000 |
| Exon duplicability | 0.223 | 0.0074 | 0.0042 | 0.0000 | 0.1189 | 0.0000 | 0.0910 | 0.0006 | 0.0003 | 0.0006 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 0.349 | 0.1699 | 0.0769 | 0.0075 | 0.0084 | 0.0716 | 0.0024 | 0.0001 | 0.0002 | 0.0027 | 0.0094 | 0.0000 |
| % intrinsically disordered regions | 0.251 | 0.1008 | 0.1240 | 0.0006 | 0.0001 | 0.0014 | 0.0009 | 0.0000 | 0.0001 | 0.0003 | 0.0229 | 0.0000 |
| % Pfam domain | 0.283 | 0.0321 | 0.0972 | 0.0002 | 0.0001 | 0.1488 | 0.0005 | 0.0003 | 0.0001 | 0.0000 | 0.0042 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 0.884 | 0.303 | 0.298 | 0.008 | 0.009 | 0.222 | 0.004 | 0.000 | 0.000 | 0.003 | 0.037 | 0.000 |
| expression level | 0.726 | 0.543 | 0.039 | 0.143 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| exon length | 0.559 | 0.343 | 0.056 | 0.125 | 0.006 | 0.012 | 0.014 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Other features | 0.519 | 0.132 | 0.004 | 0.018 | 0.206 | 0.003 | 0.144 | 0.001 | 0.000 | 0.009 | 0.001 | 0.000 |
| Compactness features | 0.221 | 0.007 | 0.011 | 0.002 | 0.023 | 0.001 | 0.036 | 0.110 | 0.001 | 0.030 | 0.000 | 0.000 |
| Splicing features | 0.081 | 0.001 | 0.002 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.071 | 0.000 | 0.000 | 0.004 |
Table S7.
The Pearson’s coefficient of correlation between each of the eleven principal components and dN/dS, dN, and dS in the within-gene analysis.
| Principal component | Pearson correlation coefficient | ||
|---|---|---|---|
|
| |||
| dN/dS | dN | dS | |
| Component 1 | −0.0763a,*** | −0.0450*** | 0.0515*** |
| Component 2 | 0.0745*** | 0.1481*** | 0.1011*** |
| Component 3 | 0.0504*** | 0.0519*** | 0.0034 |
| Component 4 | 0.1210*** | 0.2049*** | 0.1123*** |
| Component 5 | 0.0398*** | 0.1307*** | 0.1278*** |
| Component 6 | 0.0745*** | 0.1205*** | 0.0596*** |
| Component 7 | −0.1415*** | −0.2097*** | −0.0941*** |
| Component 8 | −0.0391*** | −0.0388 *** | 0.0060 |
| Component 9 | 0.0291*** | 0.0663*** | 0.0499*** |
| Component 10 | −0.1976*** | −0.2229*** | −0.0236** |
| Component 11 | 0.0561*** | 0.1260*** | 0.0970*** |
Notes:
Statistical significance.
P < 0.05;
P < 0.01;
P < 10−3.
Footnotes
Author Contributions
Conceived and designed the experiments: FCC. Analyzed the data: GCTW. Wrote the first draft of the manuscript: GCTW. Contributed to the writing of the manuscript: FCC. Agree with manuscript results and conclusions: GCTW and FCC. Jointly developed the structure and arguments for the paper: GCTW and FCC. Made critical revisions and approved final version: FCC. All authors reviewed and approved of the final manuscript.
Funding
Feng-Chi Chen was supported by the intramural funding of National Health Research Institutes and the National Science Council under contract NSC98-2311-B-400-002-MY3.
Competing Interests
The authors declare that they have no conflicts of interests.
Disclosures and Ethics
As a requirement of publication author(s) have provided to the publisher signed confirmation of compliance with legal and ethical obligations including but not limited to the following: authorship and contributorship, conflicts of interest, privacy and confidentiality and (where applicable) protection of human and animal research subjects. The authors have read and confirmed their agreement with the ICMJE authorship and conflict of interest criteria. The authors have also confirmed that this article is unique and not under consideration or published in any other publication, and that they have permission from rights holders to reproduce any copyrighted material. Any disclosures are made in this section. The external blind peer reviewers report no conflicts of interest.
References
- 1.Li WH. Molecular Evolution. Sunderland, MA: Sinauer Associates, Inc; 1997. [Google Scholar]
- 2.Pal C, Papp B, Lercher MJ. An integrated view of protein evolution. Nature Reviews Genetics. 2006;7:337–48. doi: 10.1038/nrg1838. [DOI] [PubMed] [Google Scholar]
- 3.Drummond DA, Bloom JD, Adami C, Wilke CO, Arnold FH. Why highly expressed proteins evolve slowly. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:14338–43. doi: 10.1073/pnas.0504070102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Popescu CE, Borza T, Bielawski JP, Lee RW. Evolutionary rates and expression level in. Chlamydomonas Genetics. 2006;172:1567–76. doi: 10.1534/genetics.105.047399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park SG, Choi SS. Expression breadth and expression abundance behave differently in correlations with evolutionary rates. BMC Evolutionary Biology. 2010;10:241. doi: 10.1186/1471-2148-10-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang L, Gaut BS. Factors that contribute to variation in evolutionary rate among Arabidopsis genes. Molecular Biology and Evolution. 2011;28:2359–69. doi: 10.1093/molbev/msr058. [DOI] [PubMed] [Google Scholar]
- 7.Liao B-Y, Scott NM, Zhang J. Impacts of gene essentiality, expression pattern, and gene compactness on the evolutionary rate of mammalian proteins. Molecular Biology and Evolution. 2006;23:2072–80. doi: 10.1093/molbev/msl076. [DOI] [PubMed] [Google Scholar]
- 8.Liao B-Y, Weng M-P, Zhang J. Impact of extracellularity on the evolutionary rate of mammalian proteins. Genome Biology and Evolution. 2010;2:39–43. doi: 10.1093/gbe/evp058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tang CSM, Epstein RJ. A structural split in the human genome. PLoS One. 2007;2:e603. doi: 10.1371/journal.pone.0000603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang L, Lu HHS, Chung W-y, Yang J, Li W-H. Patterns of segmental duplication in the human genome. Molecular Biology and Evolution. 2005;22:135–41. doi: 10.1093/molbev/msh262. [DOI] [PubMed] [Google Scholar]
- 11.Duret L, Galtier N. Biased gene conversion and the evolution of mammalian genomic landscapes. Annual Review of Genomics and Human Genetics. 2009;10:285–311. doi: 10.1146/annurev-genom-082908-150001. [DOI] [PubMed] [Google Scholar]
- 12.Galtier N, Duret L, Glemin S, Ranwez V. GC-biased gene conversion promotes the fixation of deleterious amino acid changes in primates. Trends in Genetics. 2009;25:1–5. doi: 10.1016/j.tig.2008.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Lin YS, Hsu WL, Hwang JK, Li WH. Proportion of solvent-exposed amino acids in a protein and rate of protein evolution. Molecular Biology and Evolution. 2007;24:1005–11. doi: 10.1093/molbev/msm019. [DOI] [PubMed] [Google Scholar]
- 14.Ramsey DC, Scherrer MP, Zhou T, Wilke CO. The relationship between relative solvent accessibility and evolutionary rate in protein evolution. Genetics. 2011;188:479–88. doi: 10.1534/genetics.111.128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown CJ, Johnson AK, Daughdrill GW. Comparing models of evolution for ordered and disordered proteins. Molecular Biology and Evolution. 2010;27:609–21. doi: 10.1093/molbev/msp277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brown CJ, Takayama S, Campen AM, et al. Evolutionary rate heterogeneity in proteins with long disordered regions. Journal of Molecular Evolution. 2002;55:104–10. doi: 10.1007/s00239-001-2309-6. [DOI] [PubMed] [Google Scholar]
- 17.Chen FC, Pan CL, Lin HY. Independent effects of alternative splicing and structural constraint on the evolution of mammalian coding exons. Molecular Biology and Evolution. 2011;29:187–93. doi: 10.1093/molbev/msr182. [DOI] [PubMed] [Google Scholar]
- 18.Wolf YI, Gopich IV, Lipman DJ, Koonin EV. Relative contributions of intrinsic structural-functional constraints and translation rate to the evolution of protein-coding genes. Genome Biology and Evolution. 2010;2:190–99. doi: 10.1093/gbe/evq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kazi JU, Kabir NN, Soh JW. Bioinformatic prediction and analysis of eukaryotic protein kinases in the rat genome. Gene. 2008;410:147–53. doi: 10.1016/j.gene.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Hanks S, Quinn A, Hunter T. The protein kinase family: conserved features and deduced phylogeny of the catalytic domains. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 21.Hallegger M, Llorian M, Smith CWJ. Alternative splicing: global insights. FEBS Journal. 2010;277:856–66. doi: 10.1111/j.1742-4658.2009.07521.x. [DOI] [PubMed] [Google Scholar]
- 22.Keren H, Lev-Maor G, Ast G. Alternative splicing and evolution: diversification, exon definition and function. Nature Reviews Genetics. 2010;11:345–55. doi: 10.1038/nrg2776. [DOI] [PubMed] [Google Scholar]
- 23.Chen FC, Chaw SM, Tzeng YH, Wang SS, Chuang TJ. Opposite evolutionary effects between different alternative splicing patterns. Molecular Biology and Evolution. 2007;24:1443–6. doi: 10.1093/molbev/msm072. [DOI] [PubMed] [Google Scholar]
- 24.Chen FC, Wang SS, Chen CJ, Li WH, Chuang TJ. Alternatively and constitutively spliced exons are subject to different evolutionary forces. Molecular Biology and Evolution. 2006;23:675–82. doi: 10.1093/molbev/msj081. [DOI] [PubMed] [Google Scholar]
- 25.Barbazuk WB, Fu Y, McGinnis KM. Genome-wide analyses of alternative splicing in plants: opportunities and challenges. Genome Research. 2008;18:1381–92. doi: 10.1101/gr.053678.106. [DOI] [PubMed] [Google Scholar]
- 26.Kim E, Goren A, Ast G. Alternative splicing: current perspectives. BioEssays. 2008;30:38–47. doi: 10.1002/bies.20692. [DOI] [PubMed] [Google Scholar]
- 27.Kim E, Magen A, Ast G. Different levels of alternative splicing among eukaryotes. Nucleic Acids Research. 2007;35:125–31. doi: 10.1093/nar/gkl924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang BB, Brendel V. Genomewide comparative analysis of alternative splicing in plants. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:7175–80. doi: 10.1073/pnas.0602039103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Filichkin SA, Priest HD, Givan SA, et al. Genome-wide mapping of a lternative splicing in Arabidopsis thaliana. Genome Research. 2010;20:45–58. doi: 10.1101/gr.093302.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soltis PS, Soltis DE. The role of hybridization in plant speciation. Annual Review of Plant Biology. 2009;60:561–88. doi: 10.1146/annurev.arplant.043008.092039. [DOI] [PubMed] [Google Scholar]
- 31.Mable BK. ‘Why polyploidy is rarer in animals than in plants’: myths and mechanisms. Biological Journal of the Linnean Society. 2004;82:453–66. [Google Scholar]
- 32.Vision TJ, Brown DG, Tanksley SD. The origins of genomic duplications in. Arabidopsis Science. 2000;290:2114–7. doi: 10.1126/science.290.5499.2114. [DOI] [PubMed] [Google Scholar]
- 33.Lockton S, Gaut BS. Plant conserved non-coding sequences and paralogue evolution. Trends in Genetics. 2005;21:60–5. doi: 10.1016/j.tig.2004.11.013. [DOI] [PubMed] [Google Scholar]
- 34.Dyson HJ, Wright PE. Intrinsically unstructured proteins and their functions. Nature Reviews Molecular Cell Biology. 2005;6:197–208. doi: 10.1038/nrm1589. [DOI] [PubMed] [Google Scholar]
- 35.Uversky VN. Intrinsically disordered proteins from A to Z. The International Journal of Biochemistry and Cell Biology. 2011;43:1090–103. doi: 10.1016/j.biocel.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 36.Uversky VN, Dunker AK. Understanding protein non-folding. Biochimica et Biophysica Acta (BBA)—Proteins and Proteomics. 2010;1804:1231–64. doi: 10.1016/j.bbapap.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Carmel L, Koonin EV. A universal nonmonotonic relationship between gene compactness and expression levels in multicellular eukaryotes. Genome Biology and Evolution. 2009;1:382–90. doi: 10.1093/gbe/evp038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ren X-Y, Vorst O, Fiers MWEJ, Stiekema WJ, Nap JP. In plants, highly expressed genes are the least compact. Trends in Genetics. 2006;22:528–32. doi: 10.1016/j.tig.2006.08.008. [DOI] [PubMed] [Google Scholar]
- 39.Drummond DA, Raval A, Wilke CO. A single determinant dominates the rate of yeast protein evolution. Molecular Biology and Evolution. 2006;23:327–37. doi: 10.1093/molbev/msj038. [DOI] [PubMed] [Google Scholar]
- 40.Plotkin JB, Fraser HB. Assessing the determinants of evolutionary rates in the presence of noise. Molecular Biology and Evolution. 2007;24:1113–21. doi: 10.1093/molbev/msm044. [DOI] [PubMed] [Google Scholar]
- 41.Kersey PJ, Staines DM, Lawson D, et al. Ensembl Genomes: an integrative resource for genome-scale data from non-vertebrate species. Nucleic Acids Research. 2012;40:D91–7. doi: 10.1093/nar/gkr895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Youens-Clark K, Buckler E, Casstevens T, et al. Gramene database in 2010: updates and extensions. Nucleic Acids Research. 2011;39:D1085–94. doi: 10.1093/nar/gkq1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–7. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leinonen R, Sugawara H, Shumway M. The Sequence Read Archive. Nucleic Acids Research. 2011;39:D19–21. doi: 10.1093/nar/gkq1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Roberts A, Pimentel H, Trapnell C, Pachter L. Identification of novel transcripts in annotated genomes using RNA-Seq. Bioinformatics. 2011;27:2325–9. doi: 10.1093/bioinformatics/btr355. [DOI] [PubMed] [Google Scholar]
- 46.Langmead B, Trapnell C, Pop M, Salzberg S. Ultrafast and memory- efficient alignment of short DNA sequences to the human genome. Genome Biology. 2009;10:R25. doi: 10.1186/gb-2009-10-3-r25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trapnell C, Pachter L, Salzberg SL. TopHat: discovering splice junctions with RNA-Seq. Bioinformatics. 2009;25:1105–11. doi: 10.1093/bioinformatics/btp120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li H, Handsaker B, Wysoker A, et al. The sequence alignment/map format and SAM tools. Bioinformatics. 2009;25:2078–9. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z. PAML 4: a program package for phylogenetic analysis by maximum likelihood. Molecular Biology and Evolution. 2007;24:1586–91. doi: 10.1093/molbev/msm088. [DOI] [PubMed] [Google Scholar]
- 50.Yang Z, Nielsen R. Estimating synonymous and nonsynonymous substitution rates under realistic evolutionary models. Molecular Biology and Evolution. 2000;17:32–43. doi: 10.1093/oxfordjournals.molbev.a026236. [DOI] [PubMed] [Google Scholar]
- 51.Nekrutenko A, Makova KD, Li WH. The KA/KS ratio test for assessing the protein-coding potential of genomic regions: an empirical and simulation study. Genome Research. 2002;12:198–202. doi: 10.1101/gr.200901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng J, Randall AZ, Sweredoski MJ, Baldi P. SCRATCH: a protein structure and structural feature prediction server. Nucleic Acids Research. 2005;33:W72–6. doi: 10.1093/nar/gki396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward JJ, McGuffin LJ, Bryson K, Buxton BF, Jones DT. The DISOPRED server for the prediction of protein disorder. Bioinformatics. 2004;20:2138–9. doi: 10.1093/bioinformatics/bth195. [DOI] [PubMed] [Google Scholar]
- 54.Finn RD, Mistry J, Tate J, et al. The Pfam protein families database. Nucleic Acids Research. 2010;38:D211–22. doi: 10.1093/nar/gkp985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Camacho C, Coulouris G, Avagyan V, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; 2011. Available at: http://www.R-project.org. [Google Scholar]
- 57.Yang H. In plants, expression breadth and expression level distinctly and non-linearly correlate with gene structure. Biology Direct. 2009;4:45. doi: 10.1186/1745-6150-4-45. discussion 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Camiolo S, Rau D, Porceddu A. Mutational biases and selective forces shaping the structure of Arabidopsis genes. PLoS One. 2009;4:e6356. doi: 10.1371/journal.pone.0006356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woody JL, Severin AJ, Bolon Y-T, et al. Gene expression patterns are correlated with genomic and genic structure in soybean. Genome. 2011;54:10–8. doi: 10.1139/G10-090. [DOI] [PubMed] [Google Scholar]
- 60.Charlesworth B. Fundamental concepts in genetics: Effective population size and patterns of molecular evolution and variation. Nature Reviews Genetics. 2009;10:195–205. doi: 10.1038/nrg2526. [DOI] [PubMed] [Google Scholar]
- 61.Templeton AR. Population genetics and microevolutionary theory. Hoboken, NJ: John Wiley & Sons, Inc; 2006. [Google Scholar]
- 62.Tsai IJ, Bensasson D, Burt A, Koufopanou V. Population genomics of the wild yeast Saccharomyces paradoxus: Quantifying the life cycle. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:4957–62. doi: 10.1073/pnas.0707314105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lundemo S, Falahati-Anbaran M, StenØIen HK. Seed banks cause elevated generation times and effective population sizes of Arabidopsis thaliana in northern Europe. Molecular Ecology. 2009;18:2798–811. doi: 10.1111/j.1365-294X.2009.04236.x. [DOI] [PubMed] [Google Scholar]
- 64.Drummond DA, Wilke CO. Mistranslation-induced protein misfolding as a dominant constraint on coding-sequence evolution. Cell. 2008;134:341–52. doi: 10.1016/j.cell.2008.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slotte T, Bataillon T, Hansen TT, St Onge K, Wright SI, Schierup MH. Genomic determinants of protein evolution and polymorphism in Arabidopsis. Genome Biology and Evolution. 2011;3:1210–9. doi: 10.1093/gbe/evr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen FC, Liao BY, Pan CL, Lin HY, Chang AYF. Assessing determinants of exonic evolutionary rates in mammals. Molecular Biology and Evolution. 2012 doi: 10.1093/molbev/mss116. Accepted. [DOI] [PubMed] [Google Scholar]
- 67.Lavner Y, Kotlar D. Codon bias as a factor in regulating expression via translation rate in the human genome. Gene. 2005;345:127–138. doi: 10.1016/j.gene.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 68.Hiraoka Y, Kawamata K, Haraguchi T, Chikashige Y. Codon usage bias is correlated with gene expression levels in the fission yeast Schizosaccharomyces pombe. Genes to Cells. 2009;14:499–509. doi: 10.1111/j.1365-2443.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 69.Duret L, Mouchiroud D. Expression pattern and, surprisingly, gene length shape codon usage in Caenorhabditis, Drosophila, and Arabidopsis. Proceedings of the National Academy of Sciences of the United States of America. 1999;96:4482–7. doi: 10.1073/pnas.96.8.4482. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) The classification of the ASE/CSE exon type; (B) Two examples of how the weighted exon frequency is calculated.
The projection patterns of the eleven principal components in the within-gene analysis.
The linear correlation between difference in the percent of Pfam domain ( ΔPD) and difference in d
The linear correlation between difference in the percent of intrinsically disordered region (ΔPIDR) and difference in dN/dS (ΔLn(dN/dS), upper panel) or difference in dN (ΔLn (dN), lower panel) for (A) the within-gene comparison; and (B) the between-gene comparison.
The percent of variances in dN/dS (upper panel), dN (middle panel), and dS (lower panel) explained by the principal components for (A) the within-gene comparison; and (B) the between-gene comparison.
Notes: Only the five most important components are shown here. The leftmost bar in each panel indicates the total variances explained by all of the nine components. Note that in this analysis, percent of intrinsically disordered region (PIDR) and percent of domain (PD) are excluded.
Table S1.
The within-gene variance in exonic dN/dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 2 | Comp 9 | Comp 10 | Comp 3 | Comp 4 | Comp 5 | Comp 6 | Comp 7 | Comp 8 | Comp 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 8.00 | 6.47 | 1.09 | 0.27 | 0.11 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.01 | 0.00 | |
| ASE/CSE exon type | 0.913 | 0.4536 | 0.4589 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Weighted exon frequency (WEF) | 0.909 | 0.4536 | 0.4556 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Average exonic expression level | 0.423 | 0.2722 | 0.0196 | 0.1247 | 0.0014 | 0.0045 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| 5′ intron length | 0.101 | 0.0907 | 0.0000 | 0.0005 | 0.0001 | 0.0002 | 0.0009 | 0.0033 | 0.0016 | 0.0036 | 0.0003 | 0.0000 |
| 3′ intron length | 0.020 | 0.0065 | 0.0000 | 0.0032 | 0.0001 | 0.0000 | 0.0042 | 0.0016 | 0.0000 | 0.0039 | 0.0001 | 0.0000 |
| Exon length | 0.753 | 0.5962 | 0.0305 | 0.1172 | 0.0051 | 0.0033 | 0.0001 | 0.0000 | 0.0000 | 0.0006 | 0.0000 | 0.0000 |
| G+C content | 0.055 | 0.0324 | 0.0011 | 0.0103 | 0.0022 | 0.0002 | 0.0043 | 0.0025 | 0.0009 | 0.0013 | 0.0002 | 0.0000 |
| Exon duplicability | 0.025 | 0.0000 | 0.0142 | 0.0005 | 0.0000 | 0.0000 | 0.0002 | 0.0022 | 0.0071 | 0.0004 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 1.426 | 1.3478 | 0.0338 | 0.0086 | 0.0318 | 0.0009 | 0.0003 | 0.0002 | 0.0003 | 0.0000 | 0.0027 | 0.0000 |
| % intrinsically disordered regions | 2.043 | 1.9310 | 0.0458 | 0.0032 | 0.0623 | 0.0005 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0002 | 0.0000 |
| % Pfam domain | 1.328 | 1.2830 | 0.0294 | 0.0016 | 0.0069 | 0.0004 | 0.0000 | 0.0000 | 0.0000 | 0.0001 | 0.0066 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 4.798 | 4.562 | 0.109 | 0.014 | 0.101 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.009 | 0.000 |
| Splicing features | 1.822 | 0.907 | 0.915 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Exon length | 0.753 | 0.596 | 0.031 | 0.117 | 0.005 | 0.003 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 | 0.000 |
| Expression level | 0.423 | 0.272 | 0.020 | 0.125 | 0.001 | 0.005 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Compactness features | 0.121 | 0.097 | 0.000 | 0.004 | 0.000 | 0.000 | 0.005 | 0.005 | 0.002 | 0.008 | 0.000 | 0.000 |
| Other features | 0.080 | 0.032 | 0.015 | 0.011 | 0.002 | 0.000 | 0.005 | 0.005 | 0.008 | 0.002 | 0.000 | 0.000 |
Table S2.
The within-gene variance in exonic dN explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 2 | Comp 9 | Comp 3 | Comp 10 | Comp 7 | Comp 6 | Comp 4 | Comp 5 | Comp 11 | Comp 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 13.49 | 9.38 | 2.33 | 0.7 | 0.52 | 0.24 | 0.21 | 0.05 | 0.02 | 0.02 | 0.02 | 0.00 | |
| ASE/CSE exon type | 1.650 | 0.6580 | 0.9809 | 0.0000 | 0.0010 | 0.0000 | 0.0002 | 0.0000 | 0.0000 | 0.0001 | 0.0100 | 0.0000 |
| Weighted exon frequency (WEF) | 1.643 | 0.6580 | 0.9739 | 0.0000 | 0.0010 | 0.0000 | 0.0002 | 0.0001 | 0.0000 | 0.0001 | 0.0100 | 0.0000 |
| Average exonic expression level | 1.000 | 0.3948 | 0.0419 | 0.3234 | 0.2361 | 0.0031 | 0.0000 | 0.0003 | 0.0000 | 0.0001 | 0.0000 | 0.0000 |
| 5′ intron length | 0.235 | 0.1316 | 0.0000 | 0.0014 | 0.0088 | 0.0002 | 0.0760 | 0.0081 | 0.0018 | 0.0066 | 0.0000 | 0.0000 |
| 3′ intron length | 0.113 | 0.0094 | 0.0000 | 0.0084 | 0.0016 | 0.0002 | 0.0817 | 0.0001 | 0.0084 | 0.0032 | 0.0000 | 0.0000 |
| Exon length | 1.430 | 0.8648 | 0.0652 | 0.3038 | 0.1716 | 0.0110 | 0.0132 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| G+C content | 0.136 | 0.0470 | 0.0023 | 0.0266 | 0.0088 | 0.0048 | 0.0279 | 0.0045 | 0.0086 | 0.0050 | 0.0000 | 0.0000 |
| Exon duplicability | 0.080 | 0.0000 | 0.0303 | 0.0014 | 0.0005 | 0.0000 | 0.0074 | 0.0354 | 0.0004 | 0.0043 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 2.167 | 1.9552 | 0.0722 | 0.0224 | 0.0452 | 0.0694 | 0.0000 | 0.0015 | 0.0006 | 0.0005 | 0.0000 | 0.0000 |
| % intrinsically disordered regions | 3.071 | 2.8012 | 0.0979 | 0.0084 | 0.0276 | 0.1358 | 0.0004 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 1.964 | 1.8612 | 0.0629 | 0.0042 | 0.0182 | 0.0151 | 0.0027 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 7.203 | 6.618 | 0.233 | 0.035 | 0.091 | 0.220 | 0.003 | 0.002 | 0.001 | 0.001 | 0.000 | 0.000 |
| Splicing features | 3.294 | 1.316 | 1.955 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.020 | 0.000 |
| Exon length | 1.430 | 0.865 | 0.065 | 0.304 | 0.172 | 0.011 | 0.013 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Expression level | 1.000 | 0.395 | 0.042 | 0.323 | 0.236 | 0.003 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Compactness features | 0.348 | 0.141 | 0.000 | 0.010 | 0.010 | 0.000 | 0.158 | 0.008 | 0.010 | 0.010 | 0.000 | 0.000 |
| Other features | 0.215 | 0.047 | 0.033 | 0.028 | 0.009 | 0.005 | 0.035 | 0.040 | 0.009 | 0.009 | 0.000 | 0.000 |
Table S3.
The within-gene variance in exonic dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 3 | Comp 2 | Comp 1 | Comp 7 | Comp 6 | Comp 9 | Comp 4 | Comp 10 | Comp 11 | Comp 5 | Comp 8 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 3.12 | 1.48 | 0.44 | 0.32 | 0.26 | 0.23 | 0.19 | 0.1 | 0.07 | 0.03 | 0.00 | 0.00 | |
| ASE/CSE exon type | 0.226 | 0.0030 | 0.1852 | 0.0224 | 0.0003 | 0.0000 | 0.0000 | 0.0001 | 0.0000 | 0.0150 | 0.0000 | 0.0000 |
| Weighted exon frequency (WEF) | 0.225 | 0.0030 | 0.1839 | 0.0224 | 0.0003 | 0.0005 | 0.0000 | 0.0001 | 0.0000 | 0.0149 | 0.0000 | 0.0000 |
| Average exonic expression level | 0.783 | 0.6719 | 0.0079 | 0.0134 | 0.0000 | 0.0014 | 0.0878 | 0.0000 | 0.0009 | 0.0000 | 0.0000 | 0.0000 |
| 5′ intron length | 0.170 | 0.0252 | 0.0000 | 0.0045 | 0.0941 | 0.0370 | 0.0004 | 0.0088 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| 3′ intron length | 0.151 | 0.0044 | 0.0000 | 0.0003 | 0.1011 | 0.0002 | 0.0023 | 0.0422 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| Exon length | 0.633 | 0.4884 | 0.0123 | 0.0294 | 0.0164 | 0.0000 | 0.0825 | 0.0005 | 0.0032 | 0.0000 | 0.0000 | 0.0000 |
| G+C content | 0.134 | 0.0252 | 0.0004 | 0.0016 | 0.0346 | 0.0205 | 0.0072 | 0.0428 | 0.0014 | 0.0000 | 0.0000 | 0.0000 |
| Exon duplicability | 0.182 | 0.0015 | 0.0057 | 0.0000 | 0.0091 | 0.1628 | 0.0004 | 0.0022 | 0.0000 | 0.0000 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 0.245 | 0.1288 | 0.0136 | 0.0666 | 0.0000 | 0.0067 | 0.0061 | 0.0030 | 0.0202 | 0.0000 | 0.0000 | 0.0000 |
| % intrinsically disordered regions | 0.235 | 0.0784 | 0.0185 | 0.0954 | 0.0005 | 0.0005 | 0.0023 | 0.0002 | 0.0396 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 0.136 | 0.0518 | 0.0119 | 0.0634 | 0.0034 | 0.0002 | 0.0011 | 0.0000 | 0.0044 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Expression level | 0.783 | 0.672 | 0.008 | 0.013 | 0.000 | 0.001 | 0.088 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 |
| Exon length | 0.633 | 0.488 | 0.012 | 0.029 | 0.016 | 0.000 | 0.082 | 0.001 | 0.003 | 0.000 | 0.000 | 0.000 |
| Structural-functional features | 0.617 | 0.259 | 0.044 | 0.225 | 0.004 | 0.007 | 0.010 | 0.003 | 0.064 | 0.000 | 0.000 | 0.000 |
| Splicing features | 0.451 | 0.006 | 0.369 | 0.045 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.030 | 0.000 | 0.000 |
| Compactness features | 0.321 | 0.030 | 0.000 | 0.005 | 0.195 | 0.037 | 0.003 | 0.051 | 0.000 | 0.000 | 0.000 | 0.000 |
| Other features | 0.315 | 0.027 | 0.006 | 0.002 | 0.044 | 0.183 | 0.008 | 0.045 | 0.001 | 0.000 | 0.000 | 0.000 |
Table S4.
The between-gene variance in exonic dN/dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 3 | Comp 4 | Comp 7 | Comp 8 | Comp 5 | Comp 2 | Comp 10 | Comp 6 | Comp 11 | Comp 9 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 13.678 | 13.088 | 0.237 | 0.157 | 0.070 | 0.039 | 0.030 | 0.020 | 0.017 | 0.015 | 0.003 | 0.002 | |
| ASE/CSE exon type | 0.046 | 0.0340 | 0.0001 | 0.0008 | 0.0000 | 0.0000 | 0.0000 | 0.0097 | 0.0000 | 0.0000 | 0.0015 | 0.0000 |
| Weighted exon frequency (WEF) | 0.057 | 0.0446 | 0.0002 | 0.0009 | 0.0000 | 0.0000 | 0.0000 | 0.0097 | 0.0000 | 0.0001 | 0.0015 | 0.0000 |
| Average exonic expression level | 1.332 | 1.2329 | 0.0967 | 0.0006 | 0.0002 | 0.0000 | 0.0001 | 0.0000 | 0.0000 | 0.0000 | 0.0000 | 0.0011 |
| 5′ intron length | 0.324 | 0.2365 | 0.0004 | 0.0471 | 0.0367 | 0.0000 | 0.0024 | 0.0002 | 0.0000 | 0.0008 | 0.0000 | 0.0000 |
| 3′ intron length | 0.201 | 0.1036 | 0.0008 | 0.0604 | 0.0320 | 0.0002 | 0.0029 | 0.0001 | 0.0000 | 0.0005 | 0.0000 | 0.0000 |
| Exon length | 1.863 | 1.7945 | 0.0612 | 0.0012 | 0.0001 | 0.0019 | 0.0021 | 0.0001 | 0.0004 | 0.0004 | 0.0000 | 0.0010 |
| G+C content | 0.070 | 0.0004 | 0.0222 | 0.0324 | 0.0003 | 0.0006 | 0.0081 | 0.0000 | 0.0002 | 0.0052 | 0.0000 | 0.0001 |
| Exon duplicability | 0.160 | 0.1354 | 0.0013 | 0.0023 | 0.0003 | 0.0000 | 0.0141 | 0.0001 | 0.0000 | 0.0070 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 2.509 | 2.4524 | 0.0302 | 0.0098 | 0.0001 | 0.0116 | 0.0004 | 0.0001 | 0.0042 | 0.0005 | 0.0000 | 0.0001 |
| % intrinsically disordered regions | 3.983 | 3.9533 | 0.0179 | 0.0012 | 0.0000 | 0.0002 | 0.0001 | 0.0001 | 0.0101 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 3.133 | 3.1008 | 0.0057 | 0.0001 | 0.0002 | 0.0242 | 0.0001 | 0.0000 | 0.0018 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 9.625 | 9.507 | 0.054 | 0.011 | 0.000 | 0.036 | 0.001 | 0.000 | 0.016 | 0.001 | 0.000 | 0.000 |
| Exon length | 1.863 | 1.794 | 0.061 | 0.001 | 0.000 | 0.002 | 0.002 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Expression level | 1.332 | 1.233 | 0.097 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.001 |
| Compactness features | 0.593 | 0.340 | 0.062 | 0.109 | 0.069 | 0.002 | 0.008 | 0.000 | 0.000 | 0.002 | 0.000 | 0.001 |
| Other features | 0.230 | 0.136 | 0.024 | 0.035 | 0.001 | 0.001 | 0.022 | 0.000 | 0.000 | 0.012 | 0.000 | 0.000 |
| Splicing features | 0.103 | 0.079 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.019 | 0.000 | 0.000 | 0.003 | 0.000 |
Table S5.
The between-gene variance in exonic dN explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 1 | Comp 3 | Comp 4 | Comp 8 | Comp 9 | Comp 2 | Comp 6 | Comp 10 | Comp 5 | Comp 7 | Comp 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 17.973 | 15.941 | 1.269 | 0.267 | 0.207 | 0.121 | 0.059 | 0.050 | 0.042 | 0.008 | 0.005 | 0.003 | |
| ASE/CSE exon type | 0.077 | 0.0457 | 0.0003 | 0.0014 | 0.0001 | 0.0000 | 0.0286 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0013 |
| Weighted exon frequency (WEF) | 0.091 | 0.0590 | 0.0010 | 0.0016 | 0.0000 | 0.0000 | 0.0285 | 0.0002 | 0.0000 | 0.0000 | 0.0000 | 0.0013 |
| Average exonic expression level | 2.082 | 1.5039 | 0.5183 | 0.0011 | 0.0002 | 0.0583 | 0.0001 | 0.0001 | 0.0001 | 0.0000 | 0.0000 | 0.0000 |
| 5′ intron length | 0.380 | 0.2908 | 0.0022 | 0.0797 | 0.0002 | 0.0000 | 0.0005 | 0.0033 | 0.0000 | 0.0008 | 0.0025 | 0.0000 |
| 3′ intron length | 0.241 | 0.1272 | 0.0042 | 0.1027 | 0.0010 | 0.0010 | 0.0002 | 0.0015 | 0.0000 | 0.0009 | 0.0022 | 0.0000 |
| Exon length | 2.583 | 2.1880 | 0.3275 | 0.0021 | 0.0105 | 0.0511 | 0.0004 | 0.0013 | 0.0011 | 0.0006 | 0.0000 | 0.0000 |
| G+C content | 0.206 | 0.0005 | 0.1197 | 0.0553 | 0.0029 | 0.0075 | 0.0000 | 0.0175 | 0.0006 | 0.0022 | 0.0000 | 0.0000 |
| Exon duplicability | 0.204 | 0.1647 | 0.0072 | 0.0040 | 0.0000 | 0.0000 | 0.0002 | 0.0242 | 0.0000 | 0.0038 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 3.239 | 2.9830 | 0.1619 | 0.0167 | 0.0622 | 0.0031 | 0.0003 | 0.0017 | 0.0104 | 0.0001 | 0.0000 | 0.0000 |
| % intrinsically disordered regions | 4.933 | 4.8077 | 0.0961 | 0.0021 | 0.0012 | 0.0002 | 0.0002 | 0.0000 | 0.0252 | 0.0000 | 0.0000 | 0.0000 |
| % Pfam domain | 3.935 | 3.7704 | 0.0307 | 0.0001 | 0.1290 | 0.0001 | 0.0001 | 0.0000 | 0.0046 | 0.0000 | 0.0000 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 12.107 | 11.561 | 0.289 | 0.019 | 0.192 | 0.003 | 0.001 | 0.002 | 0.040 | 0.000 | 0.000 | 0.000 |
| exon length | 2.583 | 2.188 | 0.328 | 0.002 | 0.010 | 0.051 | 0.000 | 0.001 | 0.001 | 0.001 | 0.000 | 0.000 |
| expression level | 2.082 | 1.504 | 0.518 | 0.001 | 0.000 | 0.058 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| Compactness features | 0.621 | 0.418 | 0.006 | 0.182 | 0.001 | 0.001 | 0.001 | 0.005 | 0.000 | 0.002 | 0.005 | 0.000 |
| Other features | 0.410 | 0.165 | 0.127 | 0.059 | 0.003 | 0.007 | 0.000 | 0.042 | 0.001 | 0.006 | 0.000 | 0.000 |
| Splicing features | 0.169 | 0.105 | 0.001 | 0.003 | 0.000 | 0.000 | 0.057 | 0.000 | 0.000 | 0.000 | 0.000 | 0.003 |
Table S6.
The between-gene variance in exonic dS explained by the principal components broken down to eleven exon features (upper half); or to six feature categories (lower half).
| Var. explained | Comp 3 | Comp 1 | Comp 9 | Comp 6 | Comp 8 | Comp 5 | Comp 7 | Comp 2 | Comp 4 | Comp 10 | Comp 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exon features | (Sub)total | |||||||||||
| 2.991 | 1.329 | 0.411 | 0.298 | 0.247 | 0.239 | 0.198 | 0.112 | 0.073 | 0.043 | 0.038 | 0.004 | |
| ASE/CSE exon type | 0.040 | 0.0003 | 0.0011 | 0.0000 | 0.0008 | 0.0001 | 0.0002 | 0.0000 | 0.0355 | 0.0002 | 0.0000 | 0.0018 |
| Weighted exon frequency (WEF) | 0.041 | 0.0011 | 0.0014 | 0.0000 | 0.0009 | 0.0000 | 0.0000 | 0.0000 | 0.0354 | 0.0002 | 0.0000 | 0.0018 |
| Average exonic expression level | 0.726 | 0.5426 | 0.0388 | 0.1430 | 0.0006 | 0.0003 | 0.0005 | 0.0003 | 0.0001 | 0.0002 | 0.0001 | 0.0000 |
| 5′ intron length | 0.113 | 0.0023 | 0.0074 | 0.0001 | 0.0153 | 0.0002 | 0.0160 | 0.0584 | 0.0006 | 0.0133 | 0.0000 | 0.0000 |
| 3′ intron length | 0.107 | 0.0043 | 0.0033 | 0.0024 | 0.0082 | 0.0011 | 0.0197 | 0.0513 | 0.0003 | 0.0169 | 0.0000 | 0.0000 |
| Exon length | 0.559 | 0.3432 | 0.0564 | 0.1254 | 0.0063 | 0.0121 | 0.0141 | 0.0002 | 0.0004 | 0.0003 | 0.0010 | 0.0000 |
| G+C content | 0.296 | 0.1248 | 0.0000 | 0.0183 | 0.0872 | 0.0034 | 0.0529 | 0.0004 | 0.0000 | 0.0087 | 0.0005 | 0.0000 |
| Exon duplicability | 0.223 | 0.0074 | 0.0042 | 0.0000 | 0.1189 | 0.0000 | 0.0910 | 0.0006 | 0.0003 | 0.0006 | 0.0000 | 0.0000 |
| % solvent-accessible amino acid residues | 0.349 | 0.1699 | 0.0769 | 0.0075 | 0.0084 | 0.0716 | 0.0024 | 0.0001 | 0.0002 | 0.0027 | 0.0094 | 0.0000 |
| % intrinsically disordered regions | 0.251 | 0.1008 | 0.1240 | 0.0006 | 0.0001 | 0.0014 | 0.0009 | 0.0000 | 0.0001 | 0.0003 | 0.0229 | 0.0000 |
| % Pfam domain | 0.283 | 0.0321 | 0.0972 | 0.0002 | 0.0001 | 0.1488 | 0.0005 | 0.0003 | 0.0001 | 0.0000 | 0.0042 | 0.0000 |
| Exon feature category | Subtotal | |||||||||||
| Structural-functional features | 0.884 | 0.303 | 0.298 | 0.008 | 0.009 | 0.222 | 0.004 | 0.000 | 0.000 | 0.003 | 0.037 | 0.000 |
| expression level | 0.726 | 0.543 | 0.039 | 0.143 | 0.001 | 0.000 | 0.001 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 |
| exon length | 0.559 | 0.343 | 0.056 | 0.125 | 0.006 | 0.012 | 0.014 | 0.000 | 0.000 | 0.000 | 0.001 | 0.000 |
| Other features | 0.519 | 0.132 | 0.004 | 0.018 | 0.206 | 0.003 | 0.144 | 0.001 | 0.000 | 0.009 | 0.001 | 0.000 |
| Compactness features | 0.221 | 0.007 | 0.011 | 0.002 | 0.023 | 0.001 | 0.036 | 0.110 | 0.001 | 0.030 | 0.000 | 0.000 |
| Splicing features | 0.081 | 0.001 | 0.002 | 0.000 | 0.002 | 0.000 | 0.000 | 0.000 | 0.071 | 0.000 | 0.000 | 0.004 |
Table S7.
The Pearson’s coefficient of correlation between each of the eleven principal components and dN/dS, dN, and dS in the within-gene analysis.
| Principal component | Pearson correlation coefficient | ||
|---|---|---|---|
|
| |||
| dN/dS | dN | dS | |
| Component 1 | −0.0763a,*** | −0.0450*** | 0.0515*** |
| Component 2 | 0.0745*** | 0.1481*** | 0.1011*** |
| Component 3 | 0.0504*** | 0.0519*** | 0.0034 |
| Component 4 | 0.1210*** | 0.2049*** | 0.1123*** |
| Component 5 | 0.0398*** | 0.1307*** | 0.1278*** |
| Component 6 | 0.0745*** | 0.1205*** | 0.0596*** |
| Component 7 | −0.1415*** | −0.2097*** | −0.0941*** |
| Component 8 | −0.0391*** | −0.0388 *** | 0.0060 |
| Component 9 | 0.0291*** | 0.0663*** | 0.0499*** |
| Component 10 | −0.1976*** | −0.2229*** | −0.0236** |
| Component 11 | 0.0561*** | 0.1260*** | 0.0970*** |
Notes:
Statistical significance.
P < 0.05;
P < 0.01;
P < 10−3.