Abstract
Since its introduction in early 1950s, electroencephalography (EEG) has been widely used in the neonatal intensive care units (NICU) for assessment and monitoring of brain function in preterm and term babies. Most common indications are the diagnosis of epileptic seizures, assessment of brain maturity, and recovery from hypoxic-ischemic events. EEG recording techniques and the understanding of neonatal EEG signals have dramatically improved, but these advances have been slow to penetrate through the clinical traditions. The aim of this presentation is to bring theory and practice of advanced EEG recording available for neonatal units.
In the theoretical part, we will present animations to illustrate how a preterm brain gives rise to spontaneous and evoked EEG activities, both of which are unique to this developmental phase, as well as crucial for a proper brain maturation. Recent animal work has shown that the structural brain development is clearly reflected in early EEG activity. Most important structures in this regard are the growing long range connections and the transient cortical structure, subplate. Sensory stimuli in a preterm baby will generate responses that are seen at a single trial level, and they have underpinnings in the subplate-cortex interaction. This brings neonatal EEG readily into a multimodal study, where EEG is not only recording cortical function, but it also tests subplate function via different sensory modalities. Finally, introduction of clinically suitable dense array EEG caps, as well as amplifiers capable of recording low frequencies, have disclosed multitude of brain activities that have as yet been overlooked.
In the practical part of this video, we show how a multimodal, dense array EEG study is performed in neonatal intensive care unit from a preterm baby in the incubator. The video demonstrates preparation of the baby and incubator, application of the EEG cap, and performance of the sensory stimulations.
Keywords: Neuroscience, Issue 60, neurophysiology, preterm baby, neonatal, EEG, evoked response, high density EEG, FbEEG, sensory evoked response, neonatal intensive care unit
Protocol
1. Preparing the baby and the cot/incubator for an EEG study
Schedule the EEG study so that it does not co-incide with the other NICU procedures, such as blood sampling or ultrasound examination, as they may interfere with both the EEG devices and baby's vigilance state.
Start recording immediately after feeding, so the baby is most likely to fall into sleep during EEG recording. It may be practical to apply all recording electrodes prior to feeding and/or other care procedures, so that the EEG can commence immediately thereafter.
Make sure that there are no unnecessary electric devices connected to the baby.
Place all wires related to the EEG recording as far apart as possible from the other wires in the cot. Turn off bed heating, if possible treatment wise.
In a case of electrical interference, you may try to identify the interfering device by turning them off (sometimes even unplugging from mains one at a time may be helpful).
Make sure that baby's head is not touching damp or wet textiles, which may increase coupling of interference from external noise sources to the baby.
2. Application of the dense array EEG cap
Remove interfering wax and oil from baby's scalp by swiping it with a cloth wetted with diluted alcohol or baby shampoo. Assess visually the head size of the baby to select the optimal size of the EEG cap in order to make sure that the cap sits firmly at vertex, and that the temporal electrodes are located at an appropriate level above ear lobes (five different sizes of these caps are available to fit from extremely young preterm to fullterm neonates).
If possible, two persons should work together to place the EEG cap, one taking care of the cap, and the other one holding baby's head and possible intubation or nasal CPAP tubes. If the patient has a CPAP, remove it for the 10-20 seconds that it takes to place the EEG cap. The holding straps of the nasal CPAP are wrapped over the EEG cap later.
(optional) If the baby has an iv line in the head, you will need to disconnect it, and pass it through the outlet made for it in the cap (available in the caps used in the present study). Otherwise, you will need to use head net and single electrode placement, which is demonstrated on the website of the NEMO study (see http://www.nemo-europe.com/en/educational-tools.php).
Hold the baby so that his/her face is directed towards you. Take the cap into both hands, and by using your fingers, push the middle of the cap inside out, so that the central (Cz and Pz electrodes) will first touch the scalp.
Roll the cap gently over the head so that the cap is almost sitting in its final position. Finally, adjust the cap symmetrically in both left-right and anterior-posterior directions. Check symmetry by making sure that the Cz electrode is in the middle (left-right) at the line that connects both ears. When pulling the cap over forehead, it is practical to keep baby's head in place by pressing your thumbs against his/her forehead above the eyes.
Fasten the chin strap to a tightness that keeps the cap well in place, and yet allows unrestricted breathing. Do also check now that the cap size is optimal. The cap shown in this video may look little loose. However, it extends well over the forehead and occipital areas, and yet it is well enough in contact with the scalp at centroparietal areas. Five different sizes of these caps are available to fit from extremely young preterm to full-term neonates, and the cap shown in this video will fit this baby for a few weeks from now on.
Apply the electrode gel through the electrode holes with a syringe connected to a thick blunt gel needle ("tip"). A bended tip may be useful to reach contacts that are difficult to access directly. Fill the electrode cups so that no gel will leak out, as it may form bridges with neighbouring electrodes or even wet the pillow possibly causing artefact. A more viscous gel formula may be practical in this regard, and allows even gelling before cap placement (see ref 1).
Check the impedances and EEG signal quality, and prepare the scalp either by gentle mechanical rubbing or by using the so called SurePrep method as needed2,3 (http://www.helsinki.fi/science/eeg/videos/sureprep/). Observe the quality of skin contact simultaneously from the impedance values given by the EEG software, or by looking at the EEG trace quality. Start from the reference and ground, then prepare one hemisphere at a time to minimize moving of the baby.
Start EEG recording when the impedance levels are acceptable, usually below 15kohms, or when the signal does look clean enough without filtering. Note that impedances often improve over time, so give some minutes time for the impedances to settle down.
3. Application of the polygraphic sensors
Record muscle tone (electromyography; EMG) by attaching two EMG electrodes under the chin. Prepare the skin gently before electrode application.
Record heart beat by applying two ECG electrodes over the chest or on the shoulders. Shoulder attachment is useful if there is a need to identify potential epileptic body movements. Prepare the skin gently before electrode application.
Record eye movements (electro-oculogram, EOG) either with frontal EEG electrodes (then no additional electrodes are needed), or with separate EOG electrodes attached near the lateral corners of the eyes. In the youngest premies, however, it may be sometimes needed to use piezo sensors attached to eyelids (not shown in this video), because their retina may not be polarized enough to cause detectable electric fields. Prepare the skin gently before electrode application.
Monitor respiration movements with a stretch-sensitive transducer belt that is wrapped around the trunk over abdomen or chest. Make sure that the transducer is tight enough to show respiratory movements in the recording, and yet loose enough to allow unrestricted respiration. In babies with spontaneous breathing, special care is needed not to restrain breathing movements (see also http://www.nemo-europe.com/en/educational-tools.php).
If clinically needed, add also a movement-sensitive piezo sensor to any body part that is expected to show unusual movements.
Position the EEG-synchronized video camera so that the whole baby is well seen in the picture. During specific parts of the EEG study, one may choose to focus on selected body parts, such as hands or feet during their tactile stimulation.
4. Recording
During the entire recording session, keep an eye on the signal quality, and make corrections if any signal deteriorates. Follow the baby as well, and add annotations of any event (eg. clinical sign, movement, drug administration) that may be of interest during later offline EEG interpretation.
Should artefacts occur during the recording, troubleshoot them and make corrections to the recording immediately. Many artefacts may permanently compromise the later use of EEG signal.
Adjust the recording length individually so that the EEG study includes at least one epoch of all vigilance states: awake, active sleep and quiet sleep. In most cases, this will take 40 to 90 minutes.
Perform sensory stimulations (see section 5 below) when the baby is well asleep. It is preferable to wait until quiet sleep period.
In the end of the EEG recording session, remove the cap and other sensors, and clean the excess gel from baby's scalp by wiping with a wet cloth.
Clean the EEG cap and other accessories first mechanically, then sterilize them according to hospital instructions.
5. Sensory stimulations
Note that all sensory responses in the young preemies have a long (several seconds) refractory period, and the responses decline rapidly with short interstimulus intervals or in the presence of other ongoing EEG activity in the sensory cortex. Hence, deliver sensory stimuli at moments when the EEG has been relatively silent for at least few seconds, which is easiest to do during quiet sleep exhibiting trace discontinue activity4-6. If possible, use stimulation devices that automatically generate a mark (trigger) into the EEG trace. Perform first the tactile stimulations, then the visual stimuli, and at last the auditory stimuli, which may wake up the baby.
To study the visual evoked responses, give single flashes with a device integrated to the EEG system. You may also show any transient light, such as moving a torch beam over the face. A response is readily generated even from distance, such as through the transparent incubator walls. Observe the response with at least one second duration in several occipital and parietal electrodes.
To study the somatosensory evoked responses, apply gentle tactile stimuli to the palm or sole of the baby. It is helpful to use a device that automatically generates a mark (trigger) into the EEG trace. Observe the response in the contralateral centro-temporal (C and T) electrodes after hand stimulation, and in the midline (Cz) electrodes after foot stimulation. To assist later analysis, add manual annotation to EEG recording any time when you see a spontaneous limb movement, because it produces responses similar to stimulated ones.
To study auditory responses, you may use almost any sound with relatively low intensity,such as hand clapping near baby's head. Avoid using the traditional horn stimulation, because its high sound intensity will lead to both auditory activation and sleep arousal, with often ensuing movement artefacts.
6. Analysis
Start preliminary analysis during the EEG recording already. Add annotations of clinical events that are not obvious from the EEG signal (eg. care procedures, unusual movements or drug administration). Make note also of all EEG artefacts (eg. sucking, rocking or tapping), which are difficult to identify afterwards. Finally, add your preliminary considerations as they arise online, as they may often involve insights that are difficult to perceive later. Make sure that you have recorded both active and quiet sleep, and that the number and quality of sensory stimuli are sufficient.
The actual, thorough review of neonatal EEG is done offline in a workstation where EEG findings can be processed and described in more detail.
7. Representative Results
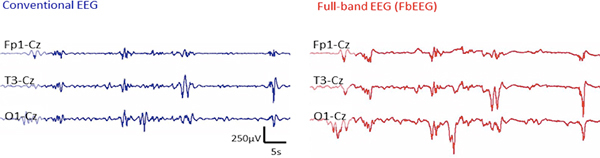 Figure 1. Comparison of the recording with conventional EEG (left) and an unfiltered, Full-band EEG (FbEEG; right). Note the prominent slow fluctuations in the FbEEG signal [see also refs 7-9], which are absent in the conventional EEG. For details, see Discussion.
Figure 1. Comparison of the recording with conventional EEG (left) and an unfiltered, Full-band EEG (FbEEG; right). Note the prominent slow fluctuations in the FbEEG signal [see also refs 7-9], which are absent in the conventional EEG. For details, see Discussion.
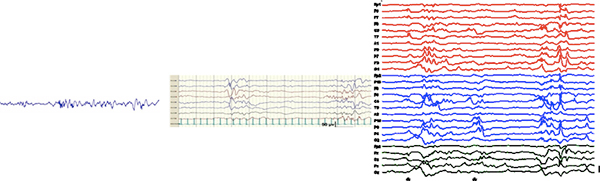 Figure 2. Comparison of the increase in information obtained by adding the number of electrodes. A high density EEG (right) makes it possible to analyse and/or follow cortical areas separately. This spatial information is negligible in the conventional 8 channel (middle) recording, and completely lost in the common one channel EEG monitoring (left). Please click here to see a larger version of this figure.
Figure 2. Comparison of the increase in information obtained by adding the number of electrodes. A high density EEG (right) makes it possible to analyse and/or follow cortical areas separately. This spatial information is negligible in the conventional 8 channel (middle) recording, and completely lost in the common one channel EEG monitoring (left). Please click here to see a larger version of this figure.
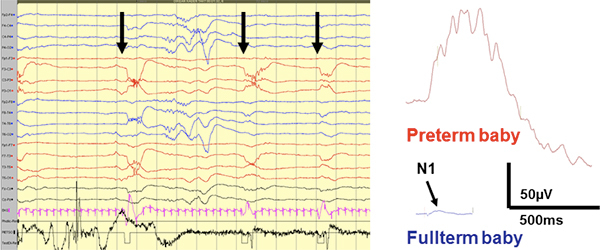 Figure 3. Left: An example is shown from single trial responses to tactile sensory stimulations of hands as seen in the raw EEG trace. Right: Comparison of the preterm and fullterm somatosensory responses (both C4-Fz derivation) demonstrates the magnitude of preterm cortical reactions. The preterm response is shown from a single trial trace, while the fullterm response is generated by averaging, since it would not be distinguished at single trial level. In the fullterm trace, the arrow depicts the N1 response that is conventionally used as the representative measure for clinical diagnosis. For further details, see refs 4,6,10,11. Please click here to see a larger version of this figure.
Figure 3. Left: An example is shown from single trial responses to tactile sensory stimulations of hands as seen in the raw EEG trace. Right: Comparison of the preterm and fullterm somatosensory responses (both C4-Fz derivation) demonstrates the magnitude of preterm cortical reactions. The preterm response is shown from a single trial trace, while the fullterm response is generated by averaging, since it would not be distinguished at single trial level. In the fullterm trace, the arrow depicts the N1 response that is conventionally used as the representative measure for clinical diagnosis. For further details, see refs 4,6,10,11. Please click here to see a larger version of this figure.
Discussion
Recording of neonatal EEG in the way shown here is safe, and hence doable from any baby and in any condition that allows handling associated with routine care procedures1. The intensive care unit is a challenging environment to the sensitive EEG devices. A key to a technically good quality recording is a proper use of well-functioning dense array EEG cap, such as the one shown in this video. NICU environment is unique in that the study subjects are critically ill, vulnerable babies, which undergo demanding care and diagnostic procedures. To ensure not only safety of the patient, but also the future of EEG studies in your NICU, it is necessary to have a close collaboration with your key NICU contact, as well as a high level of trust with all NICU staff involved in patient care.
Recent work in both human babies5-7,10 and animal models with rat pups13-15 has emphasized the dominance of infraslow frequencies in the EEG. While they are readily seen with the FbEEG technique (see Fig 1 and refs 8,9), they are ignored or distorted in the conventional EEG (using an AC coupled amplifier) that permanently cuts them at the time of signal collection, because AC coupled amplifiers act as highpass (low cut) filters. A faithful recording of these infraslow activities requires a DC stable recording setting that consists of a DC coupled amplifier (see Acknowledgements), Ag/AgCl electrodes, chloride-containing gel16, as well as an adequate elimination of epithelial potential (for details, see refs 2,3,8, as well as http://www.nemo-europe.com/en/educational-tools.php -> Setting up EEG hardware -> Skin prepping). It is notable in clinical context, that the caps used in our presentation are fully compliant with FbEEG recording, while many other clinical EEG caps are unsuitable because of their inappropriate electrode materials (eg. tin) 16 and/or poor mechanical stability due to the cut and electrode holder design (for details, see also refs 1,8).
Adequate assessment or monitoring of the early preterm brain must be based on a thorough understanding of the nature and specific characteristics of the immature brain activity itself (see above). Such an approach is largely lacking in the current clinical practice and literature. To meet this need, the protocol for a multimodal neurophysiological assessment of preterm babies was developed in our laboratory. The present paper bridges together the latest knowledge of developmental neurobiology, the relevant advances in neurophysiological techniques, as well as the pressing need for novel clinical research. Our work hences opens the window for translational studies to be performed in a bidirectional manner (from bench to bedside and back). In addition, clear demonstration of the protocol aims to open a venue for larger scale collection of clinically relevant datasets, including studies to define the elusive criteria of normality.
Our clinical experience in the Helsinki University Hospital has shown that i) multimodal studies of this kind have rapidly become an integral part of clinical routine, ii) that they have significantly increased the interest in neonatal EEG studies, and iii) that the developments in recording techniques shown here have made performance of such recordings as readily attainable as any limited, conventional EEG. Most importantly, understanding the relationships between developing brain function and structure makes it possible to assess brain maturation at a time when the baby is not yet able to communicate with the outside world 17. Better brain care at an early stage of development is likely to lead to a permanent increase in health and in overall quality of life of the preterm baby.
Disclosures
No conflicts of interest declared.
Acknowledgments
We want to thank Mr. Jyri Ojala for the technical production of the film, including its animations, graphical design, as well as all technical editing. We also want to thank the parents who gave permission to have their baby be starring in this video, as well as the nurses (especially Mr. Jarmo Mäki) for the help in preparation of film production. This work has received practical and/or financial support from Helsinki University Hospital, Helsinki University of Applied Sciences (Metropolia), Juselius Foundation, Erkko Foundation, as well as the Foundation of Pediatrics (Lastentautien tutkimussäätiö) and from the European Community's Seventh Framework Programme European Community FP7-PEOPLE-2009-IOF, grant agreement n°254235.
The manufacturer of the dense array EEG caps suitable for recording preterm babies is ANT B.V. For contact information, please visit http://www.ant-neuro.com. Notably, the NicOne EEG amplifier (CareFusion, Madison, USA) used in our video is not a genuine DC-coupled amplifier. Following manufacturers do currently (October, 2011) provide DC-coupled, clinically suitable EEG amplifiers: CareFusion (their newest amplifier), ANT Neuro (www.ant-neuro.com), EGI (www.egi.com), BrainProducts (www.BrainProducts.com), Neuroscan (www.neuroscan.com), as well as SACS (www.sacs.se).
References
- Vanhatalo S, Metsäranta M, Andersson S. High fidelity recording of brain activity in the extremely preterm babies: feasibility study in the incubator. Clin. Neurophys. 2008;119:439–445. doi: 10.1016/j.clinph.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Stjerna S, Alatalo P, Mäki J, Vanhatalo S. Evaluation of an easy, standardized, and clinically practical method (SurePrep) for the preparation of electrode-skin contact in EEG recordings. Physiological Measures. 2010;31:889–901. doi: 10.1088/0967-3334/31/7/002. [DOI] [PubMed] [Google Scholar]
- Julkunen P, Pääkkönen A, Hukkanen T, Könönen M, Tiihonen P, Vanhatalo S, Karhu J. Efficient reduction of stimulus artefact in TMS-EEG by epithelial short circuiting by mini-punctures Clin. Neurophys. 2008;119:475–481. doi: 10.1016/j.clinph.2007.09.139. [DOI] [PubMed] [Google Scholar]
- Hrbek A, Karlberg P, Olsson T. Development of visual and somatosensory evoked responses in pre-term newborn infants. Electroencephalogr. Clin. Neurophysiol. 1973;34:225–232. doi: 10.1016/0013-4694(73)90249-6. [DOI] [PubMed] [Google Scholar]
- Milh M, Kaminska A, Huon C, Lapillonne A, Ben-Ari Y, Khazipov R. Rapid cortical oscillations and early motor activity in premature human neonate. Cereb Cortex. 2007;17:1582–1594. doi: 10.1093/cercor/bhl069. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Jousmäki V, Andersson S, Metsäranta M. An easy and practical method for routine, bedside testing of somatosensory systems in extremely low birth weight infants (ELBW) Pediatric Research. 2009;66:710–713. doi: 10.1203/PDR.0b013e3181be9d66. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Palva M, Andersson S, Rivera C, Voipio J, Kaila K. Slow endogenous activity transients and developmental expression of K-Clcotransporter 2 in the immature human cortex. Eur. J. Neurosci. 22:2799–2804. doi: 10.1111/j.1460-9568.2005.04459.x. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Voipio J, Kaila K. Full-Band EEG (FbEEG): an emerging standard in electroencephalography. Clin. Neurophys. 2005;116:1–8. doi: 10.1016/j.clinph.2004.09.015. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Voipio J, Kaila K. Chapter 38. Infraslow EEG activity. In: Lopes da Silva F, editor. Niedermeyer's Electroencephalography: Basic principles, clinical applications and related fields. 5th edition. Baltimore-Munich: Williams & Wilkins; 2011. [Google Scholar]
- Vanhatalo S, Kaila K. Chapter 15. Spontaneous and evoked activity in the early human brain. In: Lagercrantz H, Hanson MA, Ment LR, Peebles DM, editors. The Newborn Brain: Neuroscience & Clinical Applications. 2nd edition. Vol. 11. Cambridge: University Press; 2009. pp. 229–243. [Google Scholar]
- Vanhatalo S, Lauronen L. Neonatal SEP: back to bedside with basic science. Seminars in Fetal and Neonatal Medicine. 2006;11:464–470. doi: 10.1016/j.siny.2006.07.009. [DOI] [PubMed] [Google Scholar]
- Vanhatalo S, Kaila K. Ontogenesis of EEG activity: from phenomenology to physiology. Seminars in Fetal and Neonatal Medicine. 2006;11:471–478. doi: 10.1016/j.siny.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Colonnese MT, Kaminska A, Minlebaev M, Milh M, Bloem B, Lescure S, Moriette G, Chiron C, Ben-Ari Y, Khazipov R. A conserved switch in sensory processing prepares developing neocortex for vision. Neuron. 2010;67:480–498. doi: 10.1016/j.neuron.2010.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khazipov R, Luhmann HJ. Early patterns of electrical activity in the developing cerebral cortex of humans and rodents. Trends Neurosci. 2006;29:414–418. doi: 10.1016/j.tins.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Seelke AM, Blumberg MS. Developmental appearance and disappearance of cortical events and oscillations in infant rats. Brain Res. 2010;1324:34–42. doi: 10.1016/j.brainres.2010.01.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tallgren P, Vanhatalo S, Kaila K, Voipio J. Evaluation of commercially available electrodes and gels for recording of slow EEG potentials. Clin. Neurophys. 2005;116:799–806. doi: 10.1016/j.clinph.2004.10.001. [DOI] [PubMed] [Google Scholar]
- White B. The newborn intensive care unit environment of care: How we got here, where we're headed, and why. Seminars in Fetal and Neonatal. 2011;35:2–7. doi: 10.1053/j.semperi.2010.10.002. [DOI] [PubMed] [Google Scholar]


