Abstract
There are numerous studies indicating that a moderate consumption of red wine provides certain health benefits, such as the protection against neurodegenerative diseases. This protective effect is most likely due to the presence of phenolic compounds in wine. Wine polyphenolic compounds are well known for the antioxidant properties. Oxidative stress is involved in many forms of cellular and molecular deterioration. This damage can lead to cell death and various neurodegenerative disorders, such as Parkinson's or Alzheimer's diseases. Extensive investigations have been undertaken to determine the neuroprotective effects of wine-related polyphenols. In this review we present the neuroprotective abilities of the major classes of wine-related polyphenols.
1. Introduction
Aging is a risk factor common to a number of neurodegenerative diseases, including Alzheimer's disease (AD) and Parkinson's disease. Moreover, associated with the population aging, the occurrence of these neurodegenerative disorders is also likely to augment [1]. In addition to the possible involvement in aging, the common characteristic of most degenerative diseases is that they result from neuronal death. Oxidative stress may play a crucial role in progressive neuronal death [2].
Free radicals are oxidative molecules that occur naturally in the environment but can also be generated in vivo. Reactive oxygen species (ROS) are produced by immune cells in order to sustain their antibacterial and antifungal functions [3]. When ROS are overproduced, they are taken in charge by various enzymatic pathways for inactivation (superoxide dismutase, catalase, cytochromes, etc.) [4, 5]. Although these enzymatic pathways can be overstepped, ROS accumulate and can react with the different cell molecules such as lipids, proteins, carbohydrates, and nucleic acids. These interactions with the ROS apply an oxidative stress to cells [6]. Some tissues, particularly the brain, are highly exposed to oxidative damages because of their elevated oxygen consumption and the induced generation of large amounts of reactive oxygen species [7, 8].
Oxidative stress resulting in ROS generation and inflammation is responsible for many forms of cellular and molecular deterioration such as mitochondrial collapsing, DNA damage, and protein, carbohydrate, and lipid oxidation [9]. This damage can lead to early cell aging, cell death, and various chronic pathologies like neurodegenerative disorders, cardiovascular illnesses, cancers, or type 2 diabetes [2, 6, 10, 11]. Difficulty in treating these diseases and better understanding of their development and causes highlight the usefulness of antioxidants as prevention treatments.
A number of epidemiological studies have shown that the consumption of a diet rich in antioxidants can influence the incidence of neurodegenerative disorders [12]. Orgogozo et al. have shown a positive correlation between a moderate consumption of red wine and a decreased incidence of dementia [13, 14]. This protective effect is most likely due to the presence of phenolic compounds in wine. Wine and grape vine polyphenols are mainly flavonoids (flavanols, flavonols, and anthocyanins) and nonflavonoids (phenolic acids, hydrolysable tannins, and stilbenes) [15]. Extensive investigations have been undertaken to determine the neuroprotective effects of wine polyphenols [16–19]. These polyphenols have displayed neuroprotective capacities in numerous in vitro and animal models of neurotoxicities [19]. Several neuroprotective mechanisms of action have been proposed, suggesting that polyphenols exert their activities by reducing the production and the accumulation of ROS, whose accumulation is likely to play a crucial pathological role in brain aging, reducing oxidative stress and inflammation and modulating the activity of intracellular signal transduction molecules [19–21].
In this review we investigate the neuroprotective abilities of the major classes of polyphenols in wine: flavanols, proanthocyanins, flavonols, anthocyanins, phenolic acids, tannins, and stilbenes. Each specific class of polyphenol has shown neuroprotective effects against neurodegenerative diseases. Their neuroprotective activity has been documented, and we underline the evidence suggesting that their mechanism of action involves their antioxidant activity.
2. Wine Polyphenols
Products such as wine extract, grape seed, and grape skin extracts are all known to contain a large variety of potent antioxidants in the form of polyphenols. Plant phenolic constituents are produced through two metabolic pathways, the main being that of shikimic acid which leads to cinnamic acids, whereas the polyacetate pathway induces the linkage of a second aromatic ring to the first pathway molecules [22–24].
Wines contain various water-soluble polyphenols including phenolic acids, stilbenes, tannins, flavanols, flavonols, and anthocyanins (see Figure 1). Wine phenolics are divided into two groups: flavonoid and nonflavonoid. The amounts of phenolic compounds in wines are highly variable due to varietal differences and process diversities. Indicative levels of phenolic components in wine are shown in Table 1 [15]. Due to wine processing, red wines contain more polyphenols than white wines. Red wine has more antioxidant capacity than white wine due to its phenolic content [25–27]. Phenolic red wines are mainly composed by flavonoids with 1450 mg/L for young wines and 1285 mg/L for aged ones. Phenolic white wines are composed principally of nonflavonoids with 164 mg/L for young wines and 245 mg/L for aged ones [15, 24].
Figure 1.
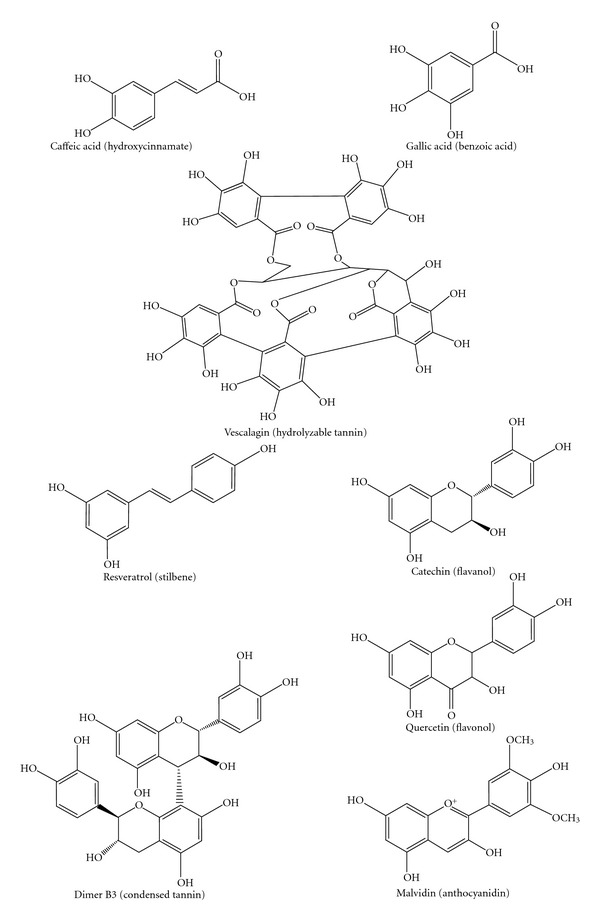
Chemical structures of some phenolic compounds from wine.
Table 1.
The levels of principal phenolic classes (mg/L) in red and white table wine [15].
| Phenol class | White wine | Red wine | ||
|---|---|---|---|---|
| Young | Aged | Young | Aged | |
| nonflavonoids | ||||
| hydroxycinnamates | 154 | 130 | 165 | 60 |
| benzoic acids | 10 | 15 | 60 | 60 |
| hydrolyzable tannins | 0 | 100 | 0 | 250 |
| stilbenes (resveratrol) | 0.5 | 0.5 | 7 | 7 |
|
| ||||
| Total mg/L | 164.5 | 245.5 | 232 | 377 |
|
| ||||
| flavonoids | ||||
| flavanol monomers | 25 | 15 | 200 | 100 |
| Condensed tannins | 20 | 25 | 750 | 1000 |
| flavonols | — | — | 100 | 100 |
| anthocyanins | — | — | 400 | 90 |
|
| ||||
| Total mg/L | 45 | 40 | 1450 | 1285 |
3. Wine Polyphenols and Neuroprotection
3.1. Flavonoids and Neuroprotection
3.1.1. Flavanols
The flavanols, also called flavan-3-ols or catechins, are the most reduced form of flavonoids. They are present in various plants and are associated with the health benefits of green tea [28, 29]. The levels in wine depend on the different grape cultivars and are typically in the range of 20–100 mg/L. Catechin is usually the major flavanol in wine [15, 30]. The condensations of flavanol in wine induce the formation of oligomers (proanthocyanidins and condensed tannins).
Flavanol intake has been associated with various beneficial health effects [31, 32], and flavanols are known to be brain-permeable substances [33]. Their transport is stereoselective involving one or more stereoselective entities and metabolizing with glucuronic acid, for example [34]. Numerous studies indicate that flavanols are of benefit for neuronal health. Catechin may protect against the brain injuries produced by endogenous neurotoxins involved in the onset of Parkinson's disease [35]. Catechin and epicatechin gallate have also shown an ability to suppress neuroinflammation and can attenuate and inhibit activation of microglia and/or astrocytes associated with the release of the mediators linked to the apoptotic death of neurons [36]. In addition, numerous studies indicate that catechin derivatives may delay the onset of neurodegenerative disorders such as Alzheimer's disease through a numerous different mechanisms such as iron chelators, radical scavengers, and modulators of pro-survival genes [31, 37–40].
3.1.2. Proanthocyanidins
Proanthocyanidins and condensed tannins are complex flavonoid polymers naturally present in cereals, legumes, and fruits [41]. They are mainly formed by the condensation of flavanol units to generate oligomers (proanthocyanidins) and polymers (condensed tannins). Their levels in wine depend on pressing techniques and grape varieties. Typically they range from 5 mg/L in white wines to 1 g/L or even higher levels in old red wines [15, 41]. They are associated with a change in wine quality such as a modification of the hue and a decrease in astringency.
Very few studies have concerned the bioavailability of proanthocyanidins. Condensed tannins should be degraded in monomeric phenols, absorbed and metabolized, as has been shown for other flavonoids [1, 2]. Numerous studies indicate that proanthocyanidins and condensed tannins might prevent both cancers and cardiovascular diseases [42, 43]. Some reports demonstrate that its biological abilities to scavenge the reactive oxygen species are associated to the degree of polyphenol oligomerization. Some of these polyphenols might have specific structures that exhibit neuroprotective effects by interacting with putative neuron-specific receptors [44]. Takahashi et al. have shown that procyanidin oligomers from grape seed exhibit higher growth-promoting activity than the monomers toward mouse hair epithelial cells in vitro and in vivo, these results indicating that the specific effect might be correlated with their structure [45]. Other research on rat brain suggests that grape seed extract enriched in proanthocyanidins might protect against pathology age-related oxidative brain damage [46].
3.1.3. Flavonols
Flavonols occur in a wide range of vegetables. There polyphenols are always found in glycoside forms in plants including grape berries, where they are present in the skin. Flavonol glycosides and aglycones are found in grape wine from trace amounts up to 200 mg/L in some red wines [15, 47, 48]. Myricetin, quercetin, and kaempferol conjugates are the major flavonols in wine [48, 49].
Primary results indicate that flavonols can pass the blood-brain barrier [50, 51]. Moreover, numerous studies indicated that flavonols, in addition to many other health benefits, contribute significantly to the protection of neuronal cells against oxidative-stress-induced neurotoxicty [52, 53]. In Alzheimer's disease, neuronal loss is preceded by the extracellular accumulation of amyloid-β peptide (Aβ). It has been shown that pretreatment of primary hippocampal cultures with quercetin significantly attenuates Aβ-induced toxicity, lipid peroxidation, protein oxidation and apoptosis [54]. A dose-response study indicated that quercetin exhibited protective capacities against Aβ-induced toxicity by modulating oxidative stress at lower doses [54]. In cerebral ischemia, calcium dysregulation is one of the main instigators of neuronal cell death and brain damage. Quercetin has been shown to exert significant protection against ischemic injury. Indeed, treatment with quercetin reduced the spectrin breakdown products caused by ischemic activation of calcium-dependent protease calpoin and inhibited the acid-mediated intracellular calcium level [55].
3.1.4. Anthocyanins
Anthocyanins act as guard systems in plants and protect them from UV damage. They form complex molecules with other phenolic molecules and strongly contribute to the color and the aging of wine [56–58]. The aglycone ring of these flavonoids is called anthocyanidin. However, nonconjugated anthocyanidins are never found in grapes or wine, except in trace quantities. In wine there are five anthocyanidins: malvidin, cyanidin, delphinidin, peonidin, and petunidin. Malvidin is the most abundant anthocyanidin in red wines [15].
Among the wine flavonoids, anthocyanins constitute one of the higher potent antioxidants correlated to their capacity to delocalize electrons and form resonating structures [59–62]. Anthocyanins present numerous health benefits such as anticarcinogenic, anti-inflammatory, or antidiabetic effects [61, 63–66]. Anthocyanins also possess beneficial neuroprotective abilities. Some of them have the ability to cross the blood-brain barrier and diffuse through the central nervous system [67, 68]. Anthocyanins have neuroprotective benefits in reducing age-associated oxidative stress and improving cognitive brain function [61, 69–72]. They induce significant neuroprotective effects against oxidative stress, DNA fragmentation and lipid peroxidation in mouse brain [73, 74]. Thus, it appears that the antioxidant and anti-inflammatory effects of anthocyanins contribute to its neuroprotective effect.
3.2. Nonflavonoids and Neuroprotection [73, 74]
3.2.1. Phenolic Acids
The benzoic acids are a minor component in wines. Whereas the hydroxycinnamates are the most important class of nonflavonoid phenols in grape vine and the major class of phenolics in white wine [15, 75]. The three important ones in wine are coumaric acid, caffeic acid, and ferulic acid. Amount of total hydroxycinnamates in wine are typically about 60 mg/L in reds and 130 mg/L in whites [15].
Hydroxycinnamates have an antioxidant activity by scavenging free radicals [76, 77]. Their strong antioxidant properties help to explain their beneficial role on health and in reducing disease risk. Hydroxycinnamates and other phenolic acids have received less attention. It has been shown that p-coumaric acid, hydroxycinnamates caffeic acid, and a Champagne wine extract rich in these compounds have neuroprotective effects against injury induced by 5-S-cysteinyl-dopamine in vitro [78]. Caffeic acid has been reported to have neuroprotective effects against Aβ-induced neurotoxicity in vitro and to inhibit peroxynitrite-induced neuronal injury [78–80]. Ferulic acid has been showed to protect primary neuronal cell cultures against hydroxyl- and peroxyl-radical-mediated oxidative damage [81, 82].
3.2.2. Hydrolyzable Tannins
Tannins are water-soluble polyphenols. One of the major properties of these molecules is their capacity to precipitate proteins such as gelatin from solution [83–85]. In wine, hydrolyzable tannins arise during maturation and ageing of wines in oak barrels [86]. Castalagin and vescalagine are the main representative compounds of ellagic tannins [87]. Their levels are about 100 mg/L in aged white wines, while red wine levels are about 250 mg/L after aging in oak barrels for two or more years [15, 88]. They are mainly ellagic acid and gallic acid ester derivatives with glucose or other sugars. Due to the presence of the ester linkage, they are described as being hydrolyzable. Hydrolyzable tannins are not present in Vitisvinifera but are present in other fruits such as muscadine grapes and raspberries [89]. These polyphenols are excellentantioxidantsand natural preservatives, also helping give the wine structure and texture. However, recent research on tannins has focused on their potential to impact positively on human health. Tannins have demonstrated a host of potent biological activities, antiperoxidation properties, inhibition of mutagenicity of carcinogens and tumor promotion, specific antitumor abilities in relation with tannin structures, antibacterial activity, and antiviral activity [89–91]. In vivo, ellagitannins are mainly transformed into ellagic acid and its metabolites. In fact, they could be the agent responsible for the effects of dietary ellagitannins observed in vivo [92, 93].
There are few studies whose objective has been the neuroprotective activity of hydrolyzable tannins. Ellagic acid has been reported to promote the formation of βA fibril and significant oligomer loss, in contradiction to previous results indicating that polyphenols inhibited Aβ fibril formation [94]. Nevertheless, ellagic acid reduces significantly Aβ-induced neurotoxicity in human SH-SY5Y neuroblastoma cells. These results are in agreement with the hypothesis that Aβ fibril formation may represent a protective mechanism of local Aβ clearance. Thus, ellagic acid may have therapeutic value in Alzheimer's disease.
3.2.3. Resveratrol and Other Stilbenes
Stilbenes are secondary metabolites described as phytoalexins. Stilbenes are found in grape vine and wine [95–97]. The main characteristic of stilbenes consist of diary groups on either end of an active double bond that generates the stilbene skeleton, the so-called resveratrol. Stilbenes can also be found in oligomeric and polymeric forms in wine [98]. Resveratrol is found in wine from trace amounts up to 10 mg/L typically 0.1 mg/L in white wines and 2.0 mg/L in red wines [15, 99]. It is a substance with great potential that is being investigated intensively, and its derivatives exhibit a wide range of pharmacological and biological properties [100].
Resveratrol and its derivatives have also been reported to be active against neuron cell dysfunction and death in animal models [20, 101–104]. Resveratrol can cross the blood–brain barrier and exhibit neuroprotective properties against cerebral injury [105]. Numerous mechanisms may underline resveratrol neuroprotective effects against Aβ-induced neurotoxicity [106]. Resveratrol can act by reducing the intracellular Aβ level by inducing protease degradation of the peptide in Alzheimer's disease. Resveratrol and other stilbenes have been shown to inhibit Aβ fibril formation in vitro [107, 108]. Furthermore, resveratrol has been shown to exhibit significant free-radical scavenging abilities in numerous cellular models [109–112]. Thus, overall scientific data tend to show that among stilbenoids resveratrol has effects against brain injuries in reducing brain damage in complex manner including antioxidant properties, regulation on neurovascular system, or ability to inhibit known neuropathological processes.
4. Mechanisms
4.1. Bioavailability of Wine-Related Polyphenol
It is now well established that wine polyphenols exhibit some beneficial activities on health, particularly on neurodegenerative diseases [113]. Biological activities are often measured on cultured cells or isolated tissues using polyphenols in their form present in wine (as aglycone or their sugar derivatives). However, the question of their achievable concentration after ingestion as well as the possibility of conjugate formation has been ignored by many studies [114]. These data are though crucial for understanding polyphenol bioactivity. Several studies indicate that the antioxidant effect in vitro of some polyphenols may not indicate its activity in vivo. Indeed, its alteration into metabolites and other derivative constituents constitute the true bioactive molecules [113, 115–118]. Polyphenols are extensively metabolized in different tissues such as colon, small intestine, and liver [119]. Polyphenols are absorbed through the gut barrier. Some of them who are not absorbed pass to the large intestine and undergo colonic biotransformation by the enzymes of the colonic microflora [118]. Polyphenols metabolized in the gastrointestinal tract undergo conjugation in the liver after absorption. Then, polyphenols are present in circulation as sulfated, glucuronidated, methylated, and as mixed forms. Moreover, a large proportion of polyphenols ingested are subjected to hydrolyses and degradation by colonic microflora to simple phenolic compounds. Wine polyphenols are grouped into two categories: flavonoids and nonflavonoids as described before. Chemical structure of polyphenol is a factor involved in the gut absorption and the metabolism. We report here data on the absorption and the metabolism of the main polyphenols found in wine.
Concerning flavonoids, flavanols were absorbed and eliminated at low micromolar amounts of their direct conjugates (methylated, sulfated, and glucuronidated derivatives) [120]. However, the degradation of flavan structure in colon leads to the formation of phenolic compounds [114, 121]. Because of their hydrosolubility and high molecular weight, proanthocyanidins are not absorbed in the gut. The large majority of proanthocyanidins cross without alteration through the small intestine after which they are transformed by the colonic microflora to produce simple phenolic acids such as phenylacetic and phenylpropionic derivatives [122]. However, Tsang et al. reported that administration of grapeseed procyanidins induce the formation of catechin glucuronide derivatives in rat plasma [123]. Flavonols are naturally occurred as glycosides. Results indicate that flavonols uptake induce a cleavage of the glycoside part in the small intestine followed by absorption and metabolism of the aglycone [124]. Aglycones are then conjugated by sulfation and glucuronidation as well as methylation of the catechol group [118]. A large number of colonic metabolites identified are simple phenolic acids [125]. Anthocyanins was absorbed and excreted at a low proportion of the intact glycosides after injection of wine extract [126, 127]. The anthocyanins degradation at the pH of the intestine in addition to the microflora activity in the colon are at least in part involved in the degradation of anthocyanins into more stable compound such as phenolic acids [113].
Concerning nonflavonoids, ellagitannins are not absorbed due to their large molecular size [128]. They are principally hydrolysed to ellagic acid under physiological conditions in small intestine [113]. Ellagic acid and ellagitannins reach the distal part of the small intestine and the colon, they are mainly transformated by gut microflora into urolithin derivatives [129]. The major stilbene compound found in wine is resveratrol; thus, bioavailability of resveratrol was investigated. Many investigations in animal models and humans have indicated that a low bioavailability of unconjugated resveratrol. More than 70% of the resveratrol uptaked is absorbed and readily transformed to produce essentially sulphate and glucuronide derivatives [99].
The conjugation of polyphenols has been recognized for many years, most of the biological studies have only been carried with polyphenol aglycones, and very little is known about the biological properties of conjugated derivatives. Numerous studies indicate that metabolic transformations of phenolic compounds reduce their antioxidant properties leading to less active antioxidants than the original compounds [130–132]. The formation of conjugated polyphenols and degradation products such as simple phenolic compounds will modify the properties observed in vivo in comparison to their unconjugated forms [114]. Much research effort is still needed to evaluate the biological effects of the conjugated derivatives and microbial metabolites of wine polyphenols.
4.2. Neurodegenerative Disorders and Oxidative Stress
Reactive oxygen species (ROS) are generated in living organisms due to various metabolic processes [108]. The narrow definition of ROS refers to oxygen free radicals, including superoxide radical anion, hydroxyl radical, hydroperoxyl radical and nonfree radicals, which can induce the generation of free radicals through divers chemical reactions. ROS produced in the human body can cause oxidative damage. Under oxidative stress, the excessive production of ROS may directly damage proteins, lipids, carbohydrates, DNA, and even cellular molecules involved in antioxidant defense systems. The over production of free radicals is implied in the progress of numerous diseases such as cardiovascular diseases, cancer, and neurodegenerative disorders [133–137]. To control, the levels of free radicals various defense mechanisms have been promoted in living organisms such as endogenous enzymes glutathione peroxidase, catalase, or superoxide dismutase. In addition to these endogenous mechanisms, much attention has been focused on the antioxidant role of some dietary compounds like polyphenols [27, 138, 139].
4.3. Antioxidants and Neuroprotection
The brain is characterized by its high susceptibility to oxidative stress due to its high oxygen consumption, its high fatty acids levels, and low antioxidant enzyme levels. Numerous works in the literature indicate that wine related-phenolic compounds exhibit a positive effect on nerve cells [18, 19, 80]. The mechanism proposed as explaining the effect on wine polyphenolic compounds on health can be principally summarized as scavenging intracellular ROS and inhibition of LDL oxidation [110, 140–142]. In recent years, studies on the activity of wine polyphenols have been extended to animal models of CNS disorders and injury [18, 143]. These effects are principally associated to their strong antioxidant capacities, since they can act as free-radical scavengers and hydrogen or electron, to preventing DNA damage and lipid peroxidation [144, 145]. Antioxidant polyphenols protect cell constituents from oxidative alteration and thus limit the risk of developing degenerative disorders induced by oxidative stress, such as in ischemia, Parkinson's disease or Alzheimer's disease. For example, an increasing number of reports has shown that acute chronic treatment of resveratrol exhibits neuroprotective effects against colchicine andnitropropionic acid [146] or motor impairment as well as hippocampal neuron loss [147, 148]. These properties are mainly associated to the antioxidant activity of resveratrol. Resveratrol decreases the oxidative damages, in reducing the levels of malondialdehyde, lipid peroxidation, xanthine oxidase, and nitric oxide, and in increasing the depleted glutathione levels and succinate dehydrogenase activity in rat brain [149, 150].
4.4. Effect of Wine Polyphenols on Redox Imbalance
In the living organism, free radicals are generated both enzymatically and nonenzymatically, inducing the generation of reactive oxygen species, which have a crucial role in neurodegenerative disorders. Thus, in neurodegenerative pathologies and aging, the neuronal cells of specific brain regions may be exposed to ROS attack, and apoptotic cell death occurs and progressively worsens until malfunctioning of the neural network and manifestations of neurodegenerative disorders ensue [151].
Compelling evidence supports that oxidative stress plays key role in the physiopathology of neurodegenerative disorders. ROS level augmentation induces oxidation of cellular components leading to a neurodegenerative signaling cascade, which generates cellular damages and induces cell death [152–155]. To prevent oxidative damage, mammalian cells have developed a complex antioxidant defense system converting ROS to less harmful species [156, 157]. Thus, a potential approach in the treatment of neurodegenerative disorders is the use of antioxidants. They have the capacity to scavenge ROS and to upmodulate endogenous antioxidant defenses. In brain, such compounds should have the capacity to cross the blood-brain barrier.
In cells, oxidative stress is associated to sugar, lipid, DNA, and protein damages. The imbalance between antioxidant defense mechanisms and the intracellular production of free radicals induces oxidative stress [158]. Neurons in their ability to regulate for redox imbalance have an age-related decrease, even minor cellular stresses can lead to irreversible disorders and, as such, participate to the causes of neurodegenerative pathologies [158]. The accumulation of free radicals may activate β-secretase, resulting in formation of β-amyloid, which is believed to be responsible for synaptic dysfunction and neuronal cell death in Alzheimer's disease [159, 160]. The wine polyphenols are powerful anti-oxidants that inhibit the production of free radicals [161, 162], preventing cells from free radical and cellular DNA damages [163–165]. Indeed, the overproduction of reactive nitrogen and oxygen species by phagocytes induces oxidative damage to proteins, lipoproteins, and DNA. These reactions may be harmful to cells and tissues and lead to inflammation [166, 167]. Thus, to reduce many inflammatory disorders inhibition of reactive nitrogen and oxygen species production is a popular target. Wine related polyphenols with their antioxidative capacities may have therapeutic value in the prevention of oxidative stress [168, 169]. Furthermore, results indicate that polyphenols from wine have both the antioxidative and anti-inflammatory properties [170, 171] and that they can prevent cardiovascular diseases [172, 173]. It is also thought that polyphenols act to modulate free radical-mediated lipid peroxidation of low-density lipoproteins (LDL), which is correlated to chronic diseases such as atherosclerosis [138, 174, 175].
4.5. Effect of Wine Polyphenols on NO Production
In complement to the antioxidant abilities of polyphenols, they might exert protective effects by improving endothelial function as indicated by both experimental and clinical studies [176]. As known for a long time, the endothelium have a crucial function in vascular health by regulating several vasorelaxing factors such as nitric oxide (NO) and endothelium-derived hyperpolarizing factor [177–180]. Indeed, the beneficial mechanisms of red wine polyphenolic compounds mainly involve the activation of endothelial NO, release through an increase in calcium levels and activation of the phosphoinositide-3 kinase/Akt pathway in endothelial cells [178, 179, 181]. Red wine polyphenolic compounds may also regulate NO activity at the level of endothelial nitric oxide synthase (eNOS) protein expression in endothelial cells [182] and blood vessels [183]. Chronic upregulation of eNOS by red wine polyphenolic compounds might constitute a preventive approach to reduce tissue injury associated with the risk of cerebral ischemia. For example, results indicate that resveratrol protects the spinal cord from ischemia-reperfusion injury by decreasing oxidative stress and increasing NO release. Resveratrol-induced neuroprotection is thus mediated by both antioxidant- and NO-promoting properties [184]. There is substantial evidence that polyphenols in red wine can exhibit anti-inflammatory abilities. This could be due to their capacities to scavenge NO or to decrease the NO synthase activity [110, 164, 185]. Resveratrol could also inhibit the neuronal NO synthase and the inducible NO synthase isoforms [164, 186]. Therefore, resveratrol could induce the activity of vasodilator-inducing enzyme such as the endothelial isoform eNOS. This effect may be associated to the anti-inflammatory property of resveratrol [187]. Additionally, Han et al. indicated that resveratrol analogues exert neuroprotective effects by the activation of some receptor binding sites localized at the level of the cellular plasma membrane in rat brain [188]. The polyphenol binding to this specific receptor may induce nitric oxide synthase activity in the brain [189]. However, the polyphenol prolonged action could modulate the sensitivity and the tolerance of its receptor. Similarly, low and high doses of red wine polyphenolic compounds have, respectively, pro- and antiangiogenic properties on postischemic neovascularization in vivo. Finally, results indicate that resveratrol uptake, in association with a moderate intake of wine, induces an NO upregulation effect on human platelets [190]. In addition, wine related-polyphenols may exert other effects such as decrease LDL oxidation and increase HDL to generate [190–192]. This unique dual effect of red wine polyphenolic compounds offers important perspectives for the treatment and prevention of different diseases.
4.6. Other Potent Effects of Wine Polyphenols
Because oxidative and nitrosative stress have a crucial impact in the causes of neurodegenerative diseases such as Alzheimer's and Parkinson's diseases and because antioxidant activity is the most studied effect of wine polyphenols, this paper was mainly focused on oxidative damage to neuronal molecules. Nevertheless, studies have indicated that polyphenols could exert neuronal regulation at different levels such antiamyloidogenic effects, neuroprotection through modulation of neural mediators and enzymes, and interaction with signaling pathways.
Resveratrol has been identified as a potential antiaging agent and several studies have been realized on protective abilities of stilbenes (resveratrol derivatives) against aging and specific neurodegenerative disorders [19, 107, 115, 164]. The neuronal regulation of resveratrol derivatives molecules could be defined through a number of complex biological processes involving, as previously discussed upregulation of brain-redox imbalance, interactions with signaling pathways crucial in inducing neuronal function and survival, regulation on neurovascular system, and ability to inhibit known neuropathological processes. In APP695-transfected cell lines, resveratrol, could reduce the level of secreted Aβ peptide without directly affecting any other components of the Aβ metabolism tested [193]. The decrease of the production of Aβ peptide could be related to the increase of its degradation. Also, resveratrol did not promote the Aβ peptide clearance by metalloendopeptidases. The treatment of cells with proteasome inhibitors reduced the Aβ decrease induced by resveratrol. Thus, resveratrol could affect the proteasome involved in the degradation of the Aβ peptide. It has been shown that resveratrol could have beneficial effects on cognitive function mediated by regulation on neurovascular system. A higher microvascular density in association with the increase of cerebral blood flow might ameliorate performance by direct increase of glucose and oxygen supply in brain [194]. Furthermore, a recent study reports that resveratrol quickly enhances blood flow into the brain, followed by increased brain oxygenation which has been correlated to the increased memory capacity and improved cognition [195]. Studies revealed that resveratrol and its derivatives identified in wine such as piceid and ε-viniferin glucoside inhibited in vitro the Aβ fibrils formation [107, 108]. Examination of the inhibitory data for the stilbene monomers suggests specific structure-activity relationships [196]. ε-viniferin glucoside has been shown to inhibit fibrillization of Aβ peptide and to protect PC12 cells against Aβ-induced toxicity [197]. These results together suggest that neuroprotective action of resveratrol could protect neurons against brain injuries in reducing brain damage in complex manner. In addition to resveratrol, various polyphenols present in wine protective effects against neurodegenerative diseases by regulation at different levels [115, 198, 199].
5. Conclusion
Wine polyphenols appear to be potentially neuroprotective agents by their capacity to inhibit and/or modulate several neurodegenerative processes. Their neuroprotective effects in in vitro and in vivo models of neurodegenerative disorders have been documented, and our own findings suggest that their mechanism of action involves their antioxidant activity, principally as scavenging intracellular ROS and inhibition of LDL oxidation, and also their activating effect on endothelial and inhibitory action on both neuronal and inducible nitric oxide synthase activity and subsequent NO production. On the other hand, as indicated by Singh et al., it would be unwise to extrapolate these results to human without conducting proper clinical trials in patients suffering from irreversible and extensive neuronal loss [200].
References
- 1.Ritchie K, Lovestone S. The dementias. The Lancet. 2002;360(9347):1759–1766. doi: 10.1016/S0140-6736(02)11667-9. [DOI] [PubMed] [Google Scholar]
- 2.Esposito L, Raber J, Kekonius L, et al. Reduction in mitochondrial superoxide dismutase modulates Alzheimer’s disease-like pathology and accelerates the onset of behavioral changes in human amyloid precursor protein transgenic mice. Journal of Neuroscience. 2006;26(19):5167–5179. doi: 10.1523/JNEUROSCI.0482-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hu DE, Brindle KM. Immune cell-induced synthesis of NO and reactive oxygen species in lymphoma cells causes their death by apoptosis. FEBS Letters. 2005;579(13):2833–2841. doi: 10.1016/j.febslet.2005.03.099. [DOI] [PubMed] [Google Scholar]
- 4.Allen RG, Tresini M. Oxidative stress and gene regulation. Free Radical Biology and Medicine. 2000;28(3):463–499. doi: 10.1016/s0891-5849(99)00242-7. [DOI] [PubMed] [Google Scholar]
- 5.Victor VM, Rocha M, De La Fuente M. Immune cells: free radicals and antioxidants in sepsis. International Immunopharmacology. 2004;4(3):327–347. doi: 10.1016/j.intimp.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Yen GC. Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L.) leaves. Food Chemistry. 2007;101(2):686–694. [Google Scholar]
- 7.Migliore L, Coppedè F. Environmental-induced oxidative stress in neurodegenerative disorders and aging. Mutation Research. 2009;674(1-2):73–84. doi: 10.1016/j.mrgentox.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 8.Esposito E, Rotilio D, Di Matteo V, Di Giulio C, Cacchio M, Algeri S. A review of specific dietary antioxidants and the effects on biochemical mechanisms related to neurodegenerative processes. Neurobiology of Aging. 2002;23(5):719–735. doi: 10.1016/s0197-4580(02)00078-7. [DOI] [PubMed] [Google Scholar]
- 9.Hogg N, Kalyanaraman B. Nitric oxide and lipid peroxidation. Biochimica et Biophysica Acta. 1999;1411(2-3):378–384. doi: 10.1016/s0005-2728(99)00027-4. [DOI] [PubMed] [Google Scholar]
- 10.Di Matteo V, Esposito E. Biochemical and therapeutic effects of antioxidants in the treatment of Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis. Curr Drug Target CNS Neurol Disord. 2003;2(2):95–107. doi: 10.2174/1568007033482959. [DOI] [PubMed] [Google Scholar]
- 11.Kris-Etherton PM, Hecker KD, Bonanome A, et al. Bioactive compounds in foods: their role in the prevention of cardiovascular disease and cancer. American Journal of Medicine. 2002;113(9) doi: 10.1016/s0002-9343(01)00995-0. [DOI] [PubMed] [Google Scholar]
- 12.De Rijk MC, Breteler MMB, Den Breeijen JH, et al. Dietary antioxidants and Parkinson disease: the Rotterdam Study. Archives of Neurology. 1997;54(6):762–765. doi: 10.1001/archneur.1997.00550180070015. [DOI] [PubMed] [Google Scholar]
- 13.Dartigues J-F, Letenneur L, Joly P, Helmer C, Orgogozo J-M, Commenges D. Age specific risk of dementia according to gender, education and wine consumption. Neurobiology of Aging. 2000;21:p. 64. [Google Scholar]
- 14.Orgogozo JM, Dartigues JF, Lafont S, et al. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Revue Neurologique. 1997;153(3):185–192. [PubMed] [Google Scholar]
- 15.Waterhouse AL. Wine phenolics. Annals of the New York Academy of Sciences. 2002;957:21–36. doi: 10.1111/j.1749-6632.2002.tb02903.x. [DOI] [PubMed] [Google Scholar]
- 16.Aboul-ela F, Karn J, Varani G. The structure of the human immunodeficiency virus type-1 TAR RNA reveals principles of RNA recognition by Tat protein. Journal of Molecular Biology. 1995;253(2):313–332. doi: 10.1006/jmbi.1995.0555. [DOI] [PubMed] [Google Scholar]
- 17.Sun AY, Simonyi A, Sun GY. The “French paradox” and beyond: neuroprotective effects of polyphenols. Free Radical Biology and Medicine. 2002;32(4):314–318. doi: 10.1016/s0891-5849(01)00803-6. [DOI] [PubMed] [Google Scholar]
- 18.Assunção M, Santos-Marques MJ, de Freitas V, et al. Red wine antioxidants protect hippocampal neurons against ethanol-induced damage: a biochemical, morphological and behavioral study. Neuroscience. 2007;146(4):1581–1592. doi: 10.1016/j.neuroscience.2007.03.040. [DOI] [PubMed] [Google Scholar]
- 19.Bastianetto S. Red wine consumption and brain aging. Nutrition. 2002;18(5):432–433. doi: 10.1016/s0899-9007(01)00745-6. [DOI] [PubMed] [Google Scholar]
- 20.Ramassamy C. Emerging role of polyphenolic compounds in the treatment of neurodegenerative diseases: a review of their intracellular targets. European Journal of Pharmacology. 2006;545(1):51–64. doi: 10.1016/j.ejphar.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 21.Choi D-Y, Lee Y-J, Hong JT, Lee H-J. Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease. Brain Research Bulletin. 2012;87(2-3):144–153. doi: 10.1016/j.brainresbull.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 22.Bruneton J. Part 2: Phenolic compounds. In: Doc ET, editor. Pharmacognosie. Paris, France: Lavoisier; 2009. p. 259 pages. [Google Scholar]
- 23.Haslam E. Plant Polyphenols. Cambridge, UK: Cambridge University Press; 1989. [Google Scholar]
- 24.Haslam E. Practical Polyphenolics: From Structure to Molecular Recognition & Physiological Action. Cambridge, UK: Cambridge University Press; 1998. [Google Scholar]
- 25.Frankel EN, Waterhouse AL, Teissedre PL. Principal phenolic phytochemicals in selected California wines and their antioxidant activity in inhibiting oxidation of human low-density lipoproteins. Journal of Agricultural and Food Chemistry. 1995;43(4):890–894. [Google Scholar]
- 26.Rice-Evans CA, Miller NJ. Antioxidant activities of flavonoids as bioactive components of food. Biochemical Society Transactions. 1996;24(3):790–795. doi: 10.1042/bst0240790. [DOI] [PubMed] [Google Scholar]
- 27.Rice-Evans CA, Miller NJ, Paganga G. Antioxidant properties of phenolic compounds. Trends in Plant Science. 1997;2(4):152–159. [Google Scholar]
- 28.Koo SI, Noh SK. Green tea as inhibitor of the intestinal absorption of lipids: potential mechanism for its lipid-lowering effect. Journal of Nutritional Biochemistry. 2007;18(3):179–183. doi: 10.1016/j.jnutbio.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wu D, Guo Z, Ren Z, Guo W, Meydani SN. Green tea EGCG suppresses T cell proliferation through impairment of IL-2/IL-2 receptor signaling. Free Radical Biology and Medicine. 2009;47(5):636–643. doi: 10.1016/j.freeradbiomed.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Arts ICW, Van De Putte B, Hollman PCH. Catechin contents of foods commonly consumed in The Netherlands. 2. Tea, wine, fruit juices, and chocolate milk. Journal of Agricultural and Food Chemistry. 2000;48(5):1752–1757. doi: 10.1021/jf000026+. [DOI] [PubMed] [Google Scholar]
- 31.Mandel S, Youdim MBH. Catechin polyphenols: neurodegeneration and neuroprotection in neurodegenerative diseases. Free Radical Biology and Medicine. 2004;37(3):304–317. doi: 10.1016/j.freeradbiomed.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 32.Sutherland BA, Rahman RMA, Appleton I. Mechanisms of action of green tea catechins, with a focus on ischemia-induced neurodegeneration. Journal of Nutritional Biochemistry. 2006;17(5):291–306. doi: 10.1016/j.jnutbio.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 33.Mandel S, Amit T, Reznichenko L, Weinreb O, Youdim MBH. Green tea catechins as brain-permeable, natural iron chelators-antioxidants for the treatment of neurodegenerative disorders. Molecular Nutrition and Food Research. 2006;50(2):229–234. doi: 10.1002/mnfr.200500156. [DOI] [PubMed] [Google Scholar]
- 34.Faria A, Pestana D, Teixeira D, et al. Insights into the putative catechin and epicatechin transport across blood-brain barrier. Food and Function. 2011;2(1):39–44. doi: 10.1039/c0fo00100g. [DOI] [PubMed] [Google Scholar]
- 35.Vauzour D, Ravaioli G, Vafeiadou K, Rodriguez-Mateos A, Angeloni C, Spencer JPE. Peroxynitrite induced formation of the neurotoxins 5-S-cysteinyl-dopamine and DHBT-1: implications for Parkinson’s disease and protection by polyphenols. Archives of Biochemistry and Biophysics. 2008;476(2):145–151. doi: 10.1016/j.abb.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 36.Li R, Huang YG, Fang D, Le WD. (-)-Epigallocatechin gallate inhibits lipopolysaccharide-induced microglial activation and protects against inflammation-mediated dopaminergic neuronal injury. Journal of Neuroscience Research. 2004;78(5):723–731. doi: 10.1002/jnr.20315. [DOI] [PubMed] [Google Scholar]
- 37.Weinreb O, Mandel S, Amit T, Youdim MBH. Neurological mechanisms of green tea polyphenols in Alzheimer’s and Parkinson’s diseases. Journal of Nutritional Biochemistry. 2004;15(9):506–516. doi: 10.1016/j.jnutbio.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 38.Choi YT, Jung CH, Lee SR, et al. The green tea polyphenol (-)-epigallocatechin gallate attenuates β-amyloid-induced neurotoxicity in cultured hippocampal neurons. Life Sciences. 2001;70(5):603–614. doi: 10.1016/s0024-3205(01)01438-2. [DOI] [PubMed] [Google Scholar]
- 39.Levites Y, Amit T, Youdim MBH, Mandel S. Involvement of protein kinase C activation and cell survival/cell cycle genes in green tea polyphenol (-)-epigallocatechin 3-gallate neuroprotective action. The Journal of Biological Chemistry. 2002;277(34):30574–30580. doi: 10.1074/jbc.M202832200. [DOI] [PubMed] [Google Scholar]
- 40.Patel AK, Rogers JT, Huang X. Flavanols, mild cognitive impairment, and Alzheimer's dementia. International Journal of Clinical and Experimental Medicine. 2008;1(2):181–191. [PMC free article] [PubMed] [Google Scholar]
- 41.Santos-Buelga C, Scalbert A. Proanthocyanidins and tannin-like compounds—nature, occurrence, dietary intake and effects on nutrition and health. Journal of the Science of Food and Agriculture. 2000;80(7):1094–1117. [Google Scholar]
- 42.Sato M, Maulik G, Ray PS, Bagchi D, Das DK. Cardioprotective effects of grape seed proanthocyanidin against ischemic reperfusion injury. Journal of Molecular and Cellular Cardiology. 1999;31(6):1289–1297. doi: 10.1006/jmcc.1999.0961. [DOI] [PubMed] [Google Scholar]
- 43.Aldini G, Carini M, Piccoli A, Rossoni G, Facino RM. Procyanidins from grape seeds protect endothelial cells from peroxynitrite damage and enhance endothelium-dependent relaxation in human artery: new evidences for cardio-protection. Life Sciences. 2003;73(22):2883–2898. doi: 10.1016/s0024-3205(03)00697-0. [DOI] [PubMed] [Google Scholar]
- 44.Narita K, Hisamoto M, Okuda T, Takeda S. Differential neuroprotective activity of two different grape seed extracts. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0014575.e14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takahashi T, Kamiya T, Hasegawa A, Yokoo Y. Procyanidin oligomers selectively and intensively promote proliferation of mouse hair epithelial cells in vitro and activate hair follicle growth in vivo . Journal of Investigative Dermatology. 1999;112(3):310–316. doi: 10.1046/j.1523-1747.1999.00532.x. [DOI] [PubMed] [Google Scholar]
- 46.Deshane J, Chaves L, Sarikonda KV, et al. Proteomics analysis of rat brain protein modulations by grape seed extract. Journal of Agricultural and Food Chemistry. 2004;52(26):7872–7883. doi: 10.1021/jf040407d. [DOI] [PubMed] [Google Scholar]
- 47.Singleton VL. Wine phenols. In: Linskens HF, Jackson JF, editors. Wine Analysis. Berlin, Germany: Springer; 1988. p. p. 171. [Google Scholar]
- 48.Makris DP, Kallithraka S, Kefalas P. Flavonols in grapes, grape products and wines: burden, profile and influential parameters. Journal of Food Composition and Analysis. 2006;19(5):396–404. [Google Scholar]
- 49.Burns J, Gardner PT, Matthews D, Duthie GG, Lean MEJ, Crozier A. Extraction of phenolics and changes in antioxidant activity of red wines during vinification. Journal of Agricultural and Food Chemistry. 2001;49(12):5797–5808. doi: 10.1021/jf010682p. [DOI] [PubMed] [Google Scholar]
- 50.Youdim KA, Qaiser MZ, Begley DJ, Rice-Evans CA, Abbott NJ. Flavonoid permeability across an in situ model of the blood-brain barrier. Free Radical Biology and Medicine. 2004;36(5):592–604. doi: 10.1016/j.freeradbiomed.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 51.Youdim KA, Shukitt-Hale B, Joseph JA. Flavonoids and the brain: interactions at the blood-brain barrier and their physiological effects on the central nervous system. Free Radical Biology and Medicine. 2004;37(11):1683–1693. doi: 10.1016/j.freeradbiomed.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 52.Ho JH, Chang YL. Protective effects of quercetin and vitamin C against oxidative stress-induced neurodegeneration. Journal of Agricultural and Food Chemistry. 2004;52(25):7514–7517. doi: 10.1021/jf049243r. [DOI] [PubMed] [Google Scholar]
- 53.Samhan-Arias AK, Martín-Romero FJ, Gutiérrez-Merino C. Kaempferol blocks oxidative stress in cerebellar granule cells and reveals a key role for reactive oxygen species production at the plasma membrane in the commitment to apoptosis. Free Radical Biology and Medicine. 2004;37(1):48–61. doi: 10.1016/j.freeradbiomed.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 54.Ansari MA, Abdul HM, Joshi G, Opii WO, Butterfield DA. Protective effect of quercetin in primary neurons against Aβ(1–42): relevance to Alzheimer’s disease. Journal of Nutritional Biochemistry. 2009;20(4):269–275. doi: 10.1016/j.jnutbio.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pandey AK, Hazari PP, Patnaik R, Mishra AK. The role of ASIC1a in neuroprotection elicited by quercetin in focal cerebral ischemia. Brain Research. 2011;1383:289–299. doi: 10.1016/j.brainres.2011.01.085. [DOI] [PubMed] [Google Scholar]
- 56.Francia-Aricha EM, Guerra MT, Rivas-Gonzalo JC, Santos-Buelga C. New anthocyanin pigments formed after condensation with flavanols. Journal of Agricultural and Food Chemistry. 1997;45(6):2262–2266. [Google Scholar]
- 57.De Freitas V, Sousa C, Silva AMS, Santos-Buelga C, Mateus N. Synthesis of a new catechin-pyrylium derived pigment. Tetrahedron Letters. 2004;45(51):9349–9352. [Google Scholar]
- 58.Fulcrand H, Cheminat A, Brouillard R, Cheynier V. Characterization of compounds obtained by chemical oxidation of caffeic acid in acidic conditions. Phytochemistry. 1994;35(2):499–505. [Google Scholar]
- 59.Rivero-Pérez MD, Muñiz P, González-Sanjosé ML. Contribution of anthocyanin fraction to the antioxidant properties of wine. Food and Chemical Toxicology. 2008;46(8):2815–2822. doi: 10.1016/j.fct.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 60.Kong JM, Chia LS, Goh NK, Chia TF, Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64(5):923–933. doi: 10.1016/s0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 61.Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Molecular Nutrition and Food Research. 2007;51(6):675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]
- 62.Castañeda-Ovando A, Pacheco-Hernandez Ma.d.L. MDL, Páez-Hernández ME, Rodríguez JA, Galán-Vidal CA. Chemical studies of anthocyanins: a review. Food Chemistry. 2009;113(4):859–871. [Google Scholar]
- 63.Ghosh D, Konishi T. Anthocyanins and anthocyanin-rich extracts: role in diabetes and eye function. Asia Pacific Journal of Clinical Nutrition. 2007;16(2):200–208. [PubMed] [Google Scholar]
- 64.Karlsen A, Retterstøl L, Laake P, et al. Anthocyanins inhibit nuclear factor-κB activation in monocytes and reduce plasma concentrations of pro-inflammatory mediators in healthy adults. Journal of Nutrition. 2007;137(8):1951–1954. doi: 10.1093/jn/137.8.1951. [DOI] [PubMed] [Google Scholar]
- 65.Sasaki R, Nishimura N, Hoshino H, et al. Cyanidin 3-glucoside ameliorates hyperglycemia and insulin sensitivity due to downregulation of retinol binding protein 4 expression in diabetic mice. Biochemical Pharmacology. 2007;74(11):1619–1627. doi: 10.1016/j.bcp.2007.08.008. [DOI] [PubMed] [Google Scholar]
- 66.Toufektsian MC, De Lorgeril M, Nagy N, et al. Chronic dietary intake of plant-derived anthocyanins protects the rat heart against ischemia-reperfusion injury. Journal of Nutrition. 2008;138(4):747–752. doi: 10.1093/jn/138.4.747. [DOI] [PubMed] [Google Scholar]
- 67.Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood? brain barrier. Journal of Agricultural and Food Chemistry. 2010;58(7):3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- 68.Talavéra S, Felgines C, Texier O, et al. Anthocyanin metabolism in rats and their distribution to digestive area, kidney, and brain. Journal of Agricultural and Food Chemistry. 2005;53(10):3902–3908. doi: 10.1021/jf050145v. [DOI] [PubMed] [Google Scholar]
- 69.Shih PH, Chan YC, Liao JW, Wang MF, Yen GC. Antioxidant and cognitive promotion effects of anthocyanin-rich mulberry (Morus atropurpurea L.) on senescence-accelerated mice and prevention of Alzheimer’s disease. Journal of Nutritional Biochemistry. 2010;21(7):598–605. doi: 10.1016/j.jnutbio.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 70.Papandreou MA, Dimakopoulou A, Linardaki ZI, et al. Effect of a polyphenol-rich wild blueberry extract on cognitive performance of mice, brain antioxidant markers and acetylcholinesterase activity. Behavioural Brain Research. 2009;198(2):352–358. doi: 10.1016/j.bbr.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 71.Tarozzi A, Morroni F, Hrelia S, et al. Neuroprotective effects of anthocyanins and their in vivo metabolites in SH-SY5Y cells. Neuroscience Letters. 2007;424(1):36–40. doi: 10.1016/j.neulet.2007.07.017. [DOI] [PubMed] [Google Scholar]
- 72.Varadinova MG, Docheva-Drenska DI, Boyadjieva NI. Effects of anthocyanins on learning and memory of ovariectomized rats. Menopause. 2009;16(2):345–349. doi: 10.1097/gme.0b013e3181847619. [DOI] [PubMed] [Google Scholar]
- 73.Acquaviva R, Russo A, Galvano F, et al. Cyanidin and cyanidin 3-O-β-D-glucoside as DNA cleavage protectors and antioxidants. Cell Biology and Toxicology. 2003;19(4):243–252. doi: 10.1023/b:cbto.0000003974.27349.4e. [DOI] [PubMed] [Google Scholar]
- 74.Di Giacomo C, Acquaviva R, Piva A, et al. Protective effect of cyanidin 3-O-β-d-glucoside on ochratoxin A-mediated damage in the rat. British Journal of Nutrition. 2007;98(5):937–943. doi: 10.1017/S0007114507756908. [DOI] [PubMed] [Google Scholar]
- 75.Vrhovšek U. Extraction of hydroxycinnamoyltartaric acids from berries of different grape varieties. Journal of Agricultural and Food Chemistry. 1998;46(10):4203–4208. [Google Scholar]
- 76.Nardini M, D’Aquino M, Tomassi G, Gentili V, Di Felice M, Scaccini C. Inhibition of human low-density lipoprotein oxidation by caffeic acid and other hydroxycinnamic acid derivatives. Free Radical Biology and Medicine. 1995;19(5):541–552. doi: 10.1016/0891-5849(95)00052-y. [DOI] [PubMed] [Google Scholar]
- 77.Maurya DK, Devasagayam TPA. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food and Chemical Toxicology. 2010;48(12):3369–3373. doi: 10.1016/j.fct.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 78.Vauzour D, Corona G, Spencer JPE. Caffeic acid, tyrosol and p-coumaric acid are potent inhibitors of 5-S-cysteinyl-dopamine induced neurotoxicity. Archives of Biochemistry and Biophysics. 2010;501(1):106–111. doi: 10.1016/j.abb.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 79.Sul D, Kim HS, Lee D, Joo SS, Hwang KW, Park SY. Protective effect of caffeic acid against beta-amyloid-induced neurotoxicity by the inhibition of calcium influx and tau phosphorylation. Life Sciences. 2009;84(9-10):257–262. doi: 10.1016/j.lfs.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 80.Vauzour D, Vafeiadou K, Spencer JPE. Inhibition of the formation of the neurotoxin 5-S-cysteinyl-dopamine by polyphenols. Biochemical and Biophysical Research Communications. 2007;362(2):340–346. doi: 10.1016/j.bbrc.2007.07.153. [DOI] [PubMed] [Google Scholar]
- 81.Sultana R. Ferulic acid ethyl ester as a potential therapy in neurodegenerative disorders. Biochimica et Biophysica Acta. 2012;1822(5):748–752. doi: 10.1016/j.bbadis.2011.10.015. [DOI] [PubMed] [Google Scholar]
- 82.Kanski J, Aksenova M, Stoyanova A, Butterfield DA. Ferulic acid antioxidant protection against hydroxyl and peroxyl radical oxidation in synaptosomal and neuronal cell culture systems in vitro: structure-activity studies. Journal of Nutritional Biochemistry. 2002;13(5):273–281. doi: 10.1016/s0955-2863(01)00215-7. [DOI] [PubMed] [Google Scholar]
- 83.Spencer CM, Cai Y, Martin R, et al. Polyphenol complexation-some thoughts and observations. Phytochemistry. 1988;27(8):2397–2409. [Google Scholar]
- 84.McManus JP, Davis KG, Beart JE, Gaffney SH, Lilley TH, Haslam E. Polyphenol interactions. Part 1. Introduction; some observations on the reversible complexation of polyphenols with proteins and polysaccharides. Journal of the Chemical Society. 1985;(9):1429–1438. [Google Scholar]
- 85.Adrian J C, Baxter NJ, Lilley TH, Haslam E, McDonald CJ, Williamson MP. Tannin interactions with a full-length human salivary proline-rich protein display a stronger affinity than with single proline-rich repeats. FEBS Letters. 1996;382(3):289–292. doi: 10.1016/0014-5793(96)00186-x. [DOI] [PubMed] [Google Scholar]
- 86.Castillo-Munoz N, Gomez-Alonso S, Garcia-Romero E, Hermosan-Gutierrez I. Flavonol profiles of Vitis vinifera white grape cultivars. Journal of Food Composition and Analysis. 2010;23(7):699–705. [Google Scholar]
- 87.García-Estévez I, Escribano-Bailón MT, Rivas-Gonzalo JC, Alcalde-Eon C. Development of a fractionation method for the detection and identification of oak ellagitannins in red wines. Analytica Chimica Acta. 2010;660(1-2):171–176. doi: 10.1016/j.aca.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 88.Quinn MK, Singleton VL. Isolation and identification of ellagitannins from white oak wood and an estimate of their roles in wine. American Journal of Enology and Viticulture. 1985;36, article 148 [Google Scholar]
- 89.Landete JM. Ellagitannins, ellagic acid and their derived metabolites: a review about source, metabolism, functions and health. Food Research International. 2011;44(5):1150–1160. [Google Scholar]
- 90.Quideau S, Jourdes M, Saucier C, Glories Y, Pardon P, Baudry C. DNA topoisomerase inhibitor acutissimin a and other flavano-ellagitannins in red wine. Angewandte Chemie—International Edition. 2003;42(48):6012–6014. doi: 10.1002/anie.200352089. [DOI] [PubMed] [Google Scholar]
- 91.Okuda T. Systematics and health effects of chemically distinct tannins in medicinal plants. Phytochemistry. 2005;66(17):2012–2031. doi: 10.1016/j.phytochem.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 92.Larrosa M, Tomás-Barberán FA, Espín JC. The dietary hydrolysable tannin punicalagin releases ellagic acid that induces apoptosis in human colon adenocarcinoma Caco-2 cells by using the mitochondrial pathway. Journal of Nutritional Biochemistry. 2006;17(9):611–625. doi: 10.1016/j.jnutbio.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 93.Quideau S, Deffieux D, Douat-Casassus C, Pouységu L. Plant polyphenols: chemical properties, biological activities, and synthesis. Angewandte Chemie—International Edition. 2011;50(3):586–621. doi: 10.1002/anie.201000044. [DOI] [PubMed] [Google Scholar]
- 94.Feng Y, Yang SG, Du XT, et al. Ellagic acid promotes Aβ42 fibrillization and inhibits Aβ42-induced neurotoxicity. Biochemical and Biophysical Research Communications. 2009;390(4):1250–1254. doi: 10.1016/j.bbrc.2009.10.130. [DOI] [PubMed] [Google Scholar]
- 95.Pezet R, Gindro K, Viret O, Spring JL. Glycosylation and oxidative dimerization of resveratrol are respectively associated to sensitivity and resistance of grapevine cultivars to downy mildew. Physiological and Molecular Plant Pathology. 2004;65(6):297–303. [Google Scholar]
- 96.Pezet R, Pont V, Cuenat P. Method to determine resveratrol and pterostilbene in grape berries and wines using high-performance liquid chromatography and highly sensitive fluorimetric detection. Journal of Chromatography A. 1994;663:191–197. [Google Scholar]
- 97.Pezet R, Perret C, Jean-Denis JB, Tabacchi R, Gindro K, Viret O. δ-viniferin, a resveratrol dehydrodimer: one of the major stilbenes synthesized by stressed grapevine leaves. Journal of Agricultural and Food Chemistry. 2003;51(18):5488–5492. doi: 10.1021/jf030227o. [DOI] [PubMed] [Google Scholar]
- 98.Amira-Guebailia H, Valls J, Richard T, et al. Centrifugal partition chromatography followed by HPLC for the isolation of cis-ε-viniferin, a resveratrol dimer newly extracted from a red Algerian wine. Food Chemistry. 2009;113(1):320–324. [Google Scholar]
- 99.Fernández-Mar MI, Mateos R, García-Parrilla MC, Puertas B, Cantos-Villar E. Bioactive compounds in wine: resveratrol, hydroxytyrosol and melatonin: a review. Food Chemistry. 2012;130(4):797–813. [Google Scholar]
- 100.Vang O, Ahmad N, Baile CA, et al. What is new for an old molecule? Systematic review and recommendations on the use of resveratrol. PLoS ONE. 2011;6(6) doi: 10.1371/journal.pone.0019881.e19881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Anekonda TS. Resveratrol-A boon for treating Alzheimer’s disease? Brain Research Reviews. 2006;52(2):316–326. doi: 10.1016/j.brainresrev.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 102.Gao ZB, Chen XQ, Hu GY. Inhibition of excitatory synaptic transmission by trans-resveratrol in rat hippocampus. Brain Research. 2006;1111(1):41–47. doi: 10.1016/j.brainres.2006.06.096. [DOI] [PubMed] [Google Scholar]
- 103.Andrabi SA, Spina MG, Lorenz P, Ebmeyer U, Wolf G, Horn TFW. Oxyresveratrol (trans-2,3′,4,5′-tetrahydroxystilbene) is neuroprotective and inhibits the apoptotic cell death in transient cerebral ischemia. Brain Research. 2004;1017(1-2):98–107. doi: 10.1016/j.brainres.2004.05.038. [DOI] [PubMed] [Google Scholar]
- 104.Richard T, Pawlus AD, Iglésias ML, et al. Neuroprotective properties of resveratrol and derivatives. Annals of the New York Academy of Sciences. 2011;1215(1):103–108. doi: 10.1111/j.1749-6632.2010.05865.x. [DOI] [PubMed] [Google Scholar]
- 105.Wang Q, Xu J, Rottinghaus GE, et al. Resveratrol protects against global cerebral ischemic injury in gerbils. Brain Research. 2002;958(2):439–447. doi: 10.1016/s0006-8993(02)03543-6. [DOI] [PubMed] [Google Scholar]
- 106.Pallàs M, Casadesús G, Smith MA, et al. Resveratrol and neurodegenerative diseases: activation of SIRT1 as the potential pathway towards neuroprotection. Current Neurovascular Research. 2009;6(1):70–81. doi: 10.2174/156720209787466019. [DOI] [PubMed] [Google Scholar]
- 107.Rivière C, Richard T, Quentin L, Krisa S, Mérillon JM, Monti JP. Inhibitory activity of stilbenes on Alzheimer’s β-amyloid fibrils in vitro. Bioorganic and Medicinal Chemistry. 2007;15(2):1160–1167. doi: 10.1016/j.bmc.2006.09.069. [DOI] [PubMed] [Google Scholar]
- 108.Rivière C, Papastamoulis Y, Fortin PY, et al. New stilbene dimers against amyloid fibril formation. Bioorganic and Medicinal Chemistry Letters. 2010;20(11):3441–3443. doi: 10.1016/j.bmcl.2009.09.074. [DOI] [PubMed] [Google Scholar]
- 109.Chanvitayapongs S, Draczynska-Lusiak B, Sun AY. Amelioration of oxidative stress by antioxidants and resveratrol in PC12 cells. NeuroReport. 1997;8(6):1499–1502. doi: 10.1097/00001756-199704140-00035. [DOI] [PubMed] [Google Scholar]
- 110.Bastianetto S, Quirion R. Natural extracts as possible protective agents of brain aging. Neurobiology of Aging. 2002;23(5):891–897. doi: 10.1016/s0197-4580(02)00024-6. [DOI] [PubMed] [Google Scholar]
- 111.Karlsson J, Emgård M, Brundin P, Burkitt MJ. trans-Resveratrol protects embryonic mesencephalic cells from tert-butyl hydroperoxide: electron paramagnetic resonance spin trapping evidence for a radical scavenging mechanism. Journal of Neurochemistry. 2000;75(1):141–150. doi: 10.1046/j.1471-4159.2000.0750141.x. [DOI] [PubMed] [Google Scholar]
- 112.Sinha K, Chaudhary G, Kumar Gupta Y. Protective effect of resveratrol against oxidative stress in middle cerebral artery occlusion model of stroke in rats. Life Sciences. 2002;71(6):655–665. doi: 10.1016/s0024-3205(02)01691-0. [DOI] [PubMed] [Google Scholar]
- 113.Espín JC, García-Conesa MT, Tomás-Barberán FA. Nutraceuticals: facts and fiction. Phytochemistry. 2007;68(22–24):2986–3008. doi: 10.1016/j.phytochem.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 114.Rechner AR, Kuhnle G, Bremner P, Hubbard GP, Moore KP, Rice-Evans CA. The metabolic fate of dietary polyphenols in humans. Free Radical Biology and Medicine. 2002;33(2):220–235. doi: 10.1016/s0891-5849(02)00877-8. [DOI] [PubMed] [Google Scholar]
- 115.Ebrahimi A, Schluesener H. Natural polyphenols against neurodegenerative disorders: potentials and pitfalls. Ageing Research Reviews. 2012;11(2):329–345. doi: 10.1016/j.arr.2012.01.006. [DOI] [PubMed] [Google Scholar]
- 116.Del Rio D, Costa LG, Lean MEJ, Crozier A. Polyphenols and health: what compounds are involved? Nutrition, Metabolism and Cardiovascular Diseases. 2010;20(1):1–6. doi: 10.1016/j.numecd.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 117.Manach C, Scalbert A, Morand C, Rémésy C, Jiménez L. Polyphenols: food sources and bioavailability. The American Journal of Clinical Nutrition. 2004;79(5):727–747. doi: 10.1093/ajcn/79.5.727. [DOI] [PubMed] [Google Scholar]
- 118.Scalbert A, Williamson G. Dietary intake and bioavailability of polyphenols. Journal of Nutrition. 2000;130(8):2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 119.Manach C, Williamson G, Morand C, Scalbert A, Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. The American Journal of Clinical Nutrition. 2005;81(1, supplement):230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 120.Warden BA, Smith LS, Beecher GR, Balentine DA, Clevidence BA. Catechins are bioavailable in men and women drinking black tea throughout the day. Journal of Nutrition. 2001;131(6):1731–1737. doi: 10.1093/jn/131.6.1731. [DOI] [PubMed] [Google Scholar]
- 121.Li C, Lee MJ, Sheng S, et al. Structural identification of two metabolites of catechins and their kinetics in human urine and blood after tea ingestion. Chemical Research in Toxicology. 2000;13(3):177–184. doi: 10.1021/tx9901837. [DOI] [PubMed] [Google Scholar]
- 122.Déprez S, Brezillon C, Rabot S, et al. Polymeric proanthocyanidins are catabolized by human colonic microflora into low-molecular-weight phenolic acids. Journal of Nutrition. 2000;130(11):2733–2738. doi: 10.1093/jn/130.11.2733. [DOI] [PubMed] [Google Scholar]
- 123.Tsang C, Auger C, Mullen W, et al. The absorption, metabolism and excretion of flavan-3-ols and procyanidins following the ingestion of a grape seed extract by rats. British Journal of Nutrition. 2005;94(2):170–181. doi: 10.1079/bjn20051480. [DOI] [PubMed] [Google Scholar]
- 124.Hollman PC, Katan MB. Bioavailability and health effects of dietary flavonols in man. Archives of Toxicology. 1998;20:237–248. doi: 10.1007/978-3-642-46856-8_21. [DOI] [PubMed] [Google Scholar]
- 125.Gross M, Pfeiffer M, Martini M, Campbell D, Slavin J, Potter J. The quantitation of metabolites of quercetin flavonols in human urine. Cancer Epidemiology Biomarkers and Prevention. 1996;5(9):711–720. [PubMed] [Google Scholar]
- 126.Lapidot T, Harel S, Granit R, Kanner J. Bioavailability of red wine anthocyanins as detected in human urine. Journal of Agricultural and Food Chemistry. 1998;46(10):4297–4302. [Google Scholar]
- 127.Murkovic M, Toplak H, Adam U, Pfannhauser W. Analysis of anthocyanins in plasma for determination of their bioavailability. Journal of Food Composition and Analysis. 2000;13(4):291–296. [Google Scholar]
- 128.Cerdá B, Tomás-Barberán FA, Espín JC. Metabolism of antioxidant and chemopreventive ellagitannins from strawberries, raspberries, walnuts, and oak-aged wine in humans: identification of biomarkers and individual variability. Journal of Agricultural and Food Chemistry. 2005;53(2):227–235. doi: 10.1021/jf049144d. [DOI] [PubMed] [Google Scholar]
- 129.Cerdá B, Periago P, Espín JC, Tomás-Barberán FA. Identification of urolithin A as a metabolite produced by human colon microflora from ellagic acid and related compounds. Journal of Agricultural and Food Chemistry. 2005;53(14):5571–5576. doi: 10.1021/jf050384i. [DOI] [PubMed] [Google Scholar]
- 130.Miyake Y, Shimoi K, Kumazawa S, Yamamoto K, Kinae N, Osawa T. Identification and antioxidant activity of flavonoid metabolites in plasma and urine of Eriocitrin-treated rats. Journal of Agricultural and Food Chemistry. 2000;48(8):3217–3224. doi: 10.1021/jf990994g. [DOI] [PubMed] [Google Scholar]
- 131.Shirai M, Moon JH, Tsushida T, Terao J. Inhibitory effect of a quercetin metabolite, quercetin 3-O-β-D-glucuronide, on lipid peroxidation in liposomal membranes. Journal of Agricultural and Food Chemistry. 2001;49(11):5602–5608. doi: 10.1021/jf010713g. [DOI] [PubMed] [Google Scholar]
- 132.Terao J, Yamaguchi S, Shirai M, et al. Protection by Quercetin and Quercetin 3-O-β-D-glucuronide of peroxynitrite-induced antioxidant consumption in human plasma low-density lipoprotein. Free Radical Research. 2001;35(6):925–931. doi: 10.1080/10715760100301421. [DOI] [PubMed] [Google Scholar]
- 133.Dyrks T, Dyrks E, Masters CL, Beyreuther K. Amyloidogenicity of rodent and human βA4 sequences. FEBS Letters. 1993;324(2):231–236. doi: 10.1016/0014-5793(93)81399-k. [DOI] [PubMed] [Google Scholar]
- 134.Dreher D, Junod AF. Role of oxygen free radicals in cancer development. European Journal of Cancer. 1996;32(1):30–38. doi: 10.1016/0959-8049(95)00531-5. [DOI] [PubMed] [Google Scholar]
- 135.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2006;185(2):219–226. doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 136.Jay D, Hitomi H, Griendling KK. Oxidative stress and diabetic cardiovascular complications. Free Radical Biology and Medicine. 2006;40(2):183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 137.Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidatives stress. Nature Reviews Drug Discovery. 2004;3(3):205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- 138.Tedesco I, Russo M, Russo P, et al. Antioxidant effect of red wine polyphenols on red blood cells. Journal of Nutritional Biochemistry. 2000;11(2):114–119. doi: 10.1016/s0955-2863(99)00080-7. [DOI] [PubMed] [Google Scholar]
- 139.Lotito SB, Frei B. Relevance of apple polyphenols as antioxidants in human plasma: contrasting in vitro and in vivo effects. Free Radical Biology and Medicine. 2004;36(2):201–211. doi: 10.1016/j.freeradbiomed.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 140.Frankel EN, Waterhouse AL, Kinsella JE. Inhibition of human LDL oxidation by resveratrol. The Lancet. 1993;341(8852):1103–1104. doi: 10.1016/0140-6736(93)92472-6. [DOI] [PubMed] [Google Scholar]
- 141.Jang JH, Surh YJ. Protective effect of resveratrol on β-amyloid-induced oxidative PC12 cell death. Free Radical Biology and Medicine. 2003;34(8):1100–1110. doi: 10.1016/s0891-5849(03)00062-5. [DOI] [PubMed] [Google Scholar]
- 142.Miyagi Y, Miwa K, Inoue H. Inhibition of human low-density lipoprotein oxidation by flavonoids in red wine and grape juice. American Journal of Cardiology. 1997;80(12):1627–1631. doi: 10.1016/s0002-9149(97)00755-8. [DOI] [PubMed] [Google Scholar]
- 143.Chan SL, Tabellion A, Bagrel D, Perrin-Sarrado C, Capdeville-Atkinson C, Atkinson J. Impact of chronic treatment with red wine polyphenols (RWP) on cerebral arterioles in the spontaneous hypertensive rat. Journal of Cardiovascular Pharmacology. 2008;51(3):304–310. doi: 10.1097/FJC.0b013e318163a946. [DOI] [PubMed] [Google Scholar]
- 144.Fenech M, Stockley C, Aitken C. Moderate wine consumption protects against hydrogen peroxide-induced DNA damage. Mutagenesis. 1997;12(4):289–296. doi: 10.1093/mutage/12.4.289. [DOI] [PubMed] [Google Scholar]
- 145.Giovannelli L, Testa G, De Filippo C, Cheynier V, Clifford MN, Dolara P. Effect of complex polyphenols and tannins from red wine on DNA oxidative damage of rat colon mucosa in vivo. European Journal of Nutrition. 2000;39(5):207–212. doi: 10.1007/s003940070013. [DOI] [PubMed] [Google Scholar]
- 146.Kumar A, Naidu PS, Seghal N, Padi SSV. Neuroprotective effects of resveratrol against intracerebroventricular colchicine-induced cognitive impairment and oxidative stress in rats. Pharmacology. 2007;79(1):17–26. doi: 10.1159/000097511. [DOI] [PubMed] [Google Scholar]
- 147.Sönmez Ü, Sönmez A, Erbil G, Tekmen I, Baykara B. Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neuroscience Letters. 2007;420(2):133–137. doi: 10.1016/j.neulet.2007.04.070. [DOI] [PubMed] [Google Scholar]
- 148.Zhang H, Schools GP, Lei T, Wang W, Kimelberg HK, Zhou M. Resveratrol attenuates early pyramidal neuron excitability impairment and death in acute rat hippocampal slices caused by oxygen-glucose deprivation. Experimental Neurology. 2008;212(1):44–52. doi: 10.1016/j.expneurol.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ates O, Cayli S, Altinoz E, et al. Neuroprotection by resveratrol against traumatic brain injury in rats. Molecular and Cellular Biochemistry. 2007;294(1-2):137–144. doi: 10.1007/s11010-006-9253-0. [DOI] [PubMed] [Google Scholar]
- 150.Tsai SK, Hung LM, Fu YT, et al. Resveratrol neuroprotective effects during focal cerebral ischemia injury via nitric oxide mechanism in rats. Journal of Vascular Surgery. 2007;46(2):346–353. doi: 10.1016/j.jvs.2007.04.044. [DOI] [PubMed] [Google Scholar]
- 151.Sun AY, Chen YM. Oxidative stress and neurodegenerative disorders. Journal of Biomedical Science. 1998;5(6):401–414. doi: 10.1007/BF02255928. [DOI] [PubMed] [Google Scholar]
- 152.Greenlund LJS, Deckwerth TL, Johnson EM. Superoxide dismutase delays neuronal apoptosis: a role for reactive oxygen species in programmed neuronal death. Neuron. 1995;14(2):303–315. doi: 10.1016/0896-6273(95)90287-2. [DOI] [PubMed] [Google Scholar]
- 153.Mattson MP, Mark RJ, Furukawa K, Bruce AJ. Distruption of brain cell ion homeostasis in Alzheimer’s disease by oxy radicals, and signaling pathways that protect therefrom. Chemical Research in Toxicology. 1997;10(5):507–517. doi: 10.1021/tx9601317. [DOI] [PubMed] [Google Scholar]
- 154.Bogdanov MB, Andreassen OA, Dedeoglu A, Ferrante RJ, Beal MF. Increased oxidative damage to DNA in a transgenic mouse model of Huntington’s disease. Journal of Neurochemistry. 2001;79(6):1246–1249. doi: 10.1046/j.1471-4159.2001.00689.x. [DOI] [PubMed] [Google Scholar]
- 155.Mokni M, Elkahoui S, Limam F, Amri M, Aouani E. Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochemical Research. 2007;32(6):981–987. doi: 10.1007/s11064-006-9255-z. [DOI] [PubMed] [Google Scholar]
- 156.Wu Z, Chen LJ, Long YJ. Analysis of ultrastructure and reactive oxygen species of hyperhydric garlic (Allium sativum L.) shoots. In Vitro Cellular and Developmental Biology—Plant. 2009;45(4):483–490. [Google Scholar]
- 157.Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84(2-3):131–141. doi: 10.1016/s0300-9084(02)01369-x. [DOI] [PubMed] [Google Scholar]
- 158.Aliev G, Obrenovich ME, Reddy VP, et al. Antioxidant therapy in Alzheimer’s disease: theory and practice. Mini Reviews in Medicinal Chemistry. 2008;8(13):1395–1406. doi: 10.2174/138955708786369582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer β-amyloid precursor protein depends on lipid rafts. Journal of Cell Biology. 2003;160(1):113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tamagno E, Guglielmotto M, Aragno M, et al. Oxidative stress activates a positive feedback between the γ- and β-secretase cleavages of the β-amyloid precursor protein. Journal of Neurochemistry. 2008;104(3):683–695. doi: 10.1111/j.1471-4159.2007.05072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Ghiselli A, Nardini M, Baldi A, Scaccini C. Antioxidant activity of different phenolic fractions separated from an Italian red wine. Journal of Agricultural and Food Chemistry. 1998;46(2):361–367. doi: 10.1021/jf970486b. [DOI] [PubMed] [Google Scholar]
- 162.Kasdallah-Grissa A, Mornagui B, Aouani E, et al. Resveratrol, a red wine polyphenol, attenuates ethanol-induced oxidative stress in rat liver. Life Sciences. 2007;80(11):1033–1039. doi: 10.1016/j.lfs.2006.11.044. [DOI] [PubMed] [Google Scholar]
- 163.Pace-Asciak CR, Hahn S, Diamandis EP, Soleas G, Goldberg DM. The red wine phenolics trans-resveratrol and quercetin block human platelet aggregation and eicosanoid synthesis: implications for protection against coronary heart disease. Clinica Chimica Acta. 1995;235(2):207–219. doi: 10.1016/0009-8981(95)06045-1. [DOI] [PubMed] [Google Scholar]
- 164.Bastianetto S, Zheng WH, Quirion R. Neuroprotective abilities of resveratrol and other red wine constituents against nitric oxide-related toxicity in cultured hippocampal neurons. British Journal of Pharmacology. 2000;131(4):711–720. doi: 10.1038/sj.bjp.0703626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Leonard SS, Xia C, Jiang BH, et al. Resveratrol scavenges reactive oxygen species and effects radical-induced cellular responses. Biochemical and Biophysical Research Communications. 2003;309(4):1017–1026. doi: 10.1016/j.bbrc.2003.08.105. [DOI] [PubMed] [Google Scholar]
- 166.Bagchi D, Bagchi M, Stohs SJ, et al. Free radicals and grape seed proanthocyanidin extract: importance in human health and disease prevention. Toxicology. 2000;148(2-3):187–197. doi: 10.1016/s0300-483x(00)00210-9. [DOI] [PubMed] [Google Scholar]
- 167.Hofseth LJ, Wargovich MJ. Inflammation, cancer, and targets of ginseng. Journal of Nutrition. 2007;137(1):183S–185S. doi: 10.1093/jn/137.1.183S. [DOI] [PubMed] [Google Scholar]
- 168.Ceriello A, Bortolotti N, Motz E, et al. Red wine protects diabetic patients from meal-induced oxidative stress and thrombosis activation: a pleasant approach to the prevention of cardiovascular disease in diabetes. European Journal of Clinical Investigation. 2001;31(4):322–328. doi: 10.1046/j.1365-2362.2001.00818.x. [DOI] [PubMed] [Google Scholar]
- 169.Rodrigo R, Rivera G, Orellana M, Araya J, Bosco C. Rat kidney antioxidant response to long-term exposure to flavonol rich red wine. Life Sciences. 2002;71(24):2881–2895. doi: 10.1016/s0024-3205(02)02140-9. [DOI] [PubMed] [Google Scholar]
- 170.Donnelly LE, Newton R, Kennedy GE, et al. Anti-inflammatory effects of resveratrol in lung epithelial cells: molecular mechanisms. American Journal of Physiology. 2004;287(4):L774–L783. doi: 10.1152/ajplung.00110.2004. [DOI] [PubMed] [Google Scholar]
- 171.Martín AR, Villegas I, Sánchez-Hidalgo M, De La Lastra CA. The effects of resveratrol, a phytoalexin derived from red wines, on chronic inflammation induced in an experimentally induced colitis model. British Journal of Pharmacology. 2006;147(8):873–885. doi: 10.1038/sj.bjp.0706469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.De Lorgeril M, Salen P, Guiraud A, Boucher F, De Leiris J. Resveratrol and non-ethanolic components of wine in experimental cardiology. Nutrition, Metabolism and Cardiovascular Diseases. 2003;13(2):100–103. doi: 10.1016/s0939-4753(03)80025-x. [DOI] [PubMed] [Google Scholar]
- 173.Scalbert A, Manach C, Morand C, Rémésy C, Jiménez L. Dietary polyphenols and the prevention of diseases. Critical Reviews in Food Science and Nutrition. 2005;45(4):287–306. doi: 10.1080/1040869059096. [DOI] [PubMed] [Google Scholar]
- 174.Stocker R, O’Halloran RA. Dealcoholized red wine decreases atherosclerosis in apolipoprotein E gene-deficient mice independently of inhibition of lipid peroxidation in the artery wall. The American Journal of Clinical Nutrition. 2004;79(1):123–130. doi: 10.1093/ajcn/79.1.123. [DOI] [PubMed] [Google Scholar]
- 175.Gorelik S, Ligumsky M, Kohen R, Kanner J. A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. The FASEB Journal. 2008;22(1):41–46. doi: 10.1096/fj.07-9041com. [DOI] [PubMed] [Google Scholar]
- 176.Schini-Kerth VB, Auger C, Kim JH, Etienne-Selloum N, Chataigneau T. Nutritional improvement of the endothelial control of vascular tone by polyphenols: role of NO and EDHF. Pflügers Archiv. 2010;459(6):853–862. doi: 10.1007/s00424-010-0806-4. [DOI] [PubMed] [Google Scholar]
- 177.Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proceedings of the National Academy of Sciences of the United States of America. 1987;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Oak MH, Chataigneau M, Keravis T, et al. Red wine polyphenolic compounds inhibit vascular endothelial growth factor expression in vascular smooth muscle cells by preventing the activation of the p38 mitogen-activated protein kinase pathway. Arteriosclerosis, Thrombosis, and Vascular Biology. 2003;23(6):1001–1007. doi: 10.1161/01.ATV.0000070101.70534.38. [DOI] [PubMed] [Google Scholar]
- 179.Mann GE, Rowlands DJ, Li FYL, de Winter P, Siow RCM. Activation of endothelial nitric oxide synthase by dietary isoflavones: role of NO in Nrf2-mediated antioxidant gene expression. Cardiovascular Research. 2007;75(2):261–274. doi: 10.1016/j.cardiores.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 180.Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization of canine coronary smooth muscle. British Journal of Pharmacology. 1988;93(3):515–524. doi: 10.1111/j.1476-5381.1988.tb10306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bernátová I, Pechánová O, Babál P, Kyselá S, Stvrtina S, Andriantsitohaina R. Wine polyphenols improve cardiovascular remodeling and vascular function in NO-deficient hypertension. American Journal of Physiology. 2002;282(3):H942–H948. doi: 10.1152/ajpheart.00724.2001. [DOI] [PubMed] [Google Scholar]
- 182.Wallerath T, Poleo D, Li H, Förstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: a mechanism that may contribute to its beneficial cardiovascular effects. Journal of the American College of Cardiology. 2003;41(3):471–478. doi: 10.1016/s0735-1097(02)02826-7. [DOI] [PubMed] [Google Scholar]
- 183.Diebolt M, Bucher B, Andriantsitohaina R. Wine polyphenols decrease blood pressure, improve NO vasodilatation, and induce gene expression. Hypertension. 2001;38(2):159–165. doi: 10.1161/01.hyp.38.2.159. [DOI] [PubMed] [Google Scholar]
- 184.Kiziltepe U, Turan NND, Han U, Ulus AT, Akar F. Resveratrol, a red wine polyphenol, protects spinal cord from ischemia-reperfusion injury. Journal of Vascular Surgery. 2004;40(1):138–145. doi: 10.1016/j.jvs.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 185.Luceri C, Caderni G, Sanna A, Dolara P. Red wine and black tea polyphenols modulate the expression of cycloxygenase-2, inducible nitric oxide synthase and glutathione-related enzymes in azoxymethane-induced F344 rat colon tumors. Journal of Nutrition. 2002;132(6):1376–1379. doi: 10.1093/jn/132.6.1376. [DOI] [PubMed] [Google Scholar]
- 186.Hung LM, Su MJ, Chen JK. Resveratrol protects myocardial ischemia-reperfusion injury through both NO-dependent and NO-independent mechanisms. Free Radical Biology and Medicine. 2004;36(6):774–781. doi: 10.1016/j.freeradbiomed.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 187.Baur JA, Pearson KJ, Price NL, et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature. 2006;444(7117):337–342. doi: 10.1038/nature05354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Han YS, Bastianetto S, Dumont Y, Quirion R. Specific plasma membrane binding sites for polyphenols, including resveratrol, in the rat brain. Journal of Pharmacology and Experimental Therapeutics. 2006;318(1):238–245. doi: 10.1124/jpet.106.102319. [DOI] [PubMed] [Google Scholar]
- 189.Bastianetto S, Brouillette J, Quirion R. Neuroprotective effects of natural products: interaction with intracellular kinases, amyloid peptides and a possible role for transthyretin. Neurochemical Research. 2007;32(10):1720–1725. doi: 10.1007/s11064-007-9333-x. [DOI] [PubMed] [Google Scholar]
- 190.Gresele P, Pignatelli P, Guglielmini G, et al. Resveratrol, at concentrations attainable with moderate wine consumption, stimulates human platelet nitric oxide production. Journal of Nutrition. 2008;138(9):1602–1608. doi: 10.1093/jn/138.9.1602. [DOI] [PubMed] [Google Scholar]
- 191.Di Castelnuovo A, Rotondo S, Iacoviello L, Donati MB, De Gaetano G. Meta-analysis of wine and beer consumption in relation to vascular risk. Circulation. 2002;105(24):2836–2844. doi: 10.1161/01.cir.0000018653.19696.01. [DOI] [PubMed] [Google Scholar]
- 192.Baron-Menguy C, Bocquet A, Guihot AL, et al. Effects of red wine polyphenols on postischemic neovascularization model in rats: low doses are proangiogenic, high doses anti-angiogenic. The FASEB Journal. 2007;21(13):3511–3521. doi: 10.1096/fj.06-7782com. [DOI] [PubMed] [Google Scholar]
- 193.Marambaud P, Zhao H, Davies P. Resveratrol promotes clearance of Alzheimer’s disease amyloid-β peptides. The Journal of Biological Chemistry. 2005;280(45):37377–37382. doi: 10.1074/jbc.M508246200. [DOI] [PubMed] [Google Scholar]
- 194.Oomen CA, Farkas E, Roman V, Van Der Beek EM, Luiten, P. Meerlo PG, Meerlo P. Resveratrol preserves cerebrovascular density and cognitive function in aging mice. Frontiers in Aging Neuroscience. 2009;1, article 4 doi: 10.3389/neuro.24.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Kennedy DO, Wightman EL, Reay JL, et al. Effects of resveratrol on cerebral blood flow variables and cognitive performance in humans: a double-blind, placebo-controlled, crossover investigation. The American Journal of Clinical Nutrition. 2010;91(6):1590–1597. doi: 10.3945/ajcn.2009.28641. [DOI] [PubMed] [Google Scholar]
- 196.Rivière C, Richard T, Vitrac X, Mérillon JM, Valls J, Monti JP. New polyphenols active on β-amyloid aggregation. Bioorganic and Medicinal Chemistry Letters. 2008;18(2):828–831. doi: 10.1016/j.bmcl.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 197.Richard T, Poupard P, Nassra M, et al. Protective effect of ε-viniferin on β-amyloid peptide aggregation investigated by electrospray ionization mass spectrometry. Bioorganic and Medicinal Chemistry. 2011;19(10):3152–3155. doi: 10.1016/j.bmc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 198.Ono K, Li L, Takamura Y, et al. Phenolic compounds prevent amyloid β-protein oligomerization and synaptic dysfunction by site-specific binding. The Journal of Biological Chemistry. 2012;278:14631–14643. doi: 10.1074/jbc.M111.325456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Spencer JPE, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Molecular Aspects of Medicine. 2012;33(1):83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- 200.Singh M, Arseneault M, Sanderson T, Murthy V, Ramassamy C. Challenges for research on polyphenols from foods in Alzheimer’s disease: bioavailability, metabolism, and cellular and molecular mechanisms. Journal of Agricultural and Food Chemistry. 2008;56(13):4855–4873. doi: 10.1021/jf0735073. [DOI] [PubMed] [Google Scholar]


